Flavor Chemistry of Virgin Olive Oil: An Overview
Abstract
1. Introduction
2. VOO Flavor Perception
2.1. Phenolic Compounds
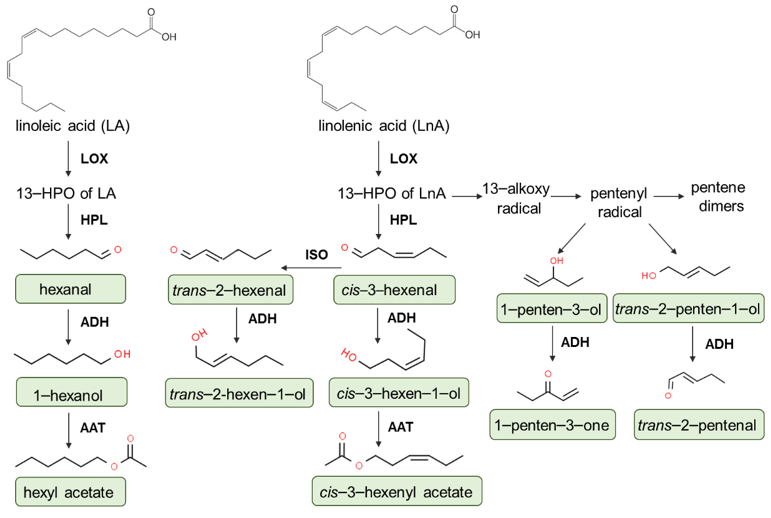
2.2. Volatile Compounds
2.3. Off-Flavors
2.4. Triglycerides and Fatty Acids
3. Factors Affecting the Flavor of VOO
3.1. Botanical, Agronomical and Technological Process
3.1.1. Variety and Ripening Degree
3.1.2. Environmental and Climatic Conditions
3.1.3. Harvest and Olive Crushing System
3.1.4. Malaxation
3.1.5. Oil Separation Process
3.2. Physico-Chemical Properties of Volatile Compounds
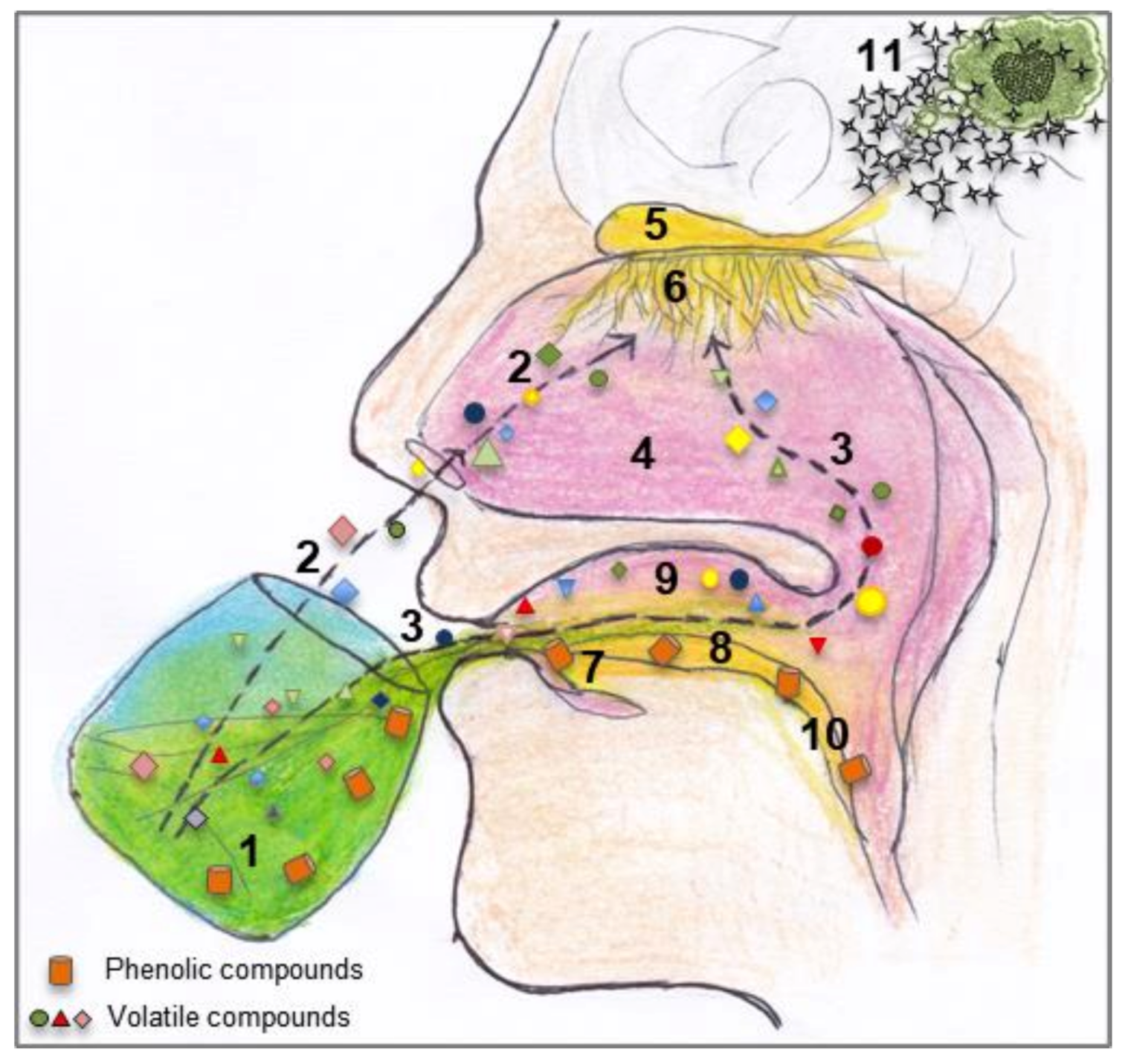
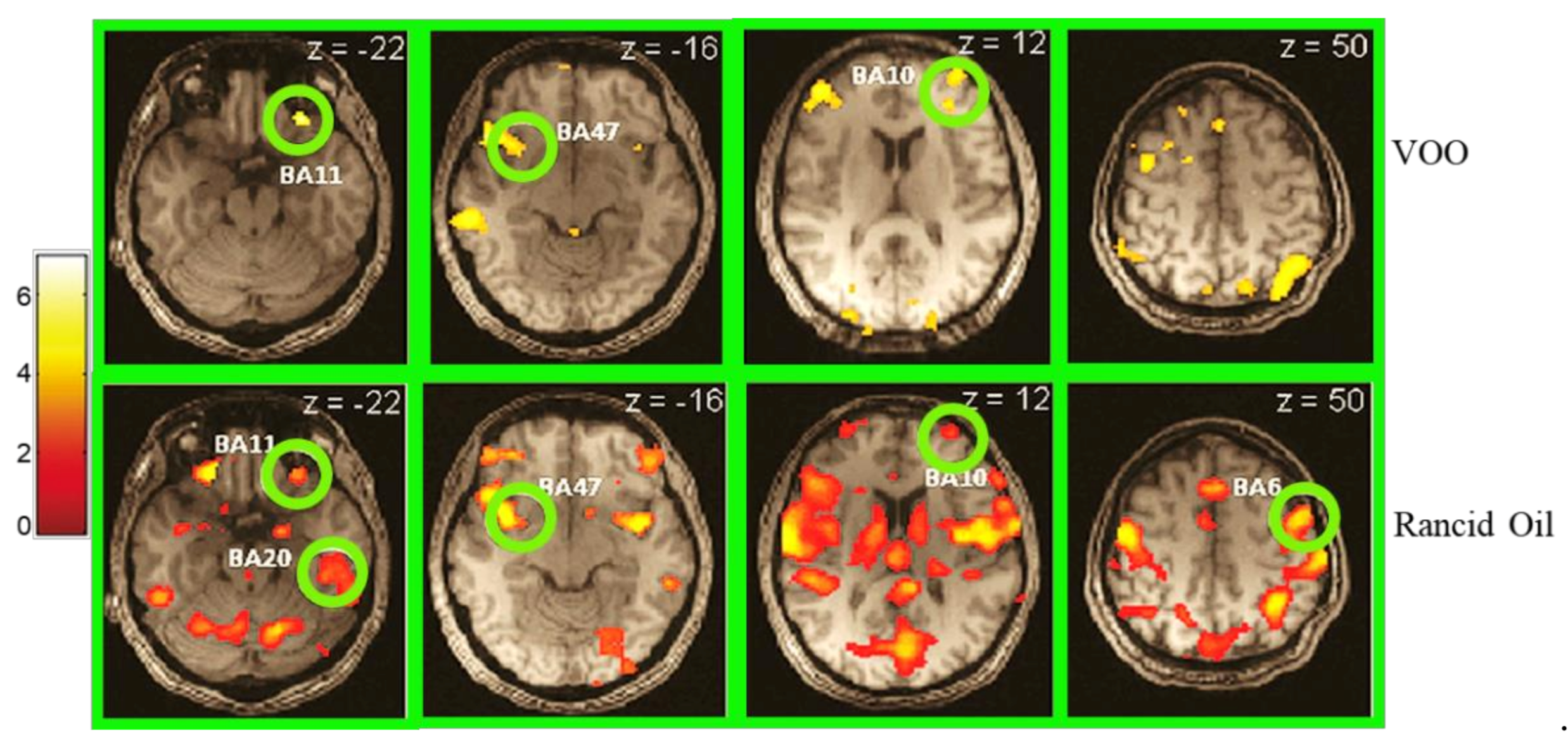
3.3. Human Physiological Factors Affecting Flavour Perception
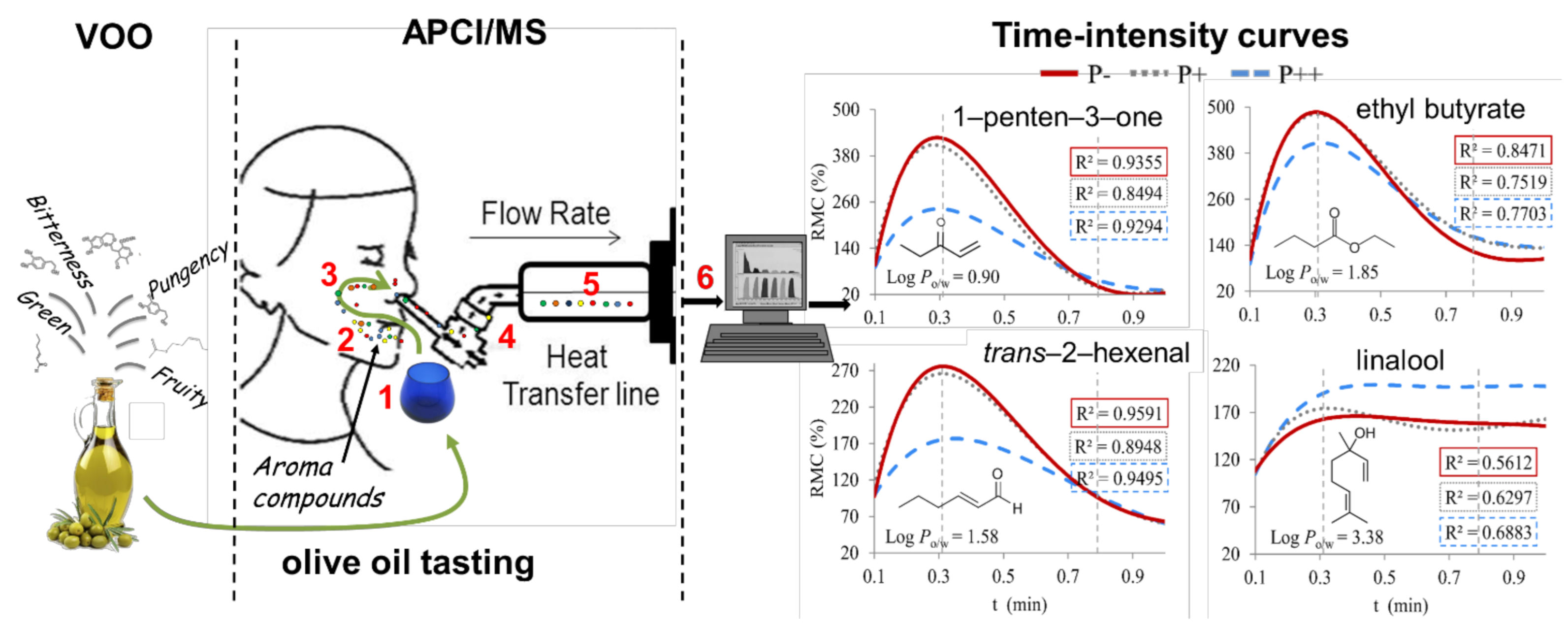
3.4. Interaction Between Food Matrix and VOO Volatile Compounds
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.L.; Sumpio, B.E. Olive Oil, the Mediterranean Diet, and Cardiovascular Health. J. Am. Coll. Surg. 2008, 207, 407–416. [Google Scholar] [CrossRef]
- Hoffman, R.; Gerber, M. The Mediterranean Diet: Health and Science; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- De Leonardis, A.; Macciola, V.; Lopez, F. The role of virgin olive oil in the traditional Mediterranean cuisine. In Virgin Olive Oil; De Leonardis, A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 259–281. [Google Scholar]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour devel-opment and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Boskou, D. Olive Oil: Minor Constituents and Health; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- The European Commission. Commission Regulation (EEC) No. 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive Residue Oil and on the Relevant Methods of Analysis; The European Commission: Brussels, Belgium, 1991; pp. 1–83. [Google Scholar]
- Stark, A.H.; Madar, Z. Olive oil as a functional food: Epidemiology and nutritional approaches. Nutr. Rev. 2002, 60, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Gallina-Toschi, T.; Fernández-Gutiérrez, A. Analytical determination of polyphenols in olive oils. J. Sep. Sci. 2005, 28, 837–858. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory properties of virgin olive oil polyphenols: Identification of deacetoxy-ligstroside aglycon as a key contributor to pungency. J. Agr. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- Fogliano, V.; Ritieni, A.; Monti, S.M.; Gallo, M.; Della Medaglia, D.; Ambrosino, M.L.; Sacchi, R. Antioxidant activity of virgin olive oil phenolic compounds in a micellar system. J. Sci. Food Agric. 1999, 79, 1803–1808. [Google Scholar] [CrossRef]
- Vitaglione, P.; Savarese, M.; Paduano, A.; Scalfi, L.; Fogliano, V.; Sacchi, R. Healthy Virgin Olive Oil: A Matter of Bitterness. Crit. Rev. Food Sci. Nutr. 2015, 55, 1808–1818. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. Biological Properties of Olive Oil Phytochemicals. Crit. Rev. Food Sci. Nutr. 2002, 42, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Lamuela-Raventos, R.M. Primary prevention of cardio-vascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Lamuela-Raventos, R.M. Primary prevention of cardio-vascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, R.; Luna, G. Characterisation of monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Biasutti, M.A. Comparative Analysis of Forms and Wikis as Tools for Online Collaborative Learning. Comput. Educ. 2017, 107, 158–171. [Google Scholar] [CrossRef]
- Caporaso, N. Virgin Olive Oils: Environmental Conditions, Agronomical Factors and Processing Technology Affecting the Chemistry of Flavor Profile. J. Food Chem. Nanotechnol. 2016, 2, 21–31. [Google Scholar] [CrossRef]
- De Santis, D.; Frangipane, M.T. Sensory Perceptions of Virgin Olive Oil: New Panel Evaluation Method and the Chemical Compounds Responsible. Nat. Sci. 2015, 7, 132–142. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2020, 105, 483–493. [Google Scholar] [CrossRef]
- Burdach, K.J.; Kroeze, J.H.A.; Köster, E.P. Nasal, retronasal, and gustatory perception: An experimental comparison. Percept. Psychophys. 1984, 36, 205–208. [Google Scholar] [CrossRef]
- Voirol, E.; Daget, N. Comparative study of nasal and retronasal olfactory perception. Lebensm. Wiss. Technol. 1986, 19, 316–319. [Google Scholar]
- Voirol, E.; Daget, N. Direct nasal and oronasal profiling of a meat flavouring: Influence of temperature, concentration and additives. Lebensm. Wiss. Technol. 1989, 22, 399–405. [Google Scholar]
- Kuo, Y.; Pangborn, R.M.; Noble, A.C. Temporal patterns of nasal, oral, and retronasal perception of citral and vanillin and interaction of these odourants with selected tastants. Int. J. Food Sci. Technol. 2007, 28, 127–137. [Google Scholar] [CrossRef]
- Linforth, R.; Martin, F.; Carey, M.; Davidson, J.; Taylor, A.J. Retronasal Transport of Aroma Compounds. J. Agric. Food Chem. 2002, 50, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Tovar, M.J.; Motilva, M.J.; Romero, M.P. Changes in the Phenolic Composition of Virgin Olive Oil from Young Trees (Olea europaea L. cv. Arbequina) Grown under Linear Irrigation Strategies. J. Agric. Food Chem. 2001, 49, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Sacchi, R. High phenolic content in extra virgin olive oil influences the release (time-intensity) of aroma compounds in mouth. Atlas of Science. Available online: https://atlasofscience.org/high-phenolic-content-in-extra-virgin-olive-oil-influences-the-release-time-intensity-of-aroma-compounds-in-mouth/ (accessed on 28 December 2020).
- Guichard, E.; Salles, C.; Morzel, M.; Le Bon, A.M. Flavour: From Food to Perception; John Wiley & Sons: Chichester, UK, 2016. [Google Scholar]
- García-González, D.L.; Vivancos, J.; Aparicio, R. Mapping Brain Activity Induced by Olfaction of Virgin Olive Oil Aroma. J. Agric. Food Chem. 2011, 59, 10200–10210. [Google Scholar] [CrossRef]
- Marciani, L.; Eldeghaidy, S.; Spiller, R.C.; Gowland, P.A.; Francis, S.T. Brain Imaging. In Food Flavour Technology; Wiley: John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 319–350. [Google Scholar]
- Frank, S.; Linder, K.; Fritsche, L.; Hege, M.A.; Kullmann, S.; Krzeminski, A.; Fritsche, A.; Schieberle, P.; Somoza, V.; Hinrichs, J.; et al. Olive oil aroma extract modulates cerebral blood flow in gustatory brain areas in humans. Am. J. Clin. Nutr. 2013, 98, 1360–1366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kako, H.; Fukumoto, S.; Kobayashi, Y.; Yokogoshi, H. Effects of direct exposure of green odour components on dopamine release from rat brain striatal slices and PC12 cells. Brain Res. Bull. 2008, 75, 706–712. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kako, H.; Yokogoshi, H. Contribution of Intracellular Ca2+ Concentration and Protein Dephosphorylation to the Induction of Dopamine Release from PC12 cells by the Green Odor Compound Hexanal. Cell. Mol. Neurobiol. 2009, 30, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.F.; Kiesewetter, H.; Nascetti, S. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Covas, M.-I.; Ruiz-Gutiérrez, V.; De La Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor Components of Olive Oil: Evidence to Date of Health Benefits in Humans. Nutr. Rev. 2006, 64, 20–30. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Fitó, M.; De La Torre, R.; Covas, M.-I. Olive oil and oxidative stress. Mol. Nutr. Food Res. 2007, 51, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Corona, G.; Spencer, J.; Dessì, M. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Heal. 2009, 25, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013, 57, 71–83. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Servili, M. The Basis of the Sensory Properties of Virgin Olive Oil. In Olive Oil Sensory Science; Wiley: John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 33–54. [Google Scholar]
- Parkinson, L.; Cicerale, S. The Health Benefiting Mechanisms of Virgin Olive Oil Phenolic Compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Farràs, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef]
- Melguizo Rodriguez, L.; Illescas-Montes, R.; Costela-Ruiz, V.J.; García-Martínez, O. Stimulation of brown adipose tissue by poly-phenols in extra virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 1–8. [Google Scholar] [CrossRef]
- Beltrán, G.; Ruano, M.T.; Jiménez, A.; Uceda, M.; Aguilera, M.P. Evaluation of virgin olive oil bitterness by total phenol content analysis. Eur. J. Lipid Sci. Technol. 2007, 109, 193–197. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T.; Pasqualone, A. Phenolic compounds in virgin olive oils: Influence of the degree of olive ripeness on organ-oleptic characteristics and shelf-life. Eur. Food Res. Technol. 2001, 212, 329–333. [Google Scholar] [CrossRef]
- Gachons, C.P.D.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B.; Beauchamp, G.K.; Breslin, P.A.S. Unusual Pungency from Extra-Virgin Olive Oil Is Attributable to Restricted Spatial Expression of the Receptor of Oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, R.; Morales, M.T.; García-González, D.L. Towards new analyses of aroma and volatiles to understand sensory perception of olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1114–1125. [Google Scholar] [CrossRef]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef]
- Olias, J.M.; Perez, A.G.; Rios, J.J.; Sanz, L.C. Aroma of virgin olive oil: Biogenesis of the” green” odor notes. J. Agric. Food Chem. 1993, 41, 2368–2373. [Google Scholar] [CrossRef]
- Sacchi, R.; Parisini, C.; Paduano, A.; Della Medaglia, D.; Savarese, M.; Ambrosino, M.L. Relationship between sensory profile and volatile compounds: Identification of sensory tipicality in PDO Italian olive oils. In Proceedings of the 11th European Symposium on Statistical Methods for the Food Industry, Benevento, Italy, 23–26 February 2010; pp. 65–72. [Google Scholar]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin olive oil odour notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Caporale, G.; Policastro, S.; Monteleone, E. Bitterness enhancement induced by cut grass odorant (cis-3-hexen-1-ol) in a model olive oil. Food Qual. Preference 2004, 15, 219–227. [Google Scholar] [CrossRef]
- Inarejos-García, A.; Santacatterina, M.; Salvador, M.D.; Fregapane, G.; Gómez-Alonso, S. PDO virgin olive oil quality—Minor components and organoleptic evaluation. Food Res. Int. 2010, 43, 2138–2146. [Google Scholar] [CrossRef]
- Morales, M.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, S.C.; Shoemaker, C.F. Volatile constituents in sensory defective virgin olive oils. Flavour Fragr. J. 2015, 31, 22–30. [Google Scholar] [CrossRef]
- Genovese, A.; Mondola, F.; Paduano, A.; Sacchi, R. Biophenolic Compounds Influence the In-Mouth Perceived Intensity of Virgin Olive Oil Flavours and Off-Flavours. Molecules 2020, 25, 1969. [Google Scholar] [CrossRef] [PubMed]
- Eldeghaidy, S.; Hollowood, T.; Marciani, L.; Head, K.; Busch, J.; Taylor, A.J.; Hort, J. Does fat alter the cortical response to flavor? Chemosens. Percept. 2012, 5, 215–230. [Google Scholar] [CrossRef]
- Drewnowski, A.; Greenwood, M. Cream and sugar: Human preferences for high-fat foods. Physiol. Behav. 1983, 30, 629–633. [Google Scholar] [CrossRef]
- Duffy, V.B.; Bartoshuk, L.M.; Lucchina, L.A. Supertesters of PROP (6-n-propylthiouracil) rate the highest creaminess to high-fat milk products. Chem. Senses 1996, 21, 598. [Google Scholar] [CrossRef][Green Version]
- Ramirez, I. Chemosensory similarities among oils: Does viscosity play a role? Chem. Senses 1994, 19, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Fat preference and adherence to a reduced-fat diet. Am. J. Clin. Nutr. 1993, 57, 373–381. [Google Scholar] [CrossRef]
- Chalé-Rush, A.; Burgess, J.R.; Mattes, R.D. Evidence for Human Orosensory (Taste?) Sensitivity to Free Fatty Acids. Chem. Senses 2007, 32, 423–431. [Google Scholar] [CrossRef]
- Chalé-Rush, A.; Burgess, J.R.; Mattes, R.D. Multiple routes of chemosensitivity to free fatty acids in humans. Am. J. Physiol. Liver Physiol. 2007, 292, G1206–G1212. [Google Scholar] [CrossRef]
- Stewart, J.E.; Newman, L.P.; Keast, R.S.J. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011, 30, 838–844. [Google Scholar] [CrossRef]
- Eldeghaidy, S.; Marciani, L.; McGlone, F.; Hollowood, T.; Hort, J.; Head, K.; Taylor, A.J.; Busch, J.; Spiller, R.C.; Gowland, P.A.; et al. The cortical response to the oral perception of fat emulsions and the effect of taster status. J. Neurophysiol. 2011, 105, 2572–2581. [Google Scholar] [CrossRef]
- Kawai, T.; Fushiki, T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R447–R454. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Feinle-Bisset, C.; Golding, M.; Delahunty, C.; Clifton, P.M.; Keast, R.S.J. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010, 104, 145–152. [Google Scholar] [CrossRef]
- Gilbertson, T.A.; Fontenot, D.T.; Liu, L.; Zhang, H.; Monroe, W.T. Fatty acid modulation of K+ channels in taste receptor cells: Gustatory cues for dietary fat. Am. J. Physiol. Physiol. 1997, 272, C1203–C1210. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Eguchi, A.; Mizushige, T.; Kitabayashi, N.; Tsuzuki, S.; Inoue, K.; Fushiki, T. Colocalization of GPR120 with phospholipase-Cβ2 and α-gustducin in the taste bud cells in mice. Neurosci. Lett. 2009, 450, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Cartoni, C.; Yasumatsu, K.; Ohkuri, T.; Shigemura, N.; Yoshida, R.; Godinot, N.; Damak, S. Taste preference for fatty acids is me-diated by GPR40 and GPR120. J. Neurosci. 2010, 30, 8376–8382. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Passilly-Degrace, P.; Patris, B.; Niot, I.; Febbraio, M.; Montmayeur, J.-P.; Besnard, P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Investig. 2005, 115, 3177–3184. [Google Scholar] [CrossRef]
- Simons, P.J.; Kummer, J.A.; Luiken, J.J.; Boon, L. Apical CD36 immunolocalization in human and porcine taste buds from circum-vallate and foliate papillae. Acta Histochem. 2011, 113, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Oral Detection of Short-, Medium-, and Long-Chain Free Fatty Acids in Humans. Chem. Senses 2008, 34, 145–150. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Love-Gregory, L.; Klein, S.; Abumrad, N.A. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 2012, 53, 561–566. [Google Scholar] [CrossRef]
- Keller, K.L.; Liang, L.C.; Sakimura, J.; May, D.; Van Belle, C.; Breen, C.; Driggin, E.; Tepper, B.J.; Lanzano, P.C.; Deng, L.; et al. Common Variants in the CD36 Gene Are Associated with Oral Fat Perception, Fat Preferences, and Obesity in African Americans. Obesity 2012, 20, 1066–1073. [Google Scholar] [CrossRef]
- de Roos, K.B. Effect of texture and microstructure on flavour retention and release. Int. Dairy J. 2003, 13, 593–605. [Google Scholar] [CrossRef]
- Nahon, D.F.; Harrison, M.; Roozen, J.P. Modeling Flavor Release from Aqueous Sucrose Solutions, Using Mass Transfer and Partition Coefficients. J. Agric. Food Chem. 2000, 48, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- van Ruth, S.M.; O’Connor, C.H.; Delahunty, C.M. Relationships between temporal release of aroma compounds in a model mouth system and their physico-chemical characteristics. Food Chem. 2000, 71, 393–399. [Google Scholar] [CrossRef]
- Linforth, R.; Taylor, A.J. Persistence of volatile compounds in the breath after their consumption in aqueous solutions. J. Agric. Food Chem. 2000, 48, 5419–5423. [Google Scholar] [CrossRef] [PubMed]
- Buffo, R.A.; Rapp, J.A.; Krick, T.; Reineccius, G.A. Persistence of aroma compounds in human breath after consuming an aqueous model aroma mixture. Food Chem. 2005, 89, 103–108. [Google Scholar] [CrossRef]
- Taylor, A.J.; Roozen, J.P. Volatile flavor release from foods during eating. Crit. Rev. Food Sci. Nutr. 1996, 36, 765–784. [Google Scholar] [CrossRef] [PubMed]
- Van Ruth, S.; Roozen, J.P. Aroma compounds of oxidised sunflower oil and its oil-in-water emulsion: Volatility and release under mouth conditions. Eur. Food Res. Technol. 2000, 210, 258–262. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; De Luca, L.; Paduano, A.; Sacchi, R. Influence of Olive Oil Phenolic Compounds on Headspace Aroma Release by Interaction with Whey Proteins. J. Agric. Food Chem. 2015, 63, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jiménez, M.; Esteban-Fernández, A.; Muñoz-González, C.; Pozo-Bayón, M.A. Interactions among Odorants, Phenolic Compounds, and Oral Components and Their Effects on Wine Aroma Volatility. Molecules 2020, 25, 1701. [Google Scholar] [CrossRef]
- Sacchi, R.; Caporaso, N.; Paduano, A.; Genovese, A. Industrial-scale filtration affects volatile compounds in extra virgin olive oil cv. Ravece. Eur. J. Lipid Sci. Technol. 2015, 117, 2007–2014. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, S.; Shoemaker, C.F.; Wang, S.C. Characterization of Volatile Compounds of Virgin Olive Oil Originating from the USA. J. Am. Oil Chem. Soc. 2014, 92, 77–85. [Google Scholar] [CrossRef]
- Boussahel, S.; Di Stefano, V.; Muscarà, C.; Cristani, M.; Melilli, M.G. Phenolic Compounds Characterization and Antioxidant Properties of Monocultivar Olive Oils from Northeast Algeria. Agriculture 2020, 10, 494. [Google Scholar] [CrossRef]
- Üçüncüoğlu, D.; Sivri-Özay, D. Geographical origin impact on volatile composition and some quality parameters of virgin olive oils extracted from the “Ayvalık” variety. Heliyon 2020, 6, 04919. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, O.; Bendini, A.; Cerretani, L.; Guerfel, M.; Baccouri, B.; Lercker, G.; Zarrouk, M.; Ben Miled, D.D. Comparative study on volatile compounds from Tunisian and Sicilian monovarietal virgin olive oils. Food Chem. 2008, 111, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tura, D.; Failla, O.; Bassi, D.; Pedò, S.; Serraiocco, A. Cultivar influence on virgin olive (Olea europea L.) oil flavor based on aromatic compounds and sensorial profile. Sci. Hortic. 2008, 118, 139–148. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Ramis-Ramos, G.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Classification of vegetable oils according to their botanical origin using sterol profiles established by direct infusion mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.; Aranda, F.; Fregapane, G. Influence of fruit ripening on ‘Cornicabra’ virgin olive oil quality A study of four successive crop seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T. Characterization of Olive Ripeness by Green Aroma Compounds of Virgin Olive Oil. J. Agric. Food Chem. 1998, 46, 1116–1122. [Google Scholar] [CrossRef]
- Toker, C.; Aksoy, U.; Ertaş, H. The effect of fruit ripening, altitude and harvest year on volatile compounds of virgin olive oil obtained from the Ayvalık variety. Flavour Fragr. J. 2015, 31, 195–205. [Google Scholar] [CrossRef]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of Olive Ripening Degree on the Oxidative Stability and Organoleptic Properties of Cv. Nostrana di Brisighella Extra Virgin Olive Oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Salvador, M.D.; La Greca, M.; Fregapane, G. Phenolic and volatile compounds od extra virgin olive oil (Olea europaea L. cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, A.S.; López-Tamames, E. Solid-Phase Microextraction in the Analysis of Virgin Olive Oil Volatile Fraction: Characterization of Virgin Olive Oils from Two Distinct Geographical Areas of Northern Italy. J. Agric. Food Chem. 2003, 51, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.; Casal, S.; Rodrigues, N.; Renard, C.M.; Pereira, J.A. Volatile changes in cv. Verdeal Transmontana olive oil: From the drupe to the table, including storage. Food Res. Int. 2018, 106, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Di Giovacchino, L.; Sestili, S.; Di Vincenzo, D. Influence of olive processing on virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 587–601. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on virgin olive oil quality. Past, present and future–An overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Influence of malaxation temperature and time on the quality of virgin olive oils. Food Chem. 2001, 72, 19–28. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Solinas, M.; Miccoli, M. Effect of extraction systems on the quality of virgin olive oil. J. Am. Oil Chem. Soc. 1994, 71, 1189–1194. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Serraiocco, A. Influenza dei sistemi di lavorazione delle olive sulla composizione dello spazio di testa degli oli. Riv. It. Sost. Grasse 1995, 72, 443–450. [Google Scholar]
- De Stefano, G.; Piacquadio, P.; Servili, M.; Di Giovacchino, L.; Sciancalepore, V. Effect of extraction systems on the phenolic composition of virgin olive oils. Fette Seifen Anstrichm. 1999, 101, 328–332. [Google Scholar] [CrossRef]
- Bubola, K.B.; Koprivnjak, O.; Sladonja, B. Influence of filtration on volatile compounds and sensory profile of virgin olive oils. Food Chem. 2012, 132, 98–103. [Google Scholar] [CrossRef]
- Haar, A.M.; Bredie, W.L.P.; Stahnke, L.H.; Jensen, B.; Refsgaard, H.H.F. Flavour release of aldehydes and diacetyl in oil/water systems. Food Chem. 2000, 71, 355–362. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Grossmann, I.; Geary, M.; Delahunty, C.M. Interactions between Artificial Saliva and 20 Aroma Compounds in Water and Oil Model Systems. J. Agric. Food Chem. 2001, 49, 2409–2413. [Google Scholar] [CrossRef] [PubMed]
- Piggott, J.R.; Schaschke, C.J. Release cells, breath analysis and in-mouth analysis in flavour research. Biomol. Eng. 2001, 17, 129–136. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food-saliva interactions: Mechanisms and implications. Trends Food Sci. Technol. 2017, 66, 125–134. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Chen, J. Food oral processing—A review. Food Hydrocoll. 2009, 23, 1–25. [Google Scholar] [CrossRef]
- Edgar, W.M. Saliva and dental health. Clinical implications of saliva: Report of a consensus meeting. Br. Dent. J. 1990, 169, 96–98. [Google Scholar] [CrossRef]
- Genovese, A.; Piombino, P.; Gambuti, A.; Moio, L. Simulation of retronasal aroma of white and red wine in a model mouth system. Investigating the influence of saliva on volatile compound concentrations. Food Chem. 2009, 114, 100–107. [Google Scholar] [CrossRef]
- Salles, C.; Chagnon, M.-C.; Feron, G.; Guichard, E.; Laboure, H.; Morzel, M.; Semon, E.; Tarrega, A.; Yven, C. In-Mouth Mechanisms Leading to Flavor Release and Perception. Crit. Rev. Food Sci. Nutr. 2010, 51, 67–90. [Google Scholar] [CrossRef]
- Neyraud, E.; Palicki, O.; Schwartz, C.; Nicklaus, S.; Feron, G. Variability of human saliva composition: Possible relationships with fat perception and liking. Arch. Oral Biol. 2012, 57, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Feron, G.; Poette, J. In-mouth mechanism leading to the perception of fat in humans: From detection to preferences. The particular role of saliva. Oléagineux Corps Gras Lipides 2013, 20, 102–107. [Google Scholar] [CrossRef]
- Friel, E.N.; Taylor, A.J. Effect of Salivary Components on Volatile Partitioning from Solutions. J. Agric. Food Chem. 2001, 49, 3898–3905. [Google Scholar] [CrossRef] [PubMed]
- van Ruth, S.M.; Roozen, J.P. Infuence of mastication and saliva on aroma release in a model mouth system. Food Chem. 2000, 71, 339–345. [Google Scholar] [CrossRef]
- Pagès-Hélary, S.; Andriot, I.; Guichard, E.; Canon, F. Retention effect of human saliva on aroma release and respective contribution of salivary mucin and α-amylase. Food Res. Int. 2014, 64, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Salas, J.J.; Sánchez, J.; Ramli, U.S.; Manaf, A.M.; Williams, M.; Harwood, J.L. Biochemistry of lipid metabolism in olive and other oil fruits. Prog. Lipid Res. 2000, 39, 151–180. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Villani, V.; Paduano, A.; Sacchi, R. Olive oil phenolic compounds affect the release of aroma compounds. Food Chem. 2015, 181, 284–294. [Google Scholar] [CrossRef]
- Odake, S.; Roozen, J.; Burger, J. Effect of saliva dilution on the release of diacetyl and 2-heptanone from cream style dressings. Food/Nahrung 1998, 42, 385–391. [Google Scholar] [CrossRef]
- Feron, G.; Ayed, C.; Qannari, E.M.; Courcoux, P.; Laboure, H.; Guichard, E. Understanding Aroma Release from Model Cheeses by a Statistical Multiblock Approach on Oral Processing. PLoS ONE 2014, 9, e93113. [Google Scholar] [CrossRef]
- Wisniewski, L.; Epstein, L.H.; Caggiula, A.R. Effect of food change on consumption, hedonics, and salivation. Physiol. Behav. 1992, 52, 21–26. [Google Scholar] [CrossRef]
- Piombino, P.; Genovese, A.; Esposito, S.; Moio, L.; Cutolo, P.P.; Chambery, A.; Severino, V.; Moneta, E.; Smith, D.P.; Owens, S.M.; et al. Saliva from Obese Individuals Suppresses the Release of Aroma Compounds from Wine. PLoS ONE 2014, 9, e85611. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Rispoli, T.; Sacchi, R. Extra virgin olive oil aroma release after interaction with human saliva from individuals with different body mass index. J. Sci. Food Agric. 2018, 98, 3376–3383. [Google Scholar] [CrossRef]
- Guichard, E. Interactions between flavour compounds and food ingredients and their influence on flavour perception. Food Rev. Int. 2002, 18, 49–70. [Google Scholar] [CrossRef]
- Naknean, P.; Meenune, M. Factors affecting retention and release of flavour compounds in food carbohydrates. Int. Food Res. J. 2010, 17, 23–34. [Google Scholar]
- Villamor, R.R.; Ross, C.F. Wine Matrix Compounds Affect Perception of Wine Aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Di Bari, V.; Yang, N.; Fisk, I. Effect of olive oil phenolic compounds on the aroma release and persistence from O/W emulsion analysed in vivo by APCI-MS. Food Res. Int. 2019, 126, 108686. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Yang, N.; Linforth, R.; Sacchi, R.; Fisk, I. The role of phenolic compounds on olive oil aroma release. Food Res. Int. 2018, 112, 319–327. [Google Scholar] [CrossRef]
- Reiners, J.; Grosch, W. Odorants of Virgin Olive Oils with Different Flavor Profiles. J. Agric. Food Chem. 1998, 46, 2754–2763. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Leone, T.; Paduano, A.; Mena, C.; Perez-Jimenez, M.A.; Sacchi, R. Use of odorant series for extra virgin olive oil aroma characterisation. J. Sci. Food Agric. 2019, 99, 1215–1224. [Google Scholar] [CrossRef]
- Rinaldi, A.; Moio, L. Salivary Protein-Tannin Interaction: The Binding behind Astringency. In Winemaking—Stabilization, Aging Chemistry and Biochemistry [Working Title]; IntechOpen: London, UK, 2020. [Google Scholar]
- Canon, F.; Paté, F.; Cheynier, V.; Sarni-Manchado, P.; Giuliani, A.; Pérez, J.; Durand, D.; Li, J.; Cabane, B. Aggregation of the Salivary Proline-Rich Protein IB5 in the Presence of the Tannin EgCG. Langmuir 2013, 29, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.J.; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple Interactions between Polyphenols and a Salivary Proline-Rich Protein Repeat Result in Complexation and Precipitation. Biochemistry 1997, 36, 5566–5577. [Google Scholar] [CrossRef] [PubMed]
- Goldner, M.; Lira, P.D.L.; Van Baren, C.; Bandoni, A. Influence of Polyphenol Levels on the Perception of Aroma in Vitis vinifera cv. Malbec wine. South Afr. J. Enol. Vitic. 2016, 32, 21–27. [Google Scholar] [CrossRef]
- Pripp, A.H.; Vreeker, R.; Van Duynhoven, J. Binding of olive oil phenolics to food proteins. J. Sci. Food Agric. 2005, 85, 354–362. [Google Scholar] [CrossRef]
- Quintero-Flórez, A.; Sánchez-Ortiz, A.; Martínez, J.J.G.; Márquez, A.J.; Maza, G.B. Interaction between extra virgin olive oil phenolic compounds and mucin. Eur. J. Lipid Sci. Technol. 2015, 117, 1569–1577. [Google Scholar] [CrossRef]
- Dawes, C. Stimulus effects on protein and electrolyte concentrations in parotid saliva. J. Physiol. 1984, 346, 579–588. [Google Scholar] [CrossRef]
| Chemical Compound | Chemical Structure | Sensory Property |
|---|---|---|
| 3,4–DHPEA–EDA (oleacein) | 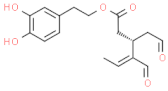 | bitter, astringent and burning (mostly on tongue) |
| 3,4–DHPEA–EA (oleuropein aglycon) | 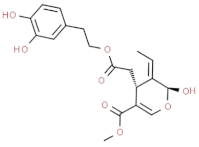 | very bitter, very astringent |
| p–HPEA–EDA (oleocanthal) | 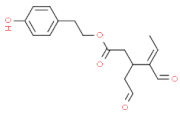 | strong burning/pungent (mostly at the back of throat); slightlybitter and astringent |
| p–HEPA–EA (ligstroside aglycon) | 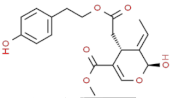 | dry mouth, burning/pungent, and not bitter |
| Sensory Defect | Chemical Compound | Chemical Structure | Odor Property |
|---|---|---|---|
| Mold-humidity | 1–octen–3–one |  | mushroom, mold, pungent |
| 1–octen–3–ol |  | mold, earthy | |
| 2–heptanol | 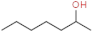 | earthy, sweety | |
| 2–heptanone | 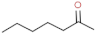 | sweet, fruity, cinnamon | |
| Winery-vinegar | ethanol | 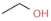 | alcohol |
| acetic acid | 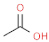 | sour, vinegary | |
| ethyl acetate | 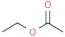 | sticky, sweet | |
| 3–methylbutanol |  | woody, whiskey, sweet | |
| Fusty | butyl acetate |  | green, fruity, pungent |
| ethyl propanoate | 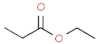 | fruit, strong | |
| ethyl butanoate | 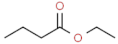 | sweet, fruity | |
| propanoic acid |  | pungent, sour | |
| Muddy-sediment | butanoic acid |  | rancid, cheese |
| pentanoic acid | 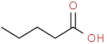 | unpleasant, pungent | |
| Rancidity | trans–2–heptenal |  | oxidized, pungent |
| trans–2–octenal |  | herbaceous, spicy | |
| trans–2–decenal |  | fishy, fatty | |
| pentanal |  | woody, bitter, oily | |
| hexanal |  | fatty, strong, green | |
| heptanal | 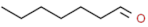 | oily, fatty, woody | |
| octanal |  | fatty, sharp | |
| nonanal | 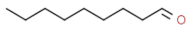 | fatty, waxy, pungent | |
| hexanoic acid |  | rancid, pungent | |
| heptanoic acid | 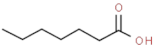 | rancid | |
| 6–methyl–5–hepten–2–one | 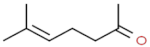 | herbaceous, pungent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, A.; Caporaso, N.; Sacchi, R. Flavor Chemistry of Virgin Olive Oil: An Overview. Appl. Sci. 2021, 11, 1639. https://doi.org/10.3390/app11041639
Genovese A, Caporaso N, Sacchi R. Flavor Chemistry of Virgin Olive Oil: An Overview. Applied Sciences. 2021; 11(4):1639. https://doi.org/10.3390/app11041639
Chicago/Turabian StyleGenovese, Alessandro, Nicola Caporaso, and Raffaele Sacchi. 2021. "Flavor Chemistry of Virgin Olive Oil: An Overview" Applied Sciences 11, no. 4: 1639. https://doi.org/10.3390/app11041639
APA StyleGenovese, A., Caporaso, N., & Sacchi, R. (2021). Flavor Chemistry of Virgin Olive Oil: An Overview. Applied Sciences, 11(4), 1639. https://doi.org/10.3390/app11041639








