Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations
Abstract
1. Introduction
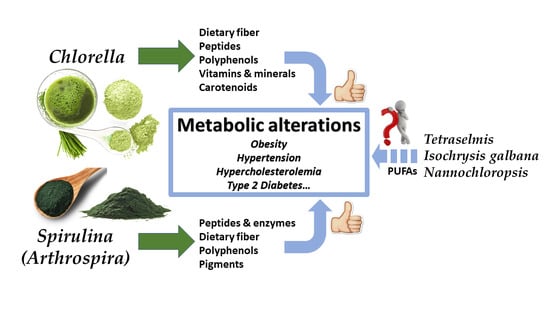
2. Microalgae
3. Spirulina (Arthrospira) and Metabolic Alterations
3.1. Dietary Fiber from Spirulina
3.2. Peptides and Enzymes from Spirulina
3.3. Unsaturated Fatty Acids from Spirulina
3.4. Polyphenols from Spirulina
3.5. Pigments from Spirulina
4. Chlorella and Metabolic Alterations
4.1. Unsaturated Fatty Acids from Chlorella
4.2. Polysaccharides from Chlorella
4.3. Peptides from Chlorella
4.4. Polyphenols from Chlorella
4.5. Vitamins and Minerals from Chlorella
4.6. Carotenoids from Chlorella
5. Other Emerging Microalgae
5.1. Tetraselmis
5.2. Isochrysis Galbana
5.3. Nannochloropsis
6. Conclusions and Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Hung, T.; Sievenpiper, J.L.; Marchie, A.; Kendall, C.W.; Jenkins, D.J.A. Fat versus carbohydrate in insulin resistance, obesity, diabetes and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 165–176. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Jáuregui, O.; Amézqueta, S.; DaSilva, G.; Medina, I.; Nogués, M.R.; Romeu, M.; et al. Mechanistically different effects of fat and sugar on insulin resistance, hypertension, and gut microbiota in rats. Am. J. Physiol. Metab. 2018, 314, E552–E563. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Komnenov, D.; Levanovich, P.E.; Rossi, N.F. Hypertension associated with fructose and high salt: Renal and sympathetic Mechanisms. Nutrients 2019, 11, 569. [Google Scholar] [CrossRef]
- Zárate, A.; Manuel-Apolinar, L.; Saucedo, R.; Hernández-Valencia, M.; Basurto, L. Hypercholesterolemia as a risk factor for cardiovascular disease: Current controversial therapeutic management. Arch. Med. Res. 2016, 47, 491–495. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/American Heart Association Conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Brown, L.; Poudyal, H.; Panchal, S.K. Functional foods as potential therapeutic options for metabolic syndrome. Obes. Rev. 2015, 16, 914–941. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Wiley-Blackwel: Hoboken, NJ, USA, 2013. [Google Scholar]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; Santa Costa De Morais, R.M.; Miranda Bernardo De Morais, A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry; Rastgoi, R., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Taminiau, B.; Walgrave, H.; Daube, G.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Spirulina protects against hepatic inflammation in aging: An Effect related to the modulation of the gut microbiota? Nutrients 2017, 9, 633. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary supplement to promote human health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for human and animal nutrition. In Handbook of Microalgal Culture; Richmon, A., Hu, Q., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 461–503. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Trepiana, J.; González-Arceo, M.; Aguirre, L.; Milton-Laskíbar, I.; González, M.; Eseberri, I.; Fernández-Quintela, A.; Portillo, M.P. Anti-obesity effects of microalgae. Int. J. Mol. Sci. 2019, 21, 41. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Kim, K.-J.; Choi, J.; Koh, E.-J.; Lee, B.-Y. Spirulina maxima extract reduces obesity through suppression of adipogenesis and activation of browning in 3t3-l1 cells and high-fat diet-induced obese mice. Nutrients 2018, 10, 712. [Google Scholar] [CrossRef]
- Heo, M.-G.; Choung, S.-Y. Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct. 2018, 9, 4906–4915. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Chen, X.; Xiong, W.; Tang, Y.; Lin, L. Spirulina platensis alleviates chronic inflammation with modulation of gut microbiota and intestinal permeability in rats fed a high-fat diet. J. Cell. Mol. Med. 2020, 24, 8603–8613. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Faghfouri, A.H.; Radkhah, N.; Foroumandi, E.; Khorshidi, M.; Rasouli, A.; Zarei, M.; Honarvar, N.M.; Hazir Karzar, N.; Ebrahimi-Mameghani, M. Spirulina supplementation and anthropometric indices: A systematic review and meta-analysis of controlled clinical trials. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, R.; Saidpour, A.; Mottaghi, A. The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: A systematic review. Complementary Ther. Med. 2019, 42, 137–144. [Google Scholar] [CrossRef]
- Pankaj, P.P. Cell suspension of Spirulina platensis partially attenuates alloxan induced alterations in carbohydrate and lipid metabolism in diabetic mice. Int. J. Pharm. Sci. Res. 2016, 7, 2805–2812. [Google Scholar] [CrossRef]
- Gargouri, M.; Magné, C.; El Feki, A. Hyperglycemia, oxidative stress, liver damage and dysfunction in alloxan-induced diabetic rat are prevented by Spirulina supplementation. Nutr. Res. 2016, 36, 1255–1268. [Google Scholar] [CrossRef]
- Aissaoui, O.; Amiali, M.; Bouzid, N.; Belkacemi, K.; Bitam, A. Effect of Spirulina platensis ingestion on the abnormal biochemical and oxidative stress parameters in the pancreas and liver of alloxan-induced diabetic rats. Pharm. Biol. 2017, 55, 1304–1312. [Google Scholar] [CrossRef]
- King, A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Gargouri, M.; Hamed, H.; Akrouti, A.; Dauvergne, X.; Magné, C.; El Feki, A. Effects of Spirulina platensis on lipid peroxidation, antioxidant defenses, and tissue damage in kidney of alloxan-induced diabetic rats. Appl. Physiol. Nutr. Metab. 2018, 43, 345–354. [Google Scholar] [CrossRef]
- Metwally, N.S.; Maghraby, A.S.; Farra, E.K.; Abd El Bak, H.H.; Farrag, A.R.H.; Foda, D.S.; Rawi, S.M. Efficiency of the algae Spirulina platensis as antidiabetic agent. World J. Pharm. Res. 2015, 4, 18–54. [Google Scholar]
- Sadek, K.M.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef]
- Oriquat, G.A.; Ali, M.A.; Mahmoud, S.A.; Eid, R.M.; Hassan, R.; Kamel, M.A. Improving hepatic mitochondrial biogenesis as a postulated mechanism for the antidiabetic effect of Spirulina platensis in comparison with metformin. Appl. Physiol. Nutr. Metab. 2019, 44, 357–364. [Google Scholar] [CrossRef]
- Shimamatsu, H. Mass production of Spirulina, an edible microalga. Hydrobiologia 2004, 512, 39–44. [Google Scholar] [CrossRef]
- Parikh, P.; Mani, U.; Iyer, U. Role of Spirulina in the control of glycemia and lipidemia in Type 2 diabetes mellitus. J. Med. Food 2001, 4, 193–199. [Google Scholar] [CrossRef]
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 2020, 177, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Dubey, J.; Mehra, S. Probiotic efficiency of Spirulina platensis—Stimulating growth of lactic acid bacteria. World J. Dairy Food Sci. 2009, 42, 160–163. [Google Scholar]
- Parada, J.L.; Zulpa de Caire, G.; Zaccaro de Mulé, M.A.C.; Storni de Cano, M.M. Lactic acid bacteria growth promoters from Spirulina platensis. Int. J. Food Microbiol. 1998, 45, 225–228. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Pakpour, S.; Wang, S.; Pan, Z.; Liu, J.; Wei, Q.; She, J.; Cang, H.; Zhang, R.X. Dose effects of orally administered Spirulina suspension on Colonic microbiota in healthy mice. Front. Cell. Infect. Microbiol. 2019, 9, 243. [Google Scholar] [CrossRef]
- Molinar-Toribio, E.; Pérez-Jiménez, J.; Ramos-Romero, S.; Lluís, L.; Sánchez-Martos, V.; Taltavull, N.; Romeu, M.; Pazos, M.; Méndez, L.; Miranda, A.; et al. Cardiovascular disease-related parameters and oxidative stress in SHROB Rats, A model for Metabolic syndrome. PLoS ONE 2014, 9, e104637. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Chang, X.-Y.; Zhao, S.; Zhang, R.-N.; Pan, Y.-L.; Zhou, X.-R. Effect of Spirulina polysaccharide on blood glucose and antioxidant activity in diabetic rats. Zhiye Jiankang 2015, 31, 3229–3235. [Google Scholar]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Ryu, B.; Ahn, G.; Yeo, I.-K.; Jeon, Y.-J. Therapeutic potential of algal natural products against metabolic syndrome: A review of recent developments. Trends Food Sci. Technol. 2020, 97, 286–299. [Google Scholar] [CrossRef]
- Ejike, C.E.; Collins, S.A.; Balasuriya, N.; Swanson, A.K.; Mason, B.; Udenigwe, C.C. Prospects of microalgae proteins in producing peptide-based functional foods for promoting cardiovascular health. Trends Food Sci. Technol. 2017, 59, 30–36. [Google Scholar] [CrossRef]
- Bermejo, R.; Talavera, E.M.; Alvarez-Pez, J.; Orte, J.C. Chromatographic purification of biliproteins from Spirulina platensis high-performance liquid chromatographic separation of their α and β subunits. J. Chromatogr. A 1997, 778, 441–450. [Google Scholar] [CrossRef]
- Li, Y.; Aiello, G.; Bollati, C.; Bartolomei, M.; Arnoldi, A.; Lammi, C. Phycobiliproteins from Arthrospira Platensis (Spirulina): A new source of peptides with dipeptidyl peptidase-IV Inhibitory activity. Nutrients 2020, 12, 794. [Google Scholar] [CrossRef]
- Nauck, M.A.; Baller, B.; Meier, J.J. Gastric Inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of Type 2 diabetes. Diabetes 2004, 53, S190–S196. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Fan, X.; Qi, P.; Zhang, X. Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Van De Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; van de Lisdonk, E.H.; Rutten, G.E.; Van Weel, C. α-glucosidase inhibitors for Patients with type 2 Diabetes: Results from a cochrane systematic review and meta-analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef] [PubMed]
- He, H.-L.; Chen, X.-L.; Wu, H.; Sun, C.-Y.; Zhang, Y.-Z.; Zhou, B.-C. High throughput and rapid screening of marine protein hydrolysates enriched in peptides with angiotensin-I-converting enzyme inhibitory activity by capillary electrophoresis. Bioresour. Technol. 2007, 98, 3499–3505. [Google Scholar] [CrossRef]
- Skeggs, L.T.; Kahn, J.R.; Shumway, N.P. The preparation and function of the hypertensin-converting enzyme. J. Exp. Med. 1956, 103, 295–299. [Google Scholar] [CrossRef]
- Suetsuna, K.; Chen, J.-R. Identification of antihypertensive peptides from peptic digest of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef]
- Carrizzo, A.; Conte Giulio, M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala Maria, C.; Aquino Rita, P.; De Lucia, M.; Madonna, M.; et al. Novel potent decameric peptide of Spirulina platensis Reduces blood pressure levels through a PI3K/AKT/eNOS-Dependent mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef]
- Li, T.-T.; Liu, Y.-Y.; Wan, X.-Z.; Huang, Z.-R.; Liu, B.; Zhao, C. Regulatory efficacy of the polyunsaturated fatty acids from microalgae Spirulina platensis on Lipid metabolism and gut microbiota in high-fat diet rats. Int. J. Mol. Sci. 2018, 19, 3075. [Google Scholar] [CrossRef]
- Wan, X.-Z.; Li, T.-T.; Zhong, R.-T.; Chen, H.-B.; Xia, X.; Gao, L.-Y.; Gao, X.-X.; Liu, B.; Zhang, H.-Y.; Zhao, C. Anti-diabetic activity of PUFAs-rich extracts of Chlorella pyrenoidosa and Spirulina platensis in rats. Food Chem. Toxicol. 2019, 128, 233–239. [Google Scholar] [CrossRef]
- Mallikarjum Gouda, K.G.; Kavitha, M.; Sarada, R. Antihyperglycemic, antioxidant and antimicrobial activities of the butanol extract from Spirulina platensis. J. Food Biochem. 2015, 39, 594–602. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; Quadros, L.; de Paula, M.; Furlong, V.B.; Abreu, P.C.; Badiale-Furlong, E. Inhibition of enzymatic and oxidative processes by phenolic extracts from Spirulina sp. and Nannochloropsis sp. Food Technol. Biotechnol. 2018, 56, 344–353. [Google Scholar] [CrossRef]
- Gómez-Zavaglia, A.; Prieto, M.A.; Jiménez-López, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Ou, Y.; Lin, L.; Pan, Q.; Yang, X.; Cheng, X. Preventive effect of phycocyanin from Spirulina platensis on alloxan-injured mice. Environ. Toxicol. Pharmacol. 2012, 34, 721–726. [Google Scholar] [CrossRef]
- Siti Halimatul Munawaroh, H.; Gumilar, G.G.; Nurjanah, F.; Yuliani, G.; Aisyah, S.; Kurnia, D.; Wulandari, A.P.; Kurniawan, I.; Ningrum, A.; Koyandev, A.K.; et al. In-vitro molecular docking analysis of microalgae extracted phycocyanin as an anti-diabetic candidate. Biochem. Eng. J. 2020, 161, 107666. [Google Scholar] [CrossRef]
- Riss, J.; Décordé, K.; Sutra, T.; Delage, M.; Baccou, J.-C.; Jouy, N.; Brune, J.-P.; Oréal, H.; Cristol, J.P.; Rouanet, J.-M. Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase Expression induced by an atherogenic diet in hamsters. J. Agric. Food Chem. 2007, 55, 7962–7967. [Google Scholar] [CrossRef]
- Ma, Q.-Y.; Fang, M.; Zheng, J.-H.; Ren, D.; Lu, J. Optimised extraction of β-carotene from Spirulina platensis and hypoglycaemic effect in streptozotocin-induced diabetic mice. J. Sci. Food Agric. 2015, 96, 1783–1789. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Habibian Dehkordi, S.; Engardeh, J.; Mahmoodnia, L.; Khaledifar, A.; Jafari, T. Effect of Chlorella supplementation on cardiovascular risk factors: A meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 1892–1901. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Takehara, I.; Masuzawa, T.; Saito, T.; Naoki, Y. Nutrigenomic studies of effects of Chlorella on Subjects with high-risk factors for lifestyle-related disease. J. Med. Food 2008, 11, 395–404. [Google Scholar] [CrossRef]
- Panahi, Y.; Pishgoo, B.; Jalalian, H.R.; Mohammadi, E.; Taghipour, H.R.; Sahebkar, A.; Abolhasani, E. Investigation of the effects of Chlorella vulgaris as an adjunctive therapy for dyslipidemia: Results of a randomised open-label clinical trial. Nutr. Diet. 2012, 69, 13–19. [Google Scholar] [CrossRef]
- Otsuki, T.; Shimizu, K.; Maeda, S. Changes in arterial stiffness and nitric oxide production with Chlorella-derived multicomponent supplementation in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 228–232. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Farhangi, M.A.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2017, 36, 1001–1006. [Google Scholar] [CrossRef]
- Itakura, H.; Kobayashi, M.; Nakamura, S. Chlorella ingestion suppresses resistin gene expression in peripheral blood cells of borderline diabetics. Clin. Nutr. ESPEN 2015, 10, e95–e101. [Google Scholar] [CrossRef]
- Rodríguez-López, M.; López-Quijada, C. Plasma-glucose and plasma-insulin in normal and alloxanized rats treated with chlorella. Life Sci. 1971, 10, 57–60. [Google Scholar] [CrossRef]
- Rodríguez-López, M. Action of the Chlorella pyrenoidosa on Experimental diabetes in rats. Pharmacology 1964, 10, 381–386. [Google Scholar] [CrossRef]
- Jong-Yuh, C.; Mei-Fen, S. Potential hypoglycemic effects of Chlorella in streptozotocin-induced diabetic mice. Life Sci. 2005, 77, 980–990. [Google Scholar] [CrossRef]
- Cherng, J.-Y.; Shih, M.-F. Improving glycogenesis in Streptozocin (STZ) diabetic mice after administration of green algae Chlorella. Life Sci. 2006, 78, 1181–1186. [Google Scholar] [CrossRef]
- Portha, B. Programmed disorders of β-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab. Res. Rev. 2005, 21, 495–504. [Google Scholar] [CrossRef]
- Jeong, H.; Kwon, H.J.; Kim, M.K. Hypoglycemic effect of Chlorella vulgaris intake in type 2 diabetic Goto-Kakizaki and normal wistar rats. Nutr. Res. Pract. 2009, 3, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-J.; Chung, H.-H.; Yeh, C.-H.; Cheng, J.; Lo, S.-H. Improvement of insulin resistance by Chlorella in FRUCTOSE-rich chow-fed rats. Phytother. Res. 2011, 25, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Cha, J.Y.; Kim, J.U. Antidiabetic Composition Containing Chlorella Extracts. Patent KR2010070154A, 25 June 2010. [Google Scholar]
- Hsieh, H.-P.; Hsu, T.-A.; Chao, Y.-S.; Chou, Y.-C.; Wang, S.T. Extracts from Chlorella containing Myristic Acid, Palmitic Acid, Palmitoleic Acid, Oleic Acid, Linoleic Acid, Linolenic Acid, and Stearic Acid, for Treating Diabetes, Obesity, and Dyslipidemia. Patent U.S20090111876A1, 1 November 2009. [Google Scholar]
- Tsai, J.S.; Liu, P.Y.; Pan Sun, B. A Composition for Reducing Insulin Resistance. Patent TWI487529B, 11 June 2015. [Google Scholar]
- Wang, P.; Cui, J. Preparing Chlorella Polysaccharides for Preventing and Treating Diabetes, and Its Application. Patent CH108164613A, 22 January 2018. [Google Scholar]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. Environ. Boil. Fishes 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Tang, C.; Ding, R.D.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The impacts of natural polysaccharides on intestinal microbiota and immune responses—A review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Raposo, M.D.J.; de Morais, A.; de Morais, R. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Leal, B.E.S. Obtenção de Oligossacarídeos Prebióticos a Partir da Hidrólise Fosfórica da Biomassa de Microalgas Utilizadas na Biomitigação de CO2 de Efluente Gasoso de Churrascaria. Master’s Thesis, Universidade Tecnológica Federal do Paraná, Curitiba, Brazil, 2015. [Google Scholar]
- Pieper, S.; Unterieser, I.; Mann, F.; Mischnick, P. A new arabinomannan from the cell wall of the chlorococcal algae Chlorella vulgaris. Carbohydr. Res. 2012, 352, 166–176. [Google Scholar] [CrossRef]
- Wan, X.-Z.; Ai, C.; Chen, Y.-H.; Gao, X.-X.; Zhong, R.-T.; Liu, B.; Chen, X.-H.; Zhao, C. Physicochemical characterization of a polysaccharide from green microalga Chlorella pyrenoidosa and its hypolipidemic activity via gut microbiota regulation in rats. J. Agric. Food Chem. 2019, 68, 1186–1197. [Google Scholar] [CrossRef]
- Sheih, I.-C.; Wu, T.-K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kim, D.; Jeon, Y.-J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.Y.; Liu, C.C.; Shen, C.R.; Lin, H.H.; Shih, M.F. Beneficial effects of Chlorella-11 peptide on blocking LPS-induced Macrophage activation and alleviating thermal injury-induced inflammation in rats. Int. J. Immunopathol. Pharmacol. 2010, 23, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Dadwar, A.; Sibi, G. In vitro antioxidant and anti-diabetic potential of green microalgae, Chlorella vulgaris. Adv. Biol. Res. 2019, 13, 76–88. [Google Scholar] [CrossRef]
- Zielinski, D.; Fraczyk, J.; Dębowski, M.; Zieliński, M.; Kaminski, Z.J.; Kregiel, D.; Jacob, C.; Kolesinska, B. Biological activity of hydrophilic extract of Chlorella vulgaris grown on post-fermentation leachate from a biogas plant supplied with stillage and maize silage. Molecules 2020, 25, 1790. [Google Scholar] [CrossRef]
- Woortman, D.V.; Fuchs, T.; Striegel, L.; Fuchs, M.; Weber, N.; Brück, T.B.; Rychlik, M. Microalgae a superior source of folates: Quantification of folates in halophile microalgae by stable isotope dilution assay. Front. Bioeng. Biotechnol. 2020, 7, 481. [Google Scholar] [CrossRef]
- Lind, M.V.; Lauritzen, L.; Kristensen, M.; Ross, A.B.; Eriksen, J.N. Effect of folate supplementation on insulin sensitivity and type 2 diabetes: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 29–42. [Google Scholar] [CrossRef]
- Meigs, J.B.; Jacques, P.F.; Selhub, J.; Singer, D.E.; Nathan, D.M.; Rifai, N.; D’Agostino, R.B.; Wilson, P.W. Fasting Plasma homocysteine levels in the insulin resistance syndrome: The Framingham offspring study. Diabetes Care 2001, 24, 1403–1410. [Google Scholar] [CrossRef]
- Homocysteine Lowering Trialists’ Collaboration Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials. Am. J. Clin. Nutr. 2005, 82, 806–812. [CrossRef] [PubMed]
- Fabregas, J.; Herrero, C. Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J. Ind. Microbiol. Biotechnol. 1990, 5, 259–263. [Google Scholar] [CrossRef]
- Askari, G.; Iraj, B.; Salehi-Abargouei, A.; Fallah, A.A.; Jafari, T. The association between serum selenium and gestational diabetes mellitus: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2015, 29, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-P.; Peng, J.; Yin, K.; Wang, J.-H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2010, 55, 150–165. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and Industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Y.; Chen, Q.; Yang, H.; Xie, X. Astaxanthin promotes Nrf2/ARE Signaling to alleviate renal fibronectin and collagen IV accumulation in diabetic rats. J. Diabetes Res. 2018, 2018, 6730315. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Boussiba, S.; Bing, W.; Yuan, J.-P.; Zarka, A.; Chen, F. Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 21, 601–604. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Ulloa, R.G.; Sineiro, J.; Feijoo, G.; Feijoo, G. Life cycle assessment of the production of bioactive compounds from Tetraselmis suecica at pilot scale. J. Clean. Prod. 2014, 64, 323–331. [Google Scholar] [CrossRef]
- Irianto, A.; Austin, B. Probiotics in aquaculture. J. Fish Dis. 2002, 25, 633–642. [Google Scholar] [CrossRef]
- Lee, M.G.; Nam, S.J.; Baek, M.J. Tetraselmis Suecica Extract Fractions for the Prevention and Treatment of Obesity and Diabetes. KR2016007979A, 6 January 2017. [Google Scholar]
- da Silvia Gorgônio, C.M.; Gómez Aranda, D.; Couri, S. Morphological and chemical aspects of Chlorella pyrenoidosa, Dunaliella tertiolecta, Isochrysis galbana and Tetraselmis gracilis microalgae. Nat. Sci. 2013, 5, 783–791. [Google Scholar] [CrossRef]
- Bonfanti, C.; Cardoso, C.; Afonso, C.; Matos, J.; García, T.; Tanni, S.; Bandarra, N.M. Potential of microalga Isochrysis galbana: Bioactivity and bioaccessibility. Algal Res. 2018, 29, 242–248. [Google Scholar] [CrossRef]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T.-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. App. Psyhol. 2014, 26, 151–161. [Google Scholar] [CrossRef]
- Liu, J.; Sommerfeld, M.; Hu, Q. Screening and characterization of Isochrysis strains and optimization of culture conditions for docosahexaenoic acid production. Appl. Microbiol. Biotechnol. 2013, 97, 4785–4798. [Google Scholar] [CrossRef]
- De los Reyes, C.; Ortega, M.J.; Rodríguez-Luna, A.; Talero, E.; Motilva, V.; Zubía, E. Molecular characterization and anti-inflammatory Activity of galactosylglycerides and galactosylceramides from the microalga Isochrysis galbana. J. Agric. Food Chem. 2016, 64, 8783–8794. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grard, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, P.S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical structure and biological activity of a highly branched (1→3,1→6)-β-d-glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef]
- Nuño, K.; Villarruel-López, A.; Pueblaperez, A.M.; Romerovelarde, E.; Puebla-Mora, A.; Ascencio, F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J. Funct. Foods 2013, 5, 106–115. [Google Scholar] [CrossRef]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef]
- Kuda, T.; Enomoto, T.; Yano, T. Effects of two storage β-1,3-glucans, laminaran from Eicenia bicyclis and paramylon from Euglena gracili, on cecal environment and plasma lipid levels in rats. J. Funct. Foods 2009, 1, 399–404. [Google Scholar] [CrossRef]
- Herrero, C.; Abalde, J.; Fábregas, J. Nutritional properties of four marine microalgae for albino rats. Environ. Boil. Fishes 1993, 5, 573–580. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Sukenik, A.; Takahashi, H.; Mokady, S. Dietary lipids from marine unicellular algae enhance the amount of liver and blood omega-3 Fatty acids in rats. Ann. Nutr. Metab. 1994, 38, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Valadez-Blanco, R.; Hernández-Carlos, B.; Torres-Ariño, A.; Guadarrama-Mendoza, P.C.; Salas-Coronado, R. Lipids rich in ω-3 polyunsaturated fatty acids from microalgae. Appl. Microbiol. Biotechnol. 2016, 100, 8667–8684. [Google Scholar] [CrossRef] [PubMed]
- Nacer, W.; Baba Ahmed, F.Z.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the anti-inflammatory and anti-oxidant effects of the microalgae Nannochloropsis gaditana in streptozotocin-induced diabetic rats. J. Diabetes Metab. Disord. 2020. [Google Scholar] [CrossRef]
- Nasirian, F.; Sarir, H.; Moradi-Kor, N. Antihyperglycemic and antihyperlipidemic activities of Nannochloropsis oculata microalgae in Streptozotocin-induced diabetic rats. Biomol. Concepts 2019, 10, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Werman, M.J.; Sukenik, A.; Mokady, S. Effects of the marine unicellular Alga Nannochloropsis. sp. to reduce the plasma and Liver cholesterol levels in male rats fed on diets with cholesterol. Biosci. Biotechnol. Biochem. 2003, 67, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; O-Nam, K.; Ko, J.-Y.; Lee, J.-H.; Kang, M.-C.; Kim, D.; Lee, J.B.; Lee, J.-S.; Jeon, Y.-J. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. Environ. Boil. Fishes 2013, 25, 1595–1606. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive properties of marine phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts from Euglena gracilis: Synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Shimada, R.; Fujita, M.; Yuasa, M.; Sawamura, H.; Watanabe, T.; Nakashima, A.; Suzuki, K. Oral administration of green algae, Euglena gracilis, inhibits hyperglycemia in OLETF rats, a model of spontaneous type 2 diabetes. Food Funct. 2016, 7, 4655–4659. [Google Scholar] [CrossRef]
| Bioactive Compounds | Arthrospira | Chlorella |
|---|---|---|
| Proteins | 60–70% | 55–60% |
| Lipids | 5% (ALA) | >10% (ω-3 PUFAs) |
| Dietary fiber | 2% | >30% |
| Minerals | 10% (P, Mg, K, Ca, Fe, Zn) | >10% (Fe, Ca, Mg, K, Zn) |
| Vitamins | E, B1, B2, B3, B9 | C, E, K1, B12, B1, B2, B6 |
| Pigments | Phycocyanine, carotenoids | 1–4% chlorophylls, carotenoids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. https://doi.org/10.3390/nu13020563
Ramos-Romero S, Torrella JR, Pagès T, Viscor G, Torres JL. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients. 2021; 13(2):563. https://doi.org/10.3390/nu13020563
Chicago/Turabian StyleRamos-Romero, Sara, Joan Ramon Torrella, Teresa Pagès, Ginés Viscor, and Josep Lluís Torres. 2021. "Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations" Nutrients 13, no. 2: 563. https://doi.org/10.3390/nu13020563
APA StyleRamos-Romero, S., Torrella, J. R., Pagès, T., Viscor, G., & Torres, J. L. (2021). Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients, 13(2), 563. https://doi.org/10.3390/nu13020563