Temperature and Concentration Dependence of Human Whole Blood and Protein Drying Droplets
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Qualitative Analysis
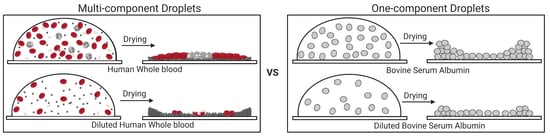
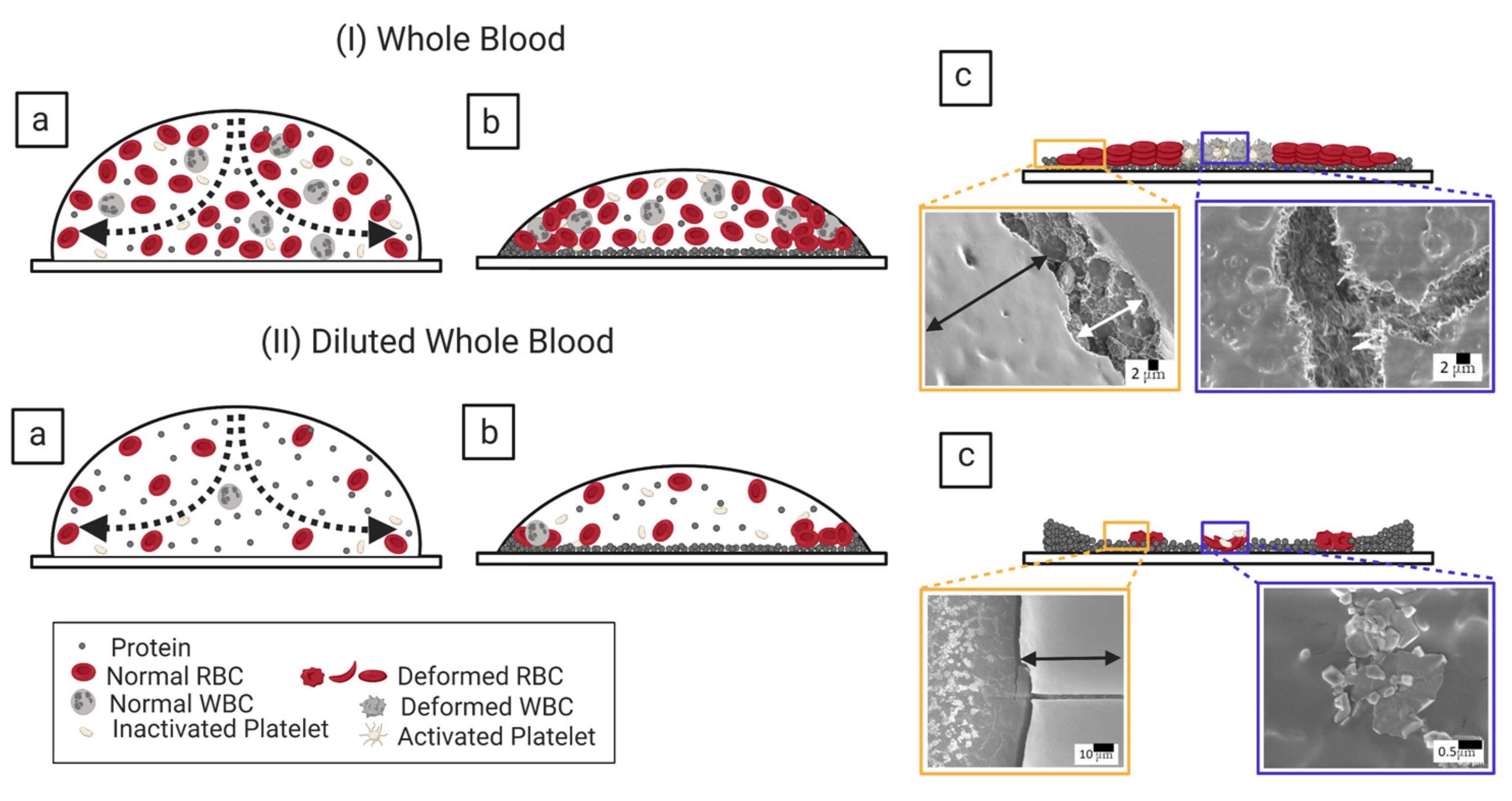
3.1.1. Whole Human Blood: The Most Complex Bio-Colloid
3.1.2. Bovine Serum Albumin Protein: The Simplest Bio-Colloid
3.2. Quantitative Analysis
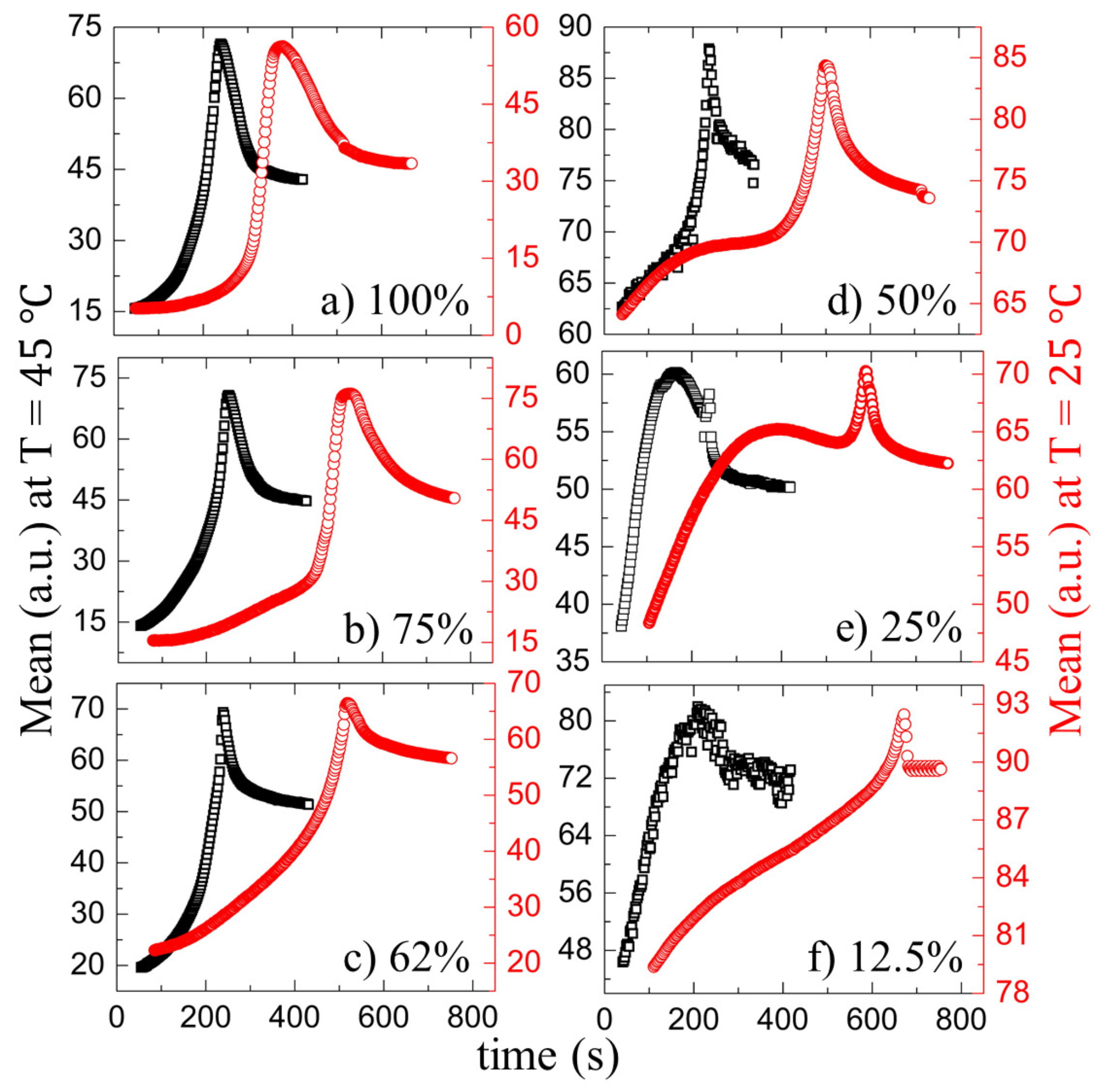
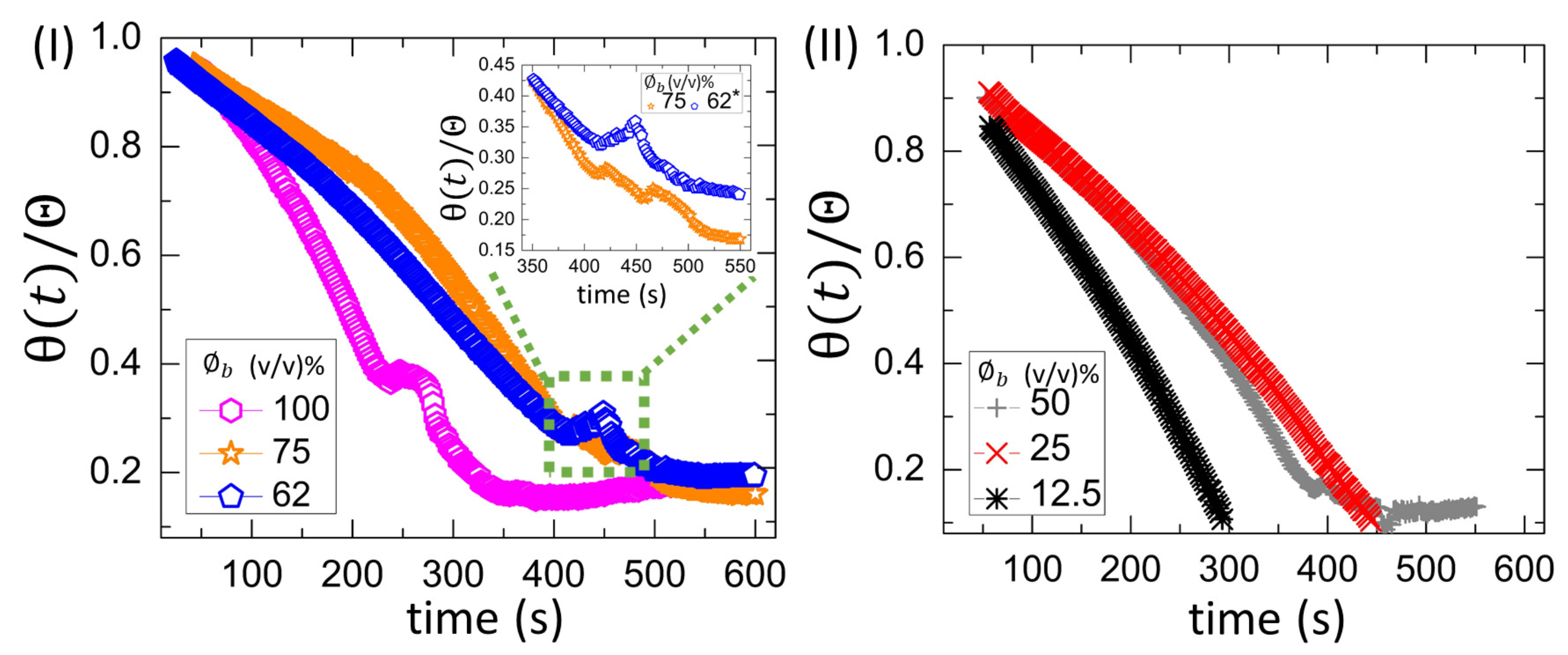
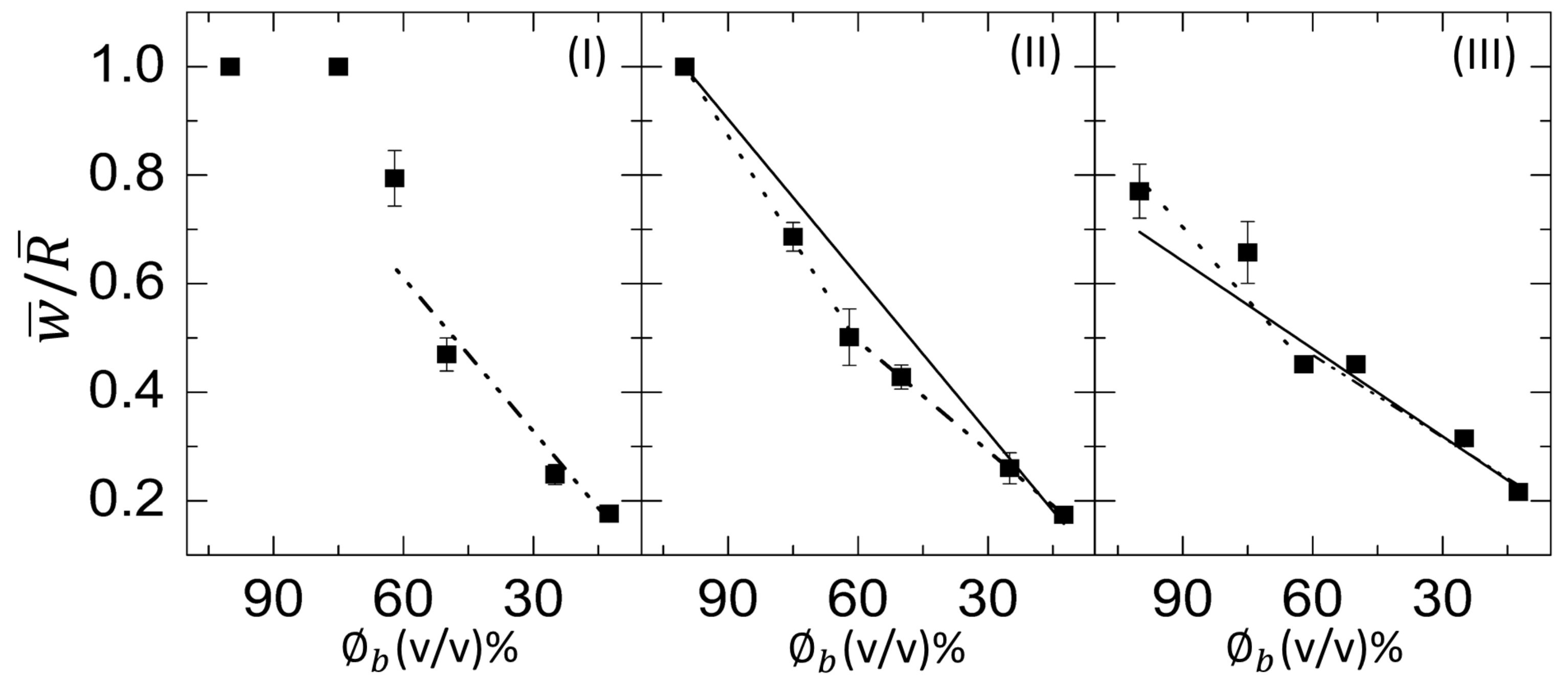
3.2.1. Drying Evolution of the Complex Bio-Colloid
3.2.2. Drying Evolution of the Simplest Bio-Colloid
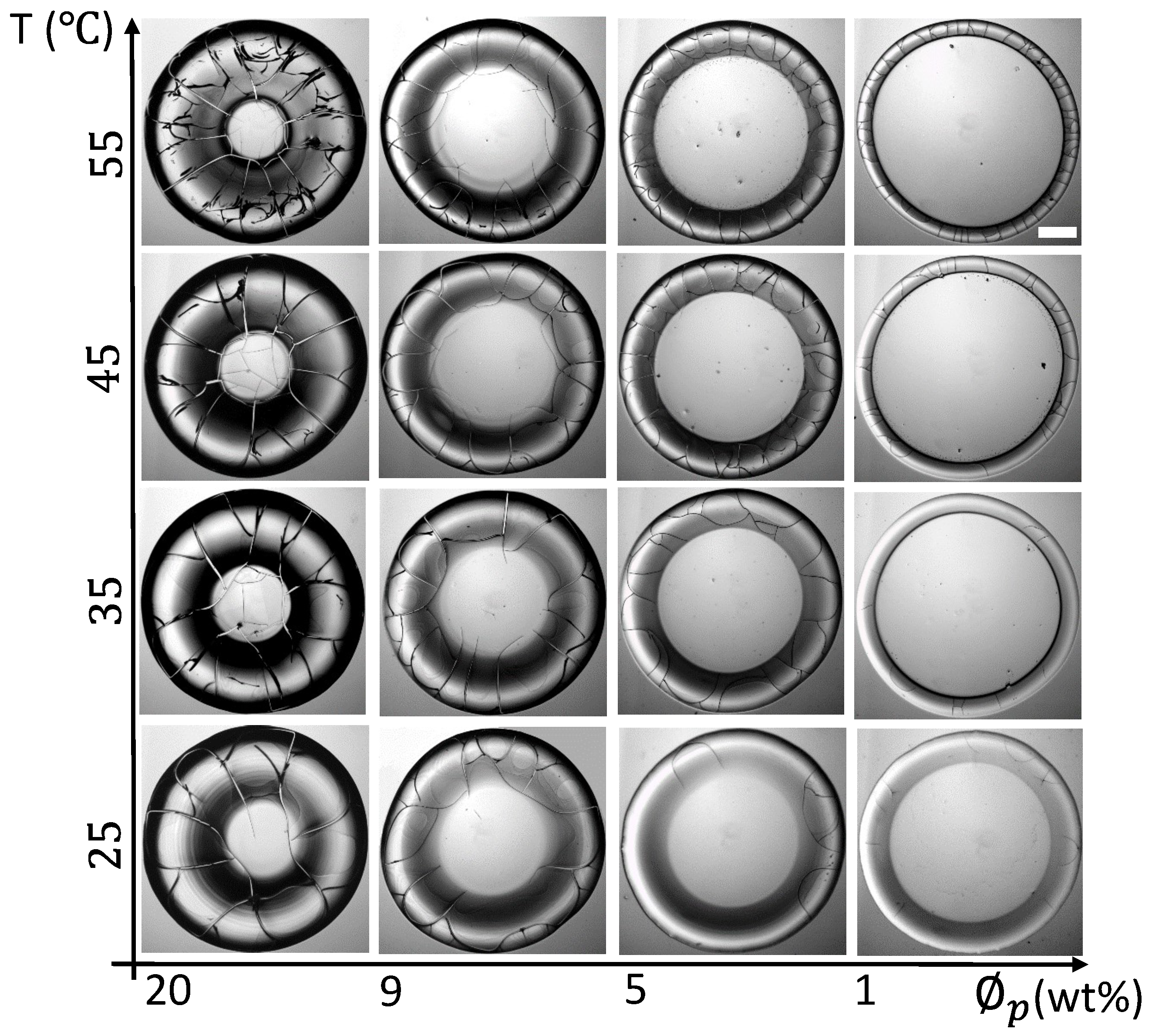
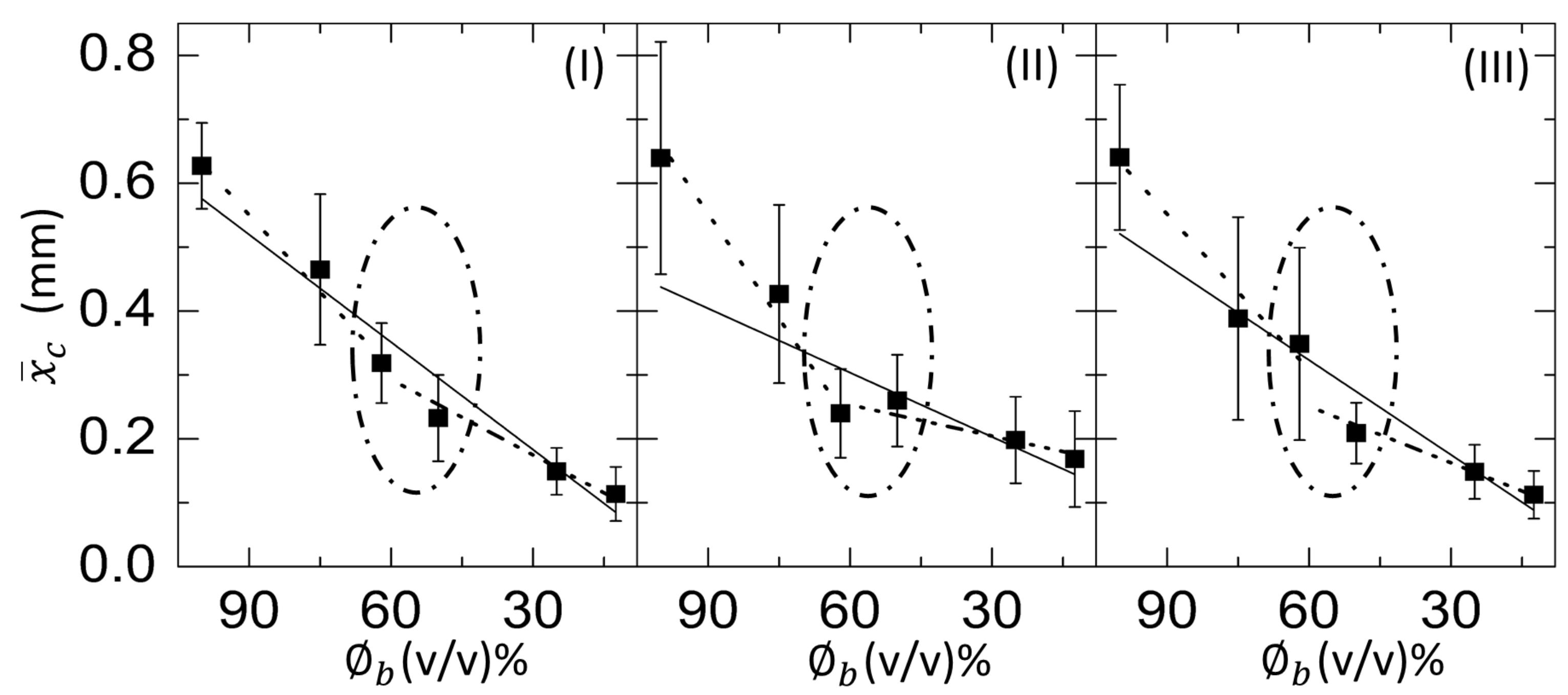
3.2.3. Morphological Patterns of the Dried Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brutin, D.; Sobac, B.; Loquet, B.; Sampol, J. Pattern formation in drying drops of blood. J. Fluid Mech. 2011, 667, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Brutin, D. Droplet Wetting and Evaporation: From Pure to Complex Fluids; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Annarelli, C.; Reyes, L.; Fornazero, J.; Bert, J.; Cohen, R.; Coleman, A.W. Ion and molecular recognition effects on the crystallisation of bovine serum albumin—Salt mixtures. Cryst. Eng. 2000, 3, 173–194. [Google Scholar] [CrossRef]
- Yakhno, T. Salt-induced protein phase transitions in drying drops. J. Colloid Interface Sci. 2008, 318, 225–230. [Google Scholar] [CrossRef]
- Chen, G.; Mohamed, G.J. Complex protein patterns formation via salt-induced self-assembly and droplet evaporation. Eur. Phys. J. E 2010, 33, 19–26. [Google Scholar] [CrossRef]
- Gorr, H.M.; Zueger, J.M.; Barnard, J.A. Lysozyme pattern formation in evaporating drops. Langmuir 2012, 28, 4039–4042. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; He, H.; Shen, W. Desiccation patterns of plasma sessile drops. ACS Sens. 2019, 4, 1701–1709. [Google Scholar] [CrossRef]
- Schena, M.; Shalon, D.; Davis, R.W.; Brown, P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995, 270, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Bel’skaya, L.V.; Sarf, E.A.; Solonenko, A.P. Morphology of dried drop patterns of saliva from a healthy individual depending on the dynamics of its surface tension. Surfaces 2019, 2, 395–414. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, R.; Shen, A.Q.; Sen, A.K. Understanding of the role of dilution on evaporative deposition patterns of blood droplets over hydrophilic and hydrophobic substrates. J. Colloid Interface Sci. 2020, 579, 541–550. [Google Scholar] [CrossRef]
- Rapis, E. A change in the physical state of a nonequilibrium blood plasma protein film in patients with carcinoma. Tech. Phys. 2002, 47, 510–512. [Google Scholar] [CrossRef]
- Muravlyova, L.; Molotov-Luchanskiy, V.B.; Bakirova, R.Y.; Zakharova, Y.E.; Klyuyev, D.A.; Bakenova, P.A.; Demidchik, L.A.; Suleimenova, S.B. Structure-forming properties of blood plasma of patients with interstitial lung diseases. World J. Med. Sci. 2014, 10, 478–483. [Google Scholar]
- Yakhno, T.A.; Sanin, A.A.; Ilyazov, R.G.; Vildanova, G.V.; Khamzin, R.A.; Astascheva, N.P.; Markovsky, M.G.; Bashirov, V.D.; Yakhno, V.G. Drying drop technology as a possible tool for detection leukemia and tuberculosis in cattle. J. Biomed. Sci. Eng. 2015, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- González-Gutiérrez, J.; Pérez-Isidoro, R.; Ruiz-Suárez, J. A technique based on droplet evaporation to recognize alcoholic drinks. Rev. Sci. Instrum. 2017, 88, 074101. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Ray, R.; Ayushman, M.; Sood, P.; Bhattacharyya, M.; Sarkar, D.; Dasgupta, S. Interfacial energy driven distinctive pattern formation during the drying of blood droplets. J. Colloid Interface Sci. 2020, 573, 307–316. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Zang, D.; Shen, W. Blood drop patterns: Formation and applications. Adv. Colloid Interface Sci. 2016, 231, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brutin, D.; Sobac, B.; Nicloux, C. Influence of substrate nature on the evaporation of a sessile drop of blood. J. Heat Transf. 2012, 134, 061101. [Google Scholar] [CrossRef]
- Sobac, B.; Brutin, D. Desiccation of a sessile drop of blood: Cracks, folds formation and delamination. Colloids Surf. A Physicochem. Eng. Asp. 2014, 448, 34–44. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Zang, D.; Shen, W. Understanding desiccation patterns of blood sessile drops. J. Mater. Chem. B 2017, 5, 8991–8998. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Shen, W. Controlling the contact angle of biological sessile drops for study of their desiccated cracking patterns. J. Mater. Chem. B 2018, 6, 5867–5875. [Google Scholar] [CrossRef]
- Lanotte, L.; Laux, D.; Charlot, B.; Abkarian, M. Role of red cells and plasma composition on blood sessile droplet evaporation. Phys. Rev. E 2017, 96, 053114. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Obayemi, J.D.; Iannacchione, G.S. Concentration-driven phase transition and self-assembly in drying droplets of diluting whole blood. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 1994; Volume 45, pp. 153–203. [Google Scholar]
- Pal, A.; Gope, A.; Iannacchione, G.S. A Comparative Study of the Phase Separation of a Nematic Liquid Crystal in the Self-assembling Drying Protein Drops. MRS Adv. 2019, 4, 1309–1314. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Athair, A.S.; Iannacchione, G.S. A comparative study of the drying evolution and dried morphology of two globular proteins in de-ionized water solutions. RSC Adv. 2020, 10, 16906–16916. [Google Scholar] [CrossRef]
- Huang, L.H.; Lin, P.H.; Tsai, K.W.; Wang, L.J.; Huang, Y.H.; Kuo, H.C.; Li, S.C. The effects of storage temperature and duration of blood samples on DNA and RNA qualities. PLoS ONE 2017, 12, e0184692. [Google Scholar] [CrossRef] [Green Version]
- Giancola, C.; De Sena, C.; Fessas, D.; Graziano, G.; Barone, G. DSC studies on bovine serum albumin denaturation effects of ionic strength and SDS concentration. Int. J. Biol. Macromol. 1997, 20, 193–204. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Pal, A.; Gope, A.; Kafle, R.; Iannacchione, G.S. Phase separation of a nematic liquid crystal in the self-assembly of lysozyme in a drying aqueous solution drop. MRS Commun. 2019, 9, 150–158. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Iannacchione, G.S. Image-Based Analysis of Patterns Formed in Drying Drops. In Pattern Recognition and Machine Intelligence; Deka, B., Maji, P., Mitra, S., Bhattacharyya, D.K., Bora, P.K., Pal, S.K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 567–574. [Google Scholar]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827. [Google Scholar] [CrossRef]
- Carreón, Y.J.; González-Gutiérrez, J.; Pérez-Camacho, M.; Mercado-Uribe, H. Patterns produced by dried droplets of protein binary mixtures suspended in water. Colloids Surf. B Biointerfaces 2018, 161, 103–110. [Google Scholar] [CrossRef]
- Carreón, Y.J.; Ríos-Ramírez, M.; Moctezuma, R.; González-Gutiérrez, J. Texture analysis of protein deposits produced by droplet evaporation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Katz, J.S.; Schroeder, C.M. Heterogeneous drying and nonmonotonic contact angle dynamics in concentrated film-forming latex drops. Phys. Rev. Fluids 2017, 2, 114304. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, M.; Ghosh, U.U.; Sarkar, D.; DasGupta, S. Surface property induced morphological alterations of human erythrocytes. Soft Matter 2018, 14, 7335–7346. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, A.; Gope, A.; Iannacchione, G. Temperature and Concentration Dependence of Human Whole Blood and Protein Drying Droplets. Biomolecules 2021, 11, 231. https://doi.org/10.3390/biom11020231
Pal A, Gope A, Iannacchione G. Temperature and Concentration Dependence of Human Whole Blood and Protein Drying Droplets. Biomolecules. 2021; 11(2):231. https://doi.org/10.3390/biom11020231
Chicago/Turabian StylePal, Anusuya, Amalesh Gope, and Germano Iannacchione. 2021. "Temperature and Concentration Dependence of Human Whole Blood and Protein Drying Droplets" Biomolecules 11, no. 2: 231. https://doi.org/10.3390/biom11020231
APA StylePal, A., Gope, A., & Iannacchione, G. (2021). Temperature and Concentration Dependence of Human Whole Blood and Protein Drying Droplets. Biomolecules, 11(2), 231. https://doi.org/10.3390/biom11020231