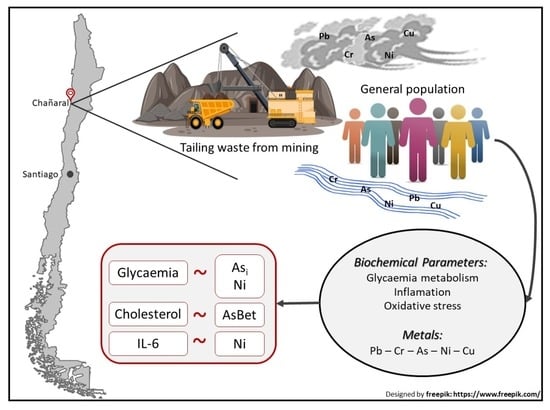
A Positive Relationship between Exposure to Heavy Metals and Development of Chronic Diseases: A Case Study from Chile
Abstract
1. Introduction
2. Material and Methods
2.1. Design
2.2. Sampling
2.3. Analysis of Metals and Metabolic Parameters
2.4. Statistical Analysis
3. Results
3.1. Description of the Participants
3.2. Associations between Biochemical Parameters and Measured Metals
3.3. Effect of Other Covariates Associated with Early Damage Parameters
4. Discussion
4.1. Findings of this Study
4.2. Toxicological Implications
4.3. Limitations and Strengths for the Public Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doumas, P.; Munoz, M.; Banni, M.; Becerra, S.; Bruneel, O.; Casiot, C.; Sappin-Didier, V. Polymetallic pollution from abandoned mines in Mediterranean regions: A multidisciplinary approach to environmental risks. Reg. Environ. Chang. 2016, 18, 677–692. [Google Scholar] [CrossRef]
- Csavina, J.; Field, J.; Taylor, M.P.; Gao, S.; Landázuri, A.; Betterton, E.A.; Sáez, A.E. A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Sci. Total Environ. 2012, 433, 58–73. [Google Scholar] [CrossRef]
- Schonfeld, S.J.; Winde, F.; Albrecht, C.; Kielkowski, D.; Liefferink, M.; Patel, M.; Sewram, V.; Stoch, L.; Whitaker, C.; Schuz, J. NoHealth effects in populations living around the uraniferous gold mine tailings in South Africa: Gaps and opportunities for research. Cancer Epidemiol. 2014, 628–632. [Google Scholar] [CrossRef]
- Yohannessen Vásquez, K.; Alvarado Orellana, S.; Mesías Monsalve, S.; Klarián Vergara, J.; Silva Zamora, C.; Vidal Muñoz, D.; Lillo, D.D.C. Exposure to fine particles by mine tailing and lung function effects in a panel of schoolchildren, Chañaral, Chile. J. Environ. Prot. 2015, 6, 118–128. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Environmental exposure to low-level lead (Pb) co-occurring with other neurotoxicants in early life and neurodevelopment of children. Environ. Res. 2019, 177, 108641. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Minería SNdGy. Catastro de Faenas Abandonadas Actualizado a 2019. Available online: https://www.sernageomin.cl/investigacion-de-faenas-abandonadas/ (accessed on 11 January 2021).
- Minería SNdGy. Anuario de la Minería de Chile 2017; Servicio Nacional de Geología y Minería: Santiago, Chile, 2017.
- Dold, B. Element flows associated with marine shore mine tailings deposits. Environ. Sci. Technol. 2006, 40, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Fariña CaC, J. NoTemporal Variation in the Diversity and Cover of Sessile Species in Rocky Intertidal Communities Affected by Copper Mine Tailing in Northern Chile. Mar. Pollut. Bull. 2001, 42, 554–568. [Google Scholar] [CrossRef]
- Castilla, J.C. Environmental impact in sandy beaches of copper mine tailings at Chañaral, Chile. Mar. Pollut. Bull. 1983, 14, 459–464. [Google Scholar] [CrossRef]
- Castilla, J.; Nealler, E. Marine environmental impact due to mining activities of El Salvador copper mine, Chile. Mar. Pollut. Bull. 1978, 9, 67–70. [Google Scholar] [CrossRef]
- Valladares, P.; Alvarado, S.; Urra, C.; Abarca, J.; Inostroza, J.; Codoceo, J.; Ruz, M. Cadmium and Lead content in Liver and Kidney tissues of Wild Turkey Vulture Cathartes aura (Linneo, 1758) from Chañaral, Atacama desert, Chile. Gayana 2013, 77, 97–104. [Google Scholar] [CrossRef]
- Cortés, S.; Lagos, L.; Burgos, S.; Adaros, H.; Ferreccio, C. Urinary Metal Levels in a Chilean Community 31 Years After the Dumping of Mine Tailings. J. Health Pollut. 2016, 6, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud. Encuesta Nacional de Salud 2016–2017. 2017. Available online: http://epi.minsal.cl/encuesta-ens/ (accessed on 11 January 2021).
- Suhaimi, N.F.; Jalaludin, J. Biomarker as a research tool in linking exposure to air particles and respiratory health. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Vattanasit, U.; Navasumrit, P.; Khadka, M.B.; Kanitwithayanun, J.; Promvijit, J.; Autrup, H.; Ruchirawat, M. Oxidative DNA damage and inflammatory responses in cultured human cells and in humans exposed to traffic-related particles. Int. J. Hyg. Environ. Health 2014, 217, 23–33. [Google Scholar] [CrossRef]
- Rajesh, B.; Hussain, R.; Giridhar, A.; Gopalakrishnan, M.; Sadasivan, S.; James, J.; Vijayan, P.P.; John, N. Knowledge and awareness about diabetes mellitus and diabetic retinopathy in suburban population of a South Indian state and its practice among the patients with diabetes mellitus: A population-based study. Indian J. Ophthalmol. 2016, 64, 272–276. [Google Scholar] [CrossRef]
- Moya, P.M.; Arce, G.J.; Leiva, C.; Vega, A.S.; Gutiérrez, S.; Adaros, H.; Muñoz, L.; Pastén, P.A. An integrated study of health, environmental and socioeconomic indicators in a mining-impacted community exposed to metal enrichment. Environ. Geochem. Health 2019, 41, 2505–2519. [Google Scholar] [CrossRef]
- Ministerio de Salud. Encuesta Nacional de Salud ENS Chile 2009–2010: III Metodología. 2010. Available online: http://www.repositoriodigital.minsal.cl/handle/2015/601 (accessed on 3 February 2021).
- Ministerio de Salud, Gobierno de Chile. Encuesta Nacional de Salud ENS Chile 2009–2010. Available online: http://web.minsal.cl/portal/url/item/bcb03d7bc28b64dfe040010165012d23.pdf (accessed on 3 February 2021).
- Prevention. CfDCa. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. 2018. Available online: https://www.cdc.gov/exposurereport/index.html (accessed on 3 February 2021).
- R Core Team (Ed.) R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Released SI. SPSS Statistics for Windows, 17.0 ed.; IBM: Chicago, IL, USA, 2008. [Google Scholar]
- Ríos, J.C.; Villarroel, L.; Torres, M.; Astaburuaga, J.P.; Leiva, C.; Cook, P.; Medel, P.; Cortés, S. Estudio de metales urinarios y plomo en sangre: Parámetros poblacionales en Antofagasta, 2018. Rev. Med. Chile 2020, 148, 746–754. [Google Scholar] [CrossRef]
- Ferreccio, C.; Huidobro, A.; Cortés, S.; Bambs, C.; Toro, P.; Van De Wyngard, V.; Acevedo, J.; Paredes, F.; Venegas, P.; Verdejo, H.; et al. Cohort Profile. Int. J. Epidemiol. 2020, 49, 760–761i. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Quintanilha, B.J.; Norde, M.M.; Pinhel, M.A.D.S.; Nonino, C.B.; Rogero, M.M. Nutritional genomics, inflammation and obesity. Arch. Endocrinol. Metab. 2020, 64, 205–222. [Google Scholar] [CrossRef]
- Liaset, B.; Øyen, J.; Jacques, H.; Kristiansen, K.; Madsen, L. Seafood intake and the development of obesity, insulin resistance and type 2 diabetes. Nutr. Res. Rev. 2019, 32, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Schmedes, M.; Balderas, C.; Aadland, E.K.; Jacques, H.; Lavigne, C.; Graff, I.E.; Eng, Ø.; Holthe, A.; Mellgren, G.; Young, J.F.; et al. The Effect of Lean-Seafood and Non-Seafood Diets on Fasting and Postprandial Serum Metabolites and Lipid Species: Results from a Randomized Crossover Intervention Study in Healthy Adults. Nutrients 2018, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Molin, M.; Ulven, S.M.; Meltzer, H.M.; Alexander, J. Arsenic in the human food chain, biotransformation and toxicology—Review focusing on seafood arsenic. J. Trace Elem. Med. Biol. 2015, 31, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Ferreccio, C.; Acevedo, J.; Castriota, F.; Cordero, J.F.; Roh, T.; Smith, A.H.; Smith, M.T.; Steinmaus, C. Socioeconomic status and the association between arsenic exposure and type 2 diabetes. Environ. Res. 2019, 172, 578–585. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Moon, K.A.; Wang, S.-L.; Silbergeld, E.; Navas-Acien, A. The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ. Health Perspect. 2017, 125, 087001. [Google Scholar] [CrossRef]
- Paul, D.S.; Harmon, A.W.; Devesa, V.; Thomas, D.J.; Stýblo, M. Molecular mechanisms of the diabetogenic effects of arsenic inhibition of insulin signaling by arsenite and methylarsonous acid. Environ. Health Perspect. 2007, 115, 734–742. [Google Scholar] [CrossRef]
- Akbari, M.; Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Quay, J.L.; Reed, W.; Samet, J.; Devlin, R.B. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappaB activation. Am. J. Respir. Cell Mol. Biol. 1998, 19, 98–106. [Google Scholar] [CrossRef]
- Tsai, D.H.; Riediker, M.; Berchet, A.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Bochud, M. Effects of short- and long-term exposures to particulate matter on inflammatory marker levels in the general population. Environ. Sci. Pollut. Res. Int. 2019, 26, 19697–19704. [Google Scholar] [CrossRef]
- Liu, G.; Sun, L.; Pan, A.; Zhu, M.; Li, Z.; Wang, Z.; Liu, X.; Ye, X.; Li, H.; Zheng, H.; et al. Nickel exposure is associated with the prevalence of type 2 diabetes in Chinese adults. Int. J. Epidemiol. 2015, 44, 240–248. [Google Scholar] [CrossRef]

| Variable | Total (n = 25) | Women (n = 19) | Men (n = 6) | Healthy Weight (n = 13) | Obese (n = 9) * |
|---|---|---|---|---|---|
| Socio demographic | |||||
| Age (years) | 48.00 (45.50–53.00) | 48.00 (46.00–51.00) | 50.50 (43.75–54.25) | 49.0 (44.0–54.5) | 47.0 (44.5–51.5) |
| Weight (kg) ~ | 78.9 ± 17.0 | 75.8 ± 17.3 | 87.0 ± 14.4 | 70.0 (57.5–79.0) | 90.0 (81.0–104.0) |
| Height (cm) | 160.5 ± 8.7 | 156.7 ± 5.9 | 170.9 ± 7.0 | 158.0 (152.5–167.0) | 158.0 (156.5–166.5) |
| Body mass index (kg/m2) ~ | 30.4 ± 5.0 | 30.6 ± 5.4 | 29.7 ± 4.2 | 27.27 (24.72–29.03) | 35.30 (33.73–37.47) |
| Time living in the area (years) | 42.00 (29.50–46.00) | 45.00 (30.00–46.00) | 32.50 (24.25–43.50) | 42.00 (29.50–46.50) | 46.0 (29.50–47.00) |
| Time living in the current house (years) | 17.92 ± 10.90 | 18.53 ± 10.87 | 16.00 ± 11.08 | 12.0 (10.0–12.0) | 12.0 (11.5–12.0) |
| Study level (years of instruction) | 11.7 ± 2.2 | 11.3 ± 1.5 | 13.2 ± 3.2 | 20.00 (8.00–24.00) | 16.00 (13.50–27.50) |
| Smokers (n, %) | 10 (44) | 6 (32) | 4 (67) | 6 (46) | 3 (33) |
| Alcohol drinkers (n, %) | 6 (24) | 2 (12) | 4 (67) | 4 (31) | 2 (22) |
| Metal levels | |||||
| As(III) (µg/L) | 0.35 (0.35–0.35) | 0.35 (0.35–0.35) | <LOD | 0.35 (0.35–0.35) | 0.35 (0.35–0.35) |
| As(V) (µg/L) | 0.35 (0.35–0.35) | 0.35 (0.35–0.35) | <LOD | 0.35 (0.35–0.35) | 0.35 (0.35–0.35) |
| Monomethylarsonic acid (µg/L) | <LOD | <LOD | <LOD | < LOD | < LOD |
| Dimethylarsinic acid (µg/L) | 12.41 ± 9.63 | 12.44 ± 9.82 | 12.32 ± 9.88 | 12.10 (4.24–17.5) | 6.35 (5.58–13.95) |
| Inorganic arsenic (µg/L) | 13.66 ± 9.67 | 13.78 ± 9.88 | 13.27 ± 9.86 | 12.80 (5.29–18.55) | 7.4 (6.63–16.67) |
| Arsenobetaine (µg/L) | 6.25 (2.72–20.60) | 6.13 (3.21–23.10) | 9.33 (0.35–19.55) | 4.91 (2.11–22.20) | 6.49 (2.07–15.25) |
| Total arsenic (µg/L) | 10.70 (5.68–30.10) | 10.70 (6.14–30.40) | 9.84 (4.85–19.80) | 10.70 (5.90–30.95) | 9.42 {3.35–21.55) |
| Chromium (µg/L) | 0.35 (0.35–0.35) | 0.35 (0.35–0.35) | 0.35 (0.35–2.19) | 0.35 (0.35–0.35) | 0.35 (0.35–0.35) |
| Nickel (µg/L) | 0.35 (0.35–1.96) | 0.35 (0.35–2.14) | 0.35 (0.35–0.70) | 0.35 (0.35–0.35) | 0.35 (0.35–2.00) |
| Copper (µg/L) | <LOD | <LOD | <LOD | < LOD | < LOD |
| Lead (µg/L) | <LOD | <LOD | <LOD | <LOD | < LOD |
| Biochemical parameters | |||||
| Basal insulin (µU/mL) ˠ | 15.84 ± 8.68 | 14.63 ± 7.72 | 19.68 ± 11.09 | 12.30 (7.90–12.30) | 19.50 (14.05–28.95) |
| Glycaemia (mg/dL) | 100.60 ± 15.02 | 98.79 ± 15.89 | 106.33 ± 11.06 | 95.00 (87.50–110.00) | 100.00 (91.00–109.00) |
| Total cholesterol (mg/dL) | 195.66 ± 39.77 | 193.14 ± 38.83 | 203.45.46 | 185.00 (161.95–220.35) | 201.40 (164.95–204.75) |
| Triglyceride (mg/dL) | 127.50 (93.00–218.00) | 125.10 (84.50–203.40) | 243.20 (133.45–370.68) | 125.10 (71.15–194.55) | 211.50 (130.20–261.30) |
| HDL (mg/dL) | 47.02 ± 8.28 | 48.44 ± 8.39 | 42.53 ± 6.63 | 47.80 (44.00–49.15) | 45.00 (41.00–51.50) |
| LDL (mg/dL) | 118.89 ± 36.00 | 120.58 ± 39.38 | 113.53 ± 24.34 | 109.10 (98.00–133.45) | 106.20 (87.55–129.15) |
| Castelli index | 4.25 ± 1.04 | 4.04 ± 0.86 | 4.88 ± 1.38 | 3.7 (3.35–4.55) | 3.9 (3.75–4.85) |
| HOMA index | 3.98 ± 2.22 | 3.58 ± 1.89 | 5.26 ± 2.88 | 2.90 (1.61–4.33) | 5.64 (3.31–7.34) |
| Clinical parameters | |||||
| IL-6 (pg/mL) | 2.59 (1.50–3.78) | 2.83 (1.55–3.96) | 2.27 (1.33–3.21) | 2.35 (1.38–3.45) | 3.20 (1.52–5.23) |
| 8-OHdG (ng/mL) | 6.61 ± 0.18 | 6.63 ± 0.20 | 6.56 ± 0.13 | 6.62 (6.58–6.77) | 6.53 (6.43–6.80) |
| Variable | Dimethylarsenate (µg/L) | Inorganic Arsenic (µg/L) | Arsenobetaine (µg/L) | Total Arsenic (µg/L) | Nickel (µg/L) |
|---|---|---|---|---|---|
| Basal insuline (µU/mL) | |||||
| Normal | 13.20 ± 11.20 | 14.25 ± 11.16 | 6.19 (3.72–35.85) | 10.45 (5.03–36.63) | 0.35 (0.35–0.95) |
| Altered | 11.44 ± 7.56 | 12.91 ± 7.83 | 7.41 (1,92–16.90) | 11.40 (6.14–19.10) | 0.35 (0.35–2.03) |
| p-value | 0.661 a | 0.739 a | 0.609 b | 0.647 b | 0.767 b |
| Glycemia (mg/dL) | |||||
| Normal | 8.96 ± 6.44 | 10.01 ± 6.45 | 4.77 (2.24–27.85) | 8.42 (3.96–30.25) | <LOD |
| Altered | 15.63 ± 11.13 | 17.03 ± 11.10 | 7.41 (3.38–20.00) | 12.70 (6.56–29.75) | 1.88 (0.35– 2.55) |
| p-value | 0.083 a | 0.069 a | 0.728 b | 0.376 b | 0.022 b |
| Total, cholesterol (mg/dL) | |||||
| Normal | 12.10 (0.35–15.80) | 12.55 ± 11.31 | 4.53 (0.35–9.27) | 9.42 (5.18–21.55) | 0.35 (0.35 - 2.09) |
| Altered | 12.95 (6.19–22.33) | 14.87 ± 7.83 | 13.90 (6.31–44.45) | 15.90 (5.44–31.68) | 0.35 (0.35–1.50) |
| p-value | 0.406 b | 0.560 a | 0.016 b | 0.437 b | 0.769 b |
| Triglyceride (mg/dL) | |||||
| Normal | 12.57 ± 11.24 | 13.58 ± 11.24 | 4.91 (3.21–31.10) | 10.70 (4.48–36.1) | 0.35 (0.35–2.36) |
| Altered | 12.21 ± 7.06 | 13.78 ± 7.26 | 9.91 (2.15–19.25) | 11.06 (5.91–28.15) | 0.35 (0.35–1.92) |
| p-value | 0.928 a | 0.960 a | 0.723 b | 0.849 b | 0.849 b |
| HDL (mg/dL) | |||||
| Normal | 10.26 (0.35–20.13) | 12.12 ± 9.96 | 11.58 (0.35–33.15) | 8.96 (2.20–30.83) | < LOD |
| Altered | 12.20 (6.35–10.20) | 14.68 ± 9.68 | 6.13 (3.92–12.40) | 10.70 (6.64–27.40) | 0.35 (0.35–2.14) |
| p-value | 0.643 b | 0.528 a | 0.978 b | 0.428 b | 0.261 b |
| LDL (mg/dL) | |||||
| Normal | 14.04 ± 13.26 | 15.74 ± 14.00 | 4.62 (0.35–12.40) | 11.40 (3.78–27.40) | 0.35 (0.35–2.14) |
| Altered | 11.80 ± 7.89 | 12.85 ± 7.79 | 6.95 (3.74–28.40) | 10.45 (5.91–30.83) | 0.35 (0.35–0.73) |
| p-value | 0.611 a | 0.513 a | 0.220 b | 1.000 b | 0.458 b |
| Castelli index (mg/dL) | |||||
| Normal | 11.90 ± 19.32 | 13.35 ± 10.53 | 4.77 (1.75–24.20) | 11.05 (5.88–30.83) | 0.35 (0.35–0.80) |
| Altered | 13.10 ± 9.08 | 14.05 ± 8.94 | 7.41 (3.21–16.90) | 10.200 (5.21–19.10) | 0.35 (0.35–2.03) |
| p-value | 0.764 a | 0.861 a | 0.467 b | 0.767 b | 0.687 b |
| HOMA index | |||||
| Normal | 14.62 ± 10.05 | 15.73 ± 9.83 | 40.60 (3.77–71.23) | 32.95 (5.88–44.28) | 0.35 (0.35–0.95) |
| Altered | 11.73 ± 9.65 | 13.01 ± 9.79 | 6.13 (2.22–12.40) | 10.20 (5.21–19.10) | 0.35 (0.35–2.03) |
| p-value | 0.533 a | 0.558 a | 0.059 b | 0.176 b | 0.687 b |
| IL-6 (µg/mL) | |||||
| Below median | 9.03 (1.66–18.15) | 11.57 ± 8.72 | 6.37 (0.74–30.20) | 15.24 ± 14.38 | < LOD |
| Above median | 12.20 (8.20–15.80) | 15.59 ± 10.43 | 6.13 (4.23–15.25) | 21.25 ±21.25 | 1.88 (0.35–2.55) |
| p-value | 0.470 b | 0.309 a | 0.979 b | 0.406 a | 0.022 b |
| 8-OHdG (ng/mL) | |||||
| Below mean | 15,15 ± 6.68 | 16.65 ± 6.58 | 12.40 (6.25–27.50) | 15.70 (6.14–29.80) | 0.35 (0.35–2.14) |
| Above mean | 10.28 ± 11.19 | 11.31 ± 11.21 | 4.58 (0.35–9.39) | 8.59 (4.31–31.83) | 0.35 (0.35–0.35) |
| p-value | 0.216 a | 0.175 a | 0.058 b | 0.501 b | 0.291 b |
| Dependent Variable | Explanatory Variables | Coefficients | Model | ||||
|---|---|---|---|---|---|---|---|
| β | Standard Error | p-Value | R2 | Adjusted R2 | p-Value | ||
| Glycemia | Age (years) | −0.49 | 0.40 | 0.904 | 0.72 | 0.60 | 0.002 |
| Sex | 14.50 | 5.06 | 0.012 | ||||
| BMI | 1.93 | 4.85 | 0.686 | ||||
| Dimethylarsinic acid (µg/L) | −6.47 | 2.73 | 0.032 | ||||
| Inorganic arsenic (µg/L) | 6.68 | 2.65 | 0.024 | ||||
| Nickel (µg/L) | 6.87 | 2.37 | 0.011 | ||||
| IL-6 | Age (years) | −0.05 | 0.06 | 0.419 | 0.36 | 0.21 | 0.088 |
| Sex | −0.004 | 0.83 | 0.999 | ||||
| BMI | 0.81 | 0.75 | 0.295 | ||||
| Nickel (µg/L) | 0.85 | 0.32 | 0.017 | ||||
| Cholesterol | Age (years) | −1.88 | 1.21 | 0.137 | 0.28 | 0.11 | 0.214 |
| Sex | 20.91 | 19.06 | 0.288 | ||||
| BMI | 8.80 | 17.70 | 0.625 | ||||
| Arsenobetaine (µg/L) | 1.10 | 0.66 | 0.11 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, S.; Zúñiga-Venegas, L.; Pancetti, F.; Covarrubias, A.; Ramírez-Santana, M.; Adaros, H.; Muñoz, L. A Positive Relationship between Exposure to Heavy Metals and Development of Chronic Diseases: A Case Study from Chile. Int. J. Environ. Res. Public Health 2021, 18, 1419. https://doi.org/10.3390/ijerph18041419
Cortés S, Zúñiga-Venegas L, Pancetti F, Covarrubias A, Ramírez-Santana M, Adaros H, Muñoz L. A Positive Relationship between Exposure to Heavy Metals and Development of Chronic Diseases: A Case Study from Chile. International Journal of Environmental Research and Public Health. 2021; 18(4):1419. https://doi.org/10.3390/ijerph18041419
Chicago/Turabian StyleCortés, Sandra, Liliana Zúñiga-Venegas, Floria Pancetti, Alejandra Covarrubias, Muriel Ramírez-Santana, Héctor Adaros, and Luis Muñoz. 2021. "A Positive Relationship between Exposure to Heavy Metals and Development of Chronic Diseases: A Case Study from Chile" International Journal of Environmental Research and Public Health 18, no. 4: 1419. https://doi.org/10.3390/ijerph18041419
APA StyleCortés, S., Zúñiga-Venegas, L., Pancetti, F., Covarrubias, A., Ramírez-Santana, M., Adaros, H., & Muñoz, L. (2021). A Positive Relationship between Exposure to Heavy Metals and Development of Chronic Diseases: A Case Study from Chile. International Journal of Environmental Research and Public Health, 18(4), 1419. https://doi.org/10.3390/ijerph18041419