Antioxidant-Rich Woodfordia fruticosa Leaf Extract Alleviates Depressive-Like Behaviors and Impede Hyperglycemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Collection and Identification of Plant
2.3. Extraction and Fractionation
2.4. Quantitative Phytochemical Analysis
2.4.1. Assessment of Total Phenolic Content (TPC)
2.4.2. Assessment of Total Flavonoid Content (TFC)
2.5. Determination of In Vitro Antioxidant Activity
2.5.1. DPPH Free Radical Scavenging Activity
2.5.2. Ferric Reducing Antioxidant Power (FRAP)
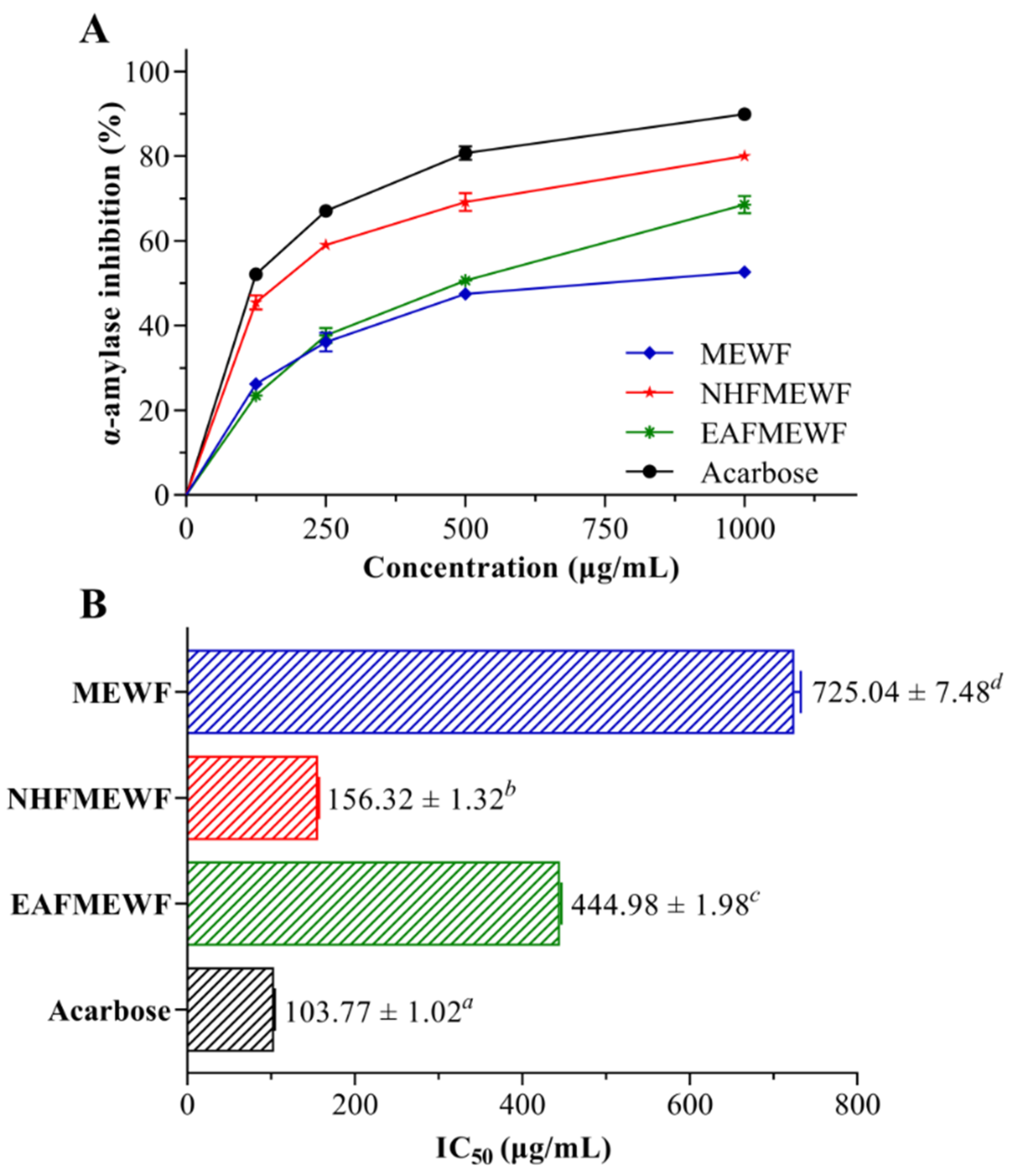
2.6. In Vitro Antidiabetic Activity (α-Amylase Inhibition Assay)
2.7. In Vivo Studies
2.7.1. Experimental Animals and Ethical Statements
2.7.2. Acute Toxicity Studies
2.7.3. Experimental Design
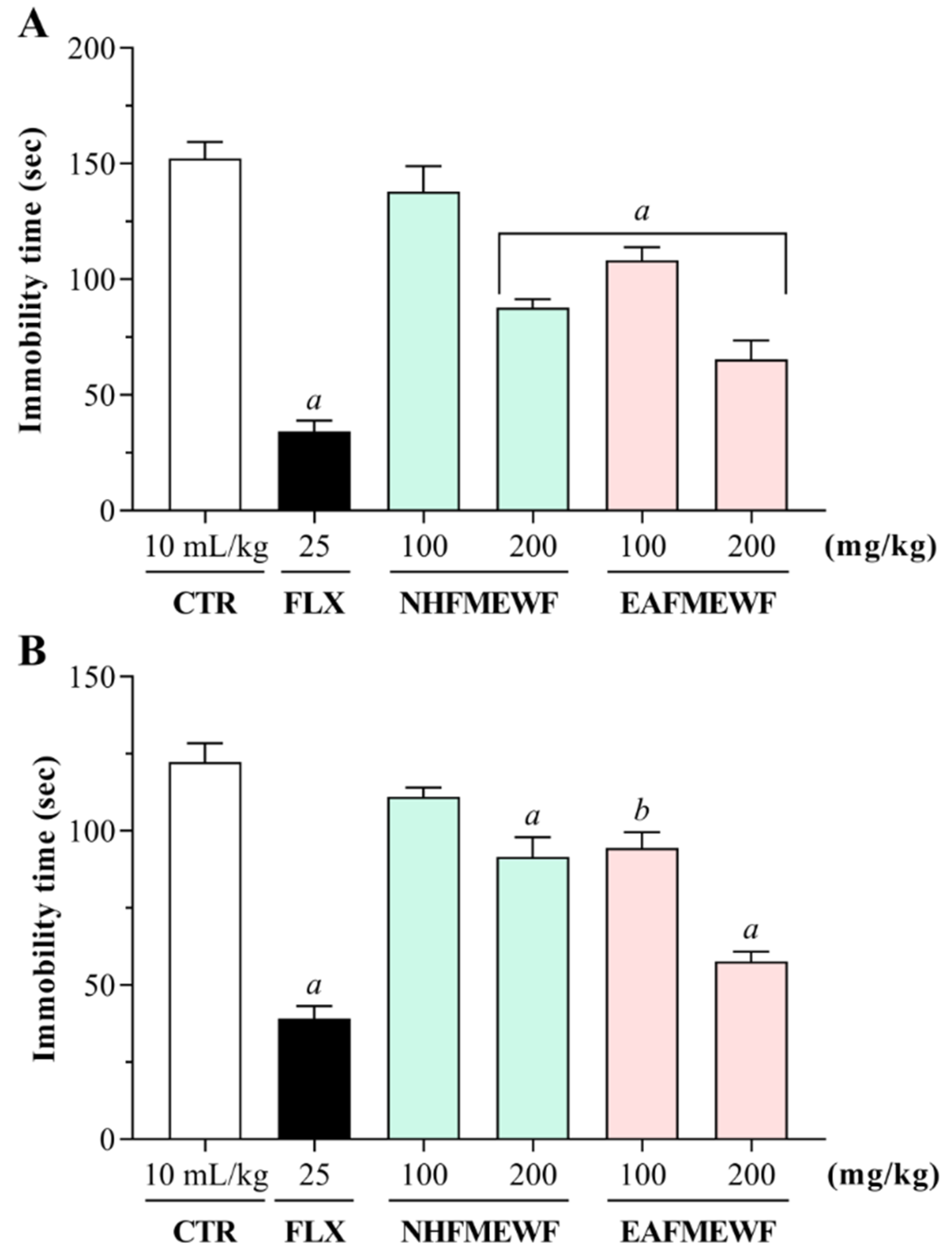
2.7.4. Antidepressant Activity
Forced Swimming Test
Tail Suspension Test (TST)
2.8. In Silico Studies
2.8.1. Molecular Docking
Selection of Compounds
Ligand Preparation
Receptor Preparation
Grid Generation and Receptor–Ligand Docking
2.8.2. Predictions of ADME/Tox Profiles
2.9. Statistical Analysis
3. Results
3.1. Total Phenolic and Flavonoid Contents
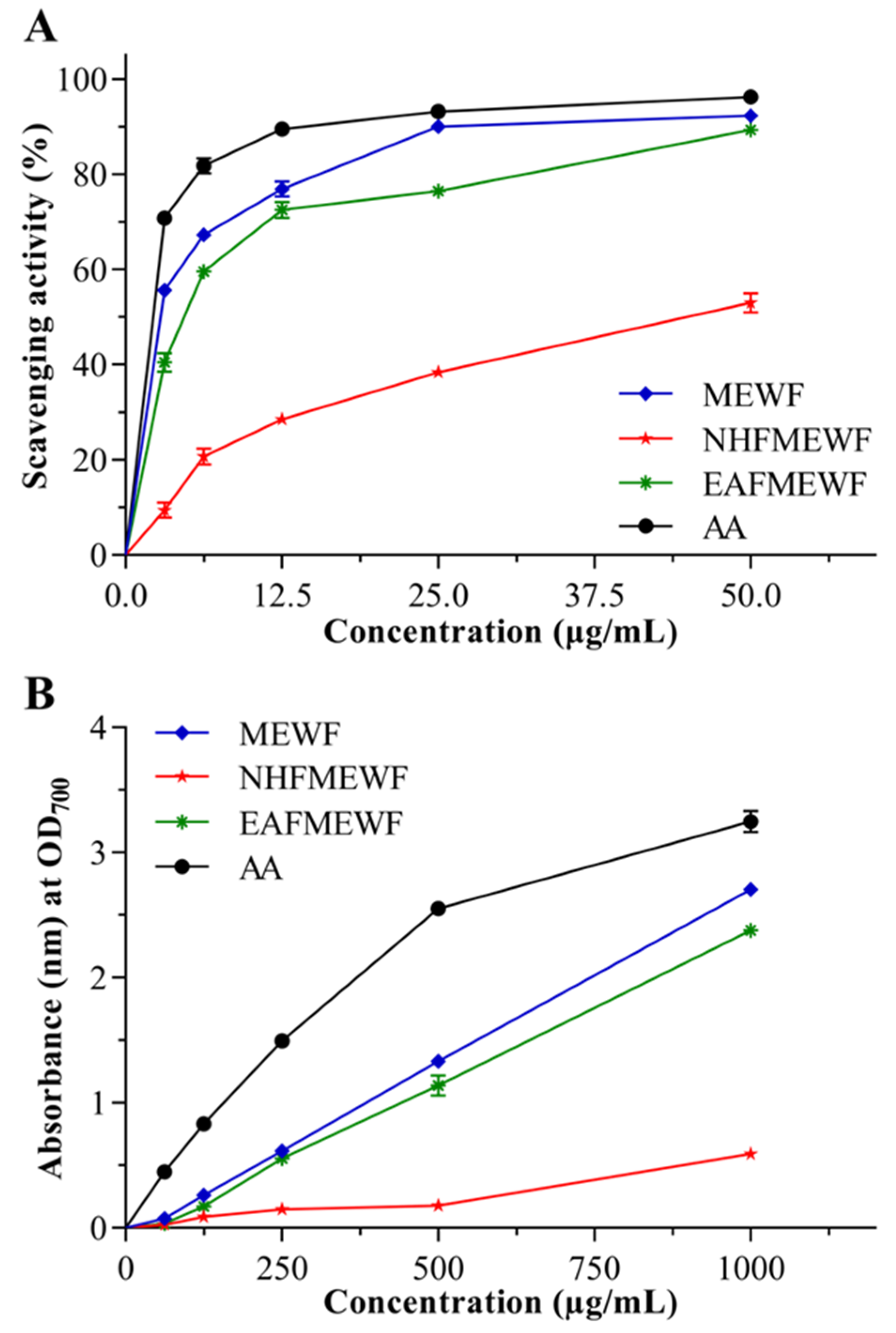
3.2. In Vitro Antioxidant Activity
3.2.1. DPPH Radical Scavenging Activity
3.2.2. Ferric Reducing Power Capacity
3.3. In Vitro Antidiabetic Activity (α-Amylase Inhibitory Activity)
3.4. In Vivo Studies
3.4.1. Acute Toxicity Study
3.4.2. Antidepressant Activity
Forced Swimming Test (FST)
Tail Suspension Test (TST)
3.5. In Silico Studies
3.5.1. Molecular Docking
3.5.2. ADME/Tox Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MEWF | methanol extract of W. fruticosa leaves |
| NHFMEWF | n-hexane fraction of methanol extract of W. fruticosa leaves |
| EAFMEWF | ethyl acetate fraction of methanol extract of W. fruticosa leaves |
| ADME/Tox | absorption, distribution, metabolism, excretion, and toxicity |
| DM2 | type 2 diabetes mellitus |
| PPHG | postprandial hyperglycemia |
| HPA | human pancreatic α-amylase |
| ROS | reactive oxygen species |
| OS | oxidative stress |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| MDD | major depressive disorder; 5-HT, 5-hydroxytryptamine |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| DNA | deoxyribonucleic acid; RT, room temperature |
| OD | optical density; Rpm, rotation per minute; p.o., orally; b.w., bodyweight |
| SERT3 | serotonin transporter 3 |
| DAT | dopamine transporter |
| MAO-A | monoamine oxidase A |
| nNOS | neuronal nitric oxide synthase |
| OPLS | optimized potentials for liquid simulations |
| RO5 | rule of Five |
| ANOVA | analysis of variance |
| SPSS | statistical package for social science |
| GABA | gamma-aminobutyric acid; i.p., intraperitoneal |
| GI | gastrointestinal |
| P-gp | p-glycoprotein |
| CYP | cytochrome P450 enzymes |
References
- Yi, F.; Li, L.; Xu, L.J.; Meng, H.; Dong, Y.M.; Liu, H.B.; Xiao, P.G. In silico approach in reveal traditional medicine plants pharmacological material basis. Chin. Med. 2018, 13, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. J. Acute Dis. 2013, 2, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Capasso, R.; Izzo, A.A.; Pinto, L.; Bifulco, T.; Vitobello, C.; Mascolo, N. Phytotherapy and quality of herbal medicines. Fitoterapia 2000, 71 (Suppl. 1), 58–65. [Google Scholar] [CrossRef]
- Chauhan, J.; Srivastava, S.; Srivastava, S. Chemical constituents of Woodfordia fruticosa Linn. J. Indian Chem. Soc. 1979, 56, 1041. [Google Scholar]
- Bulle, M.; Kota, S.; Rathakatla, D.; Aileni, M.; Kokkirala, V.R.; Gadidasu, K.K.; Abbagani, S. An Efficient in vitro Leaf-based Regeneration and Evaluation of Genetic Fidelity Using ISSR Markers in Woodfordia fruticosa (L.) Kurz. J. Herbs Spices Med. Plants 2012, 18, 178–190. [Google Scholar] [CrossRef]
- Nitha, A.; Ansil, P.N.; Prabha, S.P.; Wills, P.J.; Latha, M.S. Preventive and curative effect of Woodfordia fruticosa Kurz flowers on thioacetamide induced oxidative stress in rats. Asian Pac. J. Trop. Biomed. 2012, 2, 757–764. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, H. The Antimicrobial activity of essential oil and plant extracts of Woodfordia fruticosa. Arch. Appl. Sci. Res. 2010, 2, 302–309. [Google Scholar]
- Das, P.K.; Goswami, S.; Chinniah, A.; Panda, N.; Banerjee, S.; Sahu, N.P.; Achari, B. Woodfordia fruticosa: Traditional uses and recent findings. J. Ethnopharmacol. 2007, 110, 189–199. [Google Scholar] [CrossRef]
- Chougale, A.D.; Padul, M.V.; Arfeen, S.; Kakad, S.L. Antibacterial activity directed fractionation of Woodfordia fruticosa Kurz. leaves. J. Med. Plants 2009, 8, 75–81. [Google Scholar]
- Dubey, D.; Patnaik, R.; Ghosh, G.; Padhy, R.N. In Vitro Antibacterial Activity, Gas Chromatography-Mass Spectrometry Analysis of Woodfordia fruticosa Kurz. Leaf Extract and Host Toxicity Testing With In Vitro Cultured Lymphocytes From Human Umbilical Cord Blood. Osong Public Health Res. Perspect. 2014, 5, 298–312. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, P.A.; Ghatak, A.A.; Desai, N.S. Evaluation of radical scavenging potential and total phenol content in Woodfordia fruticosa from different altitudes. J. Plant. Biochem. Biotechnol. 2012, 21, 17–22. [Google Scholar] [CrossRef]
- Arya, A.; Abdullah, M.A.; Haerian, B.S.; Mohd, M.A. Screening for hypoglycemic activity on the leaf extracts of nine medicinal plants: In-Vivo evaluation. Eur. J. Chem. 2012, 9, 1196–1205. [Google Scholar]
- Bhujbal, S.S.; Providencia, C.A.; Nanda, R.K.; Hadawale, S.S.; Yeola, R.R. Effect of Woodfordia fruticosa on dexamethasone induced insulin resistance in mice. Braz. J. Pharmacogn. 2012, 22, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.Z.; Rana, M.S.; Hossain, S.; Ferdous, S.; Dutta, E.; Dutta, M.; Emran, T.B. In vivo neuroprotective, antinociceptive, anti-inflammatory potential in Swiss albino mice and in vitro antioxidant and clot lysis activities of fractionated Holigarna longifolia Roxb. bark extract. J. Complement. Integr. Med. 2019, 17, 1–9. [Google Scholar] [CrossRef]
- Bristy, T.A.; Barua, N.; Montakim Tareq, A.; Sakib, S.A.; Etu, S.T.; Chowdhury, K.H.; Jyoti, M.A.; Aziz, M.; Ibn, A.; Reza, A. Deciphering the pharmacological properties of methanol extract of Psychotria calocarpa leaves by in vivo, in vitro and in silico approaches. Pharmaceuticals 2020, 13, 183. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Al Mahmud, Z.; Qais, N.; Bachar, S.C.; Hasan, C.M.; Emran, T.B.; Uddin, M.M.N. Phytochemical investigations and antioxidant potential of leaf of Leea macrophylla (Roxb.). BMC Res. Notes 2017, 10, 245. [Google Scholar] [CrossRef]
- Al Mahmud, Z.; Emran, T.B.; Qais, N.; Bachar, S.C.; Sarker, M.; Uddin, M.M.N. Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb). J. Basic Clin. Physiol. Pharmacol. 2016, 27, 63–70. [Google Scholar] [CrossRef]
- Rakib, A.; Ahmed, S.; Islam, M.A.; Uddin, M.M.N.; Paul, A.; Chy, M.N.U.; Emran, T.B.; Seidel, V. Pharmacological studies on the antinociceptive, anxiolytic and antidepressant activity of Tinospora crispa. Phytother. Res. 2020, 34, 2978–2984. [Google Scholar] [CrossRef]
- Ziech, D.; Franco, R.; Georgakilas, A.G.; Georgakila, S.; Malamou-Mitsi, V.; Schoneveld, O.; Pappa, A.; Panayiotidis, M.I. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem. Biol. Interact. 2010, 188, 334–339. [Google Scholar] [CrossRef]
- Liebert, M.A.; Jones, D.P. Clinical Measures of the Balance. Antioxidants Redox Signal. 2006, 8, 1–16. [Google Scholar]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Shankar, K.; Mehendale, H.M. Oxidative Stress, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 3, pp. 735–737. [Google Scholar]
- Sen, S.; Chakraborty, R. The role of antioxidants in human health. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Publications: Washington, DC, USA, 2011; pp. 1–37. [Google Scholar]
- Reinehr, T. Type 2 diabetes mellitus in children and adolescents WJD 5th Anniversary Special Issues (2): Type 2 diabetes rent healthcare practices. World J. Diabetes 2013, 4, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 1–23. [Google Scholar] [CrossRef]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Cañizo-Gómez, F.J.D. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef] [Green Version]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yousef, M.; Sabico, S.L.; Chrousos, G.P. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (riyadh cohort 2): A decade of an epidemic. BMC Med. 2011, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.Q.; Li, M.; Zhu, F.; Liu, F.L.; Huang, J.B. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Kim, K.T.; Rioux, L.E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as alpha-Amylase and -Glucosidase Inhibitors and their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [Green Version]
- Ponnusamy, S.; Ravindran, R.; Zinjarde, S.; Bhargava, S.; Kumar, A.R. Evaluation of Traditional Indian Antidiabetic Medicinal Plants for Human Pancreatic Amylase Inhibitory Effect in Vitro. Evid. Based Complement. Altern. Med. 2010, 2011, 10. [Google Scholar]
- Picot, C.M.N.; Subratty, A.H.; Mahomoodally, M.F. Inhibitory Potential of Five Traditionally Used Native Antidiabetic Medicinal Plants on alpha-Amylase, alpha-Glucosidase, Glucose Entrapment, and Amylolysis Kinetics In Vitro. Adv. Pharmacol. Sci. 2014, 2014, 7. [Google Scholar]
- Zhao, C.; Yang, C.; Tang, S.; Wai, C.; Zhang, Y.; Maria, P.; Paoli, P.; Wu, Y.; Cheang, W.S.; Liu, B.; et al. Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2019, 59, 830–847. [Google Scholar] [CrossRef]
- Nickavar, B.; Yousefian, N. Evaluation of a alpha-amylase inhibitory activities of selected antidiabetic medicinal plants. J. Verbrauch. Lebensm. 2011, 6, 191–195. [Google Scholar] [CrossRef]
- Schmidt, H.D.; Shelton, R.C.; Duman, R.S.J.N. Functional biomarkers of depression: Diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 2011, 36, 2375–2394. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic review of gut microbiota and major depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duman, R.S.; Voleti, B.J. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, J.; Brijesh, S.J.B. Pharmacotherapy, Phytochemistry and pharmacology of anti-depressant medicinal plants: A review. Biomed. Pharmacother. 2018, 104, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model—Are we there yet? Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Pathophysiology of depression and innovative treatments: Remodeling glutamatergic synaptic connections. Dialogues Clin. Neurosci. 2014, 16, 11. [Google Scholar]
- Lee, S.-Y.; Lee, S.-J.; Han, C.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Oxidative/nitrosative stress and antidepressants: Targets for novel antidepressants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 224–235. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. Multiple antidepressant potential modes of action of curcumin: A review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J. Psychopharmacol. 2012, 26, 1512–1524. [Google Scholar] [CrossRef] [Green Version]
- Inserra, A.; Mastronardi, C.A.; Rogers, G.; Licinio, J.; Wong, M.-L. Neuroimmunomodulation in Major Depressive Disorder: Focus on Caspase 1, Inducible Nitric Oxide Synthase, and Interferon-Gamma. Mol. Neurobiol. 2019, 56, 4288–4305. [Google Scholar] [CrossRef] [Green Version]
- Yanik, M.; Erel, O.; Kati, M. The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatr. 2004, 16, 200–203. [Google Scholar] [CrossRef]
- Bouayed, J. Polyphenols: A Potential New Strategy for the Prevention and Treatment of Anxiety and Depression. Curr. Nutr. Food Sci. 2010, 6, 13–18. [Google Scholar] [CrossRef]
- Jahan, I.; Tona, M.R.; Sharmin, S.; Sayeed, M.A.; Tania, F.Z.; Paul, A.; Chy, M.; Uddin, N.; Rakib, A.; Emran, T.B. GC-MS phytochemical profiling, pharmacological properties, and in silico studies of Chukrasia velutina leaves: A novel source for bioactive agents. Molecules 2020, 25, 3536. [Google Scholar] [CrossRef] [PubMed]
- VanWagenen, B.C.; Larsen, R.; Cardellina, J.H.; Randazzo, D.; Lidert, Z.C.; Swithenbank, C. Ulosantoin, a Potent Insecticide from the Sponge Ulosa ruetzleri. J. Org. Chem. 1993, 58, 335–337. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Storms, R.; Tsang, A. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal. Biochem. 2006, 351, 146–148. [Google Scholar] [CrossRef]
- Hasanat, A.; Kabir, M.S.; Ansari, M.A.; Chowdhury, T.A.; Hossain, M.M.; Islam, M.N.; Ahmed, S.; Chy, M.N.; Adnan, M.; Kamal, A.M. Ficus cunia Buch.-Ham. ex Roxb.(leaves): An experimental evaluation of the cytotoxicity, thrombolytic, analgesic and neuropharmacological activities of its methanol extract. J. Basic Clin. Physiol. Pharmacol. 2019, 8, 30. [Google Scholar] [CrossRef]
- Porsolt, R.; Bertin, A.; Jalfre, M.J. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmocodyn. Ther. 1977, 229, 327. [Google Scholar]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Kadota, S.; Takamori, Y.; Nyein, K.N.; Kikuchi, T.; Tanaka, K.; Ekimoto, H. Constituents of the leaves of Woodfordia fruticosa Kurz. I. Isolation, structure, and proton and carbon-13 nuclear magnetic resonance signal assignments of woodfruticosin (woodfordin C), an inhibitor of deoxyribonucleic acid topoisomerase II. Chem. Pharm. Bull. 1990, 38, 2687–2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Chou, T.; Nitta, A.; Miyamoto, K.-i.; Koshiura, R.; Okuda, T. Woodfordin C, a macro-ring hydrolyzable tannin dimer with antitumor activity, and accompanying dimers from Woodfordia fruticosa flowers. Chem. Pharm. Bull. 1990, 38, 1211–1217. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Chou, T.; Nitta, A.; Okuda, T. Woodfordins A, B and C, dimeric hydrolyzable tannins from Woodfordia fruticosa flowers. Heterocycles 1989, 29, 2267–2271. [Google Scholar]
- Janet, C.; Barbee, R.; Bielitzki, J.; Clayton, L.; Donovan, J.; Hendriksen, C.; Kohn, D.; Lipman, N.; Locke, P.; Melcher, J. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Anjaneyulu, B.; Babu Rao, V.; Ganguly, A.; Govindachari, T.; Joshi, B.; KamaY, V.; Manmade, A.; Mohamed, P.; Rahimtula, A.; Saksena, A. Chemical investigation of some Indian plants. Indian J. Chem. 1965, 3, 237–238. [Google Scholar]
- Matsuda, H.; Shimoda, H.; Morikawa, T.; Yoshikawa, M. Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): Structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorg. Med. Chem. Lett. 2001, 11, 1839–1842. [Google Scholar] [CrossRef]
- Dan, S.; Dan, S. Chemical examination of the leaves of Woodfordia fruticosa. J. Indian Chem. Soc. 1984, 61, 726–727. [Google Scholar]
- Kalidhar, S.; Parthasarathy, M.; Sharma, P. Norbergenin, a New c-Glycoside from Woodfordia-Fruticosa kurz. Council Scientific Industrial Research Publ & Info Directorate; Elsevier Science B.V.: Amsterdam, The Netherlands; New Delhi, India, 1981; Volume 20, pp. 720–721. [Google Scholar]
- Hiralal Ghante, M.; Bhusari, K.P.; Duragkar, N.J.; Ghiware, N.B. Pharmacological evaluation for anti-asthmatic and anti-inflammatory potential of Woodfordia fruticosa flower extracts. Pharm. Biol. 2014, 52, 804–813. [Google Scholar] [CrossRef]
- Arya, A.; Al-Obaidi, M.M.J.; Karim, R.B.; Taha, H.; Khan, A.K.; Shahid, N.; Sayem, A.S.; Looi, C.Y.; Mustafa, M.R.; Mohd, M.A. Extract of Woodfordia fruticosa flowers ameliorates hyperglycemia, oxidative stress and improves β-cell function in streptozotocin–nicotinamide induced diabetic rats. J. Ethnopharmacol. 2015, 175, 229–240. [Google Scholar] [CrossRef]
- Tareq, A.M.; Farhad, S.; Uddin, A.N.; Hoque, M.; Nasrin, M.S.; Uddin, M.M.R.; Hasan, M.; Sultana, A.; Munira, M.S.; Lyzu, C. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon 2020, 6, e04061. [Google Scholar] [CrossRef]
- Saoji, A.; Saoji, A.; Deshmukh, V. Presence of Lawsone in Ammania bacciferra Linn. and Woodfordia fruticosa Salisb. Curr. Sci. 1972, 41, 192. [Google Scholar]
- Srivastava, S.; Sultan, M.; Chauhan, J. Anthocyanin pigment from the flowers of Woodfordia fruticosa. Proc. Natl. Acad. Sci. India 1977, 47, 35–36. [Google Scholar]
- Yoshida, T.; Chou, T.; Haba, K.; Okano, Y.; Shingu, T.; Miyamoto, K.-i.; Koshiura, R.; Okuda, T. Camelliin B and nobotanin I, macrocyclic ellagitannin dimers and related dimers, and their antitumor activity. Chem. Pharm. Bull. 1989, 37, 3174–3176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Chou, T.; Matsuda, M.; Yasuhara, T.; Yazaki, K.; Hatano, T.; Nitta, A.; Okuda, T. Woodfordin D and oenothein A, trimeric hydrolyzable tannins of macro-ring structure with anti-tumor activity. Chem. Pharm. Bull. 1991, 39, 1157–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Chou, T.; Nitta, A.; Okuda, T. Tannins and related polyphenols of Lythraceous plants. III. Hydrolyzable tannin oligomers with macrocyclic structures, and accompanying tannins from Woodfordia fruticosa Kurz. Chem. Pharm. Bull. 1992, 40, 2023–2030. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.A.; Singh, A.; Mindala, D.P.; Meena, S.; Vij, B.; Yadav, A.K.; Roy, S.; Nandi, U.; Katare, A.K.; Jaglan, S.; et al. Preclinical development of gastro-protective botanical candidate from Woodfordia fruticosa (Linn.) Kurz: Chemical standardization, efficacy, pharmacokinetics and safety pharmacology. J. Ethnopharmacol. 2019, 241, 112023. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pK a prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative catalytic anions differentially modulate human α-amylase activity and specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Son, S.Y.; Ma, J.; Kondou, Y.; Yoshimura, M.; Yamashita, E.; Tsukihara, T. Structure of human monoamine oxidase A at 2.2-Å resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. USA 2008, 105, 5739–5744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Li, H.; Tang, W.; Martásek, P.; Roman, L.J.; Poulos, T.L.; Silverman, R.B. 2-Aminopyridines with a Truncated Side Chain To Improve Human Neuronal Nitric Oxide Synthase Inhibitory Potency and Selectivity. J. Med. Chem. 2015, 58, 5548–5560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Repasky, M.P.; Shelley, M.; Friesner, R.A. Flexible Ligand Docking with Glide; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Chapter 8. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings; Elsevier Science, B.V.: Amsterdam, The Netherlands, 1997; Volume 23, pp. 3–25. [Google Scholar]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Zakharov, A.; Filimonov, D.; Poroikov, V. QSAR Modelling of Rat Acute Toxicity on the Basis of PASS Prediction. Mol. Inform. 2011, 30, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yang, S.; Xu, H.; Tao, X. Molecular docking analysis of triptoquinones from genus Tripterygium with iNOS and in silico ADMET prediction. SN Appl. Sci. 2019, 1, 1533. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Banu, N.; Alam, N.; Islam, M.N.; Islam, S.; Sakib, S.A.; Hanif, N.B.; Chowdhury, M.R.; Tareq, A.M.; Chowdhury, K.H.; Jahan, S.; et al. Insightful Valorization on Biological Activities of Pani Heloch Leaves through Experimental and Computer-Aided Mechanisms. Molecules 2020, 25, 5153. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn. Mag. 2011, 7, 325–333. [Google Scholar] [PubMed] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.A.; bin Imran, T.; Islam, S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J. Biol. Sci. 2013, 20, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Guha, B.; Arman, M.; Islam, M.N.; Tareq, S.M.; Rahman, M.M.; Sakib, S.A.; Mutsuddy, R.; Tareq, A.M.; Emran, T.B.; Alqahtani, A.M. Unveiling pharmacological studies provide new insights on Mangifera longipes and Quercus gomeziana. Saudi J. Biol. Sci. 2021, 28, 183–190. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Rakib, A.; Mitra, S.; Tareq, A.T.; Emran, T.B.; Ud-Daula, S.A.F.M.; Amin, M.N.; Simal-Gandara, J. The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives. Plants 2021, 10, 63. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Etoundi, C.B.; Kuaté, D.; Ngondi, J.L.; Oben, J. Anti-Amylase, Anti-Lipase and Antioxidant Effects of Aqueous Extracts of Some Cameroonian Spices. J. Nat. Prod. 2010, 3, 165–171. [Google Scholar]
- Wang, Y.; Huang, S.; Shao, S.; Qian, L.; Xu, P. Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: Antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Ind. Crop Prod. 2012, 37, 520–526. [Google Scholar] [CrossRef]
- Gulati, V.; Harding, I.H.; Palombo, E.A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement. Altern. Med. 2012, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Grover, N.; Patni, V. Phytochemical characterization using various solvent extracts and GC-MS analysis of methanolic extract of Woodfordia fruticosa (L.) Kurz. leaves. Int. J. Pharm. Pharm. Sci. 2013, 5, 291–295. [Google Scholar]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent Advances in Understanding the Anti-Diabetic Actions of Dietary Flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human α-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Ramachandran, N. Polyphenols of the flowers and leaves of Woodfordia fructicosa. Indian J. Pharm. 1976, 38, 110–111. [Google Scholar]
- Yao, A.M.; Ma, F.F.; Zhang, L.L.; Feng, F. Effect of aqueous extract and fractions of Zhi-Zi-Hou-Pu decoction against depression in inescapable stressed mice: Restoration of monoamine neurotransmitters in discrete brain regions. Pharm. Biol. 2013, 51, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.H.; Cheng, X.M.; Shen, J.S.; Wang, Z.T. Antidepressant effect of Yueju-Wan ethanol extract and its fractions in mice models of despair. J. Ethnopharmacol. 2008, 117, 339–344. [Google Scholar] [CrossRef]
- Subhan, F.; Karim, N.; Gilani, A.H.; Sewell, R.D. Terpenoid content of Valeriana wallichii extracts and antidepressant-like response profiles. Phytother. Res. 2010, 24, 686–691. [Google Scholar] [CrossRef]
- Shen, Y.-H.; Zhou, Y.; Zhang, C.; Liu, R.-H.; Su, J.; Liu, X.-H.; Zhang, W.-D. Antidepressant effects of methanol extract and fractions of Bacopa monnieri. Pharm. Biol. 2009, 47, 340–343. [Google Scholar] [CrossRef] [Green Version]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Capra, J.C.; Dalmarco, J.B.; Pizzolatti, M.G.; Rodrigues, A.L.; Psychiatry, B. Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: Involvement of the monoaminergic system. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 642–650. [Google Scholar] [CrossRef]
- Jang, D.-P.; Lee, S.-H.; Park, C.-W.; Lee, S.-Y.; Kim, Y.-B.; Cho, Z.-H. Effects of fluoxetine on the rat brain in the forced swimming test: A [F-18] FDG micro-PET imaging study. Neurosci. Lett. 2009, 451, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Stukalin, Y.; Lan, A.; Einat, H.J.N.; Reviews, B. Revisiting the validity of the mouse tail suspension test: Systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci. Biobehav. Rev. 2020, 112, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Page, M.E.; Detke, M.J.; Dalvi, A.; Kirby, L.G.; Lucki, I.J.P. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacol. 1999, 147, 162–167. [Google Scholar] [CrossRef]
- Petreanu, M.; Maia, P.; da Rocha Pittarello, J.L.; Loch, L.C.; Delle Monache, F.; Perez, A.L.; Solano-Arias, G.; Cechinel Filho, V.; de Souza, M.M.; Niero, R.J.N.-S. Antidepressant-like effect and toxicological parameters of extract and withanolides isolated from aerial parts of Solanum capsicoides All.(Solanaceae). Arch. Pharmacol. 2019, 392, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Can, Ö.D.; Turan, N.; Demir Özkay, Ü.; Öztürk, Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017, 190, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Raj, V.; Arya, J.; Balakrishnan, S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur. J. Pharmacol. 2012, 682, 118–125. [Google Scholar] [CrossRef]
- Dhingra, D.; Chhillar, R. Antidepressant-like activity of ellagic acid in unstressed and acute immobilization-induced stressed mice. Pharmacol. Rep. 2012, 64, 796–807. [Google Scholar] [CrossRef]
- Gerzson, M.F.B.; Victoria, F.N.; Radatz, C.S.; de Gomes, M.G.; Boeira, S.P.; Jacob, R.G.; Alves, D.; Jesse, C.R.; Savegnago, L. In vitro antioxidant activity and in vivo antidepressant-like effect of α-(phenylselanyl) acetophenone in mice. Pharmacol. Biochem. Behav. 2012, 102, 21–29. [Google Scholar] [CrossRef]
- Barauna, S.C.; Delwing-Dal Magro, D.; Brueckheimer, M.B.; Maia, T.P.; Sala, G.A.B.N.; Döhler, A.W.; Harger, M.C.; de Melo, D.F.M.; de Gasper, A.L.; Alberton, M.D.; et al. Antioxidant and antidepressant-like effects of Eugenia catharinensis D. Legrand in an animal model of depression induced by corticosterone. Metab. Brain Dis. 2018, 33, 1985–1994. [Google Scholar] [CrossRef]
- Maes, M.; Fišar, Z.; Medina, M.; Scapagnini, G.; Nowak, G.; Berk, M. New drug targets in depression: Inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 2012, 20, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Herken, H.; Gurel, A.; Selek, S.; Armutcu, F.; Ozen, M.E.; Bulut, M.; Kap, O.; Yumru, M.; Savas, H.A.; Akyol, O. Adenosine Deaminase, Nitric Oxide, Superoxide Dismutase, and Xanthine Oxidase in Patients with Major Depression: Impact of Antidepressant Treatment. Arch. Med. Res. 2007, 38, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Zafir, A.; Ara, A.; Banu, N. In vivo antioxidant status: A putative target of antidepressant action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rakib, A.; Islam, M.A.; Khanam, B.H.; Faiz, F.B.; Paul, A.; Chy, M.N.U.; Bhuiya, N.M.A.; Uddin, M.M.N.; Ullah, S.A. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clin Phytosci. 2019, 5, 36. [Google Scholar] [CrossRef]
- Dalmagro, A.P.; Camargo, A.; Zeni, A.L.B. Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice. Metab. Brain Dis. 2017, 32, 1963–1973. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Alorainy, M.S.; El-Ashmawy, I.M.; Fat’hi, S.J.P. Potential antidepressant constituents of Nigella sativa seeds. Pharmacogn. Mag. 2016, 12 (Suppl. 1), S27. [Google Scholar] [CrossRef] [Green Version]
- Chhillar, R.; Dhingra, D.J.F. Antidepressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress. Fundam. Clin. Pharmacol. 2013, 27, 409–418. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided Drug Des. 2012, 7, 146–157. [Google Scholar] [CrossRef]
- de Ruyck, J.; Brysbaert, G.; Blossey, R.; Lensink, M.F. Molecular docking as a popular tool in drug design, an in silico travel. Adv. Appl. Bioinform. Chem. 2016, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Owens, M.J.; Nemeroff, C.B. The serotonin transporter and depression. Depress Anxiety 1998, 8, 5–12. [Google Scholar] [CrossRef]
- Dailly, E.; Chenu, F.; Renard, C.E.; Bourin, M. Dopamine, depression and antidepressants. Fundam. Clin. Pharmacol. 2004, 18, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Daws, L.C. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol. Ther. 2009, 121, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzoor, S.; Hoda, N. A comprehensive review of monoamine oxidase inhibitors as Anti-Alzheimer’s disease agents: A review. Eur. J. Med. Chem. 2020, 206, 112787. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.; Johnson, S.; Ou, X.-M. Monoamine oxidases in major depressive disorder and alcoholism. Drug Discov. Ther. 2012, 6, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: From neurotransmitter imbalance to impaired neurogenesis. J. Neural Transm. 2018, 125, 53–66. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for improved antioxidant and antidepressant-like activity. Drug Deliv. 2012, 19, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Joca, S.R.; Sartim, A.G.; Roncalho, A.L.; Diniz, C.F.; Wegener, G.J.C. Nitric oxide signalling and antidepressant action revisited. Cell Tissue Res. 2019, 377, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.G.; Hu, Y.; Hua, Y.; Hu, M.; Luo, C.X.; Han, X.; Zhu, X.J.; Wang, B.; Xu, J.S.; Zhu, D.Y. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J. Neurochem. 2007, 103, 1843–1854. [Google Scholar] [CrossRef]
- Rakib, A.; Ahmed, S.; Islam, M.A.; Haye, A.; Uddin, S.N.; Uddin, M.M.N.; Hossain, M.K.; Paul, A.; Emran, T.B. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci. Nutr. 2020, 8, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Ignácio, Z.M.; Réus, G.Z.; Abelaira, H.M.; de Moura, A.B.; de Souza, T.G.; Matos, D.; Goldim, M.P.; Mathias, K.; Garbossa, L.; Petronilho, F.; et al. Acute and chronic treatment with quetiapine induces antidepressant-like behavior and exerts antioxidant effects in the rat brain. Metab. Brain Dis. 2017, 32, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sahakian, D.; de Morais, S.; Xu, J.; Polzer, R.; Winter, S. The Role of Absorption, Distribution, Metabolism, Excretion and Toxicity in Drug Discovery. Curr. Top. Med. Chem. 2005, 3, 1125–1154. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov. Today 2001, 6, 357–366. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Wilkinson, B. Drug discovery beyond the ‘rule-of-five’. Curr. Opin. Biotechnol. 2007, 18, 478–488. [Google Scholar] [CrossRef]
- Quinn, R.J.; Carroll, A.R.; Pham, N.B.; Baron, P.; Palframan, M.E.; Suraweera, L.; Pierens, G.K.; Muresan, S. Developing a drug-like natural product library. J. Nat. Prod. 2008, 71, 464–468. [Google Scholar] [CrossRef]
- Egbert, M.; Whitty, A.; Keserü, G.M.; Vajda, S. Why Some Targets Benefit from beyond Rule of Five Drugs. Am. Chem. Soc. 2019, 62, 10005–10025. [Google Scholar] [CrossRef]
- Lin, J.H.; Yamazaki, M. Role of P-Glycoprotein in Pharmacokinetics. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2017, 19, 38–54. [Google Scholar] [CrossRef]
- Rahman, J.; Tareq, A.M.; Hossain, M.M.; Sakib, S.A.; Islam, M.N.; Ali, M.H.; Uddin, A.B.M.N.; Hoque, M.; Nasrin, M.S.; Emran, T.B.; et al. Biological Evaluation, DFT Calculations and Molecular Docking Studies on the Antidepressant and Cytotoxicity Activities of Cycas pectinata Buch.-Ham. Compounds. Pharmaceuticals 2020, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Emon, N.U.; Shahriar, S.; Richi, F.T.; Haque, M.R.; Islam, M.N.; Sakib, S.A.; Ganguly, A. Pharmacological and computer-aided studies provide new insights into Millettia peguensis Ali (Fabaceae). Saudi Pharma. J. 2020, 28, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.I.; Barua, N.; Tareq, A.M.; Alam, N.; Prova, R.J.; Mamun, M.N.; Sayeed, M.A.; Chowdhury, M.A.U.; Emran, T.B. Possible neuropharmacological effects of Adenia trilobata (Roxb.) in the Swiss Albino mice model. Future J. Pharm. Sci. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Jyoti, M.A.; Barua, N.; Hossain, M.S.; Hoque, M.; Bristy, T.A.; Mahmud, S.; Kamruzzaman; Adnan, M.; Chy, M.N.U.; Paul, A.; et al. Unravelling the biological activities of the Byttneria pilosa leaves using experimental and computational approaches. Molecules 2020, 25, 4737. [Google Scholar] [CrossRef] [PubMed]
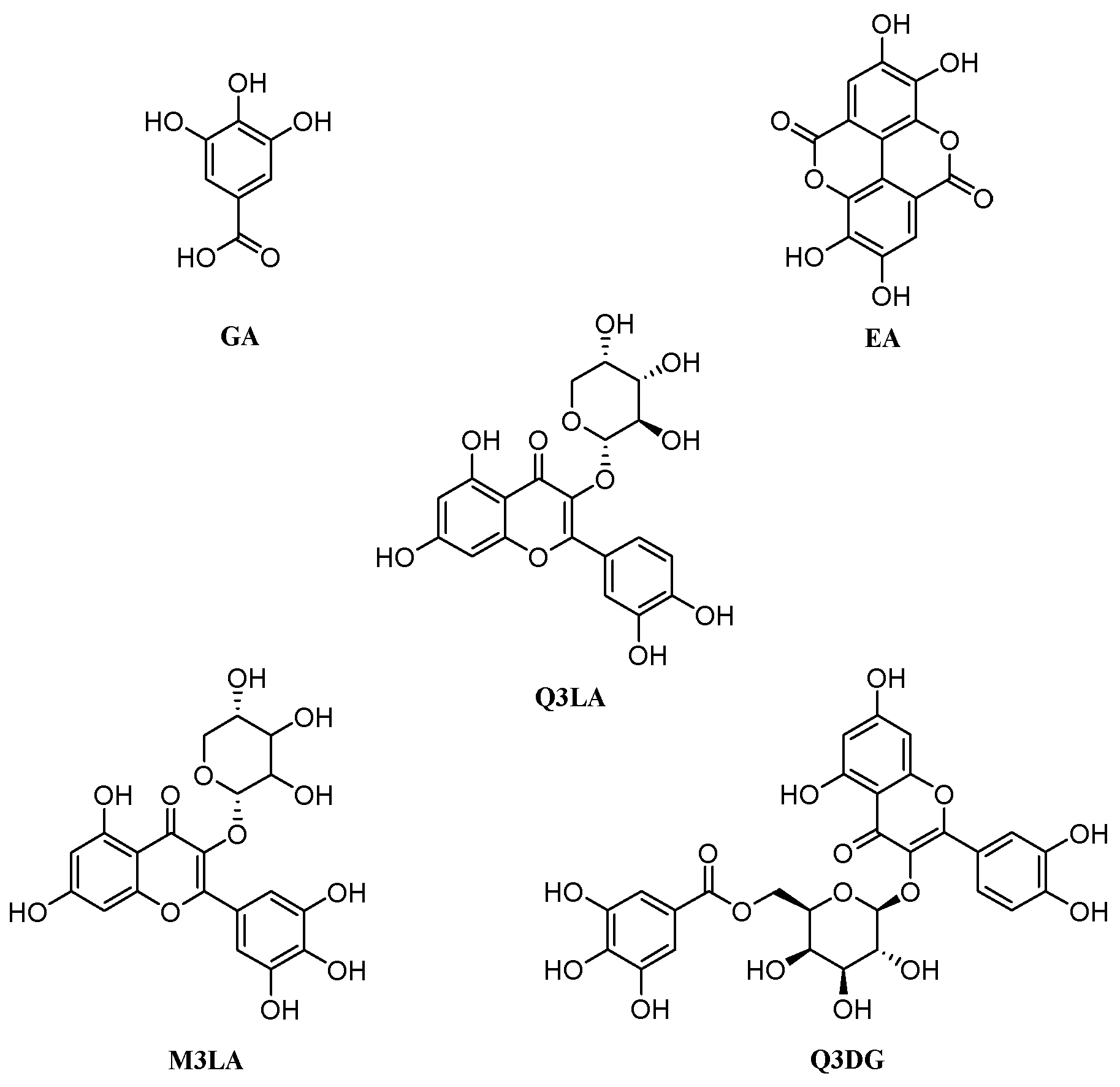
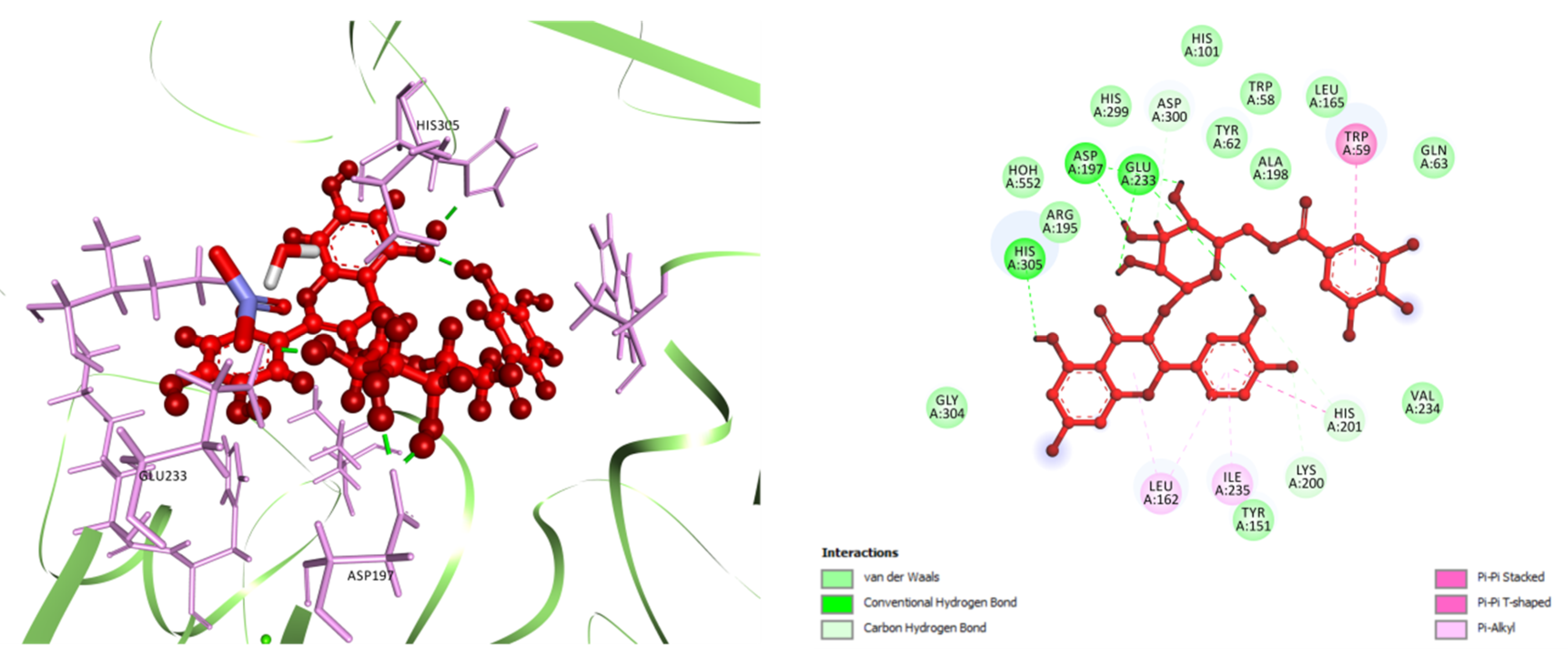
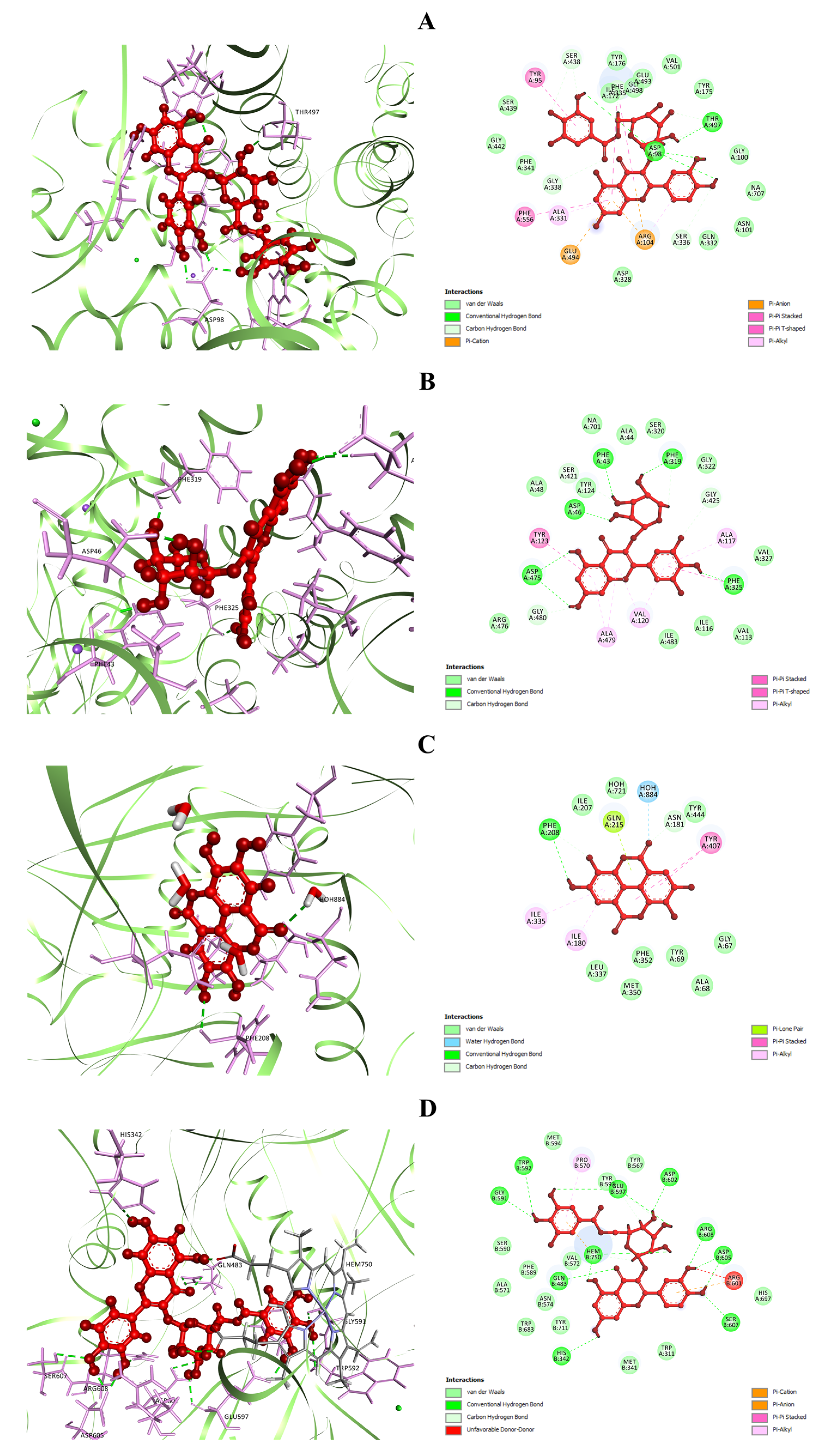
| Sl. No. | Compound Name | Plant Parts | Molecular Formula | Molecular Weight (g/mol) | Compound CID | Compound Class | References |
|---|---|---|---|---|---|---|---|
| 1 | Woodfordin A | Flowers | C75H56O48 | 1725.2 | 16130308 | Tannin | [6,71,72] |
| 2 | Woodfordin I | Leaves | C75H52O49 | 1737.2 | 16130412 | Tannin | [5,6] |
| 3 | Woodfordin B | Flowers | C75H54O48 | 1723.2 | 16130309 | Tannin | [6,71,72] |
| 4 | Woodfordin C | Flowers | C75H52O48 | 1721.2 | 16131173 | Tannin | [6,71,72] |
| 5 | Woodfordin D | Flowers | C109H76O70 | 2505.7 | 16131182 | Tannin | [6,73] |
| 6 | Octacosanol | Stems | C28H58O | 410.8 | 68406 | Fatty alcohol | [4,6,74] |
| 7 | β-sitosterol | Stems | C29H50O | 414.7 | 222284 | Phytosterols | [4,6,74,75] |
| 8 | Hecogenin | Flowers | C27H42O4 | 430.6 | 91453 | Triterpenoid | [4,6,75,76] |
| 9 | Meso-inositol | Flowers | C6H12O6 | 180.16 | 892 | Phytosterols | [4,6,75,76] |
| 10 | Lupeol | Leaves | C30H50O | 426.7 | 259846 | Triterpenoid | [6,75,76] |
| 11 | Betulin | Leaves | C30H50O2 | 442.7 | 72326 | Triterpenoid | [6,75,76] |
| 12 | Betulinic acid | Leaves | C30H48O3 | 456.7 | 64971 | Triterpenoid | [6,75,76] |
| 13 | Oleanolic acid | Leaves | C30H48O3 | 456.7 | 10494 | Triterpenoid | [6,75,76] |
| 14 | Ursolic acid | Leaves | C30H48O3 | 456.7 | 64945 | Triterpenoid | [6,76] |
| 15 | Gallic acid | Leaves, flowers and stems | C7H6O5 | 170.12 | 370 | Phenolic acid | [6,73,77,78,79] |
| 16 | Ellagic acid | Leaves and flowers | C14H6O8 | 302.19 | 5281855 | Phenolic acid | [6,79,80] |
| 17 | Bergenin | Stems | C14H16O9 | 328.27 | 66065 | Glycoside | [6,77] |
| 18 | Norbergenin | Stems | C13H14O9 | 314.24 | 73192 | Glycoside | [6,77] |
| 19 | Chrysophanol-8-O-β-D glucopyranoside | Flowers | C21H20O9 | 416.4 | 442731 | Glycoside | [4,6] |
| 20 | Lawsone | Leaves | C10H6O3 | 174.15 | 6755 | Naphthoquinone | [6,81] |
| 21 | Quercetin 3-rhamnoside | Flowers | C21H20O11 | 448.4 | 5280459 | Glycoside | [4,6] |
| 22 | Quercetin 3-β-L-arabinoside | Flowers and leaves | C20H18O11 | 434.3 | 10252339 | Glycoside | [6,80] |
| 23 | Quercetin 3-O-β-L-arabinopyranoside | Leaves | C26H28O15 | 580.5 | 21722036 | Glycoside | [6,73] |
| 24 | Quercetin 3-O-β-D-xylopyranoside | Leaves | C20H18O11 | 434.3 | 5320861 | Glycoside | [6,73] |
| 25 | Quercetin 3-O-(6”-galloyl)- β-D- galactopyranoside | Leaves | C28H24O16 | 616.5 | 5491814 | Glycoside | [6,73] |
| 26 | Quercetin 3-O-β-D-galactoside | Flowers and leaves | C21H20O12 | 464.4 | 5281643 | Glycoside | [6,73] |
| 27 | Myricetin 3-O-α-L-arabinopyranoside | Leaves | C37H58O10 | 662.8 | 24721386 | Glycoside | [6,73] |
| 28 | Quercetin 3-O-(6”-galloyl)- β-D- galactopyranoside | Leaves | C28H24O16 | 616.5 | 5491814 | Glycoside | [6,77,79] |
| 29 | Naringenin 7-O-glucoside | Flowers | C21H22O10 | 434.4 | 92794 | Glycoside | [4,6,79] |
| 30 | Kaempferol 3-O-glucoside | Flowers | C21H20O11 | 448.4 | 5282102 | Myricetin glycosides, trihydroxyflavone | [4,6,79] |
| 31 | Pelargonidin 3,5-diglucoside | Flowers | C27H31ClO15 | 631 | 167642 | Anthocyanidin pigment | [6,80] |
| 32 | Cyanidin 3,5-diglucoside | Flowers | C27H31O16 | 611.5 | 441688 | Anthocyanidin pigment | [6,82] |
| 33 | 1,2,3,6-tetra-O-galloyl- β-D-glucose | Flowers | C34H28O22 | 788.6 | 5153644 | Tannin | [6,71,72,83,84,85] |
| 34 | 1,2,4,6-tetra-O-galloyl-β-D-glucose | Flowers | C34H28O22 | 788.6 | 14464350 | Tannin | [6,71,72,83,84,85] |
| 35 | 1,2,3,4,6-penta-O-galloyl-β-D-glucose | Flowers | C41H32O26 | 940.7 | 65238 | Tannin | [6,71,72,83,84,85] |
| 36 | Tellimagrandin I | Flowers | C34H26O22 | 786.6 | 442690 | Tannin | [6,71,72,83,84,85] |
| 37 | Gemin D | Flowers | C27H22O18 | 634.5 | 471119 | Tannin | [6,71,72,83,84,85] |
| 38 | Heterophylliin A | Flowers | C34H26O22 | 786.6 | 471120 | Tannin | [6,71,72,83,84,85] |
| 39 | Oenothein B | Flowers | C68H50O44 | 1571.1 | 16132398 | Tannin | [6,71,72,83,84,85] |
| 40 | Isoschimawalin A | Flowers | C55H34O35 | 1254.8 | 16130370 | Tannin | [6,85] |
| 41 | Woodfordin E | Flowers | C75H56O48 | 1725.2 | 16130308 | Tannin | [6,85] |
| 42 | Woodfordin F | Flowers | − | − | − | Tannin | [6,73,85] |
| 43 | Woodfordin G | Flowers | − | − | − | Tannin | [6,73,85] |
| 44 | Woodfordin H | Flowers | − | − | − | Tannin | [6,73,85] |
| 45 | Woodfruticosin | Leaves | C75H52O48 | 1721.2 | 16131173 | Tannin | [6,71,72] |
| 46 | Oenothein-C | Flowers | C34H24O22 | 784.5 | 9962370 | Flavanone | [86] |
| 47 | Naringenin | Flowers | C15H12O5 | 272.25 | 932 | Flavanone | [79] |
| 48 | Kaempferol | Flowers | C15H10O6 | 286.24 | 5280863 | Flavonoid | [79] |
| 49 | Quercetin | Flowers | C15H10O7 | 302.23 | 5280343 | Flavonoid | [79] |
| Compound(s) | Abbreviation | PubChem CID | Molecular Formula |
|---|---|---|---|
| Gallic acid | GA | 370 | C7H6O5 |
| Methyl tri-O-methylgallate | MTMG | 15956 | C11H14O5 |
| Ellagic acid | EA | 5281855 | C14H6O8 |
| Quercetin 3-O-α-L-arabinopyranoside | Q3LA | 5481224 | C20H18O11 |
| Myricetin 3-O-α-L-arabinopyranoside | M3LA | 44259439 | C20H18O12 |
| Quercetin 3-O-(6″-galloyl)-β-D-galactopyranoside | Q3DG | 5491814 | C28H24O16 |
| Sample(s) | Total Phenolic Content (mg GAE/gm of Dried Extract) | Total Flavonoid Content (mg QE/gm of Dried Extract) | IC50 (µg/mL) |
|---|---|---|---|
| MEWF | 254.42 ± 1.53 a | 41.44 ± 0.99 c | 1.86 ± 0.16 a |
| NHFMEWF | 16.63 ± 1.99 c | 371.10 ± 1.99 a | 47.03 ± 0.57 c |
| EAFMEWF | 238.04 ± 1.01 b | 97.11 ± 0.67 b | 4.30 ± 0.28 b |
| AA | - | - | 0.22 ± 0.02 a |
| Protein(s) | HPA | SERT3 | DAT | MAO-A | nNOS |
|---|---|---|---|---|---|
| PDB ID(s) | 3BAJ | 5I6X | 4M48 | 2Z5Y | 4UH5 |
| Compound(s) | Docking Scores (kcal/mol) | ||||
| GA | −5.136 | −5.859 | −4.838 | −7.86 | −4.688 |
| MTMG | −3.429 | −4.782 | −6.015 | −6.532 | −3.699 |
| EA | −5.08 | −6.415 | −7.658 | −7.951 | −5.329 |
| Q3LA | −6.84 | −8.398 | −9.794 | - | −5.378 |
| M3LA | −6.105 | −7.378 | −10.796 | - | −5.912 |
| Q3DG | −7.41 | −8.678 | −10.62 | - | −8.449 |
| Standard | −7.752 | −9.426 | −7.159 | −6.782 | - |
| Compound(s) | Lipinski Rules | Lipinski’s Violation(s) | ||||
|---|---|---|---|---|---|---|
| MW | HBA | HBD | LogP | nRB | ||
| Acceptable Range | <500 | ≤10 | ≤5 | ≤5 | ≤10 | |
| GA | 170.12 | 5 | 4 | 0.21 | 1 | 0 |
| MTMG | 226.23 | 5 | 0 | 1.78 | 5 | 0 |
| EA | 302.19 | 8 | 4 | 1 | 0 | 0 |
| Q3LA | 434.35 | 11 | 7 | 0 | 3 | 2 |
| M3LA | 450.35 | 12 | 8 | −0.48 | 3 | 2 |
| Q3DG | 616.48 | 16 | 10 | −0.11 | 7 | 3 |
| Compound(s) | Absorption | Metabolism | |||||
|---|---|---|---|---|---|---|---|
| GI Absorption | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | |
| GA | High | No | No | No | No | No | Yes |
| MTMG | High | No | No | No | No | No | No |
| EA | High | No | Yes | No | No | No | No |
| Q3LA | Low | No | No | No | No | No | No |
| M3LA | Low | No | No | No | No | No | No |
| Q3DG | Low | No | No | No | No | No | No |
| Compound(s) | Toxicological Parameters | ||||
|---|---|---|---|---|---|
| Ames Toxicity | Hepatoxicity | Skin Sensitization | Rat Oral Acute Toxicity | ||
| LD50 (mg/kg) | Toxicity Classification | ||||
| GA | No | No | No | 1606 | Class 4 in AD |
| MTMG | Yes | No | No | 2815 | Class 5 in AD |
| EA | No | No | No | 1712 | Class 4 in AD |
| Q3LA | No | No | No | 2745 | Class 5 in AD |
| M3LA | No | No | No | 2748 | Class 5 in AD |
| Q3DG | No | No | No | 3501 | Class 5 in AD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayab, M.A.; Chowdhury, K.A.A.; Jabed, M.; Mohammed Tareq, S.; Kamal, A.T.M.M.; Islam, M.N.; Uddin, A.M.K.; Hossain, M.A.; Emran, T.B.; Simal-Gandara, J. Antioxidant-Rich Woodfordia fruticosa Leaf Extract Alleviates Depressive-Like Behaviors and Impede Hyperglycemia. Plants 2021, 10, 287. https://doi.org/10.3390/plants10020287
Tayab MA, Chowdhury KAA, Jabed M, Mohammed Tareq S, Kamal ATMM, Islam MN, Uddin AMK, Hossain MA, Emran TB, Simal-Gandara J. Antioxidant-Rich Woodfordia fruticosa Leaf Extract Alleviates Depressive-Like Behaviors and Impede Hyperglycemia. Plants. 2021; 10(2):287. https://doi.org/10.3390/plants10020287
Chicago/Turabian StyleTayab, Mohammed Abu, Kazi Ashfak Ahmed Chowdhury, Md. Jabed, Syed Mohammed Tareq, A. T. M. Mostafa Kamal, Mohammad Nazmul Islam, A. M. Kafil Uddin, Mohammad Adil Hossain, Talha Bin Emran, and Jesus Simal-Gandara. 2021. "Antioxidant-Rich Woodfordia fruticosa Leaf Extract Alleviates Depressive-Like Behaviors and Impede Hyperglycemia" Plants 10, no. 2: 287. https://doi.org/10.3390/plants10020287










