Extracellular Vesicles Do Not Mediate the Anti-Inflammatory Actions of Mouse-Derived Adipose Tissue Mesenchymal Stem Cells Secretome
Abstract
:1. Introduction
2. Results
2.1. Characterization of EV
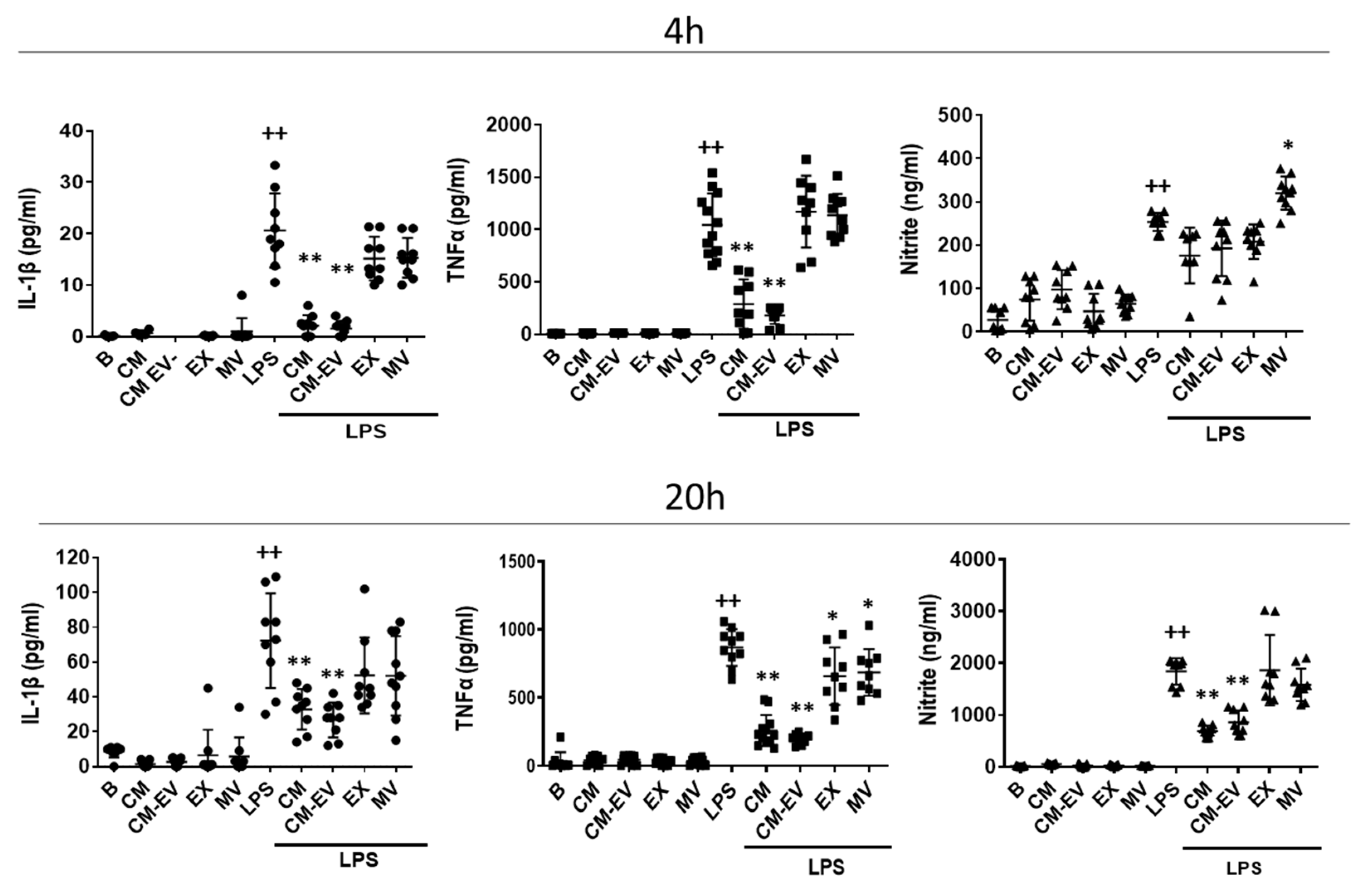
2.2. Release of Pro-Inflammatory Mediators
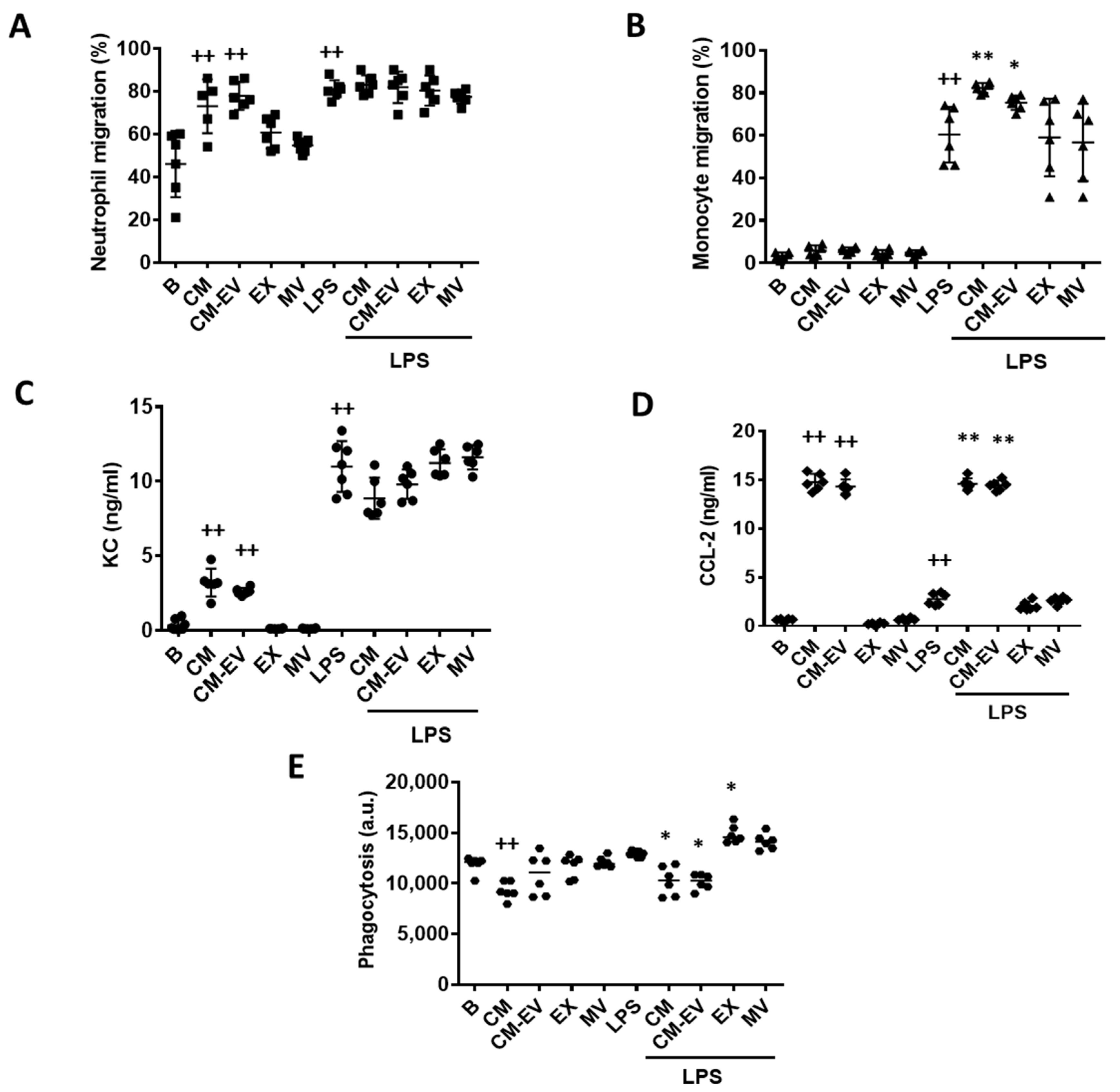
2.3. Cell Migration
2.4. Phagocytic Activity
2.5. Expression of Cluster of Differentiation 14 (CD14) and Toll-Like Receptor 4 (TLR4)
2.6. Nuclear Factor κB (NF-κB) Translocation
2.7. Adenosine Triphosphate (ATP) Release
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation of ASC and Preparation of CM
4.3. Isolation and Culture of Peritoneal Macrophages
4.4. Isolation and Culture of Neutrophils and Monocytes
4.5. Isolation of EV from ASC CM
4.6. MTT Assay
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Determination of Nitric Oxide
4.9. Cell Migration
4.10. Phagocytosis
4.11. Immunofluorescence
4.12. Determination of ATP Concentration
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Lie, P.; Miao, T.; Yu, M.; Lu, Q.; Feng, T.; Li, J.; Zu, T.; Liu, X.; Li, H. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol. Med. Rep. 2015, 12, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abumaree, M.; Al, J.M.; Pace, R.A.; Kalionis, B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012, 8, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mal, N.; Kiedrowski, M.; Chacko, M.; Askari, A.T.; Popovic, Z.B.; Koc, O.N.; Penn, M.S. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007, 21, 3197–3207. [Google Scholar] [CrossRef]
- Ma, O.K.F.; Chan, K.H. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J. Stem Cells 2016, 8, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Adreini, A.; Santarlasci, V.; Mazzinghi, B. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Uccelli, A.; Pistoia, V.; Moretta, L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007, 28, 219–226. [Google Scholar] [CrossRef]
- Tyndall, A.; Walker, U.A.; Cope, A.; Dazzi, F.; Bari, C.; Fibbe, W. Immunomodulatory properties of mesenchymal stem cells: A review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res. Ther. 2007, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010, 19, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tofiño-Vian, M.; Guillen, M.I.; Alcaraz, M.J. Extracellular vesicles: A new therapeutic strategy for joint conditions. Biochem. Pharmacol. 2018, 153, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, H.S.; Hong, I.S. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Biancone, L.; Bruno, S.; Deregibus, M.C.; Tetta, C.; Camussi, G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol. Dial. Transplant. 2012, 27, 3037–3042. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; del Caz, M.D.P.; Castejón, M.A.; Alcaraz, M.J. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid. Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef] [Green Version]
- Tofiño-Vian, M.; Guillen, M.I.; Del Caz, M.D.P.; Silvestre, A.; Alcaraz, M.J. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Carceller, M.C.; Guillen, M.I.; Ferrandiz, M.L.; Alcaraz, M.J. Paracrine in vivo inhibitory effects of adipose tissue-derived mesenchymal stromal cells in the early stages of the acute inflammatory response. Cytotherapy 2015, 17, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zent, C.S.; Elliott, M.R. Maxed out macs: Physiologic cell clearance as a function of macrophage phagocytic capacity. FEBS J. 2017, 284, 1021–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef]
- Ulevitch, R.J.; Tobias, P.S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 1995, 13, 437–457. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Sakaki, H.; Tsukimoto, M.; Harada, H.; Moriyama, Y.; Kojima, S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2y11 receptor. PLoS ONE 2013, 8, e59778. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 2002, 20, S1–S13. [Google Scholar]
- Abdollahi-Roodsaz, S.; Joosten, L.A.; Roelofs, M.F.; Radstake, T.R.; Matera, G.; Popa, C.; van der Meer, J.W.; Netea, M.G.; van den Berg, W.B. Inhibition of toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007, 56, 2957–2967. [Google Scholar] [CrossRef]
- Beutler, B.A. The role of tumor necrosis factor in health and disease. J. Rheumatol. 1999, 26 (Suppl. 57), 16–21. [Google Scholar]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Sperlagh, B.; Hasko, G.; Nemeth, Z.; Vizi, E.S. ATP released by LPS increases nitric oxide production in RAW 264.7 macrophage cell line via P2z/P2x7 receptors. Neurochem. Int. 1998, 33, 209–215. [Google Scholar] [CrossRef]
- Perregaux, D.G.; Gabel, C.A. Post-translational processing of murine IL-1: Evidence that ATP-induced release of IL-1alpha and IL-1beta occurs via a similar mechanism. J. Immunol. 1998, 160, 2469–2477. [Google Scholar]
- Yang, F.; Feng, C.; Zhang, X.; Lu, J.; Zhao, Y. The diverse biological functions of neutrophils, beyond the defense against infections. Inflammation 2017, 40, 311–323. [Google Scholar] [CrossRef]
- Liew, P.X.; Kubes, P. The neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, J.; Kim, M.Y.; Bae, Y.S.; Ryu, S.H.; Lee, T.G.; Kim, J.H. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J. Proteome Res. 2010, 9, 1754–1762. [Google Scholar] [CrossRef]
- Dewald, O.; Zymek, P.; Winkelmann, K.; Koerting, A.; Ren, G.; Abou-Khamis, T.; Michael, L.H.; Rollins, B.J.; Entman, M.L.; Frangogiannis, N.G. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 2005, 96, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiang, Y.; Li, Y.; Wang, J.; Fan, L.; Scott, M.J.; Xiao, G.; Li, S.; Billiar, T.R.; Wilson, M.A.; et al. TLR4 signaling augments monocyte chemotaxis by regulating G protein-coupled receptor kinase 2 translocation. J. Immunol. 2013, 191, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Fullerton, J.N.; O’Brien, A.J.; Gilroy, D.W. Pathways mediating resolution of inflammation: When enough is too much. J. Pathol. 2013, 231, 8–20. [Google Scholar] [CrossRef]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Fierabracci, A.; Del, F.A.; Muraca, M. The immunoregulatory activity of mesenchymal stem cells: ‘state of art’ and ‘future avenues’. Curr. Med. Chem. 2016, 23, 3014–3024. [Google Scholar] [CrossRef] [PubMed]
- Monsel, A.; Zhu, Y.G.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, F.Y.; Xu, D.; Brint, E.K.; O’Neill, L.A. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Essani, A.E.; Szekanecz, Z.; Fox, D.A.; Shahrara, S. TLRs, future potential therapeutic targets for RA. Autoimmun. Rev. 2017, 16, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ospelt, C.; Gay, S. TLRs and chronic inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Misko, T.P.; Schilling, R.J.; Salvemini, D.; Moore, W.M.; Currie, M.G. A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 1993, 214, 11–16. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carceller, M.C.; Guillén, M.I.; Gil, M.L.; Alcaraz, M.J. Extracellular Vesicles Do Not Mediate the Anti-Inflammatory Actions of Mouse-Derived Adipose Tissue Mesenchymal Stem Cells Secretome. Int. J. Mol. Sci. 2021, 22, 1375. https://doi.org/10.3390/ijms22031375
Carceller MC, Guillén MI, Gil ML, Alcaraz MJ. Extracellular Vesicles Do Not Mediate the Anti-Inflammatory Actions of Mouse-Derived Adipose Tissue Mesenchymal Stem Cells Secretome. International Journal of Molecular Sciences. 2021; 22(3):1375. https://doi.org/10.3390/ijms22031375
Chicago/Turabian StyleCarceller, María Carmen, María Isabel Guillén, María Luisa Gil, and María José Alcaraz. 2021. "Extracellular Vesicles Do Not Mediate the Anti-Inflammatory Actions of Mouse-Derived Adipose Tissue Mesenchymal Stem Cells Secretome" International Journal of Molecular Sciences 22, no. 3: 1375. https://doi.org/10.3390/ijms22031375





