Association between the Mediterranean Diet and Metabolic Syndrome with Serum Levels of miRNA in Morbid Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Variables and Information about Lifestyle
2.3. Biochemical Determinations
2.4. MiRNA Determinations
2.5. Statistical Analysis
3. Results
3.1. Anthropometric and Biochemical Characteristics
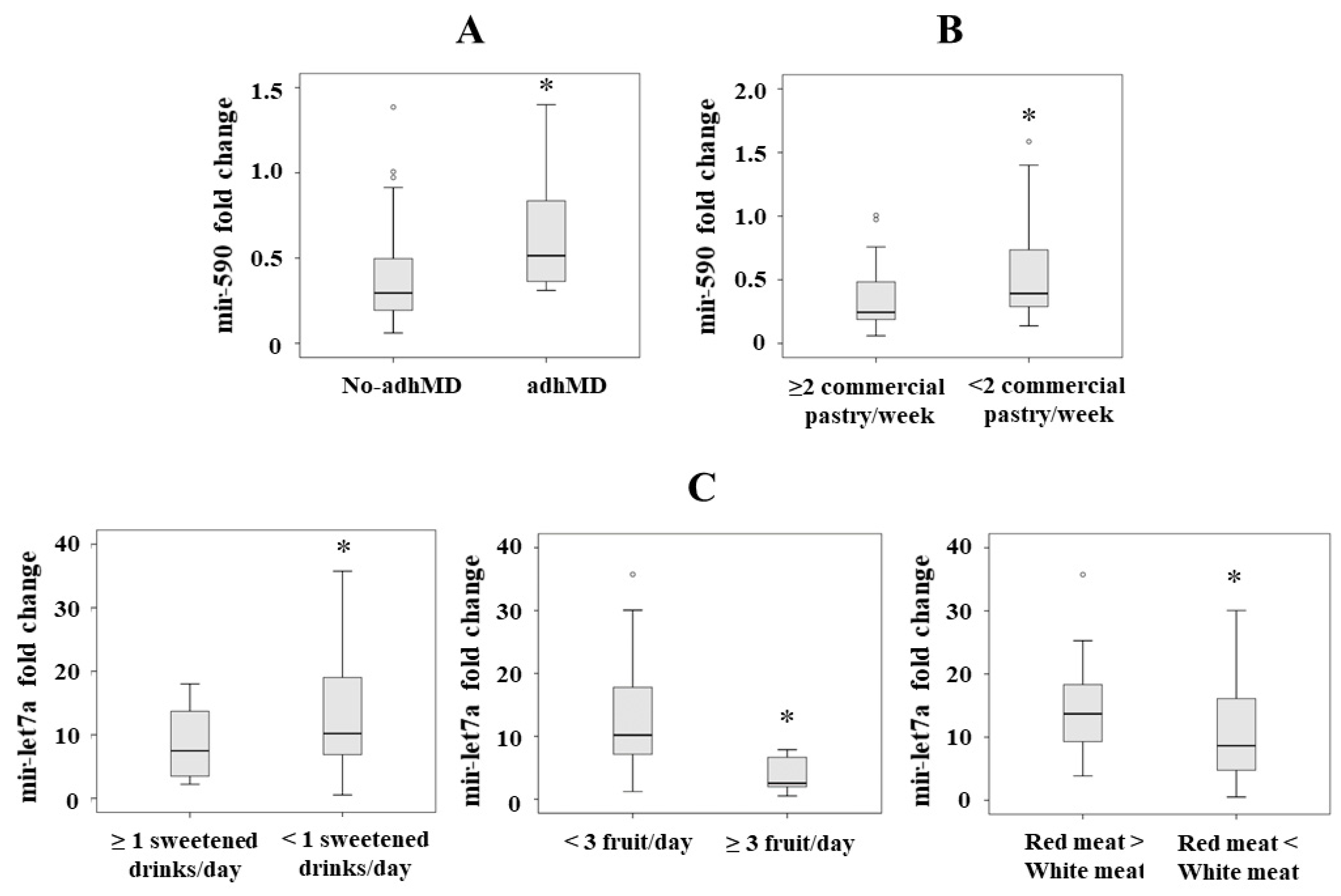
3.2. Serum miRNA Levels Regarding Adherence to the MD
3.3. Serum miRNA Levels with Respect to Individual MEDAS Items
3.3.1. Mir-590
3.3.2. Mir-Let7a
3.4. Relationship between Adherence to the MD and Metabolic Syndrome
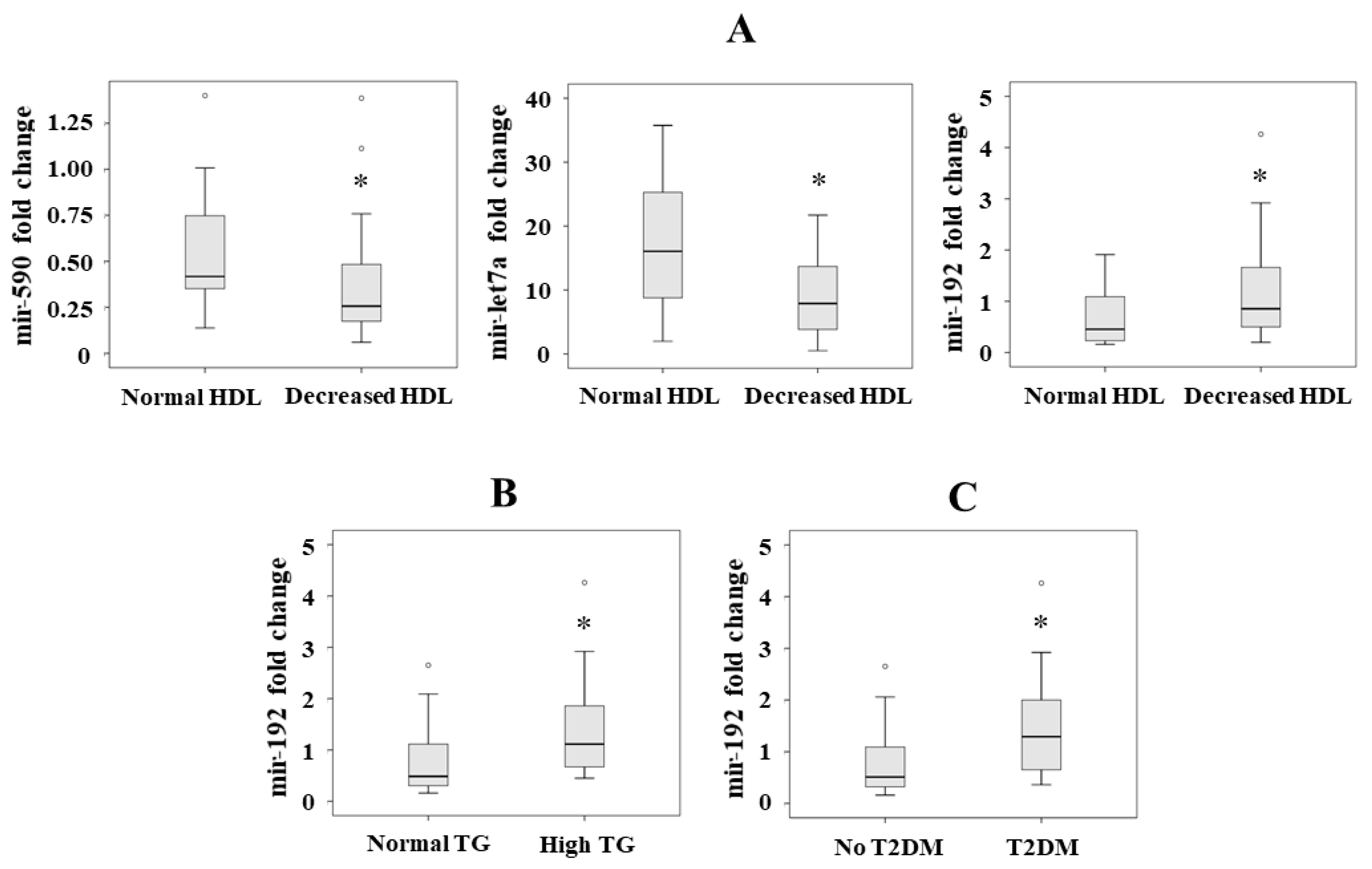
3.5. Serum miRNA Levels with Respect to Metabolic Syndrome
3.6. Association between Serum miRNA Levels and the Components of the Metabolic Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willett, W.C. The Mediterranean diet: Science and practice. Publ. Health Nutr. 2006, 9, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A. Mediterranean diet: The past and the present. Nutr. Metab. Cardiovasc. Dis. 2001, 11, 1–4. [Google Scholar] [PubMed]
- Wright, C.M. Biographical notes on Ancel Keys and Salim Yusuf: Origins and significance of the Seven Countries Study and the INTERHEART Study. J. Clin. Lipidol. 2011, 5, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Gotsis, E.; Anagnostis, P.; Mariolis, A.; Vlachou, A.; Katsiki, N.; Karagiannis, A. Health benefits of the mediterranean diet: An update of research over the last 5 years. Angiology 2015, 66, 304–318. [Google Scholar]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health. BioFactors 2013, 39, 335–342. [Google Scholar] [CrossRef]
- Dernini, S. The erosion and the renaissance of the Mediterranean diet: A sustainable cultural resource. Quad. Mediterr. 2011, 16, 75–82. [Google Scholar]
- Dernini, S.; Berry, E.M. Mediterranean diet: From a healthy diet to a sustainable dietary pattern. Front. Nutr. 2015, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: One-year results of the PREDIMED-Plus trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean diet: Insights from the PREDIMED study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvadó, J.; Fernández-Ballart, J.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Estruch, R.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, T.Y.N.; Wareham, N.J.; Khaw, K.T.; Imamura, F.; Forouhi, N.G. Prospective association of the Mediterranean diet with cardiovascular disease incidence and mortality and its population impact in a non-Mediterranean population: The EPIC-Norfolk study. BMC Med. 2016, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kura, B.; Parikh, M.; Slezak, J.; Pierce, G.N. The influence of diet on MicroRNAs that impact cardiovascular disease. Molecules 2019, 24, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynt, A.S.; Lai, E.C. Biological principles of microRNA-mediated regulation: Shared themes amid diversity. Nat. Rev. Genet. 2008, 9, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Chua, J.H.; Armugam, A.; Jeyaseelan, K. MicroRNAs: Biogenesis, function and applications. Curr. Opin. Mol. Ther. 2009, 11, 189–199. [Google Scholar]
- Lorente-Cebrián, S.; Herrera, K.; Milagro, F.I.; Sánchez, J.; de la Garza, A.L.; Castro, H. MiRNAs and novel food compounds related to the browning process. Int. J. Mol. Sci. 2019, 20, 5998. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Gao, C. MiR-590 inhibits endothelial cell apoptosis by inactivating the TLR4/NF-κB pathway in atherosclerosis. Yonsei Med. J. 2019, 60, 298–307. [Google Scholar] [CrossRef]
- Daimiel-Ruiz, L.; Klett-Mingo, M.; Konstantinidou, V.; Micó, V.; Aranda, J.F.; García, B.; Martínez-Botas, J.; Dávalos, A.; Fernández-Hernando, C.; Ordovás, J.M.; et al. Dietary lipids modulate the expression of miR-107, an miRNA that regulates the circadian system. Mol. Nutr. Food Res. 2015, 59, 552–565. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Giardina, S.; Salas-Salvadó, J.; Arcelin, P.; Bulló, M. Chronic pistachio intake modulates circulating microRNAs related to glucose metabolism and insulin resistance in prediabetic subjects. Eur. J. Nutr. 2017, 56, 2181–2191. [Google Scholar] [CrossRef]
- Quintanilha, B.J.; Reis, B.Z.; Silva Duarte, G.B.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of micrornas and nutrition in modulating inflammation and chronic diseases. Nutrients 2017, 27, 1168. [Google Scholar] [CrossRef]
- Izzotti, A.; Cartiglia, C.; Steele, V.E.; de Flora, S. MicroRNAs as targets for dietary and pharmacological inhibitors of mutagenesis and carcinogenesis. Mutat. Res. 2012, 751, 287–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pescador, N.; Pérez-Barba, M.; Ibarra, J.M.; Corbatón, A.; Martínez-Larrad, M.T.; Serrano-Ríos, M. Serum circulating microRNA profiling for identification of potential Type 2 diabetes and obesity biomarkers. PLoS ONE 2013, 8, e77251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Boer, E.J.; Slimani, N.; van’t Veer, P.; Boeing, H.; Feinberg, M.; Leclercq, C.; Trolle, E.; Amiano, P.; Andersen, L.F.; Freisling, H.; et al. The European food consumption validation project: Conclusions and recommendations. Eur. J. Clin. Nutr. 2011, 65, S102–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biró, G.; Hulshof, K.F.; Ovesen, L.; Amorim Cruz, J.A.; EFCOSUM Group. Selection of methodology to assess food intake. Eur. J. Clin. Nutr. 2002, 56, S25–S32. [Google Scholar] [CrossRef] [Green Version]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short screener is valid for assessing mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Hebestreit, K.; Yahiaoui-Doktor, M.; Engel, C.; Vetter, W.; Siniatchkin, M.; Erickson, N.; Halle, M.; Kiechle, M.; Bischoff, S.C. Validation of the German version of the Mediterranean Diet Adherence Screener (MEDAS) questionnaire. BMC Cancer 2017, 17, 341. [Google Scholar] [CrossRef]
- Bottcher, M.R.; Marincic, P.Z.; Nahay, K.L.; Baerlocher, B.E.; Willis, A.W.; Park, J.; Gaillard, P.; Greene, M.W. Nutrition knowledge and Mediterranean diet adherence in the southeast United States: Validation of a field-based survey instrument. Appetite 2017, 111, 166–176. [Google Scholar] [CrossRef]
- Papadaki, A.; Johnson, L.; Toumpakari, Z.; England, C.; Rai, M.; Toms, S.; Penfold, C.; Zazpe, I.; Martinez-Gonzalez, M.A.; Feder, G. Validation of the English version of the 14-Item Mediterranean diet adherence screener of the PREDIMED study, in people at high cardiovascular risk in the UK. Nutrients 2018, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.J.; Lee, H.; Yoon, Y.; Kim, H.M.; Chu, S.H.; Lee, J.W. Development and Validation of a Questionnaire to Measure Adherence to the Mediterranean Diet in Korean Adults. Nutrients 2020, 12, 1102. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi-roshan, M.; Salari, A.; Ggholipour, M.; Naghshbandi, M. Dietary adherence in people with cardiovascular risk factors living in Northern Iran. J. Babol. Univ. Med. Sci. 2017, 19, 62–68. [Google Scholar]
- Gnagnarella, P.; Draga, D.; Misotti, A.M.; Sieri, S.; Spaggiari, L.; Cassano, E.; Baldini, F.; Soldati, L.; Maisonneuve, P. Validation of a short questionnaire to record adherence to the Mediterranean diet: An Italian experience. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Abu-Saad, K.; Endevelt, R.; Goldsmith, R.; Shimony, T.; Nitsan, L.; Shahar, D.R.; Keinan-Boker, L.; Ziv, A.; Kalter-Leibovici, O. Adaptation and predictive utility of a Mediterranean diet screener score. Clin. Nutr. 2019, 38, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, M.J.; Rodrigues, A.M.; Salvador, C.; Dias, S.S.; de Sousa, R.D.; Mendes, J.M.; Coelho, P.S.; Branco, J.C.; Lopes, C.; Martinez-Gonzalez, M.A.; et al. Validation of the Telephone-Administered Version of the Mediterranean Diet Adherence Screener (MEDAS) Questionnaire. Nutrients 2020, 12, 1511. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the validity of the 14-item Mediterranean Diet Adherence Screener (MEDAS): A cross-national study in seven European countries around the Mediterranean region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pacheco, F.; Garcia-Serrano, S.; Garcia-Escobar, E.; Gutierrez-Repiso, C.; Garcia-Arnes, J.; Valdes, S.; Gonzalo, M.; Soriguer, F.; Moreno-Ruiz, F.J.; Rodriguez-Cañete, A.; et al. Effects of obesity/fatty acids on the expression of GPR120. Mol. Nutr. Food Res. 2014, 58, 1852–1860. [Google Scholar] [CrossRef]
- Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452.
- Soriguer, F.; Rojo-Martínez, G.; Dobarganes, M.C.; García Almeida, J.M.; Esteva, I.; Beltrán, M.; Ruiz De Adana, M.S.; Tinahones, F.; Gómez-Zumaquero, J.M.; García-Fuentes, E.; et al. Hypertension is related to the degradation of dietary frying oils. Am. J. Clin. Nutr. 2003, 78, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome-A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Ross, S.A.; Davis, C.D. The emerging role of microRNAs and nutrition in modulating health and disease. Annu. Rev. Nutr. 2014, 34, 305–336. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, B.; Ross, S.A.; Zempleni, J. Nutrition, microRNAs, and human health. Adv. Nutr. 2017, 8, 105–112. [Google Scholar]
- Cannataro, R.; Caroleo, M.C.; Fazio, A.; la Torre, C.; Plastina, P.; Gallelli, L.; Lauria, G.; Cione, E. Ketogenic diet and microRNAs linked to antioxidant biochemical homeostasis. Antioxidants 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Chen, M.; Xiao, Y.; Liang, Q.; Cai, Y.; Chen, L.; Fang, M. Bioinformatics analysis of microRNAs related to blood stasis syndrome in diabetes mellitus patients. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, X.; Shao, X. A Combination of human embryonic stem cell-derived pancreatic endoderm transplant with LDHA-repressing miRNA can attenuate high-fat diet induced Type II diabetes in mice. J. Diabetes Res. 2015, 2015, 796912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Xiang, Y.; Lv, Y.; Li, D.; Yu, L.; Guo, R. miR-590-3p mediates the protective effect of curcumin on injured endothelial cells induced by angiotensin II. A Am. J. Transl. Res. 2017, 9, 289–300. [Google Scholar]
- Lin, Y.; Ding, D.; Huang, Q.; Liu, Q.; Lu, H.; Lu, Y.; Chi, Y.; Sun, X.; Ye, G.; Zhu, H.; et al. Downregulation of miR-192 causes hepatic steatosis and lipid accumulation by inducing SREBF1: Novel mechanism for bisphenol A-triggered non-alcoholic fatty liver disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 869–882. [Google Scholar] [CrossRef]
- Liu, X.L.; Cao, H.X.; Wang, B.C.; Xin, F.Z.; Zhang, R.N.; Zhou, D.; Yang, R.X.; Zhao, Z.H.; Pan, Q.; Fan, J.G.; et al. MiR-192-5p regulates lipid synthesis in non-Alcoholic fatty liver disease through SCD-1. World J. Gastroenterol. 2017, 23, 8140–8151. [Google Scholar] [CrossRef]
- García-Serrano, S.; Moreno-Santos, I.; Garrido-Sánchez, L.; Gutierrez-Repiso, C.; García-Almeida, J.M.; García-Arnés, J.; Rivas-Marín, J.; Gallego-Perales, J.L.; García-Escobar, E.; Rojo-Martinez, G.; et al. Stearoyl-CoA desaturase-1 is associated with insulin resistance in morbidly obese subjects. Mol. Med. 2011, 17, 273–280. [Google Scholar] [CrossRef]
- Gil-Zamorano, J.; Martin, R.; Daimiel, L.; Richardson, K.; Giordano, E.; Nicod, N.; García-Carrasco, B.; Soares, S.M.A.; Iglesias-Gutiérrez, E.; Lasunción, M.A.; et al. Docosahexaenoic acid modulates the enterocyte Caco-2 cell expression of MicroRNAs involved in lipid metabolism. J. Nutr. 2014, 144, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Párrizas, M.; Brugnara, L.; Esteban, Y.; González-Franquesa, A.; Canivell, S.; Murillo, S.; Gordillo-Bastidas, E.; Cussó, R.; Cadefau, J.A.; García-Roves, P.M.; et al. Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J. Clin. Endocrinol. Metab. 2015, 100, E407–E415. [Google Scholar] [CrossRef] [Green Version]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [Green Version]
- Ranka, S.; Gee, J.M.; Biro, L.; Brett, G.; Saha, S.; Kroon, P.; Skinner, J.; Hart, A.R.; Cassidy, A.; Rhodes, M.; et al. Development of a food frequency questionnaire for the assessment of quercetin and naringenin intake. Eur. J. Clin. Nutr. 2008, 62, 1131–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Moghaddam, A.A.; Woodward, M.; Huxley, R. Obesity and risk of colorectal cancer: A meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 2533–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| 1. Do you use olive oil as the principal source of fat for cooking? | 1 point given: yes |
| 2. How much olive oil do you consume per day (including that used in frying, salads, meals eaten away from home, etc.)? | 1 point given if ≥4 Tbsp 1 |
| 3. How many servings of vegetables do you consume per day? Count garnish and side servings as 1/2 point; a full serving is 200 g. | 1 point given if ≥2 |
| 4. How many pieces of fruit (including fresh-squeezed juice) do you consume per day? | 1 point given if ≥3 |
| 5. How many servings of red meat, hamburgers, or sausages do you consume per day? A full serving is 100–150 g. | 1 point given if <1 |
| 6. How many servings (12 g) of butter, margarine, or cream do you consume per day? | 1 point given if <1 |
| 7. How many carbonated and/or sugar-sweetened beverages do you consume per day? | 1 point given if <1 2 |
| 8. Do you drink wine? How much do you consume per week? | 1 point given if ≥7 cups |
| 9. How many servings (150 g) of pulses do you consume per week? | 1 point given if ≥3 |
| 10. How many servings of fish/seafood do you consume per week? (100–150 g of fish, 4–5 pieces or 200 g of seafood) | 1 point given if ≥3 |
| 11. How many times do you consume commercial (not homemade) pastry such as cookies or cake per week? | 1 point given if <2 |
| 12. How many times do you consume nuts per week? (1 serving = 30 g) | 1 point given if ≥3 |
| 13. Do you prefer to eat chicken, turkey or rabbit instead of beef, pork, hamburgers, or sausages? | 1 point given: yes |
| 14. How many times per week do you consume boiled vegetables, pasta, rice, or other dishes with a sauce of tomato, garlic, onion, or leeks sautéed in olive oil? | 1 point given if ≥2 |
| Variables | Total Population | Non-adhMD | adhMD | p * |
|---|---|---|---|---|
| N (men/women) | 58 (17/41) | 50 (15/35) | 8 (2/6) | Ns |
| Age (years) | 54.1 ± 14.4 | 53.7 ± 14.3 | 57.1 ± 15.2 | Ns |
| Weight (kg) | 111.2 ± 17.4 | 110.9 ± 17.9 | 113.2 ± 16.2 | Ns |
| BMI (kg/m2) | 44.1 ± 4.6 | 44.0 ± 4.7 | 44.4 ± 4.0 | Ns |
| Waist (cm) | 126.8 ± 13.7 | 127.3 ± 13.5 | 124.1 ± 15.6 | Ns |
| Hip (cm) | 132.5 ± 11.1 | 131.4 ± 10.2 | 137.2 ± 14.7 | Ns |
| SBP (mmHg) | 135.1 ± 14.7 | 136.6 ± 16.1 | 133.8 ± 10.0 | Ns |
| DBP (mmHg) | 81.4 ± 9.3 | 82.1 ± 9.2 | 73.6 ± 9.6 | 0.04 |
| Glucose (mg/dL) | 122.0 ± 40.2 | 124.1 ± 42.5 | 109.3 ± 17.5 | Ns |
| Cholesterol (mg/dL) | 195.9 ± 36.7 | 194.9 ± 36.8 | 202.0 ± 39.7 | Ns |
| HDL (mg/dL) | 47.6 ± 11.6 | 47.1 ± 11.9 | 50.1 ± 9.8 | Ns |
| LDL (mg/dL) | 107.0 ± 28.4 | 106.0 ± 28.1 | 112.8 ± 31.0 | Ns |
| Triglycerides (mg/dL) | 143.4 ± 65.9 | 145.5 ± 69.4 | 130.4 ± 40.0 | Ns |
| Insulin (mIU/L) | 19.1 ± 17.5 | 20.2 ± 18.6 | 12.2 ± 5.0 | Ns |
| HOMA-IR | 5.9 ± 8.2 | 6.9 ± 8.8 | 3.3 ± 1.6 | Ns |
| %Patients who met the waist circumference criterion of MS | 100 | 100 | 100 | Ns |
| %Patients who met the triglycerides criterion of MS | 37.5 | 38.1 | 25.0 | Ns |
| %Patients who met the HDL criterion of MS | 60.7 | 63.8 | 50.0 | Ns |
| %Patients who met the hypertension criterion of MS | 72.4 | 73.5 | 75.0 | Ns |
| %Patients who met the glucose or T2DM criterion of MS | 71.9 | 72.9 | 62.5 | Ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontalba-Romero, M.I.; Lopez-Enriquez, S.; Lago-Sampedro, A.; García-Escobar, E.; Pastori, R.L.; Domínguez-Bendala, J.; Álvarez-Cubela, S.; Valdes, S.; Rojo, G.; Garcia-Fuentes, E.; et al. Association between the Mediterranean Diet and Metabolic Syndrome with Serum Levels of miRNA in Morbid Obesity. Nutrients 2021, 13, 436. https://doi.org/10.3390/nu13020436
Fontalba-Romero MI, Lopez-Enriquez S, Lago-Sampedro A, García-Escobar E, Pastori RL, Domínguez-Bendala J, Álvarez-Cubela S, Valdes S, Rojo G, Garcia-Fuentes E, et al. Association between the Mediterranean Diet and Metabolic Syndrome with Serum Levels of miRNA in Morbid Obesity. Nutrients. 2021; 13(2):436. https://doi.org/10.3390/nu13020436
Chicago/Turabian StyleFontalba-Romero, María I., Soledad Lopez-Enriquez, Ana Lago-Sampedro, Eva García-Escobar, Ricardo L. Pastori, Juan Domínguez-Bendala, Silvia Álvarez-Cubela, Sergio Valdes, Gemma Rojo, Eduardo Garcia-Fuentes, and et al. 2021. "Association between the Mediterranean Diet and Metabolic Syndrome with Serum Levels of miRNA in Morbid Obesity" Nutrients 13, no. 2: 436. https://doi.org/10.3390/nu13020436
APA StyleFontalba-Romero, M. I., Lopez-Enriquez, S., Lago-Sampedro, A., García-Escobar, E., Pastori, R. L., Domínguez-Bendala, J., Álvarez-Cubela, S., Valdes, S., Rojo, G., Garcia-Fuentes, E., Labajos-Manzanares, M. T., & García-Serrano, S. (2021). Association between the Mediterranean Diet and Metabolic Syndrome with Serum Levels of miRNA in Morbid Obesity. Nutrients, 13(2), 436. https://doi.org/10.3390/nu13020436







