Light, Sleep and Performance in Diurnal Birds
Abstract
:1. Introduction
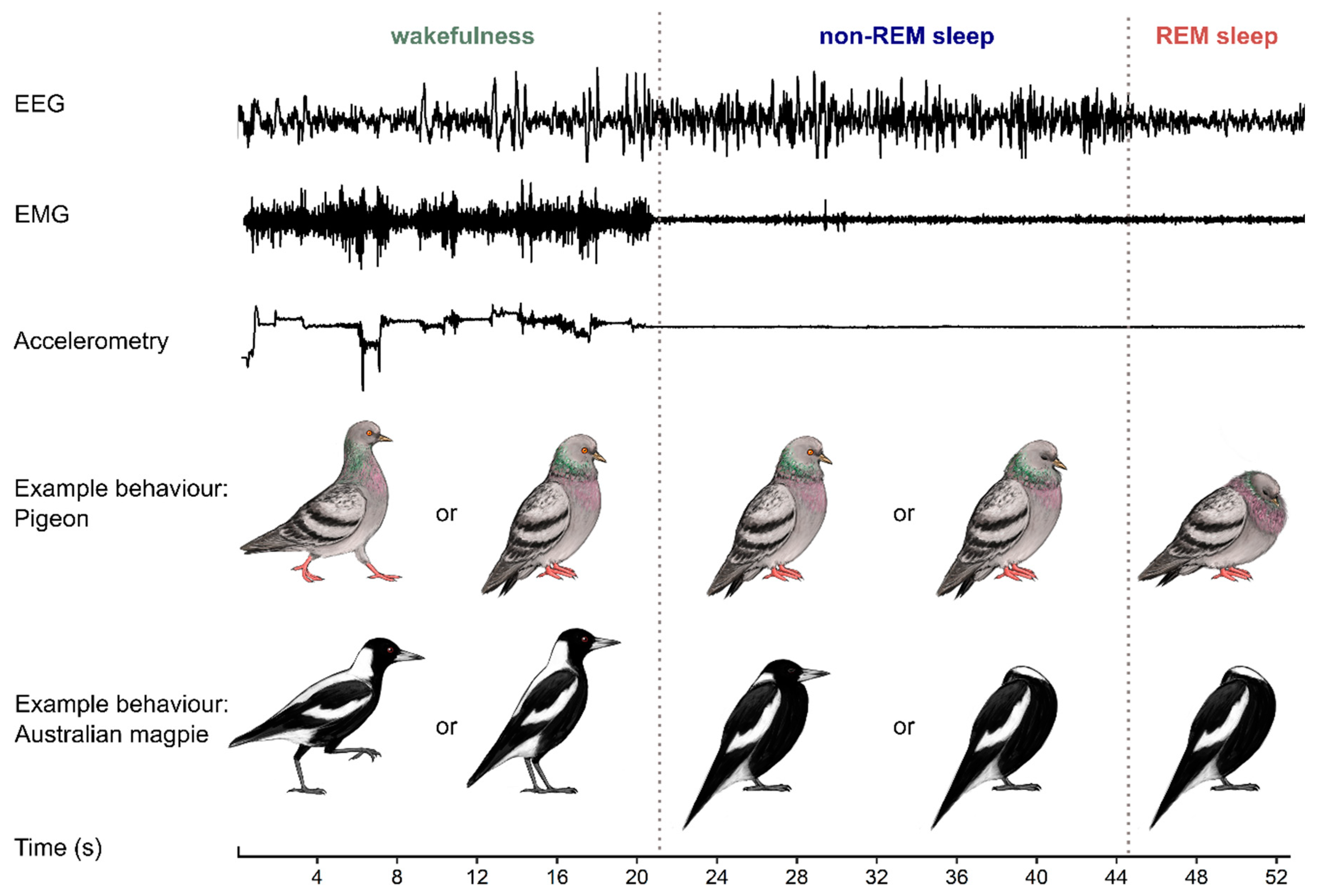
2. Characterizing Avian Sleep
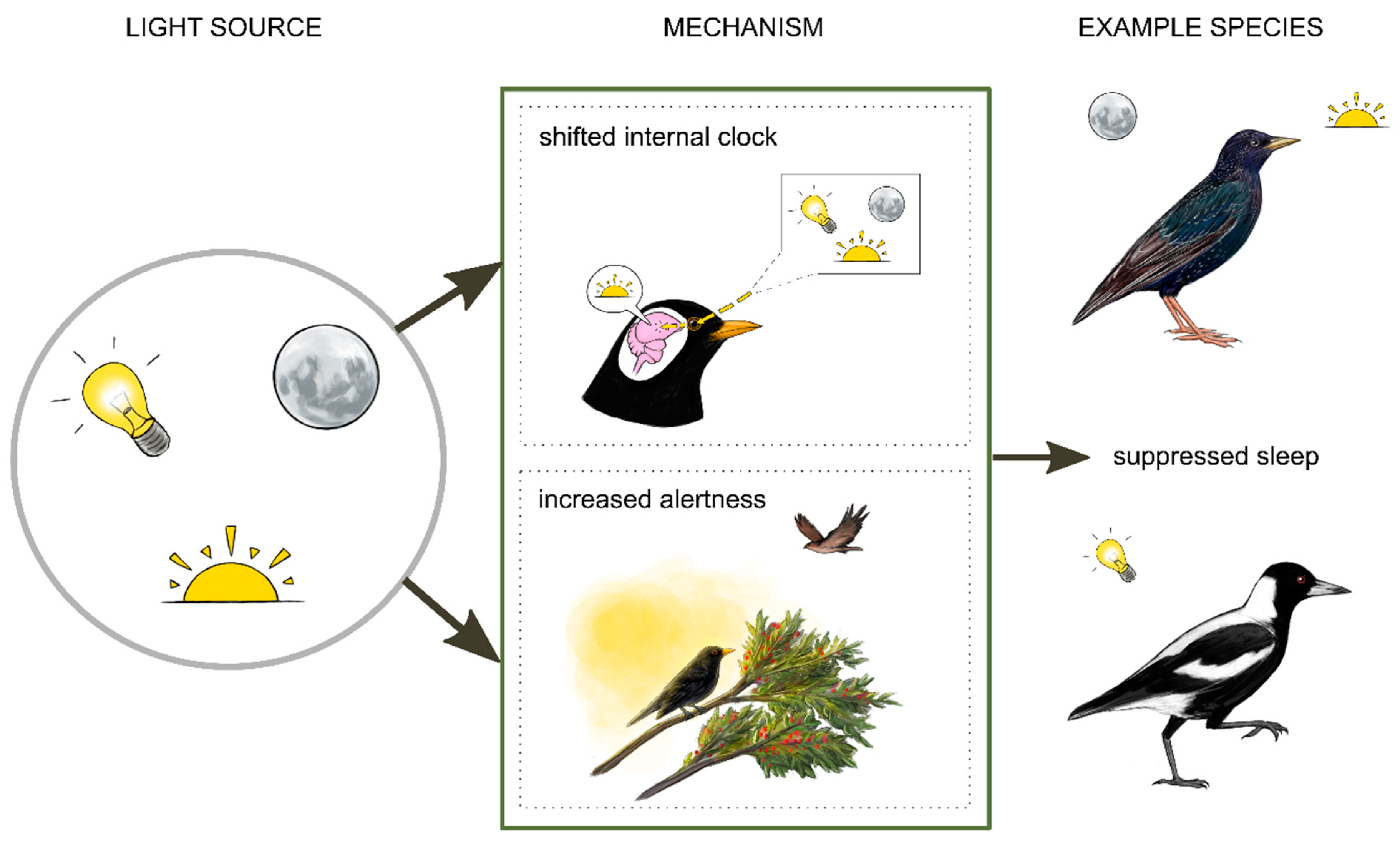
3. Light Regulates and Suppresses Sleep in Diurnal Birds
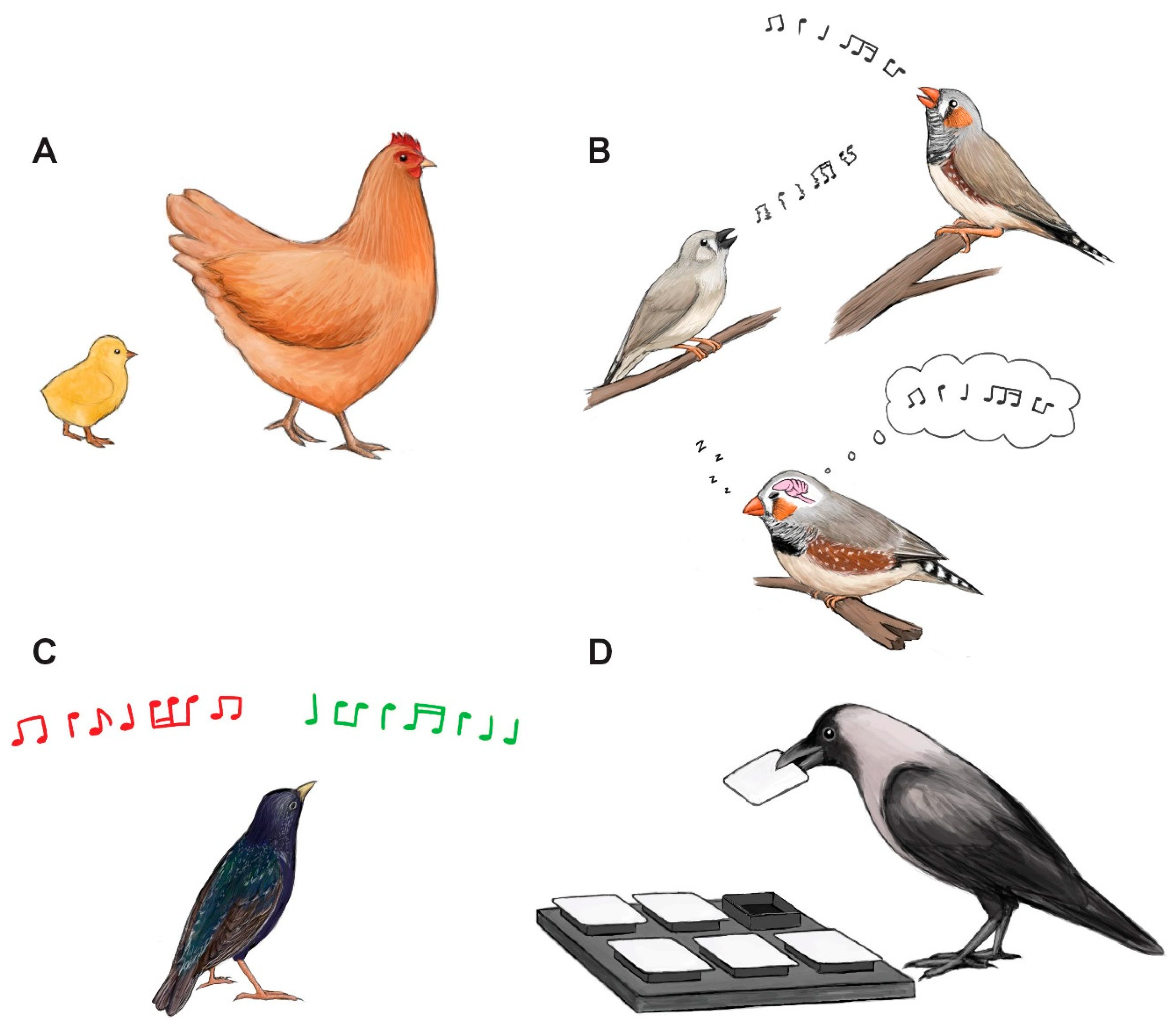
4. Sleep Affects Avian Performance
4.1. Imprinting
4.2. Song Learning
4.3. Auditory Discrimination
4.4. Spatial Learning
5. Evidence Linking Light, Sleep and Performance in Birds
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kanaya, H.J.; Park, S.; Kim, J.-H.; Kusumi, J.; Krenenou, S.; Sawatari, E.; Sato, A.; Lee, J.; Bang, H.; Kobayakawa, Y.; et al. A sleep-like state in Hydra unravels conserved sleep mechanisms during the evolutionary development of the central nervous system. Sci. Adv. 2020, 6, eabb9415. [Google Scholar] [CrossRef] [PubMed]
- Ungurean, G.; van der Meij, J.; Rattenborg, N.C.; Lesku, J.A. Evolution and plasticity of sleep. Curr. Opin. Physiol. 2020, 15, 111–119. [Google Scholar] [CrossRef]
- Lesku, J.A.; Roth, T.C.; Rattenborg, N.C.; Amlaner, C.J.; Lima, S.L. Phylogenetics and the correlates of mammalian sleep: A reappraisal. Sleep Med. Rev. 2008, 12, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Tefft, B.C. Acute sleep deprivation and culpable motor vehicle crash involvement. Sleep 2018, 41, zsy144. [Google Scholar] [CrossRef] [Green Version]
- Lima, S.L.; Rattenborg, N.C.; Lesku, J.A.; Amlaner, C.J. Sleeping under the risk of predation. Anim. Behav. 2005, 70, 723–736. [Google Scholar] [CrossRef]
- Lesku, J.A.; Rattenborg, N.C.; Valcu, M.; Vyssotski, A.L.; Kuhn, S.; Kuemmeth, F.; Heidrich, W.; Kempenaers, B. Adaptive sleep loss in polygynous pectoral sandpipers. Science 2012, 337, 1654–1658. [Google Scholar] [CrossRef] [Green Version]
- Rattenborg, N.C.; Voirin, B.; Cruz, S.M.; Tisdale, R.; Dell’Omo, G.; Lipp, H.P.; Wikelski, M.; Vyssotski, A.L. Evidence that birds sleep in mid-flight. Nat. Commun. 2016, 7, 12468. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, A.; Rattenborg, N.C.; Ruf, T.; McWilliams, S.R.; Cardinale, M.; Fusani, L. Sleeping unsafely tucked in to conserve energy in a nocturnal migratory songbird. Curr. Biol. 2019, 29, 2766–2772. [Google Scholar] [CrossRef] [Green Version]
- Siegel, J.M. Sleep viewed as a state of adaptive inactivity. Nat. Rev. Neurosci. 2009, 10, 747–753. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Swang, T.W.; Hamilton, I.M.; Best, J.A. State-dependent metabolic partitioning and energy conservation: A theoretical framework for understanding the function of sleep. PLoS ONE 2017, 12, e0185746. [Google Scholar] [CrossRef] [Green Version]
- Imeri, L.; Opp, M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derégnaucourt, S.; Mitra, P.P.; Fehér, O.; Pytte, C.; Tchernichovski, O. How sleep affects the developmental learning of bird song. Nature 2005, 433, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Kayser, M.S.; Yue, Z.F.; Sehgal, A. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science 2014, 344, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumberg, M.S. Developing sensorimotor systems in our sleep. Curr. Dir. Psychol. Sci. 2015, 24, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.P.; Stickgold, R. Sleep-dependent learning and memory consolidation. Neuron 2004, 44, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, D.; Reid, K. Fatigue, alcohol and performance impairment. Nature 1997, 388, 235. [Google Scholar] [CrossRef]
- Van Dongen, H.P.A.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Klein, B.A.; Klein, A.; Wray, M.K.; Mueller, U.G.; Seeley, T.D. Sleep deprivation impairs precision of waggle dance signaling in honey bees. Proc. Natl. Acad. Sci. USA 2010, 107, 22705–22709. [Google Scholar] [CrossRef] [Green Version]
- Kyba, C.C.M.; Kuester, T.; Sánchez de Miguel, A.; Baugh, K.; Jechow, A.; Hölker, F.; Bennie, J.; Elvidge, C.D.; Gaston, K.J.; Guanter, L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017, 3, e1701528. [Google Scholar] [CrossRef] [Green Version]
- Aulsebrook, A.E.; Jones, T.M.; Mulder, R.A.; Lesku, J.A. Impacts of artificial light at night on sleep: A review and prospectus. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.-M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing and next-morning alertness. Proc. Natl. Acad. Sci. USA 2014, 112, 1232–1237. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.H.; Lee, H.J.; Yoon, H.K.; Kang, S.G.; Bok, K.N.; Jung, K.Y.; Kim, L.; Lee, E.I. Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol. Int. 2016, 33, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wams, E.J.; Woelders, T.; Marring, I.; van Rosmalen, L.; Beersma, D.G.M.; Gordijn, M.C.M.; Hut, R.A. Linking light exposure and subsequent sleep: A field polysomnography study in humans. Sleep 2017, 40, zsx165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Ryu, S.H.; Lee, B.R.; Kim, K.H.; Lee, E.; Choi, J. Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 2015, 32, 1294–1310. [Google Scholar] [CrossRef]
- Gaston, K.J.; Duffy, J.P.; Bennie, J. Quantifying the erosion of natural darkness in the global protected area system. Conserv. Biol. 2015, 29, 1132–1141. [Google Scholar] [CrossRef]
- Gaston, K.J.; Duffy, J.P.; Gaston, S.; Bennie, J.; Davies, T.W. Human alteration of natural light cycles: Causes and ecological consequences. Oecologia 2014, 176, 917–931. [Google Scholar] [CrossRef] [Green Version]
- Van der Meij, J.; Rattenborg, N.C.; Beckers, G.J.L. Divergent neuronal activity patterns in the avian hippocampus and nidopallium. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Rattenborg, N.C.; Martinez-Gonzalez, D. Avian versus mammalian sleep: The fruits of comparing apples and oranges. Curr. Sleep Med. Rep. 2015, 1, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Emery, N.J.; Clayton, N.S. The mentality of crows: Convergent evolution of intelligence in corvids and apes. Science 2004, 306, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Vorster, A.P.; Born, J. Sleep and memory in mammals, birds and invertebrates. Neurosci. Biobehav. Rev. 2015, 50, 103–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesku, J.A.; Rattenborg, N.C. Avian sleep. Curr. Biol. 2014, 24, R12–R14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumberg, M.S.; Lesku, J.A.; Libourel, P.A.; Schmidt, M.H.; Rattenborg, N.C. What Is REM Sleep? Curr. Biol. 2020, 30, R38–R49. [Google Scholar] [CrossRef]
- Roth, T.C.; Lesku, J.A.; Amlaner, C.J.; Lima, S.L. A phylogenetic analysis of the correlates of sleep in birds. J. Sleep Res. 2006, 15, 395–402. [Google Scholar] [CrossRef]
- Canavan, S.V.; Margoliash, D. Budgerigars have complex sleep structure similar to that of mammals. PLoS Biol. 2020, 18, e3000929. [Google Scholar] [CrossRef]
- Rattenborg, N.C.; Martinez-Gonzalez, D.; Roth, T.C.; Pravosudov, V.V. Hippocampal memory consolidation during sleep: A comparison of mammals and birds. Biol. Rev. 2011, 86, 658–691. [Google Scholar] [CrossRef] [Green Version]
- Van der Meij, J.; Martinez-Gonzalez, D.; Beckers, G.J.L.; Rattenborg, N.C. Intra-“cortical” activity during avian non-REM and REM sleep: Variant and invariant traits between birds and mammals. Sleep 2019, 42, 1–13. [Google Scholar] [CrossRef]
- Rattenborg, N.C.; Martinez-Gonzalez, D.; Lesku, J.A. Avian sleep homeostasis: Convergent evolution of complex brains, cognition and sleep functions in mammals and birds. Neurosci. Biobehav. Rev. 2009, 33, 253–270. [Google Scholar] [CrossRef]
- Tobler, I. Phylogeny of sleep regulation. In Principles and Practice of Sleep Medicine, 5th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Elsevier Health Sciences: Philadelphia, PA, USA, 2011; pp. 112–125. [Google Scholar]
- Aulsebrook, A.E.; Jones, T.M.; Rattenborg, N.C.; Roth, T.C., II; Lesku, J.A. Sleep ecophysiology: Integrating neuroscience and ecology. Trends Ecol. Evol. 2016, 31, 590–599. [Google Scholar] [CrossRef]
- Lesku, J.A.; Meyer, L.C.R.; Fuller, A.; Maloney, S.K.; Dell’Omo, G.; Vyssotski, A.L.; Rattenborg, N.C. Ostriches sleep like platypuses. PLoS ONE 2011, 6, e23203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raap, T.; Pinxten, R.; Eens, M. Light pollution disrupts sleep in free-living animals. Sci. Rep. 2015, 5, 13557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulsebrook, A.E.; Lesku, J.A.; Mulder, R.A.; Goymann, W.; Vyssotski, A.L.; Jones, T.M. Streetlights disrupt night-time sleep in urban black swans. Front. Ecol. Evol. 2020, 8, 131. [Google Scholar] [CrossRef]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocrinol. 2014, 35, 76–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halford, S.; Pires, S.S.; Turton, M.; Zheng, L.; Gonzalez-Menendez, I.; Davies, W.L.; Peirson, S.N.; Garcia-Fernandez, J.M.; Hankins, M.W.; Foster, R.G. VA opsin-based photoreceptors in the hypothalamus of birds. Curr. Biol. 2009, 19, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- Dominoni, D.M. The effects of light pollution on biological rhythms of birds: An integrated, mechanistic perspective. J. Ornithol. 2015, 156, 409–418. [Google Scholar] [CrossRef]
- Honda, K.; Kondo, M.; Hiramoto, D.; Saneyasu, T.; Kamisoyama, H. Effects of continuous white light and 12h white-12h blue light-cycles on the expression of clock genes in diencephalon, liver, and skeletal muscle in chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 207, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Bian, J.; Wang, Z.; Dong, Y.; Chen, Y. Effect of monochromatic light on circadian rhythmic expression of clock genes and arylalkylamine N-acetyltransferase in chick retina. Chronobiol. Int. 2017, 34, 1149–1157. [Google Scholar] [CrossRef]
- Stuber, E.F.; Baumgartner, C.; Dingemanse, N.J.; Kempenaers, B.; Mueller, J.C. Genetic correlates of individual differences in sleep behavior of free-living great tits (Parus major). G3 Genes Genomes Genet. 2016, 6, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Russ, A.; Rüger, A.; Klenke, R. Seize the night: European blackbirds (Turdus merula) extend their foraging activity under artificial illumination. J. Ornithol. 2014, 156, 123–131. [Google Scholar] [CrossRef]
- Kempenaers, B.; Borgström, P.; Loës, P.; Schlicht, E.; Valcu, M. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 2010, 20, 1735–1739. [Google Scholar] [CrossRef] [Green Version]
- Van Hasselt, S.J.; Rusche, M.; Vyssotski, A.L.; Verhulst, S.; Rattenborg, N.C.; Meerlo, P. Sleep time in the European starling is strongly affected by night length and moon phase. Curr. Biol. 2020, 30, 1664–1671.e2. [Google Scholar] [CrossRef] [Green Version]
- Aulsebrook, A.E.; Connelly, F.; Johnsson, R.D.; Jones, T.M.; Mulder, R.A.; Hall, M.L.; Vyssotski, A.L.; Lesku, J.A. White and amber light at night disrupt sleep physiology in birds. Curr. Biol. 2020, 30, 3657–3663.e5. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.M.; Goymann, W.; Helm, B.; Partecke, J. Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): Implications of city life for biological time-keeping of songbirds. Front. Zool. 2013, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Berger, R.J.; Phillips, N.H. Constant light suppresses sleep and circadian-rhythms in pigeons without consequent sleep rebound in darkness. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 267, R945–R952. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.G.; Vyazovskiy, V.V.; Cirelli, C.; Tononi, G.; Benca, R.M. Homeostatic regulation of sleep in the white-crowned sparrow (Zonotrichia leucophrys gambelii). BMC Neurosci. 2008, 9, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Gonzalez, D.; Lesku, J.A.; Rattenborg, N.C. Increased EEG spectral power density during sleep following short-term sleep deprivation in pigeons (Columba livia): Evidence for avian sleep homeostasis. J. Sleep Res. 2008, 17, 140–153. [Google Scholar] [CrossRef]
- Rattenborg, N.C.; Obermeyer, W.H.; Vacha, E.; Benca, R.M. Acute effects of light and darkness on sleep in the pigeon (Columba livia). Physiol. Behav. 2005, 84, 635–640. [Google Scholar] [CrossRef]
- Van Hasselt, S.J.; Mekenkamp, G.J.; Vyssotski, A.L.; Piersma, T.; Rattenborg, N.C.; Meerlo, P. Sleep in barnacle geese is strongly affected by season and moon phase. J. Sleep Res. 2020, 29, 167–168. [Google Scholar]
- Steinmeyer, C.; Schielzeth, H.; Mueller, J.C.; Kempenaers, B. Variation in sleep behaviour in free-living blue tits, Cyanistes caeruleus: Effects of sex, age and environment. Anim. Behav. 2010, 80, 853–864. [Google Scholar] [CrossRef]
- Schlicht, L.; Kempenaers, B. The effects of season, sex, age and weather on population-level variation in the timing of activity in Eurasian Blue Tits Cyanistes caeruleus. Ibis 2020, 162, 1146–1162. [Google Scholar] [CrossRef] [Green Version]
- Van Hasselt, S.J.; Hut, R.A.; Allocca, G.; Vyssotski, A.L.; Piersma, T.; Rattenborg, N.C.; Meerlo, P. Cloud cover amplifies the sleep-suppressing effect of artificial light at night in geese. Environ. Pollut. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, A.L.; Hall, M.L.; Jones, T.M. The effect of variation in moonlight on nocturnal song of a diurnal bird species. Behav. Ecol. Sociobiol. 2020, 74, 109. [Google Scholar] [CrossRef]
- Stuber, E.F.; Dingemanse, N.J.; Kempenaers, B.; Mueller, J.C. Sources of intraspecific variation in sleep behaviour of wild great tits. Anim. Behav. 2015, 106, 201–221. [Google Scholar] [CrossRef]
- Ouyang, J.Q.; de Jong, M.; van Grunsven, R.H.A.; Matson, K.D.; Haussmann, M.F.; Meerlo, P.; Visser, M.E.; Spoelstra, K. Restless roosts: Light pollution affects behavior, sleep, and physiology in a free-living songbird. Glob. Chang. Biol. 2017, 23, 4987–4994. [Google Scholar] [CrossRef]
- Amichai, E.; Kronfeld-Schor, N. Artificial light at night promotes activity throughout the night in nesting common swifts (Apus apus). Sci. Rep. 2019, 9, 11052. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; He, Y.; Kou, H.; Ju, Z.; Gao, X.; Zhao, H. The effects of artificial light at night on Eurasian tree sparrow (Passer montanus): Behavioral rhythm disruption, melatonin suppression and intestinal microbiota alterations. Ecol. Indic. 2020, 108, 105702. [Google Scholar] [CrossRef]
- Dominoni, D.; Smit, J.A.H.; Visser, M.E.; Halfwerk, W. Multisensory pollution: Artificial light at night and anthropogenic noise have interactive effects on activity patterns of great tits (Parus major). Environ. Pollut. 2020, 256, 113314. [Google Scholar] [CrossRef]
- Alaasam, V.J.; Duncan, R.; Casagrande, S.; Davies, S.; Sidher, A.; Seymoure, B.; Shen, Y.; Zhang, Y.; Ouyang, J.Q. Light at night disrupts nocturnal rest and elevates glucocorticoids at cool color temperatures. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 465–472. [Google Scholar] [CrossRef]
- Batra, T.; Malik, I.; Kumar, V. Illuminated night alters behaviour and negatively affects physiology and metabolism in diurnal zebra finches. Environ. Pollut. 2019, 254, 112916. [Google Scholar] [CrossRef]
- De Jong, M.; Jeninga, L.; Ouyang, J.Q.; van Oers, K.; Spoelstra, K.; Visser, M.E. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 2016, 155, 172–179. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Caro, S.P.; Gienapp, P.; Spoelstra, K.; Visser, M.E. Early birds by light at night: Effects of light color and intensity on daily activity patterns in blue tits. J. Biol. Rhythm. 2017, 32, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Peng, X.; Ren, Z.; Liu, M.; Dang, R.; Chen, Y.; Liu, F. The effect of artificial light with different SPDs and intensities on the sleep onset of silvereyes. Biol. Rhythm Res. 2018, 50, 787–804. [Google Scholar] [CrossRef]
- Yorzinski, J.L.; Chisholm, S.; Byerley, S.D.; Coy, J.R.; Aziz, A.; Wolf, J.A.; Gnerlich, A.C. Artificial light pollution increases nocturnal vigilance in peahens. PeerJ 2015, 3, e1174. [Google Scholar] [CrossRef] [Green Version]
- Raap, T.; Pinxten, R.; Eens, M. Artificial light at night disrupts sleep in female great tits (Parus major) during the nestling period and is followed by a sleep rebound. Environ. Pollut. 2016, 215, 125–134. [Google Scholar] [CrossRef]
- Raap, T.; Sun, J.; Pinxten, R.; Eens, M. Disruptive effects of light pollution on sleep in free-living birds: Season and/or light intensity-dependent? Behav. Processes 2017, 144, 13–19. [Google Scholar] [CrossRef]
- Sun, J.; Raap, T.; Pinxten, R.; Eens, M. Artificial light at night affects sleep behaviour differently in two closely related songbird species. Environ. Pollut. 2017, 231, 882–889. [Google Scholar] [CrossRef]
- Batra, T.; Malik, I.; Prabhat, A.; Bhardwaj, S.K.; Kumar, V. Sleep in unnatural times: Illuminated night negatively affects sleep and associated hypothalamic gene expressions in diurnal zebra finches. Proc. R. Soc. B 2020, 287, 20192952. [Google Scholar] [CrossRef]
- Caorsi, V.; Sprau, P.; Zollinger, S.A.; Brumm, H. Nocturnal resting behaviour in urban great tits and its relation to anthropogenic disturbance and microclimate. Behav. Ecol. Sociobiol. 2019, 73, 19. [Google Scholar] [CrossRef] [Green Version]
- Raap, T.; Pinxten, R.; Eens, M. Cavities shield birds from effects of artificial light at night on sleep. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 449–456. [Google Scholar] [CrossRef]
- Weljie, A.M.; Meerlo, P.; Goel, N.; Sengupta, A.; Kayser, M.S.; Abel, T.; Birnbaum, M.J.; Dinges, D.F.; Sehgal, A. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc. Natl. Acad. Sci. USA 2015, 112, 2569–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raap, T.; Pinxten, R.; Eens, M. Artificial light at night causes an unexpected increase in oxalate in developing male songbirds. Conserv. Physiol. 2018, 6, coy005. [Google Scholar] [CrossRef]
- Ulgezen, Z.N.; Kapyla, T.; Meerlo, P.; Spoelstra, K.; Visser, M.E.; Dominoni, D.M. The preference and costs of sleeping under light at night in forest and urban great tits. Proc. R. Soc. B 2019, 286, 20190872. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [Green Version]
- Czeisler, C. Casting light on sleep deficiency. Nature 2013, 497, S13. [Google Scholar] [CrossRef] [PubMed]
- Dominoni, D.M.; Carmona-Wagner, E.O.; Hofmann, M.; Kranstauber, B.; Partecke, J. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J. Anim. Ecol. 2014, 83, 681–692. [Google Scholar] [CrossRef]
- Spoelstra, K.; Verhagen, I.; Meijer, D.; Visser, M.E. Artificial light at night shifts daily activity patterns but not the internal clock in the great tit (Parus major). Proc. R. Soc. B 2018, 285, 20172751. [Google Scholar] [CrossRef] [Green Version]
- Moaraf, S.; Vistoropsky, Y.; Pozner, T.; Heiblum, R.; Okuliarova, M.; Zeman, M.; Barnea, A. Artificial light at night affects brain plasticity and melatonin in birds. Neurosci. Lett. 2020, 716, 134639. [Google Scholar] [CrossRef]
- Zada, D.; Bronshtein, I.; Lerer-Goldshtein, T.; Garini, Y.; Appelbaum, L. Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat. Commun. 2019, 10, 895. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.L.; Kang, H.Y.; Xu, Q.W.; Chen, M.J.; Liao, Y.H.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Horn, G. Pathways of the past: The imprint of memory. Nat. Rev. Neurosci. 2004, 5, 108–120. [Google Scholar] [CrossRef]
- McCabe, B.J. Visual imprinting in birds: Behavior, models, and neural mechanisms. Front. Physiol. 2019, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Cipollaneto, J.; Horn, G.; McCabe, B.J. Hemispheric-asymmetry and imprinting: The effect of sequential lesions to the hyperstriatum ventrale. Exp. Brain Res. 1982, 48, 22–27. [Google Scholar]
- Horn, G.; Nicol, A.U.; Brown, M.W. Tracking memory’s trace. Proc. Natl. Acad. Sci. USA 2001, 98, 5282–5287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, C.; McCabe, B.J.; Nicol, A.U.; Grout, A.S.; Brown, M.W.; Horn, G. Dynamics effects of of a memory trace: Sleep on consolidation. Curr. Biol. 2008, 18, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honey, R.C.; Horn, G.; Bateson, P.; Walpole, M. Functionally distinct memories for imprinting stimuli: Behavioral and neural dissociations. Behav. Neurosci. 1995, 109, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.E.; McCabe, B.J.; Horn, G. Mechanisms of information-storage after imprinting in the domestic chick. Behav. Brain Res. 1987, 26, 209–210. [Google Scholar] [CrossRef]
- McCabe, B.J.; Cipollaneto, J.; Horn, G.; Bateson, P. Amnesic effects of bilateral lesions placed in the hyperstriatum ventrale of the chick after imprinting. Exp. Brain Res. 1982, 48, 13–21. [Google Scholar] [CrossRef]
- Inostroza, M.; Born, J. Sleep for preserving and transforming episodic memory. Annu. Rev. Neurosci. 2013, 36, 79–102. [Google Scholar] [CrossRef] [Green Version]
- Solodkin, M.; Cardona, A.; Corsicabrera, M. Paradoxical sleep augmentation after imprinting in the domestic chick. Physiol. Behav. 1985, 35, 343–348. [Google Scholar] [CrossRef]
- Lipkind, D.; Tchernichovski, O. Quantification of developmental birdsong learning from the subsyllabic scale to cultural evolution. Proc. Natl. Acad. Sci. USA 2011, 108, 15572–15579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brawn, T.P.; Margoliash, D. A bird’s eye view of sleep-dependent memory consolidation. In Sleep, Neuronal Plasticity and Brain Function; Meerlo, P., Benca, R.M., Abel, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 25, pp. 207–237. [Google Scholar] [CrossRef]
- Giret, N. The role of sleep in song learning processes in songbird. In Handbook of Sleep Research, 1st ed.; Dringenberg, H.C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 30, pp. 395–410. [Google Scholar]
- Dave, A.S.; Margoliash, D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science 2000, 290, 812–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahnloser, R.H.R.; Wang, C.Z.H.; Nager, A.; Naie, K. Spikes and bursts in two types of thalamic projection neurons differentially shape sleep patterns and auditory responses in a songbird. J. Neurosci. 2008, 28, 5040–5052. [Google Scholar] [CrossRef] [Green Version]
- Margoliash, D. Song learning and sleep. Nat. Neurosci. 2005, 8, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Crandall, S.R.; Adam, M.; Kinnischtzke, A.K.; Nick, T.A. HVC neural sleep activity increases with development and parallels nightly changes in song behavior. J. Neurophysiol. 2007, 98, 232–240. [Google Scholar] [CrossRef]
- Shank, S.S.; Margoliash, D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature 2009, 458, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.L.; Pytte, C.L.; Vicario, D.S. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc. Natl. Acad. Sci. USA 2006, 103, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Brawn, T.P.; Nusbaum, H.C.; Margoliash, D. Sleep consolidation of interfering auditory memories in starlings. Psychol. Sci. 2013, 24, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Brawn, T.P.; Nusbaum, H.C.; Margoliash, D. Sleep-dependent consolidation of auditory discrimination learning in adult starlings. J. Neurosci. 2010, 30, 609–613. [Google Scholar] [CrossRef]
- Brawn, T.P.; Nusbaum, H.C.; Margoliash, D. Sleep-dependent reconsolidation after memory destabilization in starlings. Nat. Commun. 2018, 9, 3093. [Google Scholar] [CrossRef]
- Margoliash, D.; Schmidt, M.F. Sleep, off-line processing, and vocal learning. Brain Lang. 2010, 115, 45–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shettleworth, S.J. Spatial memory in food-storing birds. Phil. Trans. R. Soc. B 1990, 329, 143–151. [Google Scholar]
- Brodin, A. The history of scatter hoarding studies. Phil. Trans. R. Soc. B 2010, 365, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Pravosudov, V.V.; Roth, T.C.; LaDage, L.D.; Freas, C.A. Environmental influences on spatial memory and the hippocampus in food-caching chickadees. Comp. Cogn. Behav. Rev. 2015, 10, 25–43. [Google Scholar] [CrossRef]
- Nelini, C.; Bobbo, D.; Mascetti, G.G. Local sleep: A spatial learning task enhances sleep in the right hemisphere of domestic chicks (Gallus gallus). Exp. Brain Res. 2010, 205, 195–204. [Google Scholar] [CrossRef]
- Nelini, C.; Bobbo, D.; Mascetti, G.G. Monocular learning of a spatial task enhances sleep in the right hemisphere of domestic chicks (Gallus gallus). Exp. Brain Res. 2012, 218, 381–388. [Google Scholar] [CrossRef]
- Taufique, S.K.T.; Kumar, V. Differential activation and tyrosine hydroxylase distribution in the hippocampal, pallial and midbrain brain regions in response to cognitive performance in Indian house crows exposed to abrupt light environment. Behav. Brain Res. 2016, 314, 21–29. [Google Scholar] [CrossRef]
- Yorzinski, J.L.; Ordonez, K.A.; Chema, K.T.; Ebensperger, L. Does artificial light pollution impair problem-solving success in peafowl? Ethology 2017, 123, 854–860. [Google Scholar] [CrossRef]
- Puig, M.V.; Antzoulatos, E.G.; Miller, E.K. Prefrontal dopamine in associative learning and memory. Neuroscience 2014, 282, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate of prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Taufique, S.K.T.; Prabhat, A.; Kumar, V. Constant light environment suppresses maturation and reduces complexity of new born neuron processes in the hippocampus and caudal nidopallium of a diurnal corvid: Implication for impairment of the learning and cognitive performance. Neurobiol. Learn. Mem. 2018, 147, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rattenborg, N.C.; Mandt, B.H.; Obermeyer, W.H.; Winsauer, P.J.; Huber, R.; Wikelski, M.; Benca, R.M. Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii). PLoS Biol. 2004, 2, E212. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.G.; Paletz, E.M.; Obermeyer, W.H.; Hannan, C.T.; Benca, R.M. Seasonal influences on sleep and executive function in the migratory white-crowned sparrow (Zonotrichia leucophrys gambelii). BMC Neurosci. 2010, 11, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aulsebrook, A.E.; Johnsson, R.D.; Lesku, J.A. Light, Sleep and Performance in Diurnal Birds. Clocks & Sleep 2021, 3, 115-131. https://doi.org/10.3390/clockssleep3010008
Aulsebrook AE, Johnsson RD, Lesku JA. Light, Sleep and Performance in Diurnal Birds. Clocks & Sleep. 2021; 3(1):115-131. https://doi.org/10.3390/clockssleep3010008
Chicago/Turabian StyleAulsebrook, Anne E., Robin D. Johnsson, and John A. Lesku. 2021. "Light, Sleep and Performance in Diurnal Birds" Clocks & Sleep 3, no. 1: 115-131. https://doi.org/10.3390/clockssleep3010008
APA StyleAulsebrook, A. E., Johnsson, R. D., & Lesku, J. A. (2021). Light, Sleep and Performance in Diurnal Birds. Clocks & Sleep, 3(1), 115-131. https://doi.org/10.3390/clockssleep3010008




