Asian Population Is More Prone to Develop High-Risk Myelodysplastic Syndrome, Concordantly with Their Propensity to Exhibit High-Risk Cytogenetic Aberrations
Abstract
Simple Summary
Abstract
1. Introduction
2. Results and Discussion
2.1. The Age of Onset Is Earlier and the Incidence Lower in Asia Compared to Western Population
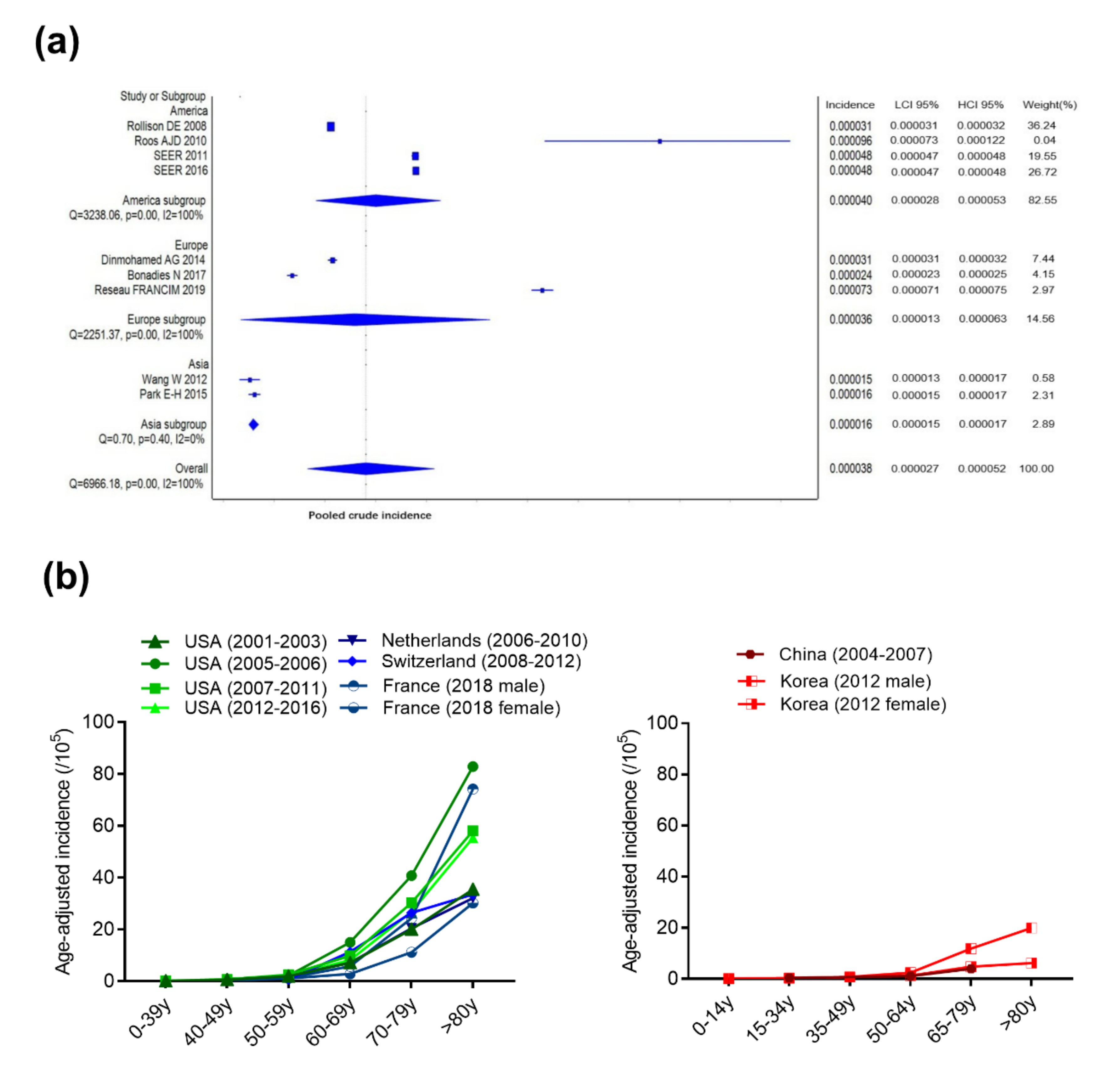
2.1.1. Incidence and Age-Adjusted Incidence
2.1.2. Age of Onset
2.1.3. Gender Ratio
2.2. Asian Population is More Prone to Develop Severe MDS Subtypes
2.2.1. Subtype Distribution
2.2.2. Cell Morphology
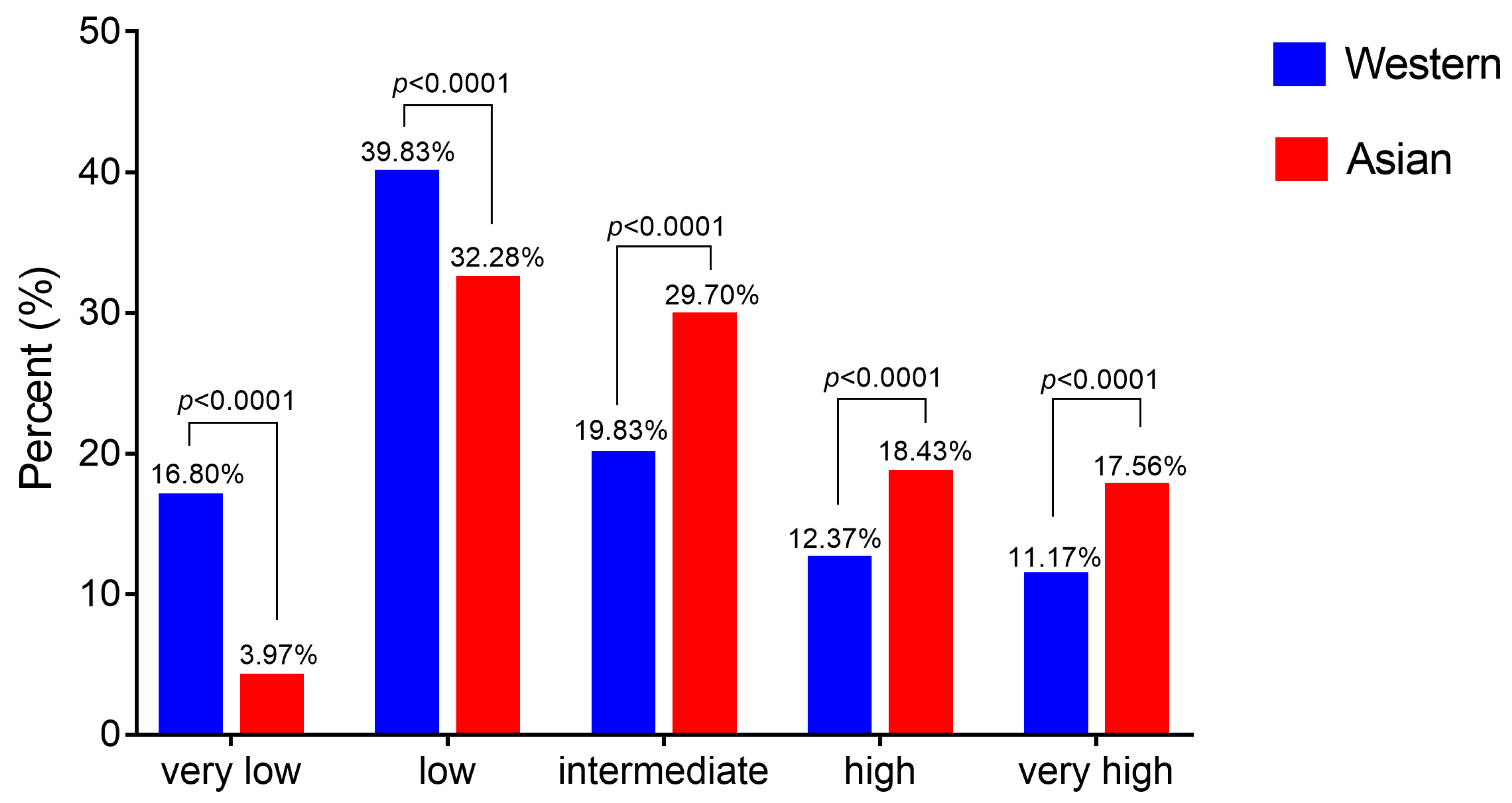
2.3. Asian MDS Patients Fall More Frequently into the Intermediate-, High- and Very High-Risk Pronostic Groups
2.4. Genetic Characteristics Are Concordant with the Prognosis Difference Observed between Asian and Western MDS Patients
2.4.1. Cytogenetics
2.4.2. Molecular Genetics
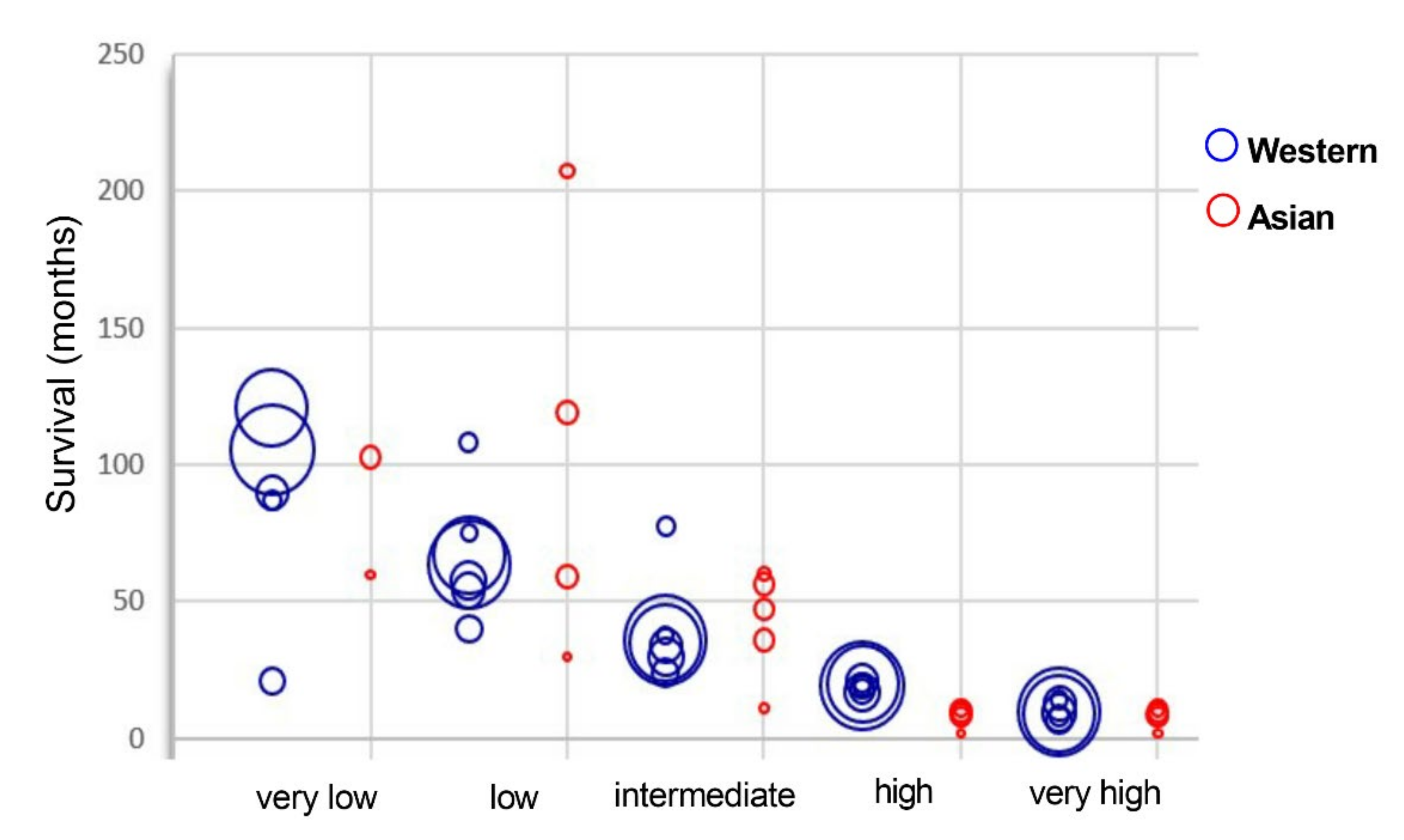
2.5. Survival Rates Are Equivalent for Asian and Western MDS Patients and Strictly Correlated to the Prognostic Groups
2.5.1. Therapeutic Options
2.5.2. Survival Rate
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeidan, A.M.; Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X. Epidemiology of Myelodysplastic Syndromes: Why Characterizing the Beast Is a Prerequisite to Taming It. Blood Rev. 2019, 34, 1–15. [Google Scholar] [CrossRef]
- List, A.; Ebert, B.L.; Fenaux, P. A Decade of Progress in Myelodysplastic Syndrome with Chromosome 5q Deletion. Leukemia 2018, 32, 1493–1499. [Google Scholar] [CrossRef]
- Adès, L.; Boehrer, S.; Prebet, T.; Beyne-Rauzy, O.; Legros, L.; Ravoet, C.; Dreyfus, F.; Stamatoullas, A.; Chaury, M.P.; Delaunay, J.; et al. Efficacy and Safety of Lenalidomide in Intermediate-2 or High-Risk Myelodysplastic Syndromes with 5q Deletion: Results of a Phase 2 Study. Blood 2009, 113, 3947–3952. [Google Scholar] [CrossRef]
- Magalhães, S.M.M.; Velloso, E.D.R.P.; Buzzini, R.; Bernardo, W.M. Part 4: Myelodysplastic Syndromes—Treatment of Low-Risk Patients with the 5q Deletion. Hematol. Transfus. Cell Ther. 2018, 40, 274–277. [Google Scholar] [CrossRef]
- Richard-Carpentier, G.; DeZern, A.E.; Takahashi, K.; Konopleva, M.Y.; Loghavi, S.; Masarova, L.; Alvarado, Y.; Ravandi, F.; Montalban Bravo, G.; Naqvi, K.; et al. Preliminary Results from the Phase II Study of the IDH2-Inhibitor Enasidenib in Patients with High-Risk IDH2-Mutated Myelodysplastic Syndromes (MDS). Blood 2019, 134, 678. [Google Scholar] [CrossRef]
- Sallman, D.A.; DeZern, A.E.; Steensma, D.P.; Sweet, K.L.; Cluzeau, T.; Sekeres, M.A.; Garcia-Manero, G.; Roboz, G.J.; McLemore, A.F.; McGraw, K.L.; et al. Phase 1b/2 Combination Study of APR-246 and Azacitidine (AZA) in Patients with TP53 Mutant Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML). Blood 2018, 132, 3091. [Google Scholar] [CrossRef]
- Matsuda, A.; Germing, U.; Jinnai, I.; Misumi, M.; Kuendgen, A.; Knipp, S.; Aivado, M.; Iwanaga, M.; Miyazaki, Y.; Tsushima, H.; et al. Difference in Clinical Features between Japanese and German Patients with Refractory Anemia in Myelodysplastic Syndromes. Blood 2005, 106, 2633–2640. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, W.-L.; Jin, J.; Xue, Y.-Q.; Cheng, X.; Chen, X.-T.; Cui, J.; Chen, Z.-M.; Cao, Q.; Yang, G.; et al. Clinical and Cytogenetic Features of 508 Chinese Patients with Myelodysplastic Syndrome and Comparison with Those in Western Countries. Leukemia 2005, 19, 767–775. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.-H.; Shin, Y.-R.; Lee, J.-S.; Kim, W.-K.; Chi, H.-S.; Park, C.-J.; Seo, E.-J.; Lee, K.-H. Application of Different Prognostic Scoring Systems and Comparison of the FAB and WHO Classifications in Korean Patients with Myelodysplastic Syndrome. Leukemia 2003, 17, 305–313. [Google Scholar] [CrossRef]
- Rollison, D.E.; Howlader, N.; Smith, M.T.; Strom, S.S.; Merritt, W.D.; Ries, L.A.; Edwards, B.K.; List, A.F. Epidemiology of Myelodysplastic Syndromes and Chronic Myeloproliferative Disorders in the United States, 2001-2004, Using Data from the NAACCR and SEER Programs. Blood 2008, 112, 45–52. [Google Scholar] [CrossRef]
- Roos, A.J.D.; Deeg, H.J.; Onstad, L.; Kopecky, K.J.; Bowles, E.J.A.; Yong, M.; Fryzek, J.; Davis, S. Incidence of Myelodysplastic Syndromes within a Nonprofit Healthcare System in Western Washington State, 2005–2006. Am. J. Hematol. 2010, 85, 765–770. [Google Scholar] [CrossRef]
- Sect_30_mds.Pdf. Available online: https://seer.cancer.gov/archive/csr/1975_2011/results_merged/sect_30_mds.pdf (accessed on 27 July 2020).
- Sect_30_mds.Pdf. Available online: https://seer.cancer.gov/csr/1975_2016/results_merged/sect_30_mds.pdf#search=mds%20incidence (accessed on 9 December 2019).
- Dinmohamed, A.G.; Visser, O.; van Norden, Y.; Huijgens, P.C.; Sonneveld, P.; van de Loosdrecht, A.A.; Jongen-Lavrencic, M. Trends in Incidence, Initial Treatment and Survival of Myelodysplastic Syndromes: A Population-Based Study of 5144 Patients Diagnosed in the Netherlands from 2001 to 2010. Eur. J. Cancer 2014, 50, 1004–1012. [Google Scholar] [CrossRef]
- Bonadies, N.; Feller, A.; Rovo, A.; Ruefer, A.; Blum, S.; Gerber, B.; Stuessi, G.; Benz, R.; Cantoni, N.; Holbro, A.; et al. Trends of Classification, Incidence, Mortality, and Survival of MDS Patients in Switzerland between 2001 and 2012. Cancer Epidemiol. 2017, 46, 85–92. [Google Scholar] [CrossRef]
- Source: Réseau FRANCIM. Available online: https://lesdonnees.e-cancer.fr/Informations/Sources/SOURCE-Reseau-FRANCIM (accessed on 9 December 2019).
- Incidence of Myelodysplastic Syndrome in Japan. J. Epidemiol. 2014, 24, 469–473. [CrossRef]
- Wang, W.; Wang, H.; Wang, X.-Q.; Lin, G.-W. First Report of Incidence of Adult Myelodysplastic Syndrome in China. Ann. Hematol. 2012, 91, 1321–1322. [Google Scholar] [CrossRef]
- Park, E.-H.; Lee, H.; Won, Y.-J.; Ju, H.Y.; Oh, C.-M.; Ingabire, C.; Kong, H.-J.; Park, B.-K.; Yoon, J.Y.; Eom, H.-S.; et al. Nationwide Statistical Analysis of Myeloid Malignancies in Korea: Incidence and Survival Rate from 1999 to 2012. Blood Res. 2015, 50, 204–217. [Google Scholar] [CrossRef]
- Bowen, D.T. Occupational and Environmental Etiology of MDS. Best Pract. Res. Clin. Haematol. 2013, 26, 319–326. [Google Scholar] [CrossRef]
- Zhang, T.T.; Sun, A.N.; Pan, J.L.; Wu, D.P.; Qiu, H.Y.; Tang, X.W.; Miao, M.; Chen, S.N. The clinical features, cytogenetic characteristics and survival analysis of 550 myelodysplastic syndromes in a single center. Zhonghua Xue Ye Xue Za Zhi 2016, 37, 864–869. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Z.; Chang, C.; Xu, F.; Wu, L.; He, Q.; Xu, Z.; Song, L.; Zhang, Z.; Zhou, L.; et al. Distinct Clinical and Experimental Characteristics in the Patients Younger than 60 Years Old with Myelodysplastic Syndromes. PLoS ONE 2013, 8, e57392. [Google Scholar] [CrossRef]
- Neukirchen, J.; Schoonen, W.M.; Strupp, C.; Gattermann, N.; Aul, C.; Haas, R.; Germing, U. Incidence and Prevalence of Myelodysplastic Syndromes: Data from the Düsseldorf MDS-Registry. Leuk. Res. 2011, 35, 1591–1596. [Google Scholar] [CrossRef]
- Mądry, K.; Machowicz, R.; Waszczuk-Gajda, A.; Drozd-Sokołowska, J.; Hołowiecka, B.S.; Wiater, E.; Mital, A.; Obara, A.; Szmigielska-Kapłon, A.; Kołkowska-Leśniak, A.; et al. Demographic, Hematologic, and Clinical Features of Myelodysplastic Syndrome Patients: Results from the First Polish Myelodysplastic Syndrome Registry. AHA 2015, 134, 125–134. [Google Scholar] [CrossRef]
- Van den Berghe, H.; Michaux, L. 5q-, Twenty-Five Years Later: A Synopsis. Cancer Genet. Cytogenet. 1997, 94, 1–7. [Google Scholar] [CrossRef]
- Berggren, D.M.; Folkvaljon, Y.; Engvall, M.; Sundberg, J.; Lambe, M.; Antunovic, P.; Garelius, H.; Lorenz, F.; Nilsson, L.; Rasmussen, B.; et al. Prognostic Scoring Systems for Myelodysplastic Syndromes (MDS) in a Population-Based Setting: A Report from the Swedish MDS Register. Br. J. Haematol. 2018, 181, 614–627. [Google Scholar] [CrossRef] [PubMed]
- McQuilten, Z.K.; Wood, E.M.; Polizzotto, M.N.; Campbell, L.J.; Wall, M.; Curtis, D.J.; Farrugia, H.; McNeil, J.J.; Sundararajan, V. Underestimation of Myelodysplastic Syndrome Incidence by Cancer Registries: Results from a Population-Based Data Linkage Study. Cancer 2014, 120, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, X.; Xu, X.; Lin, G. Cytogenetic Features and Prognosis Analysis in Chinese Patients with Myelodysplastic Syndrome: A Multicenter Study. Ann. Hematol. 2010, 89, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Xu, Z.; Zhang, Y.; Qin, T.; Zhang, T.; Cui, R.; Xiao, Z. Impacts of Cytogenetic Categories in the Revised International Prognostic Scoring System on the Prognosis of Primary Myelodysplastic Syndromes: Results of a Single-Center Study. Leuk. Lymphoma 2012, 53, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-Y.; Hou, H.-A.; Lin, T.-Y.; Lin, C.-C.; Chou, W.-C.; Tseng, M.-H.; Chiang, Y.-C.; Liu, M.-C.; Liu, C.-W.; Kuo, Y.-Y.; et al. Distinct Mutation Profile and Prognostic Relevance in Patients with Hypoplastic Myelodysplastic Syndromes (h-MDS). Oncotarget 2016, 7, 63177–63188. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Tuechler, H.; Sanz, G.; Schanz, J.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Differing Clinical Features between Japanese and Caucasian Patients with Myelodysplastic Syndromes: Analysis from the International Working Group for Prognosis of MDS. Leuk. Res. 2018, 73, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Haase, D.; Germing, U.; Schanz, J.; Pfeilstöcker, M.; Nösslinger, T.; Hildebrandt, B.; Kundgen, A.; Lübbert, M.; Kunzmann, R.; Giagounidis, A.A.N.; et al. New Insights into the Prognostic Impact of the Karyotype in MDS and Correlation with Subtypes: Evidence from a Core Dataset of 2124 Patients. Blood 2007, 110, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Gologan, R.; Georgescu, D.; Tatic, A.; Radulescu, I.; Vasilache, D. Epidemiological Data from the Registry of Patients with Myelodysplastic Syndrome in a Single Hospital Center of Romania. Leuk. Res. 2009, 33, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Chen, D.; Patnaik, M.; Gangat, N.; Begna, K.; Elliott, M.; Hogan, W.; Litzow, M.; Al-Kali, A. Clinical Outcome of Patients Diagnosed with Myelodysplastic Syndrome-Unclassifiable (MDS-U): Single Center Experience. Leuk. Lymphoma 2019, 60, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Xu, M.; Deng, J.; Liu, L.; Guo, T.; Xia, L.; Hu, Y.; Mei, H. Evaluation of Different Scoring Systems and Gene Mutations for the Prognosis of Myelodysplastic Syndrome (MDS) in Chinese Population. J. Cancer 2020, 11, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Chinese Society of Hematology, Chinese Medical Association [Chinese guidelines for diagnosis and treatment of myelodysplastic syndromes (2019)]. Zhonghua Xue Ye Xue Za Zhi 2019, 40, 89–97. [CrossRef]
- Haferlach, T. The Molecular Pathology of Myelodysplastic Syndrome. Pathobiology 2019, 86, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Voso, M.T.; Fenu, S.; Latagliata, R.; Buccisano, F.; Piciocchi, A.; Aloe-Spiriti, M.A.; Breccia, M.; Criscuolo, M.; Andriani, A.; Mancini, S.; et al. Revised International Prognostic Scoring System (IPSS) Predicts Survival and Leukemic Evolution of Myelodysplastic Syndromes Significantly Better than IPSS and WHO Prognostic Scoring System: Validation by the Gruppo Romano Mielodisplasie Italian Regional Database. J. Clin. Oncol. 2013, 31, 2671–2677. [Google Scholar] [CrossRef]
- Kawabata, H.; Tohyama, K.; Matsuda, A.; Araseki, K.; Hata, T.; Suzuki, T.; Kayano, H.; Shimbo, K.; Zaike, Y.; Usuki, K.; et al. Validation of the Revised International Prognostic Scoring System in Patients with Myelodysplastic Syndrome in Japan: Results from a Prospective Multicenter Registry. Int. J. Hematol. 2017, 106, 375–384. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Tuechler, H.; Malcovati, L.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; et al. Validation of WHO Classification-Based Prognostic Scoring System (WPSS) for Myelodysplastic Syndromes and Comparison with the Revised International Prognostic Scoring System (IPSS-R). A Study of the International Working Group for Prognosis in Myelodysplasia (IWG-PM). Leukemia 2015, 29, 1502–1513. [Google Scholar] [CrossRef]
- Mishra, A.; Corrales-Yepez, M.; Ali, N.A.; Kharfan-Dabaja, M.; Padron, E.; Zhang, L.; Epling-Burnette, P.K.; Pinilla-Ibarz, J.; Lancet, J.E.; List, A.F.; et al. Validation of the Revised International Prognostic Scoring System in Treated Patients with Myelodysplastic Syndromes. Am. J. Hematol. 2013, 88, 566–570. [Google Scholar] [CrossRef]
- Gangat, N.; Patnaik, M.M.; Begna, K.; Kourelis, T.; Knudson, R.A.; Ketterling, R.P.; Hodnefield, J.M.; Hanson, C.A.; Pardanani, A.; Tefferi, A. Evaluation of Revised IPSS Cytogenetic Risk Stratification and Prognostic Impact of Monosomal Karyotype in 783 Patients with Primary Myelodysplastic Syndromes. Am. J. Hematol. 2013, 88, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Kaivers, J.; Lauseker, M.; Hildebrandt, B.; Fenaux, P.; Pfeilstöcker, M.; Valent, P.; Platzbecker, U.; Latagliata, R.; Oliva, E.N.; Xicoy, B.; et al. The IPSS-R Has Prognostic Impact in Untreated Patients with MDS Del(5q). Leuk. Res. 2018, 72, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-T.; Hou, H.-A.; Liu, C.-Y.; Lin, C.-C.; Chou, W.-C.; Lee, F.-Y.; Liu, M.-C.; Liu, C.-W.; Tang, J.-L.; Yao, M.; et al. IPSS-R in 555 Taiwanese Patients with Primary MDS: Integration of Monosomal Karyotype Can Better Risk-Stratify the Patients. Am. J. Hematol. 2014, 89, E142–E149. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.-A.; Tsai, C.-H.; Lin, C.-C.; Chou, W.-C.; Kuo, Y.-Y.; Liu, C.-Y.; Tseng, M.-H.; Peng, Y.-L.; Liu, M.-C.; Liu, C.-W.; et al. Incorporation of Mutations in Five Genes in the Revised International Prognostic Scoring System Can Improve Risk Stratification in the Patients with Myelodysplastic Syndrome. Blood Cancer J. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.-P.; Nie, L.; Yu, M.-H.; Zhang, Y.; Qin, T.-J.; Xiao, Z.-J. Unique Cytogenetic Features of Primary Myelodysplastic Syndromes in Chinese Patients. Leuk. Res. 2009, 33, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Schanz, J.; Steidl, C.; Fonatsch, C.; Pfeilstöcker, M.; Nösslinger, T.; Tuechler, H.; Valent, P.; Hildebrandt, B.; Giagounidis, A.; Aul, C.; et al. Coalesced Multicentric Analysis of 2,351 Patients With Myelodysplastic Syndromes Indicates an Underestimation of Poor-Risk Cytogenetics of Myelodysplastic Syndromes in the International Prognostic Scoring System. J. Clin. Oncol. 2011, 29, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Avgerinou, C.; Alamanos, Y.; Zikos, P.; Lampropoulou, P.; Melachrinou, M.; Labropoulou, V.; Tavernarakis, I.; Aktypi, A.; Kaiafas, P.; Raptis, C.; et al. The Incidence of Myelodysplastic Syndromes in Western Greece Is Increasing. Ann. Hematol. 2013, 92, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Belli, C.B.; Bengió, R.; Aranguren, P.N.; Sakamoto, F.; Flores, M.G.; Watman, N.; Nucifora, E.; Prates, M.V.; Arbelbide, J.; Larripa, I. Partial and Total Monosomal Karyotypes in Myelodysplastic Syndromes: Comparative Prognostic Relevance among 421 Patients. Am. J. Hematol. 2011, 86, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Dan, L.; Zefeng, X.; Tiejun, Q.; Chengwen, L.; Naibo, H.; Lijuan, P.; Shiqiang, Q.; Bing, L.; Zhijian, X. Analysis of clinical characteristics, treatment response rate and survival of 77 myelodysplastic syndrome patients with del (5q) syndrome. Chin. J. Hematol. 2019, 40, 895–900. [Google Scholar] [CrossRef]
- Braun, T.; de Botton, S.; Taksin, A.-L.; Park, S.; Beyne-Rauzy, O.; Coiteux, V.; Sapena, R.; Lazareth, A.; Leroux, G.; Guenda, K.; et al. Characteristics and Outcome of Myelodysplastic Syndromes (MDS) with Isolated 20q Deletion: A Report on 62 Cases. Leuk. Res. 2011, 35, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Soupir, C.P.; Johari, V.; Hasserjian, R.P. Myelodysplastic Syndrome with Isolated Deletion of Chromosome 20q: An Indolent Disease with Minimal Morphological Dysplasia and Frequent Thrombocytopenic Presentation. Br. J. Haematol. 2007, 139, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Ito, Y.; Hsiao, H.-H.; Sashida, G.; Kodama, A.; Ohyashiki, J.H.; Ohyashiki, K. Risk Factor Analysis in Myelodysplastic Syndrome Patients with Del(20q): Prognosis Revisited. Cancer Genet. Cytogenet. 2006, 171, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Saumell, S.; Florensa, L.; Luño, E.; Sanzo, C.; Cañizo, C.; Hernández, J.M.; Cervera, J.; Gallart, M.A.; Carbonell, F.; Collado, R.; et al. Prognostic Value of Trisomy 8 as a Single Anomaly and the Influence of Additional Cytogenetic Aberrations in Primary Myelodysplastic Syndromes. Br. J. Haematol. 2012, 159, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Drevon, L.; Marceau, A.; Maarek, O.; Cuccuini, W.; Clappier, E.; Eclache, V.; Cluzeau, T.; Richez, V.; Berkaoui, I.; Dimicoli-Salazar, S.; et al. Myelodysplastic Syndrome (MDS) with Isolated Trisomy 8: A Type of MDS Frequently Associated with Myeloproliferative Features? A Report by the Groupe Francophone Des Myélodysplasies. Br. J. Haematol. 2018, 182, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Sloand, E.M.; Mainwaring, L.; Fuhrer, M.; Ramkissoon, S.; Risitano, A.M.; Keyvanafar, K.; Lu, J.; Basu, A.; Barrett, A.J.; Young, N.S. Preferential Suppression of Trisomy 8 Compared with Normal Hematopoietic Cell Growth by Autologous Lymphocytes in Patients with Trisomy 8 Myelodysplastic Syndrome. Blood 2005, 106, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Ganster, C.; Müller-Thomas, C.; Haferlach, C.; Strupp, C.; Ogata, K.; Germing, U.; Hildebrandt, B.; Mallo, M.; Lübbert, M.; Müller, C.; et al. Comprehensive Analysis of Isolated Der(1;7)(Q10;P10) in a Large International Homogenous Cohort of Patients with Myelodysplastic Syndromes. Genes Chromosomes Cancer 2019, 58, 689–697. [Google Scholar] [CrossRef]
- Ogawa, S. Genetics of MDS. Blood 2019, 133, 1049–1059. [Google Scholar] [CrossRef]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent Pathway Mutations of Splicing Machinery in Myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and Biological Implications of Driver Mutations in Myelodysplastic Syndromes. Blood 2013, 122, 3616–3627. [Google Scholar] [CrossRef]
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of Genetic Lesions in 944 Patients with Myelodysplastic Syndromes. Leukemia 2014, 28, 241–247. [Google Scholar] [CrossRef]
- Makishima, H.; Visconte, V.; Sakaguchi, H.; Jankowska, A.M.; Abu Kar, S.; Jerez, A.; Przychodzen, B.; Bupathi, M.; Guinta, K.; Afable, M.G.; et al. Mutations in the Spliceosome Machinery, a Novel and Ubiquitous Pathway in Leukemogenesis. Blood 2012, 119, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.-H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yin, J.; He, Q.; Zhang, F.; Huang, H.; Wu, B.; Wang, X.; Liu, H.; Yin, H.; Zeng, Y.; et al. Chinese and Europeans with Acute Myeloid Leukemia Have Discordant Mutation Topographies. Leuk. Res. 2018, 70, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.J.; Shen, D.; Shao, J.; Ding, L.; White, B.S.; Kandoth, C.; Miller, C.A.; Niu, B.; McLellan, M.D.; Dees, N.D.; et al. Clonal Diversity of Recurrently Mutated Genes in Myelodysplastic Syndromes. Leukemia 2013, 27, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; et al. Clinical Effect of Point Mutations in Myelodysplastic Syndromes. N. Engl. J. Med. 2011, 364, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Montalban-Bravo, G.; Takahashi, K.; Patel, K.; Wang, F.; Xingzhi, S.; Nogueras, G.M.; Huang, X.; Pierola, A.A.; Jabbour, E.; Colla, S.; et al. Impact of the Number of Mutations in Survival and Response Outcomes to Hypomethylating Agents in Patients with Myelodysplastic Syndromes or Myelodysplastic/Myeloproliferative Neoplasms. Oncotarget 2018, 9, 9714–9727. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, T.; Bao, X.; Wang, Q.; Zhang, L.; Hong, Y.; Zeng, Z.; Shen, H.; Wu, D.; Pan, J.; et al. Combining Gene Variants with Clinical Characteristics Improves Outcome Prediction in Chinese Patients with Myelodysplastic Syndromes. Leuk. Lymphoma 2020, 61, 919–926. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Xu, Q.; Chen, Y.; Lv, N.; Jing, Y.; Dou, L.; Bo, J.; Hou, G.; Guo, J.; et al. Implications of Mutational Spectrum in Myelodysplastic Syndromes Based on Targeted Next-Generation Sequencing. Oncotarget 2017, 8, 82475–82490. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Patnaik, M.M.; Saeed, L.; Mudireddy, M.; Idossa, D.; Finke, C.; Ketterling, R.P.; Pardanani, A.; Gangat, N. Targeted Next-Generation Sequencing in Myelodysplastic Syndromes and Prognostic Interaction between Mutations and IPSS-R. Am. J. Hematol. 2017, 92, 1311–1317. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Gallì, A.; Bacigalupo, A.; Zibellini, S.; Bernardi, M.; Rizzo, E.; Allione, B.; van Lint, M.T.; Pioltelli, P.; Marenco, P.; et al. Clinical Effects of Driver Somatic Mutations on the Outcomes of Patients With Myelodysplastic Syndromes Treated With Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2016, 34, 3627–3637. [Google Scholar] [CrossRef]
- Yoshizato, T.; Nannya, Y.; Atsuta, Y.; Shiozawa, Y.; Iijima-Yamashita, Y.; Yoshida, K.; Shiraishi, Y.; Suzuki, H.; Nagata, Y.; Sato, Y.; et al. Genetic Abnormalities in Myelodysplasia and Secondary Acute Myeloid Leukemia: Impact on Outcome of Stem Cell Transplantation. Blood 2017, 129, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Kosmider, O.; Gelsi-Boyer, V.; Cheok, M.; Grabar, S.; Della-Valle, V.; Picard, F.; Viguié, F.; Quesnel, B.; Beyne-Rauzy, O.; Solary, E.; et al. TET2 Mutation Is an Independent Favorable Prognostic Factor in Myelodysplastic Syndromes (MDSs). Blood 2009, 114, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Malcovati, L.; Papaemmanuil, E.; Bowen, D.T.; Boultwood, J.; Della Porta, M.G.; Pascutto, C.; Travaglino, E.; Groves, M.J.; Godfrey, A.L.; Ambaglio, I.; et al. Clinical Significance of SF3B1 Mutations in Myelodysplastic Syndromes and Myelodysplastic/Myeloproliferative Neoplasms. Blood 2011, 118, 6239–6246. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Gale, R.P.; Xu, Z.; Qin, T.; Fang, L.; Zhang, H.; Pan, L.; Zhang, Y.; Xiao, Z. Clinical Importance of SF3B1 Mutations in Chinese with Myelodysplastic Syndromes with Ring Sideroblasts. Leuk. Res. 2012, 36, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Jafari, P.A.; Ayatollahi, H.; Sadeghi, R.; Sheikhi, M.; Asghari, A. Prognostic Significance of SRSF2 Mutations in Myelodysplastic Syndromes and Chronic Myelomonocytic Leukemia: A Meta-Analysis. Hematology 2018, 23, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Kade, S.; Schlarmann, C.; Löffeld, P.; Morgan, M.; Krauter, J.; Wlodarski, M.W.; Kölking, B.; Wichmann, M.; Görlich, K.; et al. Frequency and Prognostic Impact of Mutations in SRSF2, U2AF1, and ZRSR2 in Patients with Myelodysplastic Syndromes. Blood 2012, 119, 3578–3584. [Google Scholar] [CrossRef]
- Wu, S.-J.; Kuo, Y.-Y.; Hou, H.-A.; Li, L.-Y.; Tseng, M.-H.; Huang, C.-F.; Lee, F.-Y.; Liu, M.-C.; Liu, C.-W.; Lin, C.-T.; et al. The Clinical Implication of SRSF2 Mutation in Patients with Myelodysplastic Syndrome and Its Stability during Disease Evolution. Blood 2012, 120, 3106–3111. [Google Scholar] [CrossRef]
- Wu, P.; Weng, J.; Li, M.; Lu, Z.; Deng, C.; Sun, Q.; Xu, R.; Geng, S.; Du, X. Co-Occurrence of RUNX1 and ASXL1 Mutations Underlie Poor Response and Outcome for MDS Patients Treated with HMAs. Am. J. Transl. Res. 2019, 11, 3651–3658. [Google Scholar]
- Chen, C.-Y.; Lin, L.-I.; Tang, J.-L.; Ko, B.-S.; Tsay, W.; Chou, W.-C.; Yao, M.; Wu, S.-J.; Tseng, M.-H.; Tien, H.-F. RUNX1 Gene Mutation in Primary Myelodysplastic Syndrome--the Mutation Can Be Detected Early at Diagnosis or Acquired during Disease Progression and Is Associated with Poor Outcome. Br. J. Haematol. 2007, 139, 405–414. [Google Scholar] [CrossRef]
- Dicker, F.; Haferlach, C.; Sundermann, J.; Wendland, N.; Weiss, T.; Kern, W.; Haferlach, T.; Schnittger, S. Mutation Analysis for RUNX1, MLL -PTD, FLT3 -ITD, NPM1 and NRAS in 269 Patients with MDS or Secondary AML. Leukemia 2010, 24, 1528–1532. [Google Scholar] [CrossRef]
- Lin, M.-E.; Hou, H.-A.; Tsai, C.-H.; Wu, S.-J.; Kuo, Y.-Y.; Tseng, M.-H.; Liu, M.-C.; Liu, C.-W.; Chou, W.-C.; Chen, C.-Y.; et al. Dynamics of DNMT3A Mutation and Prognostic Relevance in Patients with Primary Myelodysplastic Syndrome. Clin. Epigenetics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yao, D.; Qian, J.; Chen, Q.; Qian, W.; Li, Y.; Yang, J.; Wang, C.; Chai, H.; Qian, Z.; et al. Recurrent DNMT3A R882 Mutations in Chinese Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome. PLoS ONE 2011, 6, e26906. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Tang, J.-L.; Lin, C.-T.; Kuo, Y.-Y.; Li, L.-Y.; Tseng, M.-H.; Huang, C.-F.; Lai, Y.-J.; Lee, F.-Y.; Liu, M.-C.; et al. Clinical Implications of U2AF1 Mutation in Patients with Myelodysplastic Syndrome and Its Stability during Disease Progression. Am. J. Hematol. 2013, 88, E277–E282. [Google Scholar] [CrossRef] [PubMed]
- Kulasekararaj, A.G.; Smith, A.E.; Mian, S.A.; Mohamedali, A.M.; Krishnamurthy, P.; Lea, N.C.; Gäken, J.; Pennaneach, C.; Ireland, R.; Czepulkowski, B.; et al. TP53 Mutations in Myelodysplastic Syndrome Are Strongly Correlated with Aberrations of Chromosome 5, and Correlate with Adverse Prognosis. Br. J. Haematol. 2013, 160, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Jung, S.-H.; Hur, E.-H.; Choi, E.-J.; Lee, K.-H.; Yim, S.-H.; Kim, H.-J.; Kwon, Y.-R.; Jeon, Y.-W.; Lee, S.H.; et al. TP53 Mutation in Allogeneic Hematopoietic Cell Transplantation for de Novo Myelodysplastic Syndrome. Leuk. Res. 2018, 74, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Chilkulwar, A.; Saliba, R.M.; Chen, J.; Rondon, G.; Patel, K.P.; Khogeer, H.; Shah, A.R.; Randolph, B.V.; Perez, J.M.R.; et al. Prognostic Factors Influencing Survival after Allogeneic Transplantation for AML/MDS Patients with TP53 Mutations. Blood 2018, 131, 2989–2992. [Google Scholar] [CrossRef]
- Kim, M.; Yahng, S.-A.; Kwon, A.; Park, J.; Jeon, Y.-W.; Yoon, J.-H.; Shin, S.-H.; Lee, S.-E.; Cho, B.-S.; Eom, K.-S.; et al. Mutation in TET2 or TP53 Predicts Poor Survival in Patients with Myelodysplastic Syndrome Receiving Hypomethylating Treatment or Stem Cell Transplantation. Bone Marrow Transpl. 2015, 50, 1132–1134. [Google Scholar] [CrossRef]
- Cabrero, M.; Wei, Y.; Yang, H.; Ganan-Gomez, I.; Bohannan, Z.; Colla, S.; Marchesini, M.; Bravo, G.M.; Takahashi, K.; Bueso-Ramos, C.; et al. Down-Regulation of EZH2 Expression in Myelodysplastic Syndromes. Leuk. Res. 2016, 44, 1–7. [Google Scholar] [CrossRef]
- Nikoloski, G.; Langemeijer, S.M.C.; Kuiper, R.P.; Knops, R.; Massop, M.; Tönnissen, E.R.L.T.M.; van der Heijden, A.; Scheele, T.N.; Vandenberghe, P.; de Witte, T.; et al. Somatic Mutations of the Histone Methyltransferase Gene EZH2 in Myelodysplastic Syndromes. Nat. Genet. 2010, 42, 665–667. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Hanson, C.A.; Hodnefield, J.M.; Lasho, T.L.; Finke, C.M.; Knudson, R.A.; Ketterling, R.P.; Pardanani, A.; Tefferi, A. Differential Prognostic Effect of IDH1 versus IDH2 Mutations in Myelodysplastic Syndromes: A Mayo Clinic Study of 277 Patients. Leukemia 2012, 26, 101–105. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hou, H.-A.; Chou, W.-C.; Kuo, Y.-Y.; Liu, C.-Y.; Chen, C.-Y.; Lai, Y.-J.; Tseng, M.-H.; Huang, C.-F.; Chiang, Y.-C.; et al. IDH Mutations Are Closely Associated with Mutations of DNMT3A, ASXL1 and SRSF2 in Patients with Myelodysplastic Syndromes and Are Stable during Disease Evolution. Am. J. Hematol. 2014, 89, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, F.; Shan, N.; Sui, X.; Xu, H. IDH1 Mutation Is an Independent Inferior Prognostic Indicator for Patients with Myelodysplastic Syndromes. Acta Haematol. 2017, 138, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hu, C.; Yu, M.; Chen, F.; Ye, L.; Yin, X.; Zhuang, Z.; Tong, H. Prognostic Value of Isocitrate Dehydrogenase Mutations in Myelodysplastic Syndromes: A Retrospective Cohort Study and Meta-Analysis. PLoS ONE 2014, 9, e100206. [Google Scholar] [CrossRef] [PubMed]
- Tien, H.F.; Wang, C.H.; Chuang, S.M.; Chow, J.M.; Lee, F.Y.; Liu, M.C.; Chen, Y.C.; Shen, M.C.; Lin, D.T.; Lin, K.H. Cytogenetic Studies, Ras Mutation, and Clinical Characteristics in Primary Myelodysplastic Syndrome. A Study on 68 Chinese Patients in Taiwan. Cancer Genet. Cytogenet. 1994, 74, 40–49. [Google Scholar] [CrossRef]
- Rocquain, J.; Carbuccia, N.; Trouplin, V.; Raynaud, S.; Murati, A.; Nezri, M.; Tadrist, Z.; Olschwang, S.; Vey, N.; Birnbaum, D.; et al. Combined Mutations of ASXL1, CBL, FLT3, IDH1, IDH2, JAK2, KRAS, NPM1, NRAS, RUNX1, TET2 and WT1 Genes in Myelodysplastic Syndromes and Acute Myeloid Leukemias. BMC Cancer 2010, 10, 401. [Google Scholar] [CrossRef]
- Rasighaemi, P.; Ward, A.C. ETV6 and ETV7: Siblings in Hematopoiesis and Its Disruption in Disease. Crit. Rev. Oncol. Hematol. 2017, 116, 106–115. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Cluzeau, T.; Mansat-De Mas, V.; Dreyfus, F.; Beyne-Rauzy, O.; Quesnel, B.; Vey, N.; Gelsi-Boyer, V.; Raynaud, S.; et al. Impact of TET2 Mutations on Response Rate to Azacitidine in Myelodysplastic Syndromes and Low Blast Count Acute Myeloid Leukemias. Leukemia 2011, 25, 1147–1152. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Raval, A.; Kusler, B.; Gotlib, J.R.; Alizadeh, A.A.; Mitchell, B.S. Impact of TET2 Mutations on MRNA Expression and Clinical Outcomes in MDS Patients Treated with DNA Methyltransferase Inhibitors. Hematol. Oncol. 2011, 29, 157–160. [Google Scholar] [CrossRef]
- Bejar, R.; Lord, A.; Stevenson, K.; Bar-Natan, M.; Pérez-Ladaga, A.; Zaneveld, J.; Wang, H.; Caughey, B.; Stojanov, P.; Getz, G.; et al. TET2 Mutations Predict Response to Hypomethylating Agents in Myelodysplastic Syndrome Patients. Blood 2014, 124, 2705–2712. [Google Scholar] [CrossRef]
- Traina, F.; Visconte, V.; Elson, P.; Tabarroki, A.; Jankowska, A.M.; Hasrouni, E.; Sugimoto, Y.; Szpurka, H.; Makishima, H.; O’Keefe, C.L.; et al. Impact of Molecular Mutations on Treatment Response to DNMT Inhibitors in Myelodysplasia and Related Neoplasms. Leukemia 2014, 28, 78–87. [Google Scholar] [CrossRef]
- Hong, J.Y.; Seo, J.-Y.; Kim, S.-H.; Jung, H.A.; Park, S.; Kim, K.; Jung, C.W.; Kim, J.S.; Park, J.S.; Kim, H.-J.; et al. Mutations in the Spliceosomal Machinery Genes SRSF2, U2AF1, and ZRSR2 and Response to Decitabine in Myelodysplastic Syndrome. Anticancer Res. 2015, 35, 3081–3089. [Google Scholar] [PubMed]
- Zhang, Q.; Haider, M.; Al Ali, N.H.; Lancet, J.E.; Epling-Burnette, P.K.; List, A.F.; Padron, E.; Komrokji, R.S. SF3B1 Mutations Negatively Predict for Response to Immunosuppressive Therapy in Myelodysplastic Syndromes. Clin. Lymphoma Myeloma Leuk. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mossner, M.; Jann, J.-C.; Nowak, D.; Platzbecker, U.; Giagounidis, A.; Götze, K.; Letsch, A.; Haase, D.; Shirneshan, K.; Braulke, F.; et al. Prevalence, Clonal Dynamics and Clinical Impact of TP53 Mutations in Patients with Myelodysplastic Syndrome with Isolated Deletion (5q) Treated with Lenalidomide: Results from a Prospective Multicenter Study of the German MDS Study Group (GMDS). Leukemia 2016, 30, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Obeng, E.A.; Chappell, R.J.; Seiler, M.; Chen, M.C.; Campagna, D.R.; Schmidt, P.J.; Schneider, R.K.; Lord, A.M.; Wang, L.; Gambe, R.G.; et al. Physiologic Expression of Sf3b1(K700E) Causes Impaired Erythropoiesis, Aberrant Splicing, and Sensitivity to Therapeutic Spliceosome Modulation. Cancer Cell 2016, 30, 404–417. [Google Scholar] [CrossRef]
- Lehmann, S.; Bykov, V.J.N.; Ali, D.; Andrén, O.; Cherif, H.; Tidefelt, U.; Uggla, B.; Yachnin, J.; Juliusson, G.; Moshfegh, A.; et al. Targeting P53 in Vivo: A First-in-Human Study With P53-Targeting Compound APR-246 in Refractory Hematologic Malignancies and Prostate Cancer. JCO 2012, 30, 3633–3639. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Watts, J.M.; Stein, E.M.; de Botton, S.; Fathi, A.T.; Prince, G.T.; Stein, A.S.; Foran, J.M.; Stone, R.M.; Patel, P.A.; et al. Ivosidenib (AG-120) Induced Durable Remissions and Transfusion Independence in Patients with IDH1-Mutant Relapsed or Refractory Myelodysplastic Syndrome: Results from a Phase 1 Dose Escalation and Expansion Study. Blood 2018, 132, 1812. [Google Scholar] [CrossRef]
- Shirai, C.L.; White, B.S.; Tripathi, M.; Tapia, R.; Ley, J.N.; Ndonwi, M.; Kim, S.; Shao, J.; Carver, A.; Saez, B.; et al. Mutant U2AF1-Expressing Cells Are Sensitive to Pharmacological Modulation of the Spliceosome. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Duarte, F.B.; Barbosa, M.C.; dos Santos, T.E.J.; Lemes, R.P.G.; Vasconcelos, J.P.; de Vasconcelos, P.R.L.; Rocha, F.D.; Zalcberg, I.; Coutinho, D.F. Bone Marrow Fibrosis at Diagnosis Is Associated with TP53 Overexpression and Adverse Prognosis in Low-Risk Myelodysplastic Syndrome. Br. J. Haematol. 2018, 181, 547–549. [Google Scholar] [CrossRef]
- Silveira, C.G.T.; Oliveira, F.M.; Valera, E.T.; Ikoma, M.R.V.; Borgonovo, T.; Cavalli, I.J.; Tone, L.G.; Rogatto, S.R. New Recurrent Deletions in the PPARγ and TP53 Genes Are Associated with Childhood Myelodysplastic Syndrome. Leuk. Res. 2009, 33, 19–27. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, K.; Hwang, B.; Im, K.; Park, S.N.; Kim, J.-A.; Hwang, S.M.; Bang, D.; Lee, D.S. The High Frequency of the U2AF1 S34Y Mutation and Its Association with Isolated Trisomy 8 in Myelodysplastic Syndrome in Asians, but Not in Caucasians. Leuk. Res. 2017, 61, 96–103. [Google Scholar] [CrossRef]
- Kennedy, A.L.; Shimamura, A. Genetic Predisposition to MDS: Clinical Features and Clonal Evolution. Blood 2019, 133, 1071–1085. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Y.; Li, T.; Li, Y.; Xing, H.; Sun, H.; Sun, L.; Wan, D.; Liu, Y.; Xie, X.; et al. Gene Mutational Analysis by NGS and Its Clinical Significance in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukemia. Exp. Hematol. Oncol. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Pellagatti, A.; Boultwood, J. The Molecular Pathogenesis of the Myelodysplastic Syndromes. Eur. J. Haematol. 2015, 95, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.K.; Blydt-Hansen, M.; Rauh, M.J. Age-Associated TET2 Mutations: Common Drivers of Myeloid Dysfunction, Cancer and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 626. [Google Scholar] [CrossRef]
- Brissot, E.; Bernard, D.G.; Loréal, O.; Brissot, P.; Troadec, M.-B. Too Much Iron: A Masked Foe for Leukemias. Blood Rev. 2020, 39, 100617. [Google Scholar] [CrossRef]
- Harada, H.; Watanabe, M.; Suzuki, K.; Yanagita, S.; Suzuki, T.; Yoshida, Y.; Kimura, A.; Tsudo, M.; Matsuda, A.; Tohyama, K.; et al. Lenalidomide Is Active in Japanese Patients with Symptomatic Anemia in Low- or Intermediate-1 Risk Myelodysplastic Syndromes with a Deletion 5q Abnormality. Int. J. Hematol. 2009, 90, 353–360. [Google Scholar] [CrossRef]
- Du, X.; Lai, Y.-Y.; Xiao, Z.; Liu, T.; Hu, Y.; Sun, A.; Li, X.; Shen, Z.-X.; Jin, J.; Yu, L.; et al. Efficacy, Safety and Pharmacokinetics of Subcutaneous Azacitidine in Chinese Patients with Higher Risk Myelodysplastic Syndromes: Results from a Multicenter, Single-Arm, Open-Label Phase 2 Study. Asia Pac. J. Clin. Oncol. 2018, 14, 270–278. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jang, J.H.; Park, J.; Park, S.; Joo, Y.-D.; Kim, Y.-K.; Kim, H.-G.; Choi, C.W.; Kim, S.-H.; Park, S.K.; et al. A Prospective Multicenter Observational Study of Decitabine Treatment in Korean Patients with Myelodysplastic Syndrome. Haematologica 2011, 96, 1441–1447. [Google Scholar] [CrossRef]
- Wu, D.; Du, X.; Jin, J.; Xiao, Z.; Shen, Z.; Shao, Z.; Li, X.; Huang, X.; Liu, T.; Yu, L.; et al. Decitabine for Treatment of Myelodysplastic Syndromes in Chinese Patients: An Open-Label, Phase-3b Study. Adv. Ther. 2015, 32, 1140–1159. [Google Scholar] [CrossRef]
- Oki, Y.; Kondo, Y.; Yamamoto, K.; Ogura, M.; Kasai, M.; Kobayashi, Y.; Watanabe, T.; Uike, N.; Ohyashiki, K.; Okamoto, S.; et al. Phase I/II Study of Decitabine in Patients with Myelodysplastic Syndrome: A Multi-Center Study in Japan. Cancer Sci. 2012, 103, 1839–1847. [Google Scholar] [CrossRef]
- Kröger, N. Allogeneic Stem Cell Transplantation for Elderly Patients with Myelodysplastic Syndrome. Blood 2012, 119, 5632–5639. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Pidala, J.; Perez, W.S.; Deeg, H.J.; Garcia-Manero, G.; Malcovati, L.; Cazzola, M.; Park, S.; Itzykson, R.; Ades, L.; et al. Role of Reduced-Intensity Conditioning Allogeneic Hematopoietic Stem-Cell Transplantation in Older Patients with de Novo Myelodysplastic Syndromes: An International Collaborative Decision Analysis. J. Clin. Oncol. 2013, 31, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, J.E.; Scott, B.L.; Deeg, H.J. Transplantation for Myelodysplastic Syndromes 2013. Curr. Opin. Hematol. 2013, 20, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Nazha, A.; Sekeres, M.A.; Garcia-Manero, G.; Barnard, J.; Al Ali, N.H.; Roboz, G.J.; Steensma, D.P.; DeZern, A.E.; Zimmerman, C.; Jabbour, E.J.; et al. Outcomes of Patients with Myelodysplastic Syndromes Who Achieve Stable Disease after Treatment with Hypomethylating Agents. Leuk. Res. 2016, 41, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Does, M.; Raza, A.; Mayne, S.T. Myelodysplastic Syndromes: Incidence and Survival in the United States. Cancer 2007, 109, 1536–1542. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Eveillard, J.-R.; Couturier, M.-A.; Soubise, B.; Chen, J.-M.; Gao, S.; Basinko, A.; Morel, F.; Douet-Guilbert, N.; Troadec, M.-B. Asian Population Is More Prone to Develop High-Risk Myelodysplastic Syndrome, Concordantly with Their Propensity to Exhibit High-Risk Cytogenetic Aberrations. Cancers 2021, 13, 481. https://doi.org/10.3390/cancers13030481
Jiang Y, Eveillard J-R, Couturier M-A, Soubise B, Chen J-M, Gao S, Basinko A, Morel F, Douet-Guilbert N, Troadec M-B. Asian Population Is More Prone to Develop High-Risk Myelodysplastic Syndrome, Concordantly with Their Propensity to Exhibit High-Risk Cytogenetic Aberrations. Cancers. 2021; 13(3):481. https://doi.org/10.3390/cancers13030481
Chicago/Turabian StyleJiang, Yan, Jean-Richard Eveillard, Marie-Anne Couturier, Benoit Soubise, Jian-Min Chen, Sujun Gao, Audrey Basinko, Frédéric Morel, Nathalie Douet-Guilbert, and Marie-Bérengère Troadec. 2021. "Asian Population Is More Prone to Develop High-Risk Myelodysplastic Syndrome, Concordantly with Their Propensity to Exhibit High-Risk Cytogenetic Aberrations" Cancers 13, no. 3: 481. https://doi.org/10.3390/cancers13030481
APA StyleJiang, Y., Eveillard, J.-R., Couturier, M.-A., Soubise, B., Chen, J.-M., Gao, S., Basinko, A., Morel, F., Douet-Guilbert, N., & Troadec, M.-B. (2021). Asian Population Is More Prone to Develop High-Risk Myelodysplastic Syndrome, Concordantly with Their Propensity to Exhibit High-Risk Cytogenetic Aberrations. Cancers, 13(3), 481. https://doi.org/10.3390/cancers13030481