Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Virus, and Reagents
2.2. Viral Stocks Preparation and Titration
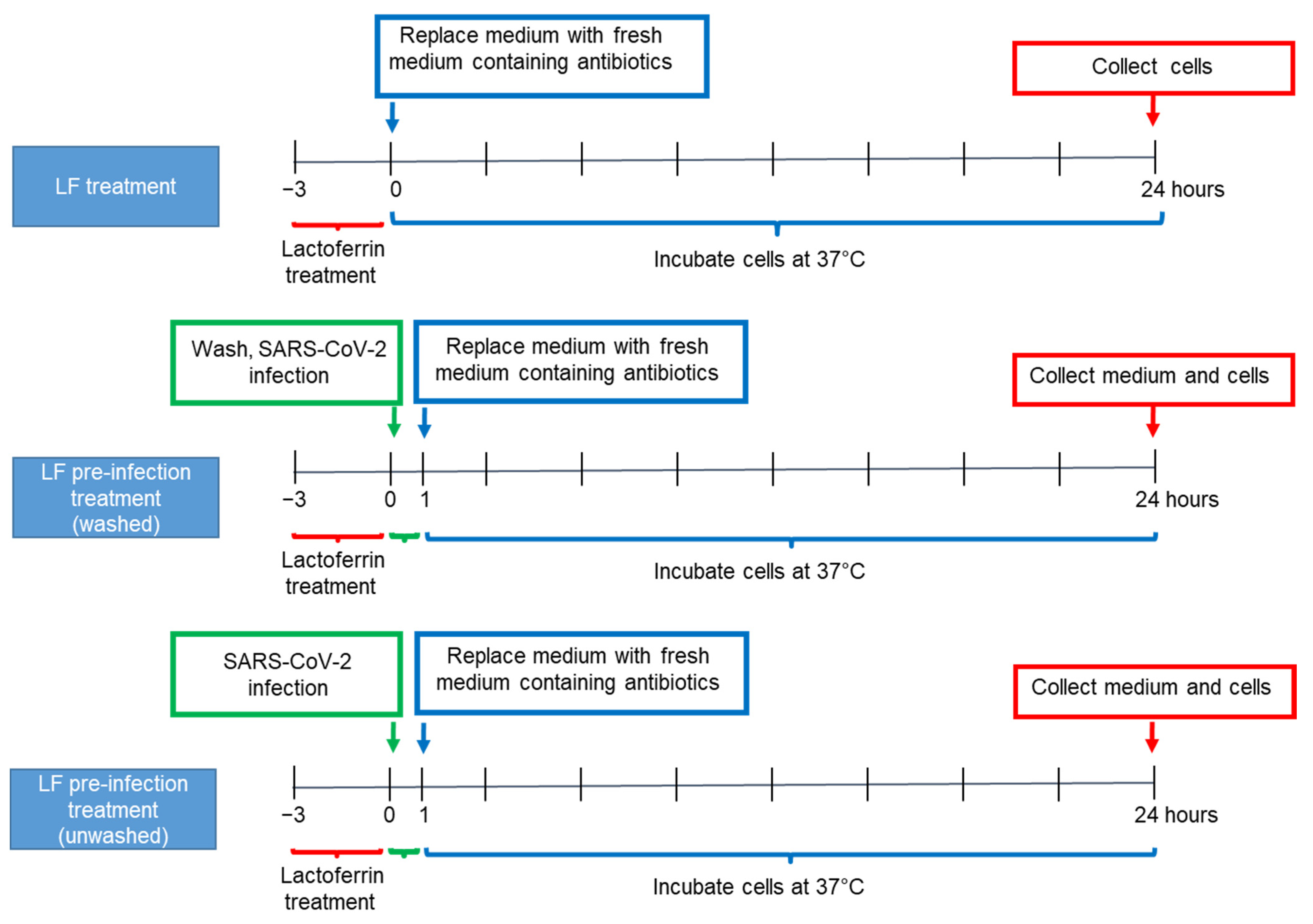
2.3. Caco-2 Cell Culture and Lactoferrin Treatments
2.4. RNA Extraction and Real-Time RT-PCR
2.5. Immunofluorescence
2.6. Statistical Analysis
3. Results
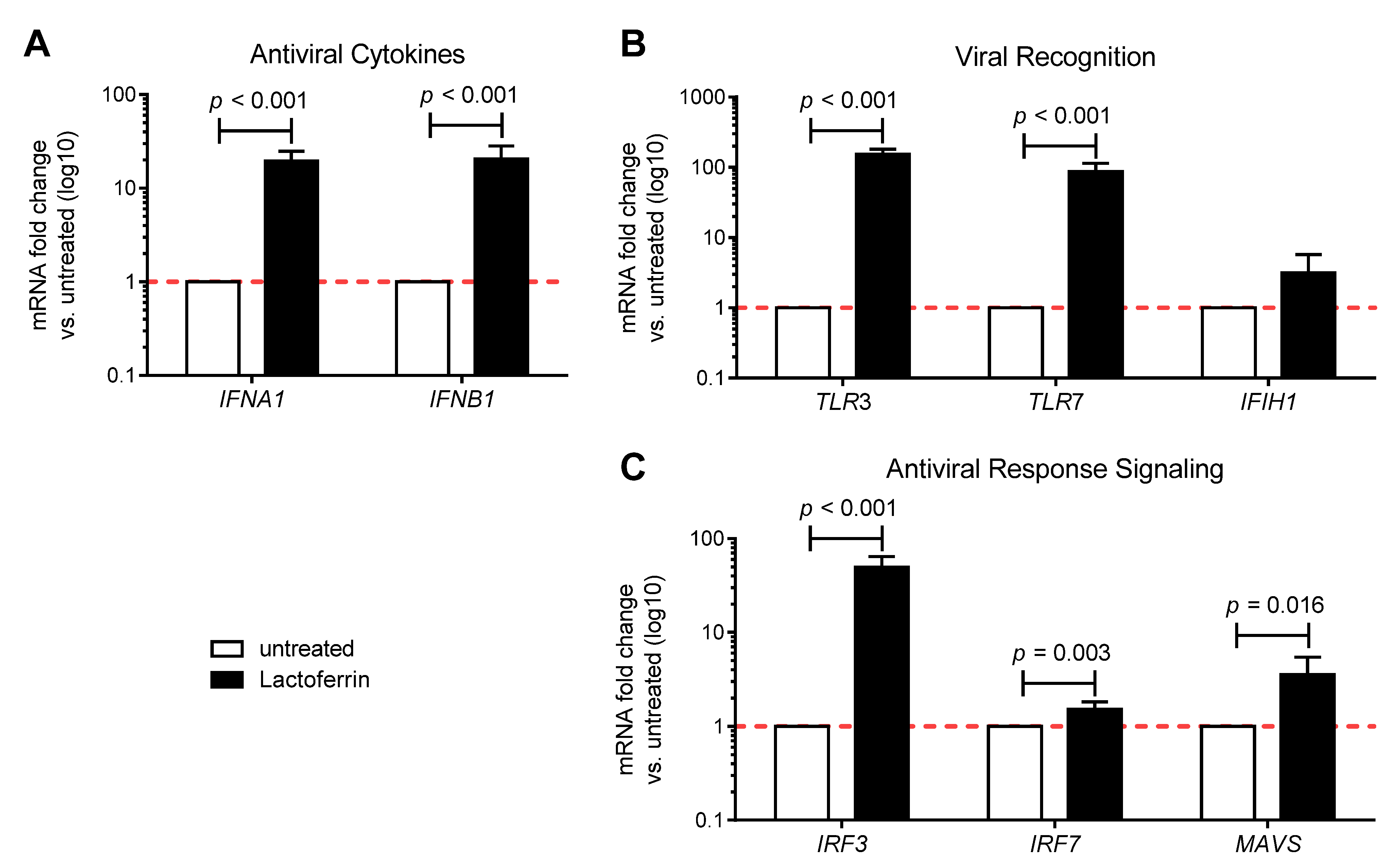
3.1. Lactoferrin Enhances the Antiviral Immune Response in Uninfected Caco-2 Intestinal Epithelial Cells
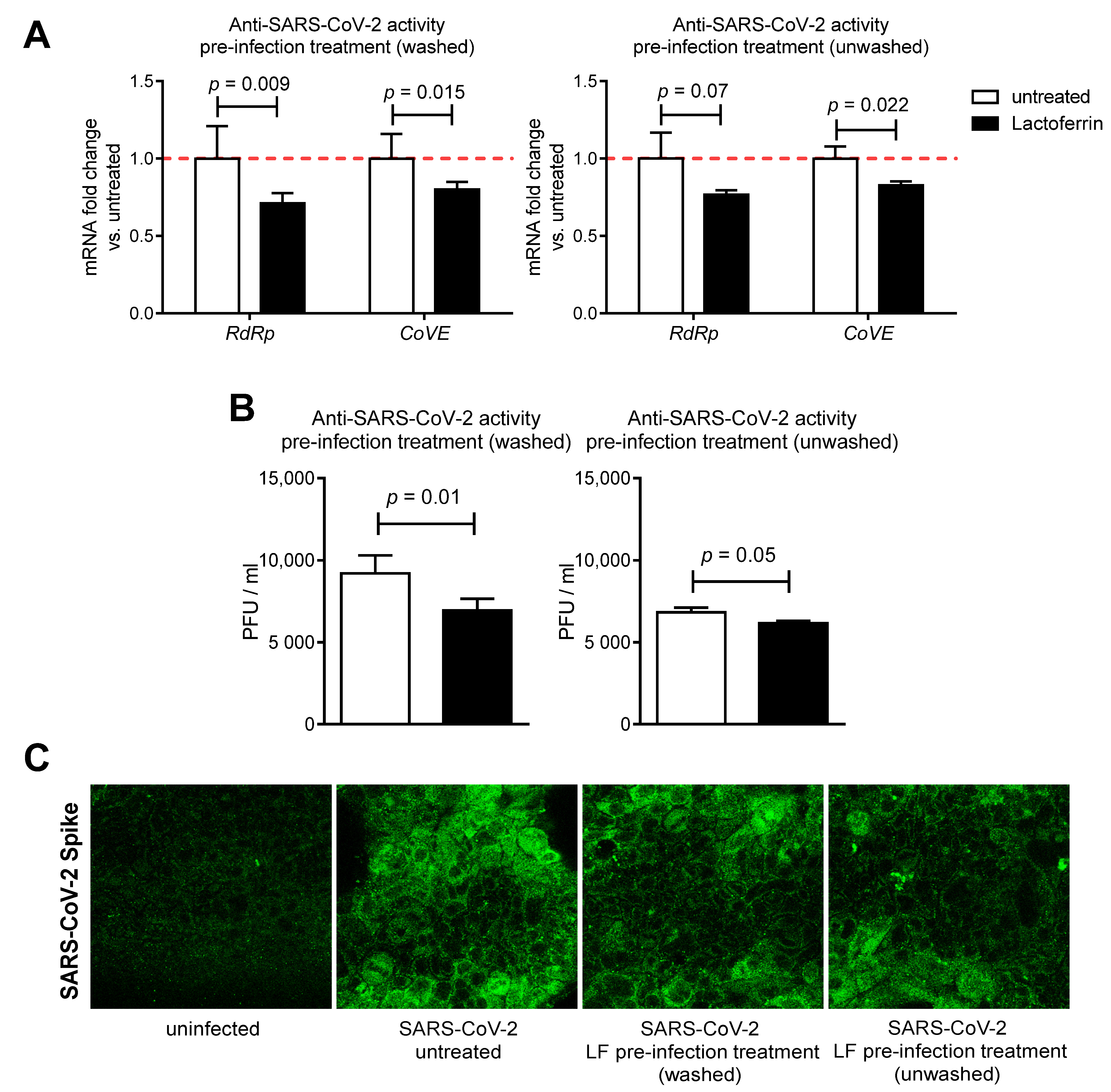
3.2. Lactoferrin Partially Inhibits SARS-CoV-2 Infection in Caco-2 Intestinal Epithelial Cells
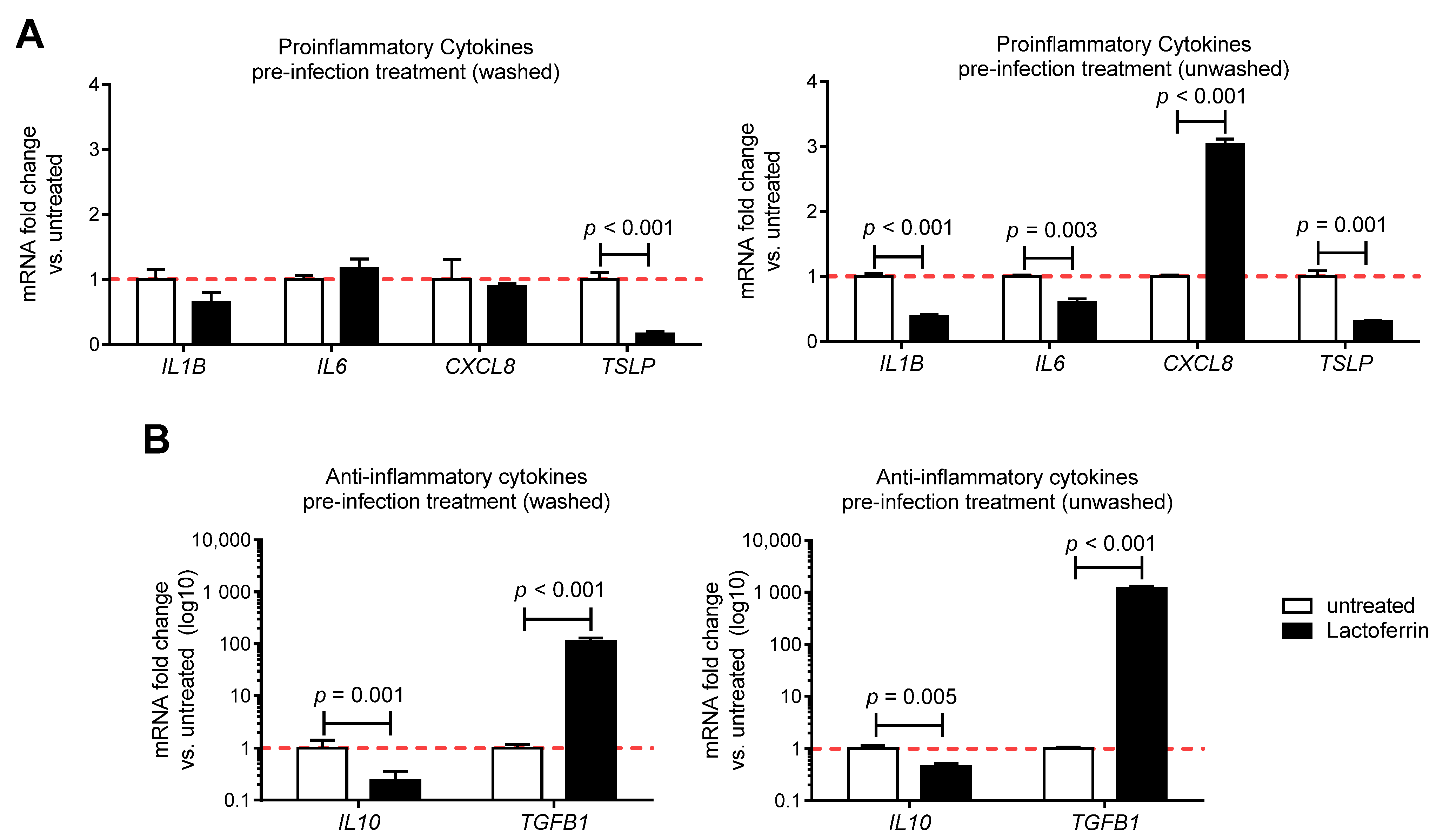
3.3. Lactoferrin Pre-Infection Treatments Modulate Cytokines Production Triggered by SARS-CoV-2 in Caco-2 Intestinal Epithelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, C. Clinical Features of Deaths in the Novel Coronavirus Epidemic in China. Rev. Med Virol. 2020, 30, e2103. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.X.; Ng, Y.L.; Tam, J.P.; Liu, D.X. Human Coronaviruses: A Review of Virus-Host Interactions. Diseases 2016, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020. [Google Scholar] [CrossRef]
- World Health Organization: Modes of Transmission. Available online: https://scholar.google.com/scholar_lookup?journal=Sci+Br&title=Modes+of+transmission+of+virus+causing+COVID%E2%80%9019:+implications+for+IPC+precaution+recommendations&publication_year=2020& (accessed on 10 December 2020).
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 Productively Infects Human Gut Enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Van Doorn, A.S.; Meijer, B.; Frampton, C.M.A.; Barclay, M.L.; de Boer, N.K.H. Systematic Review with Meta-analysis: SARS-CoV-2 Stool Testing and the Potential for Faecal-oral Transmission. Aliment Pharm. 2020. [Google Scholar] [CrossRef]
- Martinez, M.A. Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- GLANVILLE, D. Treatments and Vaccines for COVID-19: Authorised Medicines. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-vaccines-covid-19-authorised-medicines (accessed on 18 January 2021).
- Convertino, I.; Tuccori, M.; Ferraro, S.; Valdiserra, G.; Cappello, E.; Focosi, D.; Blandizzi, C. Exploring Pharmacological Approaches for Managing Cytokine Storm Associated with Pneumonia and Acute Respiratory Distress Syndrome in COVID-19 Patients. Crit. Care 2020, 24, 331. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 903. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Superti, F.; Ammendolia, M.G.; Valenti, P.; Seganti, L. Antirotaviral Activity of Milk Proteins: Lactoferrin Prevents Rotavirus Infection in the Enterocyte-like Cell Line HT-29. Med. Microbiol. Immunol. 1997, 186, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Nozaki, A.; Sugiyama, K.; Tanaka, T.; Naganuma, A.; Tanaka, K.; Sekihara, H.; Shimotohno, K.; Saito, M.; Kato, N. Characterization of Antiviral Activity of Lactoferrin against Hepatitis C Virus Infection in Human Cultured Cells. Virus Res. 2000, 66, 51–63. [Google Scholar] [CrossRef]
- Van der Strate, B.W.; Beljaars, L.; Molema, G.; Harmsen, M.C.; Meijer, D.K. Antiviral Activities of Lactoferrin. Antivir. Res. 2001, 52, 225–239. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for Prevention of Common Viral Infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef]
- Elnagdy, S.; AlKhazindar, M. The Potential of Antimicrobial Peptides as an Antiviral Therapy against COVID-19. ACS Pharm. Transl. Sci. 2020, 3, 780–782. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E.W. Antimicrobial Properties of Lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- De Haan, C.A.M.; Li, Z.; te Lintelo, E.; Bosch, B.J.; Haijema, B.J.; Rottier, P.J.M. Murine Coronavirus with an Extended Host Range Uses Heparan Sulfate as an Entry Receptor. J. Virol. 2005, 79, 14451–14456. [Google Scholar] [CrossRef]
- Milewska, A.; Zarebski, M.; Nowak, P.; Stozek, K.; Potempa, J.; Pyrc, K. Human Coronavirus NL63 Utilizes Heparan Sulfate Proteoglycans for Attachment to Target Cells. J. Virol. 2014, 88, 13221–13230. [Google Scholar] [CrossRef]
- Sano, E.; Miyauchi, R.; Takakura, N.; Yamauchi, K.; Murata, E.; Le, Q.T.; Katunuma, N. Cysteine Protease Inhibitors in Various Milk Preparations and Its Importance as a Food. Food Res. Int. 2005, 38, 427–433. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and Its Immune Cross-Reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Pišlar, A.; Mitrović, A.; Sabotič, J.; Fonović, U.P.; Nanut, M.P.; Jakoš, T.; Senjor, E.; Kos, J. The Role of Cysteine Peptidases in Coronavirus Cell Entry and Replication: The Therapeutic Potential of Cathepsin Inhibitors. PLoS Pathog. 2020, 16, e1009013. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K.; Hwang, S.-A.; Kruzel, M.L. Lactoferrin as a Natural Immune Modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Machnicki, M.; Zimecki, M.; Zagulski, T. Lactoferrin Regulates the Release of Tumour Necrosis Factor Alpha and Interleukin 6 in Vivo. Int. J. Exp. Pathol. 1993, 74, 433–439. [Google Scholar]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration, UK. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Chang, R.; Ng, T.B.; Sun, W.-Z. Lactoferrin as Potential Preventative and Adjunct Treatment for COVID-19. Int. J. Antimicrob Agents 2020, 56, 106118. [Google Scholar] [CrossRef]
- Takayama, K. In vitro and Animal Models for SARS-CoV-2 Research. Trends Pharmacol. Sci. 2020, 41, 513–517. [Google Scholar] [CrossRef]
- Molecular Assays to Diagnose COVID-19: Summary Table of Available Protocols. Available online: https://www.who.int/publications/m/item/molecular-assays-to-diagnose-covid-19-summary-table-of-available-protocols (accessed on 30 November 2020).
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharm. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Baveye, S.; Elass, E.; Mazurier, J.; Spik, G.; Legrand, D. Lactoferrin: A Multifunctional Glycoprotein Involved in the Modulation of the Inflammatory Process. Clin. Chem. Lab. Med. 1999, 37, 281–286. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Wang, H.; Luo, Y.; Wan, L.; Jiang, M.; Chu, Y. Lactoferrin for the Treatment of COVID-19 (Review). Exp. Med. 2020, 20. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-Inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. IJRHS 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Lopez, S.A.; Nonnecke, E.B.; Lönnerdal, B.L. The Lactoferrin Receptor Is Differentially Expressed across Several Human Epithelial Cell Types. FASEB J. 2012, 26, 644. [Google Scholar] [CrossRef]
- Ghio, A.J.; Carter, J.D.; Dailey, L.A.; Devlin, R.B.; Samet, J.M. Respiratory Epithelial Cells Demonstrate Lactoferrin Receptors That Increase after Metal Exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999, 276, L933–L940. [Google Scholar] [CrossRef]
- Jiang, R.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and Holo-Lactoferrin Are Both Internalized by Lactoferrin Receptor via Clathrin-Mediated Endocytosis but Differentially Affect ERK-Signaling and Cell Proliferation in Caco-2 Cells. J. Cell Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Wakabayashi, H.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Recombinant Human Intelectin Binds Bovine Lactoferrin and Its Peptides. Biol. Pharm Bull. 2008, 31, 1605–1608. [Google Scholar] [CrossRef] [PubMed]
- Curran, C.S.; Demick, K.P.; Mansfield, J.M. Lactoferrin Activates Macrophages via TLR4-Dependent and -Independent Signaling Pathways. Cell Immunol. 2006, 242, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Honda, K.; Taniguchi, T. IRFs: Master Regulators of Signalling by Toll-like Receptors and Cytosolic Pattern-Recognition Receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Zheng, J.; Wohlford-Lenane, C.; Abrahante, J.E.; Mack, M.; Sompallae, R.; McCray, P.B.; Meyerholz, D.K.; Perlman, S. IFN-I Response Timing Relative to Virus Replication Determines MERS Coronavirus Infection Outcomes. J. Clin. Investig. 2019, 129, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Van Splunter, M.; Perdijk, O.; Fick-Brinkhof, H.; Feitsma, A.L.; Floris-Vollenbroek, E.G.; Meijer, B.; Brugman, S.; Savelkoul, H.F.J.; van Hoffen, E.; van Neerven, R.J.J. Bovine Lactoferrin Enhances TLR7-Mediated Responses in Plasmacytoid Dendritic Cells in Elderly Women: Results From a Nutritional Intervention Study With Bovine Lactoferrin, GOS and Vitamin D. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Buscarini, E.; Manfredi, G.; Brambilla, G.; Menozzi, F.; Londoni, C.; Alicante, S.; Iiritano, E.; Romeo, S.; Pedaci, M.; Benelli, G.; et al. GI Symptoms as Early Signs of COVID-19 in Hospitalised Italian Patients. Gut 2020, 69, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Ianiro, G.; Privitera, G.; Lopetuso, L.R.; Vetrone, L.M.; Petito, V.; Pugliese, D.; Neri, M.; Cammarota, G.; Ringel, Y.; et al. The Thrilling Journey of SARS-CoV-2 into the Intestine: From Pathogenesis to Future Clinical Implications. Inflamm. Bowel Dis. 2020, 26, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhao, L.; Ren, F.; Guo, H. Lactoferrin Stimulates the Expression of Vitamin D Receptor in Vitamin D Deficient Mice. J. Funct. Foods 2019, 55, 48–56. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D Deficiency Aggravates COVID-19: Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of Vitamin D Level among Asymptomatic and Critically Ill COVID-19 Patients and Its Correlation with Inflammatory Markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Fabbri, A.; Infante, M.; Ricordi, C. Editorial—Vitamin D Status: A Key Modulator of Innate Immunity and Natural Defense from Acute Viral Respiratory Infections. Eur. Rev. Med. Pharm. Sci. 2020, 24, 4048–4052. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not Just the Bone. Evidence for Beneficial Pleiotropic Extraskeletal Effects. Eat Weight Disord. 2017, 22, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Redhu, N.S.; Gounni, A.S. Function and Mechanisms of TSLP/TSLPR Complex in Asthma and COPD. Clin. Exp. Allergy 2012, 42, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Fornasa, G.; Tsilingiri, K.; Caprioli, F.; Botti, F.; Mapelli, M.; Meller, S.; Kislat, A.; Homey, B.; Di Sabatino, A.; Sonzogni, A.; et al. Dichotomy of Short and Long Thymic Stromal Lymphopoietin Isoforms in Inflammatory Disorders of the Bowel and Skin. J. Allergy Clin. Immunol. 2015, 136, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Louie, M.C.; Vannella, K.M.; Wilke, C.A.; LeVine, A.M.; Moore, B.B.; Shanley, T.P. New Concepts of IL-10-Induced Lung Fibrosis: Fibrocyte Recruitment and M2 Activation in a CCL2/CCR2 Axis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L341–L353. [Google Scholar] [CrossRef] [PubMed]
| Gene | 5′-->3′ forward Sequence | 5′-->3′ Reverse Sequence | Ta °C |
|---|---|---|---|
| GAPDH | GACACCCACTCCTCCACCTTT | TTGCTGTAGCCAAATTCGTTGT | 60 |
| IFNA1 | TTCAGGGGCATCAGTCCCTA | CCGTCCATTCCTTGATTTGGTT | 60 |
| IFNB1 | TCTCCTGTTGTGCTTCTCCAC | GCCTCCCATTCAATTGCCAC | 60 |
| IL1B | CTGAGCTCGCCAGTGAAATG | TGTCCATGGCCACAACAACT | 60 |
| IL6 | GTCCAGTTGCCTTCTCCCTGG | CCCATGCTACATTTGCCGAAG | 60 |
| IL10 | GTGAAAACAAGAGCAAGGCCG | TAGAGTCGCCACCCTGATGT | 60 |
| TGFB1 | ACTGCGGATCTCTGTGTCAT | AGAGTCCCTGCATCTCAGAGT | 60 |
| CXCL8 | TGGACCCCAAGGAAAACTGG | ATTTGCTTGAAGTTTCACTGGCA | 60 |
| TSLP | AAGGCAACAGCATGGGTGAA | GGGAACATACGTGGACACCC | 60 |
| TLR3 | CCTTTTGCCCTTTGGGATGC | TGAAGTTGGCGGCTGGTAAT | 60 |
| TLR7 | CCTTGTGCGCCGTGTAAAAA | GGGCACATGCTGAAGAGAGT | 60 |
| MAVS | GCAATGCCGTTTGCTGAAGA | CGCCGCTGAAGGGTATTGAA | 60 |
| IFIH1 | GCATATGCGCTTTCCCAGTG | CTCTCATCAGCTCTGGCTCG | 60 |
| IRF3 | GAGCTGTGCTGGCGAGAAG | CTCTCCAGGAGCCTTGGTTG | 60 |
| IRF7 | CCATCGGCTTTTGGGTCTGT | TTCCCATGGTCCGGCCTC | 60 |
| CoVE | ACAGGTACGTTAATAGTTAATAGCGT | ATATTGCAGCAGTACGCACACA | 60 |
| RdRp IP2 | ATGAGCTTAGTCCTGTTG | CTCCCTTTGTTGTGTTGT | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. https://doi.org/10.3390/nu13020328
Salaris C, Scarpa M, Elli M, Bertolini A, Guglielmetti S, Pregliasco F, Blandizzi C, Brun P, Castagliuolo I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients. 2021; 13(2):328. https://doi.org/10.3390/nu13020328
Chicago/Turabian StyleSalaris, Claudio, Melania Scarpa, Marina Elli, Alice Bertolini, Simone Guglielmetti, Fabrizio Pregliasco, Corrado Blandizzi, Paola Brun, and Ignazio Castagliuolo. 2021. "Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro" Nutrients 13, no. 2: 328. https://doi.org/10.3390/nu13020328
APA StyleSalaris, C., Scarpa, M., Elli, M., Bertolini, A., Guglielmetti, S., Pregliasco, F., Blandizzi, C., Brun, P., & Castagliuolo, I. (2021). Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients, 13(2), 328. https://doi.org/10.3390/nu13020328







