Research of Curing Time and Temperature-Dependent Strengths and Fire Resistance of Geopolymer Foam Coated on an Aluminum Plate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterizations
3. Results and Discussion
4. Conclusions
- All samples showed significant improvements in compressive and flexural strength as curing conditions increased from 50 to 105 °C compared to at RT. The compressive and flexural strength of GF was most significantly improved under the curing condition of 85 °C.
- The maximum load for specimens was found after curing for 7 days at 85 °C and soaking for 2 h; surprisingly, the load of the test samples decreased after 14 and 28 days.
- The apparent density of the GF was stable at the age of 7 days.
- The aluminum plate covered with a protective GF layer showed an increased fire resistance time compared to the unprotected plate. The aluminum plate covered with a protective GF layer of 20 mm in thickness resisted the fire for the longest time. Its fire-resistance time was 30 times higher than that of the aluminum plate without the GF layer. The maximal temperature in the furnace did not exceed 600 °C.
- Temperatures at around 600 °C did not affect the GF structure but made it more stiffened.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymer Chemistry and Application Saint-Quentin: Institut Geopolymere; Institut Géopolymère: Saint-Quentin, France, 2015. [Google Scholar]
- Davidovits, J. Properties of geopolymer cements. In Proceedings of the First International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 11–14 October 1994; pp. 131–149. [Google Scholar]
- Davidovits, J. Geopolymers: Man-made rock geosynthesis and the resulting development of very early high strength cement. J. Mater. Educ. 1994, 16, 91. [Google Scholar]
- Yan, S.; Zhang, F.; Liu, J.; Ren, B.; He, P.; Jia, D.; Yang, J. Green synthesis of high porosity waste gangue microsphere/geopolymer composite foams via hydrogen peroxide modification. J. Clean. Prod. 2019, 227, 483–494. [Google Scholar] [CrossRef]
- Petlitckaia, S.; Poulesquen, A. Design of lightweight metakaolin based geopolymer foamed with hydrogen peroxide. Ceram. Int. 2019, 45, 1322–1330. [Google Scholar] [CrossRef]
- Leiva, C.; Luna-Galiano, Y.; Arenas, C.; Alonso-Fariñas, B.; Fernández-Pereira, C. A porous geopolymer based on aluminum-waste with acoustic properties. Waste Manag. 2019, 95, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Ducman, V.; Korat, L. Characterization of geopolymer fly-ash based foams obtained with the addition of Al powder or H2O2 as foaming agents. Mater. Charact. 2016, 113, 207–213. [Google Scholar] [CrossRef]
- Cilla, M.S.; Colombo, P.; Morelli, M.R. Geopolymer foams by gelcasting. Ceram. Int. 2014, 40, 5723–5730. [Google Scholar] [CrossRef]
- Bai, C.; Franchin, G.; Elsayed, H.; Conte, A.; Colombo, P. High strength metakaolin-based geopolymer foams with variable macroporous structure. J. Eur. Ceram. Soc. 2016, 36, 4243–4249. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Hamad, A.J. Size and shape effect of specimen on the compressive strength of HPLWFC reinforced with glass fibres. J. King Saud Univ. Eng. Sci. 2017, 29, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Process for the Fabrication of Sintered Panels and Panels Resulting from the Application of This Process. U.S. Patent No. 3,950,470, 13 April 1976. [Google Scholar]
- Sakkas, K.; Panias, D.; Nomikos, P.; Sofianos, A. Potassium based geopolymer for passive fire protection of concrete tunnels linings. Tunn. Undergr. Space Technol. 2014, 43, 148–156. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; van Riessen, A. Preparation of metakaolin based geopolymer coatings on metal substrates as thermal barriers. Appl. Clay Sci. 2009, 46, 265–270. [Google Scholar] [CrossRef]
- Davidovits, J. Years of successes and failures in geopolymer applications. Market trends and potential breakthroughs. In Proceedings of the Geopolymer 2002 Conference, Melbourne, Australia, 28–29 October 2002; p. 29. [Google Scholar]
- Bai, C.; Franchin, G.; Elsayed, H.; Zaggia, A.; Conte, L.; Li, H.; Colombo, P. High-porosity geopolymer foams with tailored porosity for thermal insulation and wastewater treatment. J. Mater. Res. 2017, 32, 3251–3259. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Liu, L.C.; Xu, Y.; Wang, Y.C. A new alkali-activated steel slag-based cementitious material for photocatalytic degradation of organic pollutant from waste water. J. Hazard. Mater. 2012, 209, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, K.; Sofianos, A.; Nomikos, P.; Panias, D. Behaviour of passive fire protection K-geopolymer under successive severe fire incidents. Materials 2015, 8, 6096–6104. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Lecomte, I.; Liégeois, M.; Rulmont, A.; Cloots, R.; Maseri, F. Synthesis and characterization of new inorganic polymeric composites based on kaolin or white clay and on ground-granulated blast furnace slag. J. Mater. Res. 2003, 18, 2571–2579. [Google Scholar] [CrossRef]
- Palmero, P.; Formia, A.; Antonaci, P.; Brini, S.; Tulliani, J.-M. Geopolymer technology for application-oriented dense and lightened materials. Elaboration and characterization. Ceram. Int. 2015, 41, 12967–12979. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.; Ascensão, G.; Seabra, M.; Labrincha, J. Porous biomass fly ash-based geopolymers with tailored thermal conductivity. J. Clean. Prod. 2016, 119, 99–107. [Google Scholar] [CrossRef]
- Hlaváček, P.; Šmilauer, V.; Škvára, F.; Kopecký, L.; Šulc, R. Inorganic foams made from alkali-activated fly ash: Mechanical, chemical and physical properties. J. Eur. Ceram. Soc. 2015, 35, 703–709. [Google Scholar] [CrossRef]
- Kamseu, E.; Nait-Ali, B.; Bignozzi, M.; Leonelli, C.; Rossignol, S.; Smith, D.S. Bulk composition and microstructure dependence of effective thermal conductivity of porous inorganic polymer cements. J. Eur. Ceram. Soc. 2012, 32, 1593–1603. [Google Scholar] [CrossRef]
- Kong, D.L.; Sanjayan, J.G. Damage behavior of geopolymer composites exposed to elevated temperatures. Cem. Concr. Compos. 2008, 30, 986–991. [Google Scholar] [CrossRef]
- Chiu, Y.-P.; Lu, Y.-M.; Shiau, Y.-C. Applying inorganic geopolymers added with aluminium powder to fire-resistant fillers. Mater. Res. Innov. 2015, 19, S5-642–S5-649. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Yahya, Z.; Razak, R.A. Fly Ash-based geopolymer lightweight concrete using foaming agent. Int. J. Mol. Sci. 2012, 13, 7186–7198. [Google Scholar] [CrossRef] [PubMed]
- Shi, C. Composition of Materials for Use in Cellular Lightweight Concrete and Methods Thereof. U.S. Patents No. 6,488,762, 3 December 2002. [Google Scholar]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Building, C.M.A. Thermal insulation products for buildings. In Factory Made Mineral Wool (MW) Products, Specification ed.; ROCKWOOL: Bohumin, Czech Republic, 2015; p. 52. [Google Scholar]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Félix, T.; Tahri, W.; Aguiar, J.B. Mix design, properties and cost analysis of fly ash-based geopolymer foam. Constr. Build. Mater. 2015, 80, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Mo, B.-H.; Zhu, H.; Cui, X.-m.; He, Y.; Gong, S.-Y. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A review of unconventional sustainable building insulation materials. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Kamseu, E.; Ceron, B.; Tobias, H.; Leonelli, E.; Bignozzi, M.C.; Muscio, A.; Libbra, A. Insulating behavior of metakaolin-based geopolymer materials assess with heat flux meter and laser flash techniques. J. Therm. Anal. Calorim. 2011, 108, 1189–1199. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Zheng, F.; Li, H.-G.; Lu, C.-L. An environment-friendly thermal insulation material from cotton stalk fibers. Energy Build. 2010, 42, 1070–1074. [Google Scholar] [CrossRef]
- Mallicoat, S.; Sarin, P.; Kriven, W. Novel, alkali-bonded, ceramic filtration membranes. In Proceedings of the Developments in Advanced Ceramics and Composites: A Collection of Papers Presented at the 29th International Conference on Advanced Ceramics and Composites, Cocoa Beach, FL, USA, 23–28 January 2005, Ceramic Engineering and Science Proceedings; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 298, p. 37. [Google Scholar]
- Bai, C.; Colombo, P. High-porosity geopolymer membrane supports by peroxide route with the addition of egg white as surfactant. Ceram. Int. 2017, 43, 2267–2273. [Google Scholar] [CrossRef]
- Ge, Y.; Yuan, Y.; Wang, K.; He, Y.; Cui, X. Preparation of geopolymer-based inorganic membrane for removing Ni2+ from wastewater. J. Hazard. Mater. 2015, 299, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef] [PubMed]
- López, F.J.; Sugita, S.; Tagaya, M.; Kobayashi, T. Metakaolin-based geopolymers for targeted adsorbents to heavy metal ion separation. J. Mater. Sci. Chem. Eng. 2014, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Minelli, M.; Medri, V.; Papa, E.; Miccio, F.; Landi, E.; Doghieri, F. Geopolymers as solid adsorbent for CO2 capture. Chem. Eng. Sci. 2016, 148, 267–274. [Google Scholar] [CrossRef]
- Luukkonen, T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; Lassi, U. Metakaolin geopolymer characterization and application for ammonium removal from model solutions and landfill leachate. Appl. Clay Sci. 2016, 119, 266–276. [Google Scholar] [CrossRef]
- Sharma, S.; Medpelli, D.; Chen, S.; Seo, D.-K. Calcium-modified hierarchically porous aluminosilicate geopolymer as a highly efficient regenerable catalyst for biodiesel production. Rsc Adv. 2015, 5, 65454–65461. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Lutze, W.; Pegg, I.L. High-Strength Geopolymer Composite Cellular Concrete. U.S. Patents No. 9,919,974, 20 March 2018. [Google Scholar]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Henon, J.; Alzina, A.; Absi, J.; Smith, D.S.; Rossignol, S. Potassium geopolymer foams made with silica fume pore forming agent for thermal insulation. J. Porous Mater. 2012, 20, 37–46. [Google Scholar] [CrossRef]
- Skvara, F.; Sulc, R.; Zdenek, T.; Petr, S.; Vit, S.; Zuzana, Z.C. Preparation and properties of fly ash-based geopolymer foams. Ceram. -Silik. 2014, 58, 188–197. [Google Scholar]
- Kearsley, E.P.; Wainwright, J.P. The effect of porosity on the strength of foamed concrete. Cem. Concr. Res. 2020, 23, 233–239. [Google Scholar] [CrossRef]
- Le, V.S.; Szczypinski, M.M.; Hájková, P.; Kovacic, V.; Bakalova, T.; Volesky, L.; Louda, P. Mechanical properties of geopolymer foam at high temperature. Sci. Eng. Compos. Mater. 2020, 27, 129. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and ground-granulated blast furnace slag. Constr. Build. Mater. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Yao, X.; Zhu, Y. Effects of halloysite in kaolin on the formation and properties of geopolymers. Cem. Concr. Compos. 2012, 34, 709–715. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 2nd ed.; Institute Geopolymere: Saint Quentin, France, 2008. [Google Scholar]
- Liu, Y.; Yan, C.; Zhang, Z.; Gong, Y.; Wang, H.; Qiu, X. A facile method for preparation of floatable and permeable fly ash-based geopolymer block. Mater. Lett. 2016, 185, 370–373. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the chemical foaming reaction to control the porosity of geopolymer foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Zhao, J.; Li, D.; Ng, S.; Rui, Y. Effect of calcium stearate based foam stabilizer on pore characteristics and thermal conductivity of geopolymer foam material. J. Build. Eng. 2018, 20, 21–29. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.; García, S.V.; Israde-Alcántara, I. Fabrication and characterization of thermally-insulating coconut ash-based geopolymer foam. Waste Manag. 2018, 80, 235–240. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Zhao, Y.; Jow, J.; Cai, X.; Lai, S.Y. Fly ash-based geopolymer foam technology for thermal insulation and fire protection applications. In Proceedings of the 2015 World of Coal Ash (WOCA), Nashville, TN, USA, 5–7 May 2015; pp. 5–7. [Google Scholar]
- Ranjbar, N.; Mehrali, M.; Alengaram, U.J.; Metselaar, H.S.C.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr. Build. Mater. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Aredes, F.; Campos, T.; Machado, J.; Sakane, K.; Thim, G.; Brunelli, D. Effect of cure temperature on the formation of metakaolinite-based geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M. Thermo-mechanical characteristics of geopolymer mortar. Constr. Build. Mater. 2019, 213, 100–108. [Google Scholar] [CrossRef]

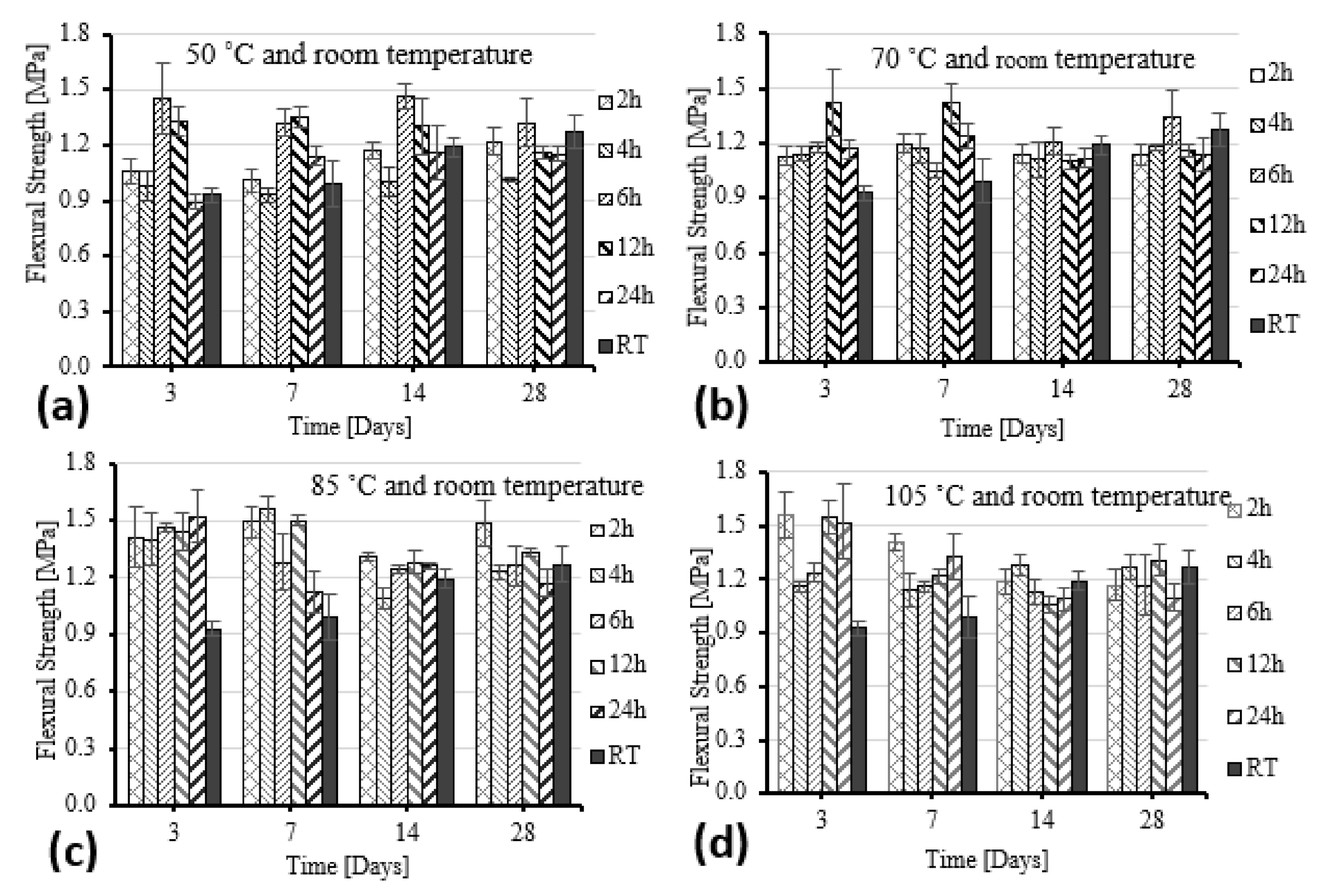
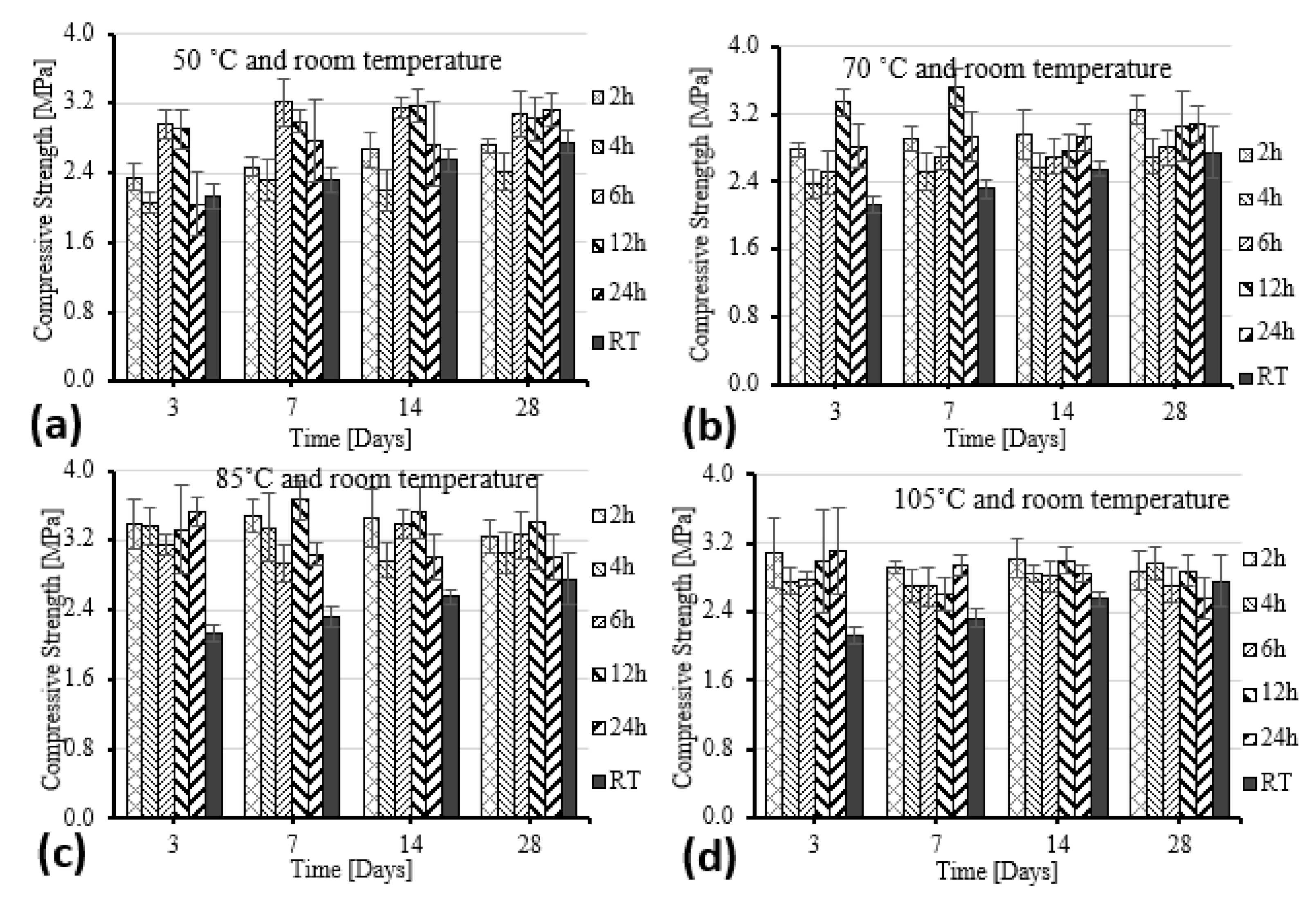
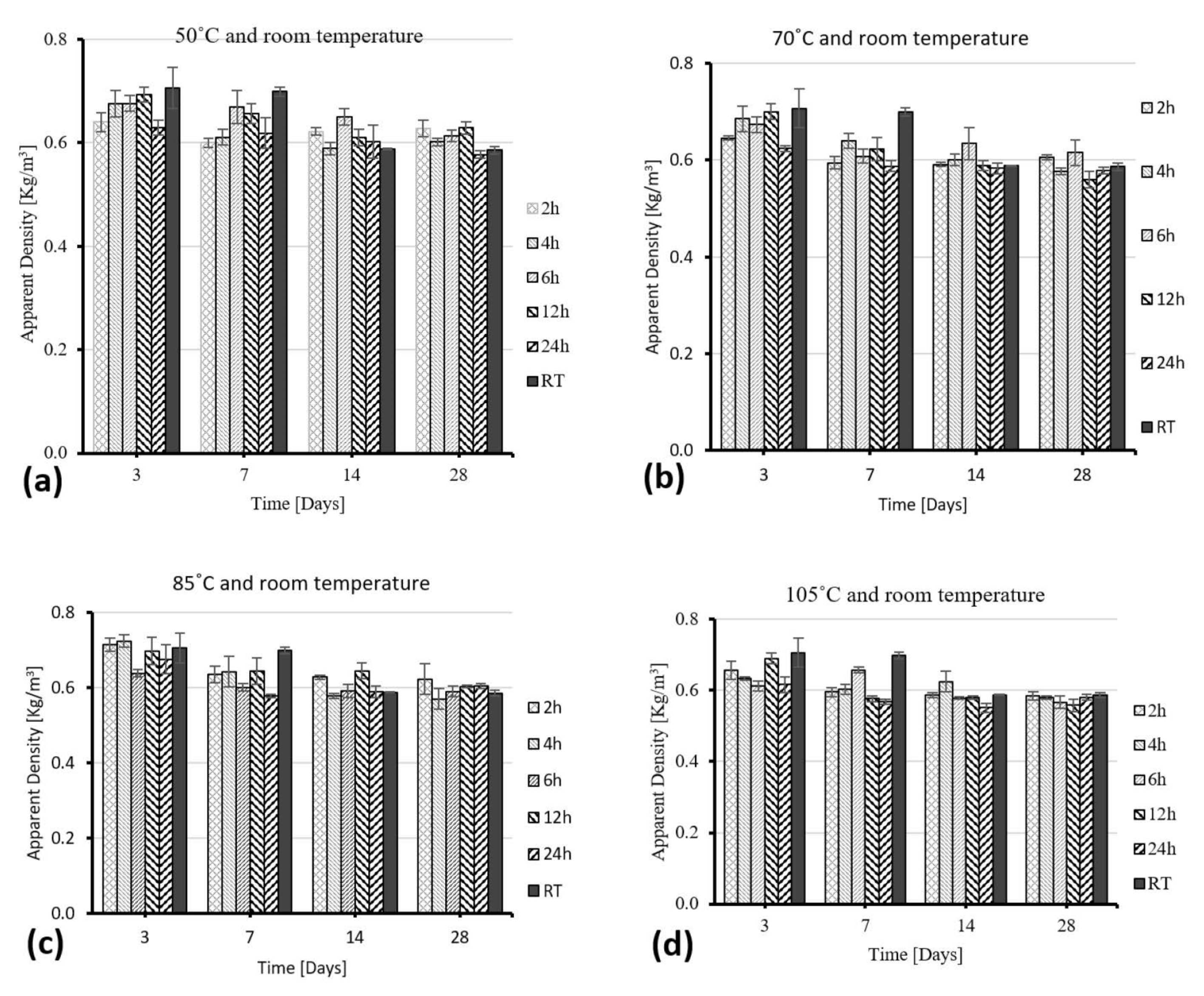
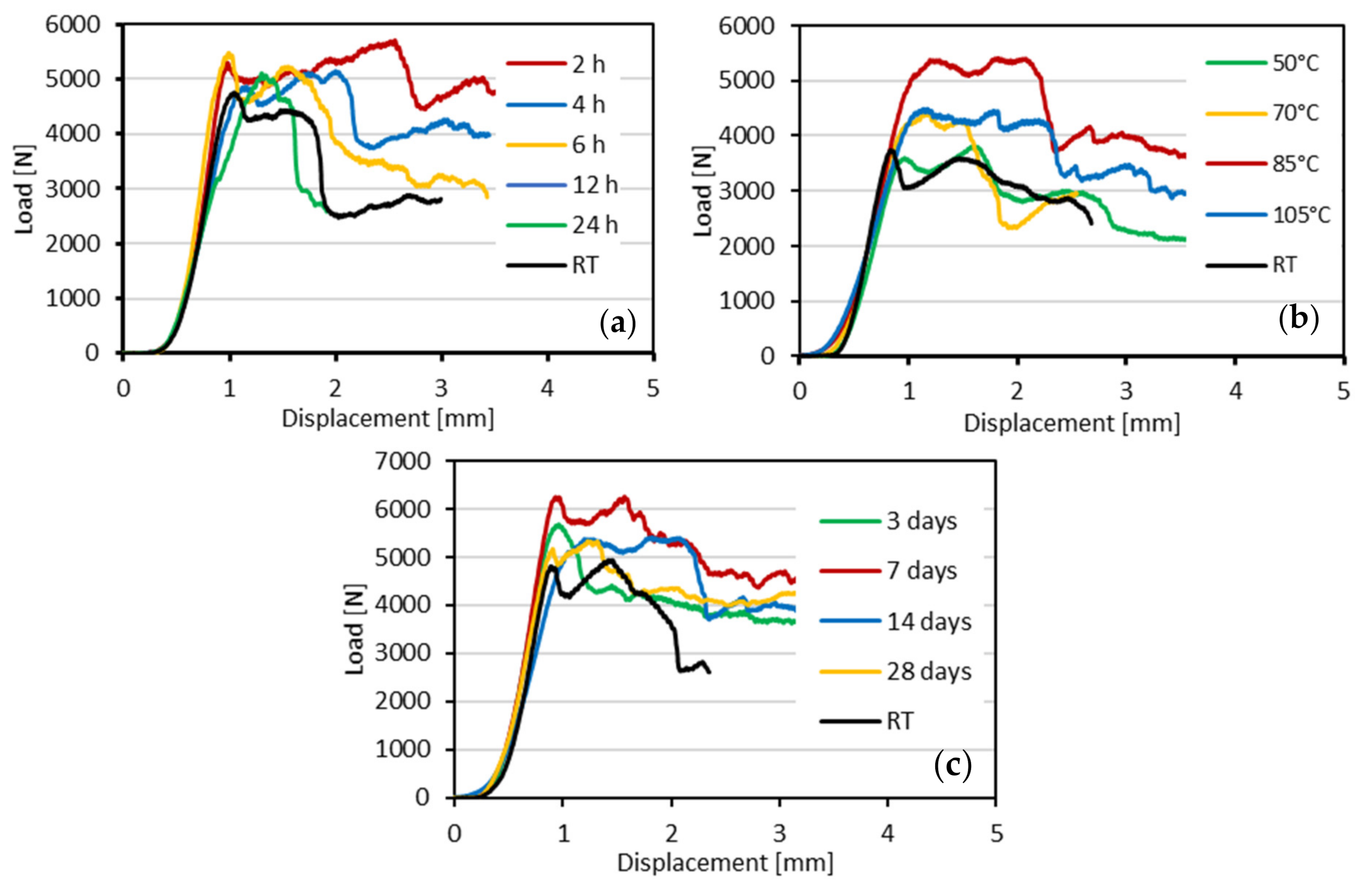
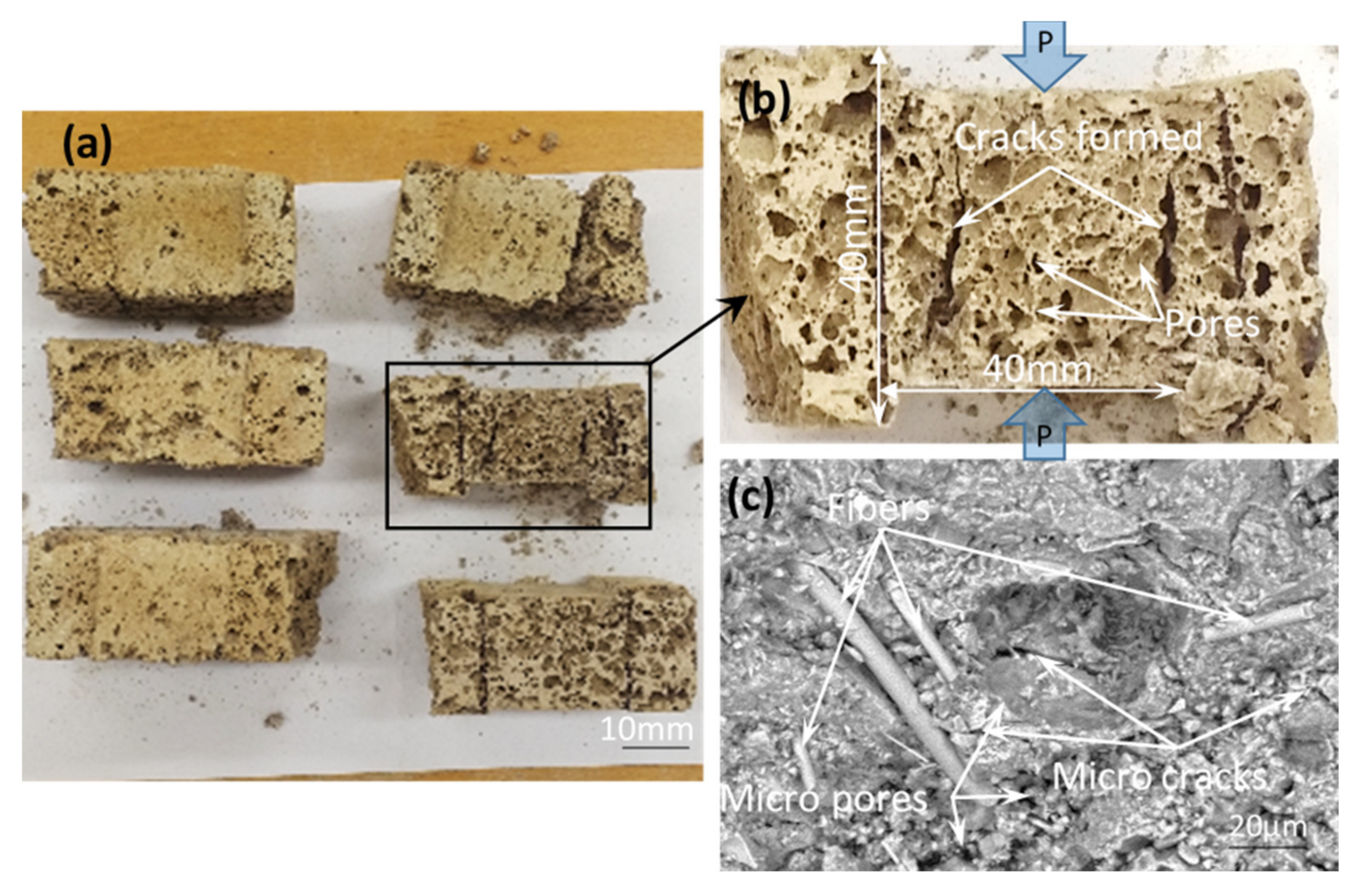
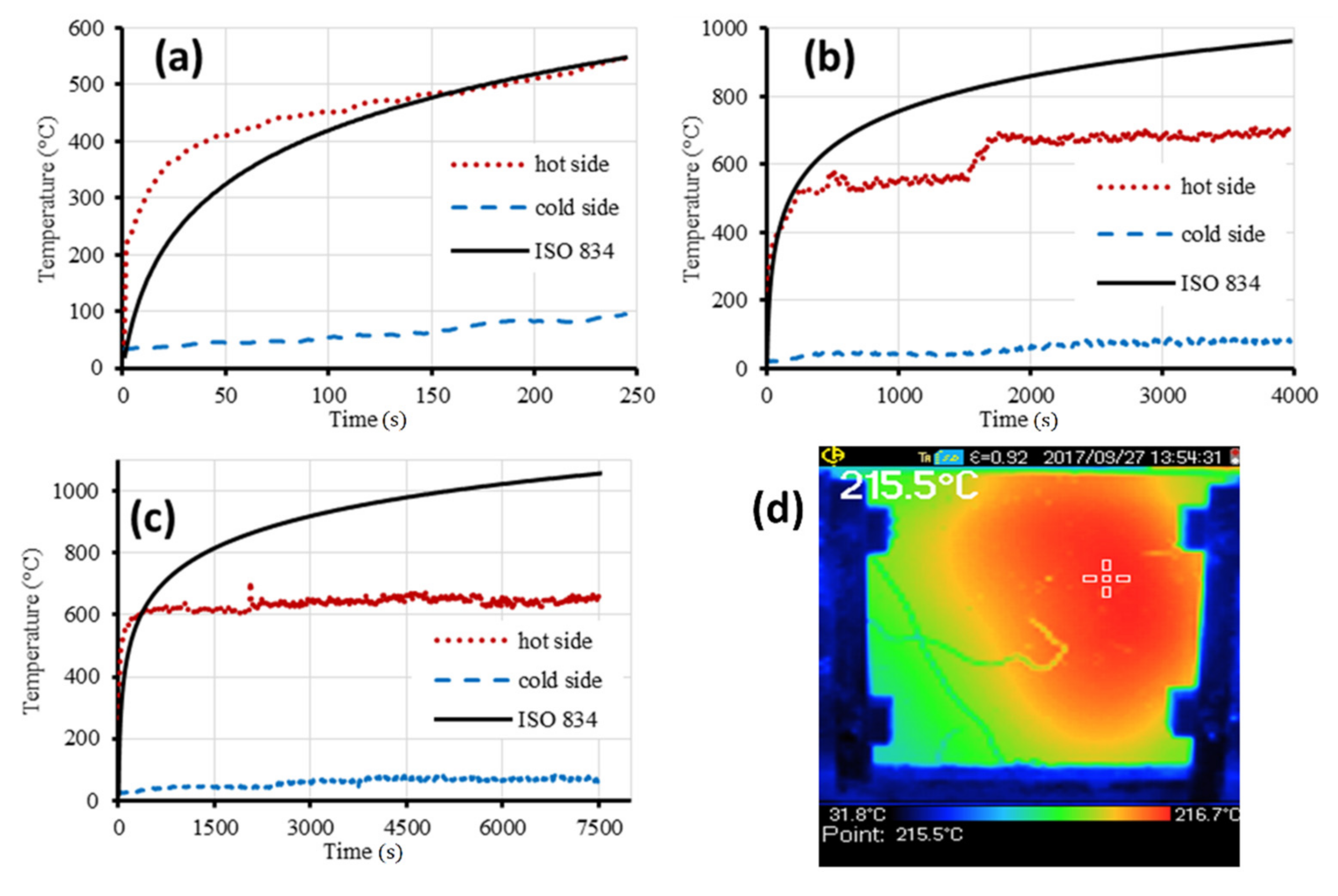
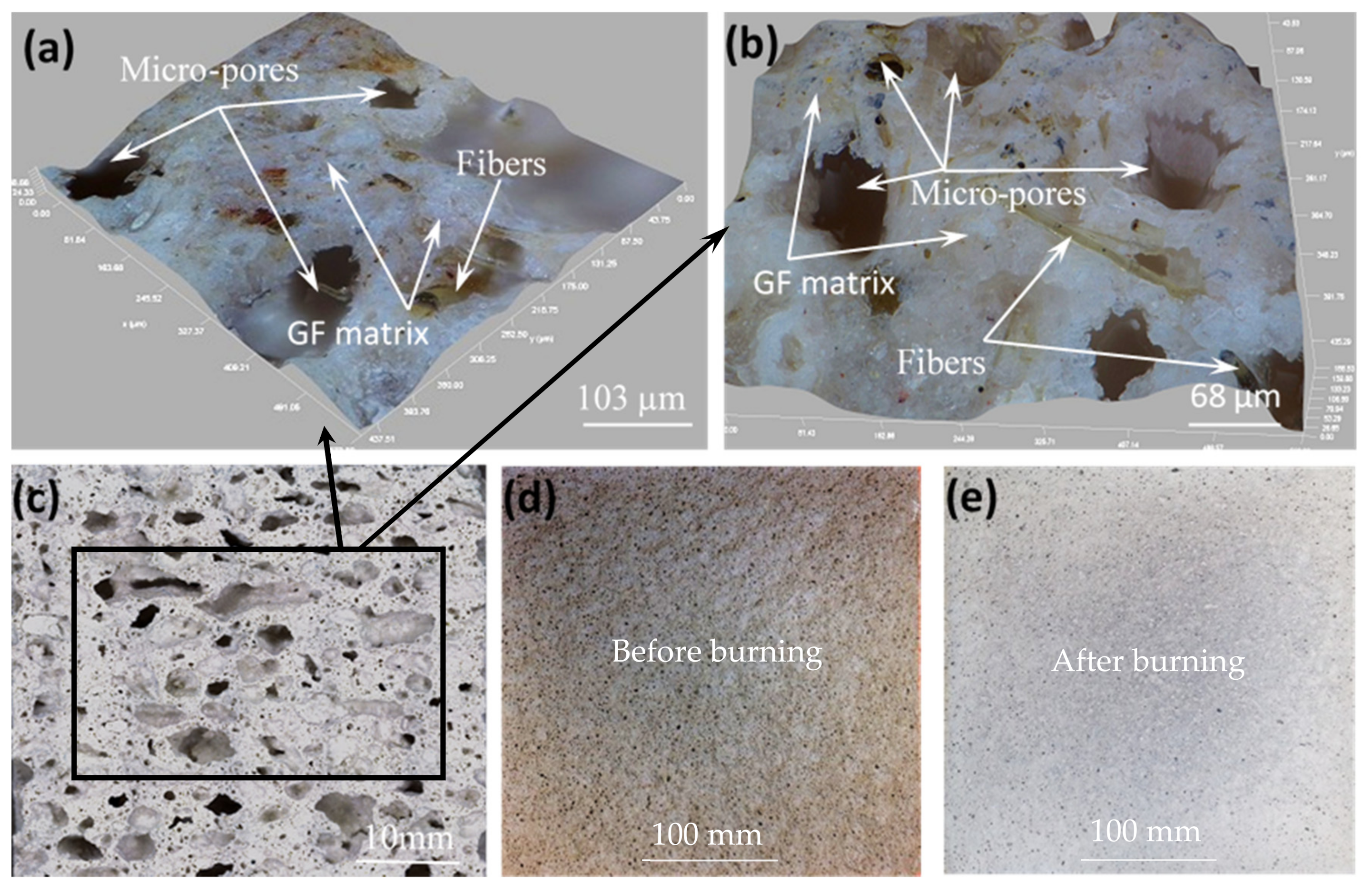
| Constituents | O | Si | Ca | Al | K | Mg | Ti | Na | Fe | Mn | S | Other | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geopolymer | 46.9 | 20.8 | 12.6 | 15.3 | 0.62 | 1.34 | 0.78 | 0.18 | 0.57 | 0.22 | 0.18 | 0.51 | 2.56 |
| Basalt ground fiber | 39.4 | 31.8 | 18.0 | 9.2 | 0.6 | 0.4 | 0.3 | 0.3 | 0.1 | – | – | – | 2.05 |
| CTI (h) | CTE (°C) | TT (Days) | CS (MPa) | FS (MPa) | D (g/cm3) | Ref. |
|---|---|---|---|---|---|---|
| 5 | 80 | 28 | 0.3–3 | – | 0.45–0.75 | [8] |
| 10 | 80 | 28 | 0.55 | – | 0.37 | [53] |
| 24 | 60 | 7 | 0.42–1.59 | – | – | [54] |
| 24 | 60 | 28 | 1.45 | – | 0.31 | [55] |
| 24 | 60 | – | 0.57–5.9 | – | 0.21–1 | [5] |
| 24 | 70 | 28 | 3.07 | – | 0.92 | [6] |
| 24 | 40 | 28 | 4.23–14.8 | – | 0.10–0.16 | |
| 24 | 70 | – | 2.9–9.3 | 2–3.6 | 0.64–1 | [7] |
| 24 | 40/75 | 28 | 2.3–30.7 | – | 0.37–0.87 | [16] |
| 24 | 75 | – | 2.19–3.11 | – | 0.4–0.51 | [9] |
| 24 | 85 | – | 0.67–0.96/ | – | 0.239–0.335 | [46] |
| 96 | 40 | 7 | 4.5 | – | 0.54 | [37] |
| 168 | 60 | – | 0.06–1.56 | – | 0.21–0.63 | [4] |
| – | RT | 28 | 1.3 | – | 0.6 | [56] |
| 2/4/6/12/24 | 20/50/70/85/105 | 3/7/14/28 | 2.75–3.5 | 0.9–1.5 | 0.56–0.62 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, V.S.; Louda, P. Research of Curing Time and Temperature-Dependent Strengths and Fire Resistance of Geopolymer Foam Coated on an Aluminum Plate. Coatings 2021, 11, 87. https://doi.org/10.3390/coatings11010087
Le VS, Louda P. Research of Curing Time and Temperature-Dependent Strengths and Fire Resistance of Geopolymer Foam Coated on an Aluminum Plate. Coatings. 2021; 11(1):87. https://doi.org/10.3390/coatings11010087
Chicago/Turabian StyleLe, Van Su, and Petr Louda. 2021. "Research of Curing Time and Temperature-Dependent Strengths and Fire Resistance of Geopolymer Foam Coated on an Aluminum Plate" Coatings 11, no. 1: 87. https://doi.org/10.3390/coatings11010087






