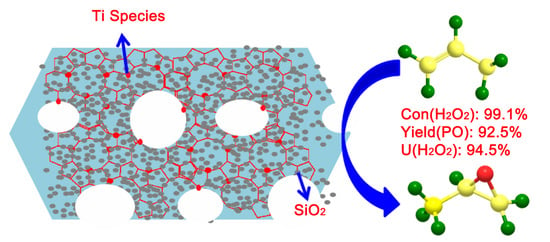
Improved Catalytic Propylene Epoxidation for Extruded Micrometer TS-1: Introducing Mesopores and Macropores Insides the Crystals
Abstract
1. Introduction
2. Results
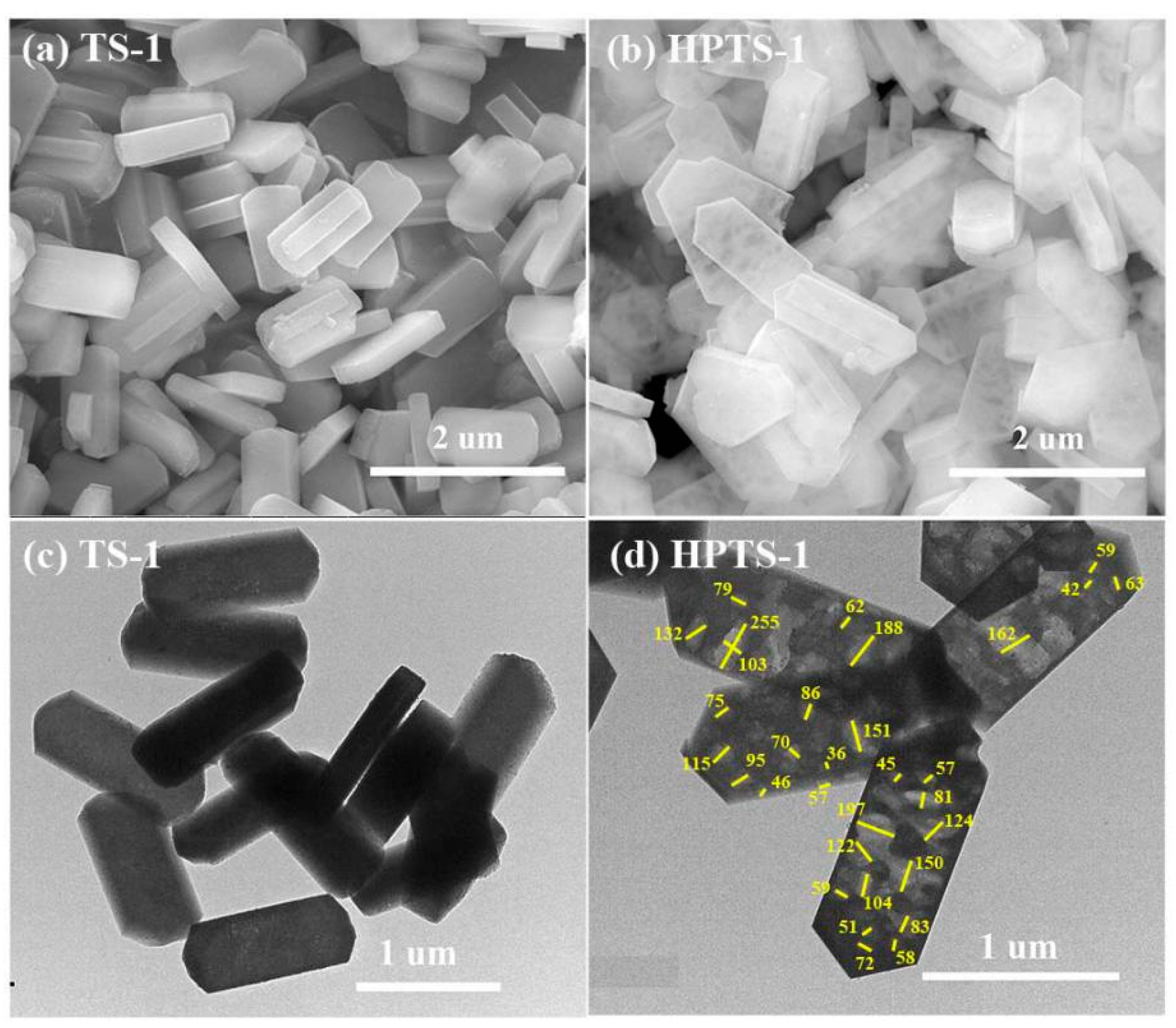
2.1. Characterization of Extruded TS-1 and HPTS-1 Samples
2.2. Catalytic Performance of Extruded TS-1 and HPTS-1 Samples in the Batch Reactor
2.3. Orthogonal Experiment Analysis of Extruded HPTS-1 Samples
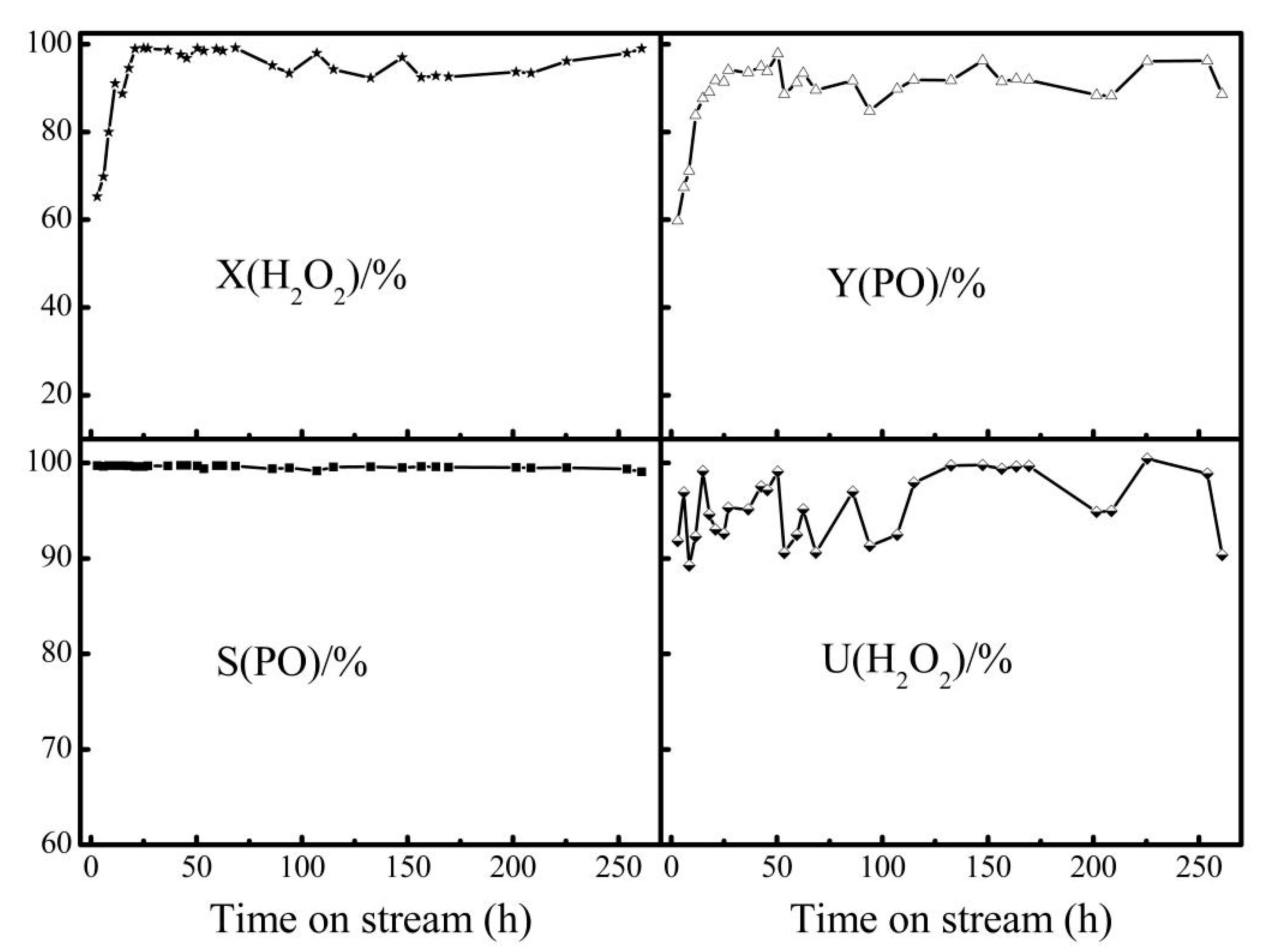
2.4. Stability and Regeneration Performance of Extruded HPTS-1 Samples
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Wu, H.; Jiang, J.; He, M.; Wu, P. Selective synthesis of propylene oxide through liquid-phase epoxidation of propylene with H2O2 over formed Ti-MWW catalyst. J. Catal. 2016, 342, 173–183. [Google Scholar] [CrossRef]
- Lu, M.; Zhao, X.; Zhou, J.; Qian, G.; Duan, X.; Yuan, W.; Zhou, X. Solvent screening and process optimization for separating propylene oxide from direct propylene epoxidation with H2 and O2. Ind. Eng. Chem. Res. 2019, 58, 395–402. [Google Scholar] [CrossRef]
- Lu, M.; Wang, G.; Zhang, Z.; Duan, X.; Yuan, W.; Zhou, X. Microporous inert membrane packed-bed reactor for propylene epoxidation with hydrogen and oxygen: Modelling and simulation. Chem. Eng. Process. 2017, 122, 425–433. [Google Scholar] [CrossRef]
- Kertalli, E.; Schouten, J.; Nijhuis, T. Direct synthesis of propylene oxide in the liquid phase under mild conditions. Appl. Catal. A Gen. 2016, 524, 200–205. [Google Scholar] [CrossRef]
- Kertalli, E.; Kosinov, N.; Schouten, J.; Nijhuis, T. Direct synthesis of propylene oxide in a packed bed membrane reactor. Chem. Eng. J. 2017, 307, 9–14. [Google Scholar] [CrossRef]
- Qi, C. The production of propylene oxide over nanometer Au catalysts in the presence of H2 and O2. Gold Bull. 2008, 41, 224–234. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, Y.; Guo, Y.; Zhang, Q.; Liu, X.; Wang, L.; Zhan, W.; Lu, G. Epoxidation of propylene by molecular oxygen over unsupported AgCux bimetallic catalyst. Rare Metals 2015, 34, 477–490. [Google Scholar] [CrossRef]
- Blanco-Brieva, G.; Frutos-Escrig, M.; Martín, H.; Campos-Martin, J.; Fierro, J. Selective decomposition of hydrogen peroxide in the epoxidation effluent of the HPPO process. Catal. Today 2012, 187, 168–172. [Google Scholar] [CrossRef]
- Song, W.; Zuo, Y.; Xiong, G.; Zhang, X.; Jin, F.; Liu, L.; Wang, X. Transformation of SiO2 in titanium silicalite-1/SiO2 extrudates during tetrapropylammonium hydroxide treatment and improvement of catalytic properties for propylene epoxidation. Chem. Eng. J. 2014, 253, 464–471. [Google Scholar] [CrossRef]
- Blanco-Brieva, G.; Capel-Sanchez, M.; Frutos, M.; Padilla-Polo, A. Campos-Martin, J.; Fierro, J. New two-step process for propene oxide production (HPPO) based on the direct synthesis of hydrogen peroxide. Ind. Eng. Chem. Res. 2008, 47, 8011–8015. [Google Scholar] [CrossRef]
- Huang, J.; Takei, T.; Ohashi, H.; Haruta, M. Propene epoxidation with oxygen over gold clusters: Role of basic salts and hydroxides of alkalis. Appl. Catal. A Gen. 2012, 435–436, 115–122. [Google Scholar] [CrossRef]
- Huang, J.; Haruta, M. Gas-phase propene epoxidation over coinage metal catalysts. Res. Chem. Intermed. 2012, 38, 1–24. [Google Scholar] [CrossRef]
- Qi, C.; Huang, J.; Bao, S.; Su, H.; Akita, T.; Haruta, M. Switching of reactions between hydrogenation and epoxidation of propene over Au/Ti-based oxides in the presence of H2 and O2. J. Catal. 2011, 281, 12–20. [Google Scholar] [CrossRef]
- Chowdhury, B.; Brave-Suárez, J.; Daté, M.; Tsubota, S.; Haruta, M. Trimethylamine as a gas-phase promoter: Highly efficient epoxidation of propylene over supported hold catalysts. Angew. Chem. 2006, 45, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Xia, C.; Zhu, B.; Li, H.; Shu, X. Green and efficient epoxidation of propylene with hydrogen peroxide (HPPO process) catalyzed by hollow TS-1 zeolite: A 1.0 kt/a pilot-scale study. Chem. Eng. J. 2016, 295, 370–375. [Google Scholar] [CrossRef]
- Russo, V.; Tesser, R.; Santacesaria, E.; Serio, M. Chemical and technical aspects of propene oxide production via hydrogen peroxide (HPPO process). Ind. Eng. Chem. Res. 2013, 52, 1168–1178. [Google Scholar] [CrossRef]
- Song, W.; Xiong, G.; Long, H.; Jin, F.; Liu, L.; Wang, X. Effect of treatment with different bases on the catalytic properties of TS-1/SiO2 extrudates in propylene epoxidation. Microporous Mesoporous Mater. 2015, 212, 48–55. [Google Scholar] [CrossRef]
- Signorile, M.; Crocellà, V.; Damin, A.; Rossi, B.; Lamberti, C.; Bonino, F.; Bordiga, S. Effect of Ti speciation on catalytic performance of TS-1 in the hydrogen peroxide to propylene oxide reaction. J. Phys. Chem. C 2018, 122, 9021–9034. [Google Scholar] [CrossRef]
- Nijhuis, T.; Makkee, M.; Moulijn, J.; Weckhuysen, B. The production of propene oxide: Catalytic processes and recent developments. Ind. Eng. Chem. Res. 2006, 45, 3447–3459. [Google Scholar] [CrossRef]
- Russo, V.; Tesser, R.; Santacesaria, E.; Di Serio, M. Kinetics of propene oxide production via hydrogen peroxide with TS-1. Ind. Eng. Chem. Res. 2014, 53, 6274–6287. [Google Scholar] [CrossRef]
- Shul’pin, G.; Sooknoi, T.; Shul’pina, L. The influence of organic additives on the regioselectivity of oxygenation of alkanes with hydrogen peroxide in the presence of TS-1. Petro. Chem. 2008, 48, 36–39. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Chen, G.; Chen, M.; Bai, R.; Jia, M.; Yu, J. Synthesis of anatase-free nano-sized hierarchical TS-1 zeolite and its excellent catalytic performance in alkenes epoxidation. J. Mater. Chem. A 2018, 20, 9473–9479. [Google Scholar] [CrossRef]
- Luo, Y.; Xiong, J.; Pang, C.; Li, G.; Hu, C. Direct hydroxylation of benzene to phenol over TS-1 catalysts. Catalysts 2018, 8, 49. [Google Scholar]
- Lu, X.; Guan, Y.; Xu, H.; Wu, H.; Wu, P. Clean synthesis of furfural oxime through liquid-phase ammoximation of furfural over titanosilicate catalysts. Green Chem. 2017, 19, 4871–4878. [Google Scholar] [CrossRef]
- Harvey, L.; Kennedy, E.; Dlugogorski, B.; Stockenhuber, M. Influence of impurities on the epoxidation of allyl alcohol to glycidol with hydrogen peroxide over titanium silicate TS-1. Appl. Catal. A Gen. 2015, 489, 241–246. [Google Scholar] [CrossRef]
- Přech, J.; Morris, R.; Čejka, J. Selective oxidation of bulky organic sulphides over layered titanosilicate catalysts. Catal. Sci. Technol. 2016, 6, 2775–2786. [Google Scholar] [CrossRef]
- Serrano, D.; Sanz, R.; Pizarro, P.; Moreno, I.; Frutos, P.; Blázquez, S. Preparation of extruded catalysts based on TS-1 zeolite for their application in propylene epoxidation. Catal. Today 2013, 143, 151–157. [Google Scholar] [CrossRef]
- Irandoust, S.; Andersson, B. Monolithic catalysts for nonautomobile applications. Catal. Rev. 1988, 30, 341–392. [Google Scholar] [CrossRef]
- Freiding, J.; Patcas, F.; Kraushaar-Czarnetzki, B. Extrusion of zeolites: Properties of catalysts with a novel aluminium phosphate sintermatrix. Appl. Catal. A Gen. 2007, 328, 210–218. [Google Scholar] [CrossRef]
- Prokof’ev, V. Masses for catalyst extrusion: Measuring and optimization of molding properties. Kinet. Catal. 2012, 53, 616–619. [Google Scholar] [CrossRef]
- Michels, N.; Mitchell, S.; Pérez-Ramírez, J. Effects of binders on the performance of shaped hierarchical MFI zeolites in methanol-to-hydrocarbons. ACS Catal. 2014, 4, 2409–2417. [Google Scholar] [CrossRef]
- Zuo, Y.; Song, W.; Wang, M.; Xu, Y.; Wang, X.; Guo, X. Epoxidation of propylene over small-crystal TS-1 extrudate in a fixed-bed reactor. Acta Phys. Chim. Sin. 2013, 29, 183–190. [Google Scholar]
- Wu, D.; Tang, M. Effects of process factors on extrusion of hierarchically porous ZSM-5 zeolite. Powder Technol. 2019, 352, 79–90. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, M.; Hong, L.; Wu, M.; Zhang, T.; Ma, M.; Song, C.; Guo, X. Role of supports in the tetrapropylammonium hydroxide treated titanium silicalite-1 extrudates. Ind. Eng. Chem. Res. 2015, 54, 1513–1519. [Google Scholar] [CrossRef]
- Liu, M.; Wei, H.; Li, B.; Song, L.; Zhao, S.; Niu, C.; Jia, C.; Wang, X.; Wen, Y. Green and efficient preparation of hollow titanium silicalite-1 by using recycled mother liquid. Chem. Eng. J. 2018, 331, 194–202. [Google Scholar] [CrossRef]
- Hu, Y.; Du, C.; Wang, T.; Luo, G. Highly efficient and greener synthesis of TS-1 in a flow system by recycling the mother liquid. Microporous Mesoporous Mater. 2019, 288, 109585. [Google Scholar] [CrossRef]
- Jiao, Y.; Adedigba, A.; He, Q.; Miedziak, P.; Brett, G.; Dummer, N.; Perdjon, M.; Liu, J.; Hutchings, G. Inter-connected and open pore hierarchical TS-1 with controlled framework titanium for catalytic cyclohexene epoxidation. Catal. Sci. Tech. 2018, 8, 2211–2217. [Google Scholar] [CrossRef]
- Huang, X.; Xue, Y.; Gao, X.; Li, B.; Wen, Y.; Wang, X. Development of an analytical method for evaluating the catalytic active sites of titanium silicalite zeolite. J. Mater. Sci. Chem. Eng. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Bordiga, S.; Damin, A.; Bonino, F.; Ricchiardi, G.; Lamberti, C.; Zecchina, A. The structure of the peroxo species in the TS-1 catalyst as investigated by resonant raman spectroscopy. Angew. Chem. Int. Ed. 2002, 41, 4734–4737. [Google Scholar] [CrossRef]
- Geobaldo, F.; Bordiga, S.; Zecchina, A.; Giamello, E.; Leofanti, G.; Petrini, G. DRS UV-Vis and EPR spectroscopy of hydroperoxo and superoxo complexes in titanium silicalite. Catal. Lett. 1992, 16, 109–115. [Google Scholar] [CrossRef]
- Bordiga, S.; Bonino, F.; Damin, A.; Lamberti, C. Adsorption properties of HKUST-1 toward hydrogen and other small molecules monitored by IR. Phys. Chem. Chem. Phys. 2007, 9, 2676–2685. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, G.; Su, J.; Li, P.; Guo, H. In situ UV raman spectroscopic study on the reaction intermediates for propylene epoxidation on TS-1. J. Phys. Chem. C 2012, 116, 9122–9131. [Google Scholar] [CrossRef]
- Lin, W.; Frei, H. Photochemical and FT-IR probing of the active site of hydrogen peroxide in Ti silicalite sieve. J. Am. Chem. Soc. 2002, 124, 9292–9298. [Google Scholar] [CrossRef]
- Prestipino, C.; Bonino, F.; Usseglio, S.; Damin, A.; Tasso, A.; Clerici, M.; Bordiga, S.; D’Acapito, F.; Zecchina, A.; Lamberti, C. Equilibria between peroxo and hydroperoxo species in the titanosilicates: An in situ high-resolution XANES investigation. ChemPhysChem 2004, 5, 1799–1804. [Google Scholar] [CrossRef]
- Sever, R.; Root, T. DFT study of solvent coordination effects on titanium-based epoxidation catalysts. Part two: Reactivity of titanium hydroperoxo complexes in ethylene epoxidation. J. Phys. Chem. B 2003, 107, 4090–4099. [Google Scholar] [CrossRef]
- Panyaburapa, W.; Nanok, T.; Limtrakul, J. Epoxidation reaction of unsaturated hydrocarbons with H2O2 over defect TS-1 investigated by ONIOM method: Formation of active sites and reaction mechanisms. J. Phys. Chem. C 2007, 111, 3433–3441. [Google Scholar] [CrossRef]


| Samples | SBET | Smic | Sext | Vtot | Vmic | Vmeso |
|---|---|---|---|---|---|---|
| m2/g | cm3/g | |||||
| TS-1 | 419 | 335 | 83.9 | 0.197 | 0.138 | 0.059 |
| E-TS-1-1 | 366 | 258 | 108 | 0.252 | 0.107 | 0.145 |
| E-TS-1-2 | 316 | 166 | 150 | 0.273 | 0.071 | 0.202 |
| HPTS-1 | 416 | 229 | 186 | 0.262 | 0.097 | 0.164 |
| E-HPTS-1-4 | 366 | 178 | 189 | 0.306 | 0.076 | 0.230 |
| E-HPTS-1-8 | 345 | 134 | 211 | 0.302 | 0.059 | 0.243 |
| Samples | Binder | X(H2O2) | Y(PO) | S(PO) | U(H2O2) | Strength | Specific Rate |
|---|---|---|---|---|---|---|---|
| % | (N/cm) | (h−1) | |||||
| TS-1 | 0 | 97.9 | 88.9 | 99.5 | 91.2 | -- | 241 |
| E-TS-1-1 | 20 | 78.9 | 78.3 | 99.5 | 99.7 | 82.1 | 243 |
| E-TS-1-2 | 30 | 48.7 | 47.7 | 99.8 | 98.0 | 190 | 214 |
| HPTS-1 | 0 | 99.7 | 98.4 | 99.0 | 99.7 | -- | 248 |
| E-HPTS-1-4 | 20 | 99.7 | 85.6 | 93.0 | 92.3 | 135 | 310 |
| E-HPTS-1-8 | 30 | 99.1 | 92.5 | 98.7 | 94.5 | 200 | 352 |
| Samples | Binder (wt%) | Sesbania Powder (wt%) | PVA (wt%) | Starch (wt%) | Strength (N/cm) | YPO (%) | Specific Rate (h−1) |
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||
| E-HPTS-1-1 | 10 | 1 | 0 | 1 | 33.5 | 86.7 | 275 |
| E-HPTS-1-2 | 10 | 3 | 5 | 3 | 42.7 | 90.4 | 275 |
| E-HPTS-1-3 | 10 | 5 | 10 | 5 | 61.2 | 89.5 | 275 |
| E-HPTS-1-4 | 20 | 1 | 5 | 5 | 135 | 85.6 | 310 |
| E-HPTS-1-5 | 20 | 3 | 10 | 1 | 151 | 89.2 | 310 |
| E-HPTS-1-6 | 20 | 5 | 0 | 3 | 143 | 88.6 | 310 |
| E-HPTS-1-7 | 30 | 1 | 10 | 3 | 186 | 90.6 | 354 |
| E-HPTS-1-8 | 30 | 3 | 0 | 5 | 200 | 92.5 | 352 |
| E-HPTS-1-9 | 30 | 5 | 5 | 1 | 171 | 86.6 | 340 |
| K1 | 137 | 355 | 377 | 356 | |||
| K2 | 429 | 394 | 349 | 372 | |||
| K3 | 557 | 375 | 398 | 396 | |||
| L1 | 267 | 263 | 268 | 263 | |||
| L2 | 263 | 272 | 263 | 270 | |||
| L3 | 270 | 265 | 269 | 268 | |||
| R1 | 140 | 13 | 16.3 | 13.3 | |||
| R2 | 2.3 | 3.0 | 2.0 | 2.33 |
| Samples | X(H2O2)/% | Y(PO)/% | S(PO)/% | U(H2O2)/% |
|---|---|---|---|---|
| E-HPTS-1-8 | 99.14 | 92.49 | 98.72 | 94.51 |
| Re-E-HPTS-1-8 | 99.60 | 96.44 | 98.95 | 97.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, F.; Zong, L.; Wang, X.; Wei, H. Improved Catalytic Propylene Epoxidation for Extruded Micrometer TS-1: Introducing Mesopores and Macropores Insides the Crystals. Catalysts 2021, 11, 113. https://doi.org/10.3390/catal11010113
Li J, Zhang F, Zong L, Wang X, Wei H. Improved Catalytic Propylene Epoxidation for Extruded Micrometer TS-1: Introducing Mesopores and Macropores Insides the Crystals. Catalysts. 2021; 11(1):113. https://doi.org/10.3390/catal11010113
Chicago/Turabian StyleLi, Jiangbo, Feifei Zhang, Lukuan Zong, Xiangyu Wang, and Huijuan Wei. 2021. "Improved Catalytic Propylene Epoxidation for Extruded Micrometer TS-1: Introducing Mesopores and Macropores Insides the Crystals" Catalysts 11, no. 1: 113. https://doi.org/10.3390/catal11010113
APA StyleLi, J., Zhang, F., Zong, L., Wang, X., & Wei, H. (2021). Improved Catalytic Propylene Epoxidation for Extruded Micrometer TS-1: Introducing Mesopores and Macropores Insides the Crystals. Catalysts, 11(1), 113. https://doi.org/10.3390/catal11010113