Two Hours of Separation Prior to Milking: Is This Strategy Stressful for Jennies and Their Foals?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Animals, Materials and Methods
2.1. Animals, Housing and Management
2.2. Experimental Design
2.3. Behaviour Assessments
2.4. Saliva Sampling
2.5. Milking
2.6. Salivary Cortisol Analysis
2.7. Data Analysis
3. Results
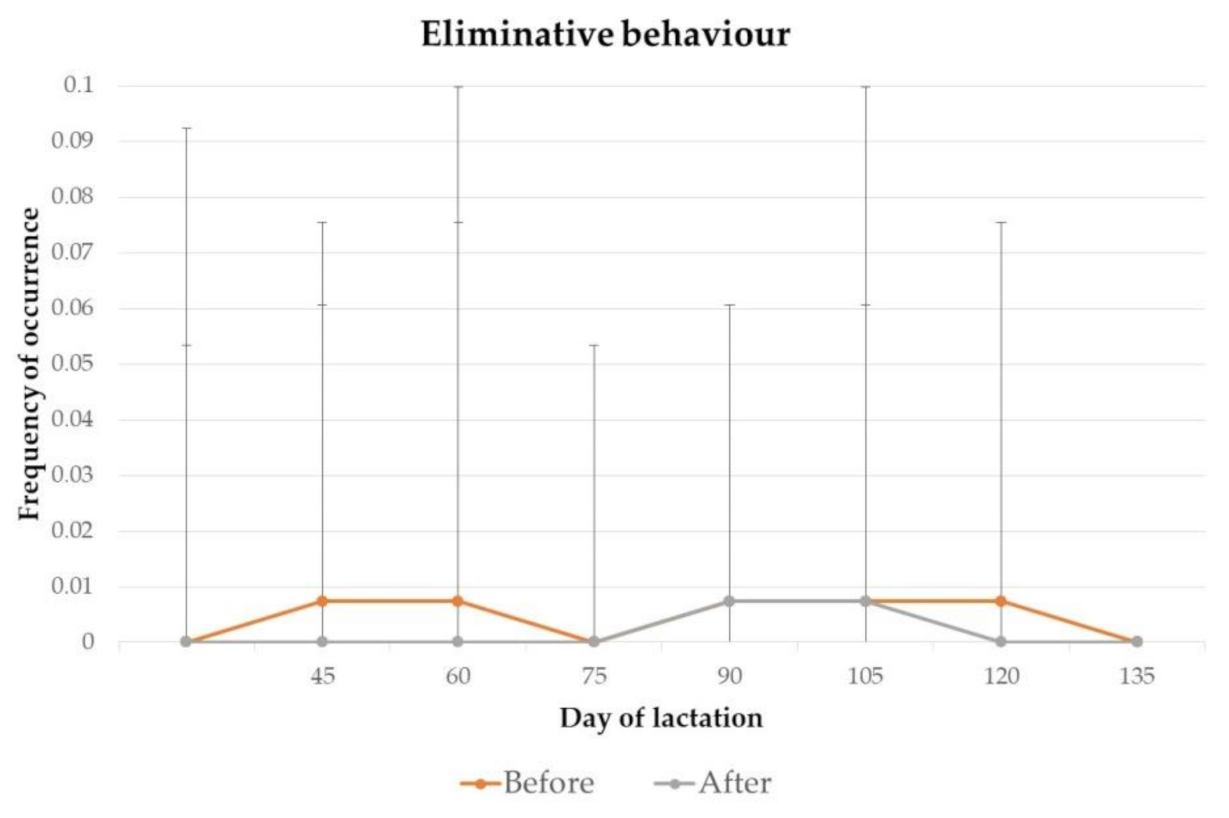
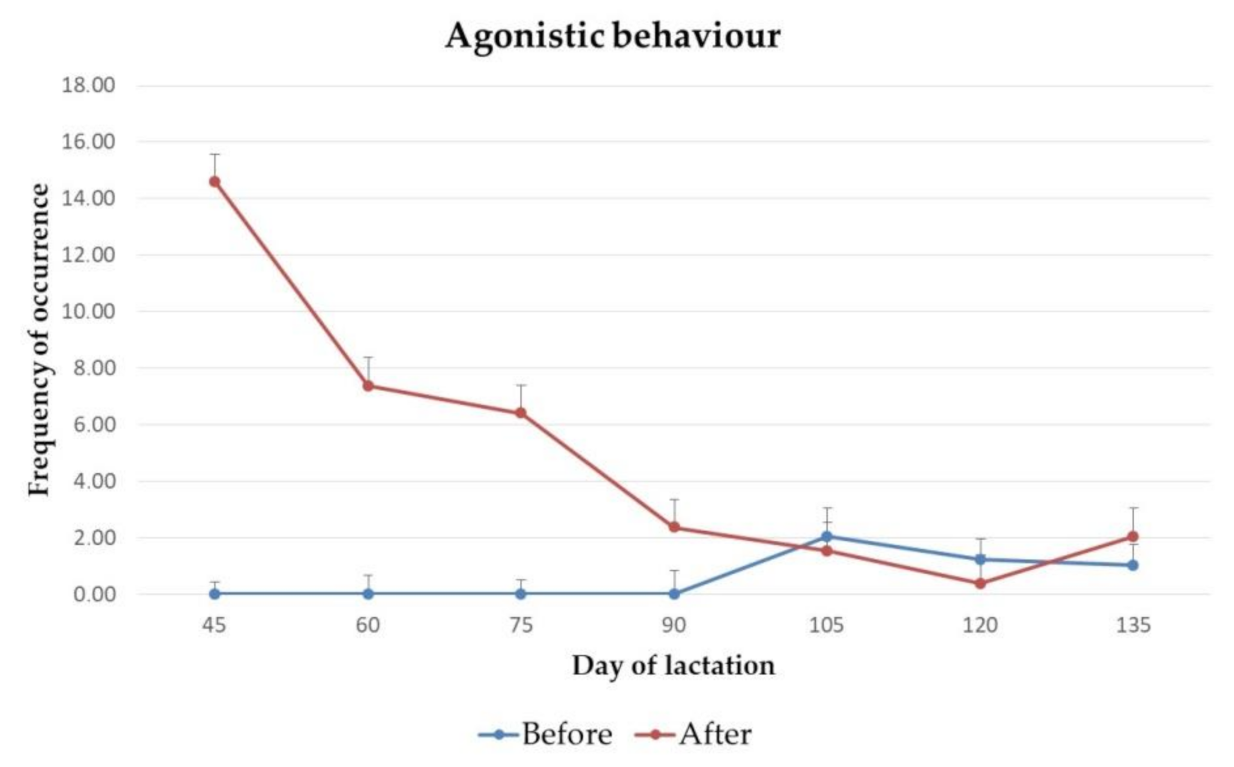
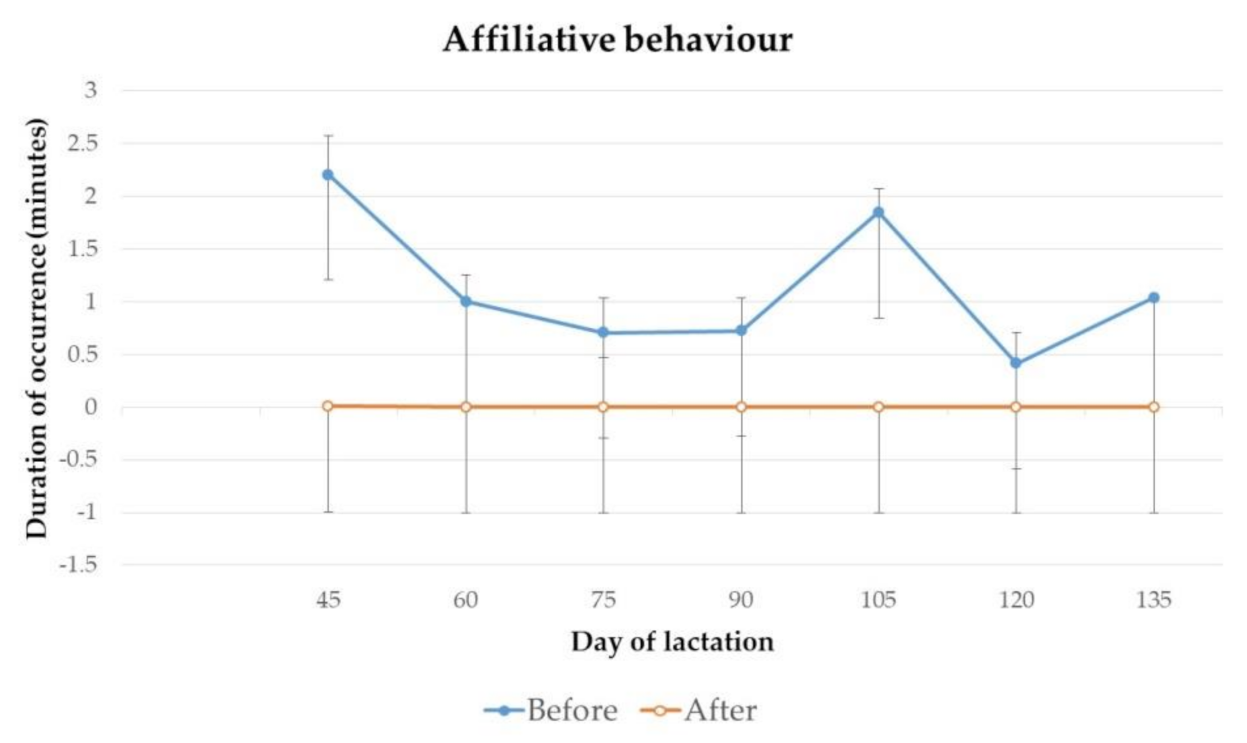
3.1. Behaviour
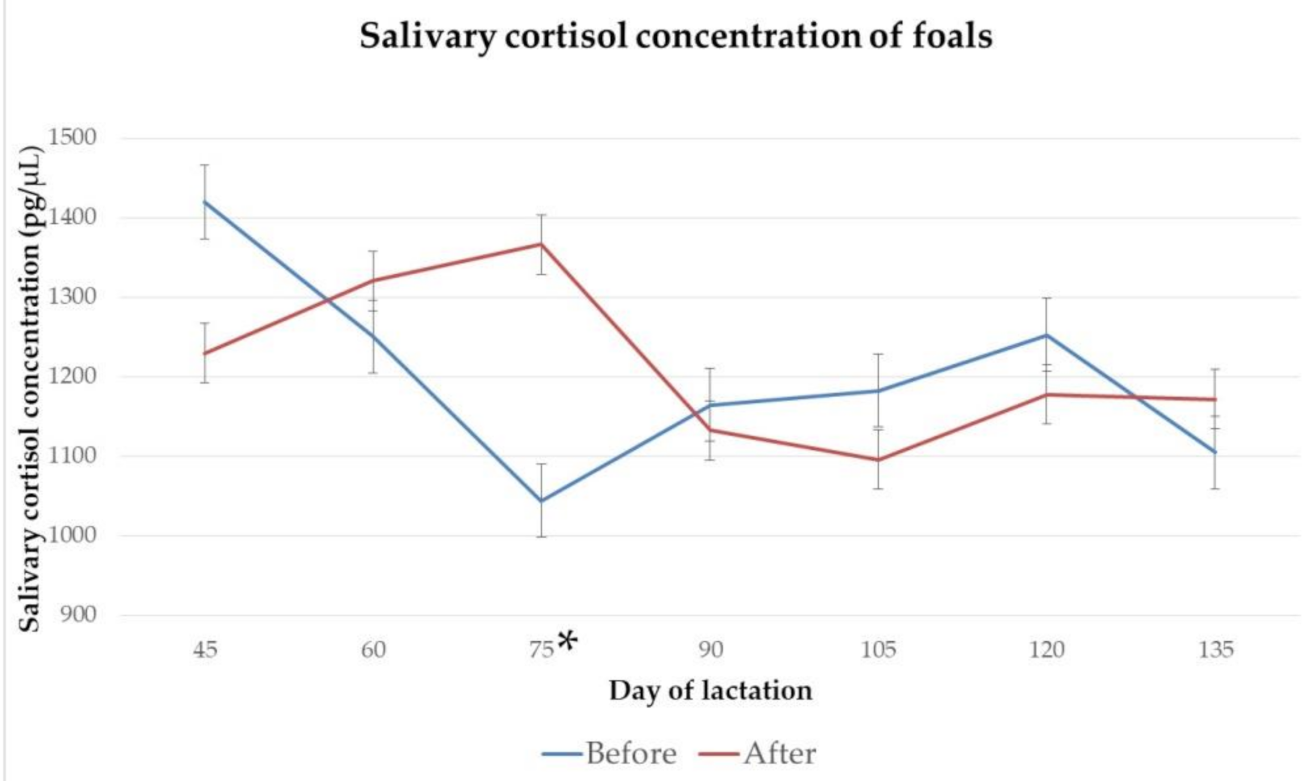
3.2. Salivary Cortisol Concentration
3.3. Milk Yield
4. Discussion
4.1. Behaviour
4.2. Salivary Cortisol Concentration
4.3. Milk Yield
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordonaro, S.; Dimauro, C.; Criscione, A.; Marletta, D.; Macciotta, N.P.P. The mathematical modeling of the lactation curve for dairy traits of the donkey (equus asinus). J. Dairy Sci. 2013, 96, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- IBGE. Produção da pecuária municipal 2012 (In portuguese). Inst. Bras. Geogr. Estatística IBGE 2012, 40, 71. [Google Scholar]
- Ochieng, F.; Alemayahu, M.; Smith, D. Improving the productivity of donkeys in Ethiopia. In Responding to the Increasing Global Demand for Animal Products: Programme and Summaries 42; Department for International Development: Addis Ababa, Ethiopia, 2004; pp. 93–94. [Google Scholar]
- Mariante, A.D.S.; Albuquerque, M.D.S.M.; Egito, A.A.; McManus, C.; Lopez, M.A.; Paiva, S.R. Present status of the conservation of livestock genetic resources in Brazil. Livest. Sci. 2009, 120, 204–212. [Google Scholar] [CrossRef] [Green Version]
- McManus, C.; Paiva, S.; Louvandini, H.; Melo, C.; Seixas, L. Jumentos no Brasil. In INCT Informaçãos Genético-Sanitária da Pecuária; 2010; pp. 1–10. [Google Scholar]
- Girardi, A.M.; Marquez, L.C.; de Toledo, C.Z.P.; de Campos Filho, E. Hematological variables of the Pêga donkey (Equus asinus) breed: Influence of age and sex. Comp. Clin. Pathol. 2015, 24, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Chiofalo, B.; Salimei, E.; Chiofalo, L. Ass’s milk: Exploitation of an alimentary resource. Riv. Folium 2001, 1, 235–241. [Google Scholar]
- Veneziano, V.; Di Loria, A.; Masucci, R.; Di Palo, R.; Brianti, E.; Gokbulut, C. Efficacy of eprinomectin pour-on against Dictyocaulus arnfieldi infection in donkeys (Equus asinus). Vet. J. 2011, 190, 414–415. [Google Scholar] [CrossRef] [Green Version]
- Souroullas, K.; Aspri, M.; Papademas, P. Donkey milk as a supplement in infant formula: Benefits and technological challenges. Food Res. Int. 2018, 109, 416–425. [Google Scholar] [CrossRef]
- D’Alessandro, A.G.; Martemucci, G. Lactation curve and effects of milking regimen on milk yield and quality, and udder health in Martina Franca jennies (Equus asinus). J. Anim. Sci. 2012, 90, 669–681. [Google Scholar] [CrossRef]
- Tesse, R.; Paglialunga, C.; Braccio, S.; Armenio, L. Adequacy and tolerance to ass’s milk in an Italian cohort of children with cow’s milk allergy. Ital. J. Pediatr. 2009, 35, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Carroccio, A.; Cavataio, F.; Montalto, G.; D’Amico, D.; Alabrese, L.; Iacono, G. Intolerance to hydrolysed cow’s milk proteins in infants: Clinical characteristics and dietary treatment. Clin. Exp. Allergy 2000, 30, 1598–1603. [Google Scholar] [CrossRef]
- De Palo, P.; Maggiolino, A.; Albenzio, M.; Caroprese, M.; Centoducati, P.; Tateo, A. Evaluation of different habituation protocols for training dairy jennies to the milking parlor: Effect on milk yield, behavior, heart rate and salivary cortisol. Appl. Anim. Behav. Sci. 2018, 204, 72–80. [Google Scholar] [CrossRef]
- D’Alessandro, A.G.; Mariano, M.; Martemucci, G. Udder characteristics and effects of pulsation rate on milking machine efficiency in donkeys. J. Dairy Res. 2015, 82, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.D.F.; Seppa, G.S.; Teixeira, L.G.; Rocha, T.G.; França, T.D.N.; Hess, T.M.; Peixoto, P.V. Mammary adenocarcinoma in a mare. Cienc. Rural 2008, 38, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Simoni, A.; Salimei, E.; Varisco, G. Struttura e Routine di Mungitura e Caratteristiche Della Produzione di Latte di Asina, Alimento Ipoallergenico per l’infanzia; Atti VI convegno società Italiana di ippologia: Campobasso, Italy, 2004; pp. 85–98. [Google Scholar]
- Burden, F.; Thiemann, A. Donkeys are different. J. Equine Vet. Sci. 2015, 35, 376–382. [Google Scholar] [CrossRef]
- Alabiso, M.; Giosuè, C.; Alicata, M.L.; Mazza, F.; Iannolino, G. The effects of different milking intervals and milking times per day in jennet milk production. Animal 2009, 3, 543–547. [Google Scholar] [CrossRef] [Green Version]
- Doreau, M.; Boulot, S. Recent knowledge on mare milk production: A review. Livest. Prod. Sci. 1989, 22, 213–235. [Google Scholar] [CrossRef]
- Henry, M.; McDonnell, S.M.; Lodi, L.D.; Gastal, E.L. Pasture mating behaviour of donkeys (Equus asinus) at natural and induced oestrus. J. Reprod. Fertil. Suppl. 1991, 44, 77–86. [Google Scholar]
- Klingel, H. Observations on Social Organization and Behaviour of African and Asiatic Wild Asses (Equus africanus and E. hemionus). Z. Tierpsychol. 1977, 44, 323–331. [Google Scholar] [CrossRef]
- Woodward, S.L. The Social System of Feral Asses (Equus asinus). Z. Tierpsychol. 1979, 49, 304–316. [Google Scholar] [CrossRef]
- McGreevy, P. Equine Behavior: A Guide for Veterinarians and Equine Scientists, 2nd ed.; W. B. Saunders: Edinburgh, UK, 2012. [Google Scholar]
- Mazzatenta, A.; Veronesi, M.C.; Vignola, G.; Ponzio, P.; Carluccio, A.; De Amicis, I. Behavior of Martina Franca donkey breed jenny-and-foal dyad in the neonatal period. J. Vet. Behav. 2019, 33, 81–89. [Google Scholar] [CrossRef]
- French, J.M. Mother-offspring relationships in donkeys. Appl. Anim. Behav. Sci. 1998, 60, 253–258. [Google Scholar] [CrossRef]
- Rashek, V.A. Details of feeding and feeding behaviour in young wild asses on Barsa-Kelmes Island (Aral Sea). Zoologicheskiĭ Zhurnal 1976, 55, 784–786. [Google Scholar]
- Smith-Funk, E.D.; Crowell-Davis, S.L. Maternal behavior of draft mares (Equus caballus) with mule foals (Equus asinus × Equus caballus). Appl. Anim. Behav. Sci. 1992, 33, 93–119. [Google Scholar] [CrossRef]
- Rossdale, P.D. Perinatal behavior in the throughbred horse. In Abnormal Behavior in Animals; Fox, M.W., Ed.; W. B. Saunders: Philadelphia, PA, USA, 1968; pp. 227–237. [Google Scholar]
- Scott, J.P. Social behavior, organisation, and leadership in a small flock of domestic sheep. Comp. Psychol. Monogr. 1945, 18, 1–29. [Google Scholar]
- Fazio, E.; Medica, P.; Grasso, L.; Messineo, C.; Ferlazzo, A. Changes of circulating β-endorphin, adrenocorticotrophin and cortisol concentrations during growth and rearing in Thoroughbred foals. Livest. Sci. 2009, 125, 31–36. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Cravana, C.; Ferlazzo, A. Adrenocortical response of jennies to milking stress. Livest. Sci. 2011, 137, 278–281. [Google Scholar] [CrossRef]
- Moberg, G.P. Biological Response to Stress: Key to Assessment of Animal Well-Being? Anim. Stress 1985, 27–49. [Google Scholar] [CrossRef]
- Yeon, S.C. Acoustic communication in the domestic horse (Equus caballus). J. Vet. Behav. Clin. Appl. Res. 2012, 7, 179–185. [Google Scholar] [CrossRef]
- Wickens, C.L.; Heleski, C.R. Crib-biting behavior in horses: A review. Appl. Anim. Behav. Sci. 2010, 128, 1–9. [Google Scholar] [CrossRef]
- Fureix, C.; Meagher, R.K. What can inactivity (in its various forms) reveal about affective states in non-human animals? A review. Appl. Anim. Behav. Sci. 2015, 171, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Zanella, A.J.; Broom, D.M.; Hunter, J.C.; Mendl, M.T. Brain opioid receptors in relation to stereotypies, inactivity, and housing in sows. Physiol. Behav. 1996, 59, 769–775. [Google Scholar] [CrossRef]
- McPhee, M.E.; Carlstead, K. The importance of maintaining natural behaviors in captive mammals. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management, 2nd ed.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 303–313. [Google Scholar]
- Beery, A.K.; Kaufer, D. Stress, social behavior, and resilience: Insights from rodents. Neurobiol. Stress 2015, 1, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Andrade, A.; Pinto, S.C.; de Oliveira, R.S. Animais de Laboratório: Criação e Experimentação; FIOCRUZ: Rio de Janeiro, Brazil, 2006; ISBN 8575410156. [Google Scholar]
- Steimer, T. The biology of fear- and anxiety-related behaviors. Dialogues Clin. Neurosci. 2002, 4, 231–249. [Google Scholar] [PubMed]
- Reis, L.S.L.S.; Pardo, P.E.; Frazatti-Gallina, N.M.; Paoli, R.L.; Oba, E.; Kronka, S.N.; Camargos, A.S. Effects of primovaccination and booster vaccination on serum cortisol and humoral immune response in cattle. Adv. Biosci. Biotechnol. 2013, 04, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Bruckmaier, R.M.; Wellnitz, O. Induction of milk ejection and milk removal in different production systems. J. Anim. Sci. 2008, 86, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheidegger, M.D.; Gerber, V.; Ramseyer, A.; Schüpbach-Regula, G.; Bruckmaier, R.M.; van der Kolk, J.H. Repeatability of the ACTH stimulation test as reflected by salivary cortisol response in healthy horses. Domest. Anim. Endocrinol. 2016, 57, 43–47. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Regan, F.H.; Hockenhull, J.; Pritchard, J.C.; Waterman-Pearson, A.E.; Whay, H.R. Behavioural repertoire of working donkeys and consistency of behaviour over time, as a preliminary step towards identifying pain-related behaviours. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Cozzi, A.; Sighieri, C.; Gazzano, A.; Nicol, C.J.; Baragli, P. Post-conflict friendly reunion in a permanent group of horses (Equus caballus). Behav. Processes 2010, 85, 185–190. [Google Scholar] [CrossRef]
- Heitor, F.; do Mar Oom, M.; Vicente, L. Social relationships in a herd of Sorraia horses. Part I. Correlates of social dominance and contexts of aggression. Behav. Processes 2006, 73, 170–177. [Google Scholar] [CrossRef]
- Schmidt, E.; Zillikens, D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun. Rev. 2010, 10, 84–89. [Google Scholar] [CrossRef]
- Schmidt, E.; Dähnrich, C.; Rosemann, A.; Probst, C.; Komorowski, L.; Saschenbrecker, S.; Schlumberger, W.; Stöcker, W.; Hashimoto, T.; Bröcker, E.B.; et al. Novel ELISA systems for antibodies to desmoglein 1 and 3: Correlation of disease activity with serum autoantibody levels in individual pemphigus patients. Exp. Dermatol. 2010, 19, 458–463. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/SHARE® 9.4: User’s Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Lebelt, D.; Schönreiter, S.; Zanella, A.J. Salivary cortisol in stallions: The relationship with plasma levels, daytime profile and changes in response to semen collection. Pferdeheilkunde 1996, 12, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol. In Encyclopedia of Stress; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2000; Volume 3, pp. 379–384. [Google Scholar]
- Schmidt, A.; Möstl, E.; Wehnert, C.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release and heart rate variability in horses during road transport. Horm. Behav. 2010, 57, 209–215. [Google Scholar] [CrossRef]
- Riad, F.D.; Read, G.F.; Walker, R.F. Variation in Endocrine Activity. J. Steroid Biochem. 1983, 19, 265–272. [Google Scholar] [CrossRef]
- Bonelli, F.; Rota, A.; Aurich, C.; Ille, N.; Camillo, F.; Panzani, D.; Sgorbini, M. Journal of Equine Veterinary Science Determination of Salivary Cortisol in Donkey Stallions. J. Equine Vet. Sci. 2019, 77, 68–71. [Google Scholar] [CrossRef]
- Hopster, H.; Van Der Werf, J.T.N.; Erkens, J.H.F.; Blokhuis, H.J. Effects of Repeated Jugular Puncture on Plasma Cortisol Concentrations in Loose-Housed Dairy Cows. J. Anim. Sci. 1999, 77, 708–714. [Google Scholar] [CrossRef]
- Aurich, J.; Wulf, M.; Ille, N.; Erber, R.; Von Lewinski, M.; Palme, R.; Aurich, C. Effects of season, age, sex and housing on salivary cortisol concentrations in horses. Domest. Anim. Endocrinol. 2015. [Google Scholar] [CrossRef]
- Schmidt, A.; Biau, S.; Möstl, E.; Becker-Birck, M.; Morillon, B. Changes in cortisol release and heart rate variability in sport horses during long-distance road transport. Domest. Anim. Endocrinol. 2010, 38, 179–189. [Google Scholar] [CrossRef]
- Schmidt, A.; Hödl, S.; Möstl, E.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release, heart rate, and heart rate variability in transport-naive horses during repeated road transport. DAE 2010, 39, 205–213. [Google Scholar] [CrossRef]
- Irvine, C.H.G.; Alexander, S.L. Factors affecting the circadian rhythm in plasma cortisol concentrations in the horse. Domest. Anim. Endocrinol. 1994, 11, 227–238. [Google Scholar] [CrossRef]
- Nagel, C.; Erber, R.; Bergmaier, C.; Wulf, M.; Aurich, J.; Möstl, E.; Aurich, C. Cortisol and progestin release, heart rate and heart rate variability in the pregnant and postpartum mare, fetus and newborn foal. THE 2012, 78, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Bohák, Z.; Szabó, F.; Beckers, J.; De Sousa, N.M.; Kutasi, O.; Nagy, K.; Szenci, O. Domestic Animal Endocrinology Monitoring the circadian rhythm of serum and salivary cortisol concentrations in the horse. Domest. Anim. Endocrinol. 2013, 45, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.L.; Irvine, C.H.G. The effect of social stress on adrenal axis activity in horses: The importance of monitoring corticosteroid-binding globulin capacity. J. Endocrinol. 1998, 157, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Dohanics, J.; Tóth, G.; Pongrácz, J. Maturation of the circadian rhythm of the adrenocortical functions in human neonates and infants. Horm. Res. 1980, 12, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Franks, R.C. Diurnal Variation of Plasma 17-Hydroxycorticosteroids in Children. J. Clin. Endocrinol. Metab. 1967, 27, 75–78. [Google Scholar] [CrossRef]
- Evans, F.D.; Christopherson, R.J.; Aherne, F.X. Development of the Circadian Rhythm of Cortisol in the Gilt from Weaning Until Puberty. Can. J. Anim. Sci. 1988, 68, 1105–1111. [Google Scholar] [CrossRef]
- Santos, I.C.B. Produção e Composição do Leite de Jumentas da Raça Pêga. Master’s thesis, Federal University of Bahia, Salvador, Brazil, 2017. [Google Scholar]
- Martini, M.; Altomonte, I.; Licitra, R.; Salari, F. Nutritional and Nutraceutical Quality of Donkey Milk. J. Equine Vet. Sci. 2018, 65, 33–37. [Google Scholar] [CrossRef]




| Affiliative | |
| Mutual grooming | Behaviour in which two donkeys use their teeth to simultaneously nibble any of each other’s body parts. |
| Licking | Licking any part of the body of another donkey. |
| Body sniffing | Sniffing the neck, withers, flank or tail of another donkey which may or may not reciprocate. |
| Approaching | Moving to within 1 m of another donkey that does not immediately move away and staying there for at least 10 s without initiating physical contact with it. |
| Touching | Touching another donkey at the neck or head, which may or may not reciprocate. |
| Agonistic | |
| Kicking | Rapid lifting of one or both hind limbs off the ground, directed towards another donkey or the observer, in an attempt to hit them, with the ears laid back. |
| Pushing | Pressing head, neck, chest or shoulder against another donkey, making them move away. |
| Chasing | Rapid movement toward another donkey and pursuit for a distance of over three body lengths, with the ears laid back, head raised and mouth closed. |
| Biting | Extension of head and neck towards another donkey, with the ears laid back, head raised and mouth open, closing teeth on its body. |
| Fighting | Pursuing another donkey for a distance of over three body lengths, with ears laid back, head raised and mouth open, attempting to close teeth on its body. |
| Abnormal | |
| Biting the stalls or structures | Grasping of structures with incisive teeth, which may be followed by simultaneous arching of the neck and sucking of air (cribbing). |
| False licking | Behaviour in which the animal slowly places its tongue on the borders of the stall or trough while keeping it still and stiff, so the action does not represent true licking. |
| Pawing | Vigorous and persistent stomping of limbs on the ground. |
| Eliminative | |
| Urinating | Elimination of urine. |
| Defecating | Elimination of faeces. |
| Vocalisations | |
| Vocalisations | Expression of vocal communication, such as whinnies, snores, snorts, groans or screams. |
| Inactivity | |
| Inactivity | Absence of movement or other actions. |
| Milk Yield (mL/day) | Cortisol before 2 h Separation (nmol/L) | Cortisol after 2 h Separation (nmol/pL) | |
|---|---|---|---|
| Milk yield (mL/day) | 1.00 | −0.131 | −0.044 |
| Cortisol before 2 h separation (nmol/L) | 1.00 | 0.432 | |
| Cortisol after 2 h separation (nmol/pL) | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Farias, S.; Montechese, A.C.D.; Bernardino, T.; Rodrigues, P.H.M.; de Araujo Oliveira, C.A.; Zanella, A.J. Two Hours of Separation Prior to Milking: Is This Strategy Stressful for Jennies and Their Foals? Animals 2021, 11, 178. https://doi.org/10.3390/ani11010178
de Souza Farias S, Montechese ACD, Bernardino T, Rodrigues PHM, de Araujo Oliveira CA, Zanella AJ. Two Hours of Separation Prior to Milking: Is This Strategy Stressful for Jennies and Their Foals? Animals. 2021; 11(1):178. https://doi.org/10.3390/ani11010178
Chicago/Turabian Stylede Souza Farias, Sharacely, Ana Carolina Dierings Montechese, Thiago Bernardino, Paulo Henrique Mazza Rodrigues, Chiara Albano de Araujo Oliveira, and Adroaldo José Zanella. 2021. "Two Hours of Separation Prior to Milking: Is This Strategy Stressful for Jennies and Their Foals?" Animals 11, no. 1: 178. https://doi.org/10.3390/ani11010178
APA Stylede Souza Farias, S., Montechese, A. C. D., Bernardino, T., Rodrigues, P. H. M., de Araujo Oliveira, C. A., & Zanella, A. J. (2021). Two Hours of Separation Prior to Milking: Is This Strategy Stressful for Jennies and Their Foals? Animals, 11(1), 178. https://doi.org/10.3390/ani11010178






