Factors Associated with the Introduction of Mycobacterium avium spp. Paratuberculosis (MAP) into Dairy Herds in Galicia (North-West Spain): The Perception of Experts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Area Description
2.2. Herds Surveyed and Data Collection
2.3. Expert Group Workshop
- -
- Initially, nine vets from different subspecialties (laboratory personnel in charge of processing MAP control program samples, practitioners, veterinarians in charge of MAP control programs, researchers specializing in epidemiology and infectious diseases in cattle, cattle association veterinarians and veterinarians from pharmaceutical laboratories) were selected, based on their experience, proximity and knowledge of the disease. Information on the background of the participants is provided as Table A1 (Appendix B).
- -
- Next, the decision trees were sent to each veterinarian, together with an appropriate explanation of the method, where each event was to be assigned a score (on an ordinal scale from 0 to 9) based on the risk that, in the opinion of each expert, this event could pose for the entry of the disease into a dairy herd [21].
- -
- Finally, a workshop for experts was held on December 2019 in the Veterinary Faculty of Lugo (Spain). During the workshop, doubts and possible misunderstandings were discussed and resolved. In addition, histograms showing the scores assigned by the participants were presented. These histograms were discussed with the entire group, and the participants had the chance to change their assigned scores if they considered that they had over- or underestimated any of the events.
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
| Position. Institution | Expertise | |
|---|---|---|
| 1 | Head of the molecular biology service. Animal health and production laboratory (Xunta de Galicia). | Around 28 years working on serology and molecular biology of infectious cattle diseases. |
| 2 | Chief vet. Sanitary Defense Group of Galicia | Responsible for the MAP control program in 4 municipalities in Galicia over the past 12 years. Previously 15 years of experience as free cattle practitioner. |
| 3 | Chief vet. Sanitary Defense Group of Galicia | Responsible for the MAP control program in 5 municipalities in Galicia over the past 15 years. |
| 4 | Professor and researcher. Animal Health. Veterinary Faculty. Zaragoza University | Around 30 years working on infectious ruminant diseases, mainly with risk analysis and transmission models. |
| 5 | Professor and researcher. Animal Health. Veterinary Faculty. Autonomous University of Barcelona | Around 20 years working on infectious cattle diseases mainly with risk and spatial analysis. |
| 6 | Professor and researcher. Animal Health. Veterinary Faculty. Santiago de Compostela University | Around 35 years working on infectious cattle diseases mainly with diagnostic techniques and transmission models. |
| 7 | Head of Dairy Herd Improvement Program, Galicia | Around 33 years of experience with dairy cattle. Has worked as a practitioner, nutritionist, head of a farming cooperative and during the last 23 years as head of the Dairy Herd Improvement Program in Galicia. |
| 8 | Cattle field technician. Pharmaceutical laboratory | Around 19 years of experience with dairy cattle. Free practitioner and has spent the last 5 years as a dairy cattle field technician at a pharmaceutical laboratory. |
| 9 | Free cattle practitioner | Around 9 years of experience as a free dairy cattle practitioner. |
References
- Lambrecht, R.S.; Collins, M.T. Mycobacterium paratuberculosis factors that influence mycobactin dependence. Diagn. Microbiol. Infect. Dis. 1992, 15, 239–246. [Google Scholar] [CrossRef]
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and dormancy of Mycobacterium avium subsp. paratuberculosis in the environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, E.K.B.; More, S.J. Direct and indirect effects of Johne’s disease on farm and animal productivity in an Irish dairy herd. Ir. Vet. J. 2009, 62, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozsvari, L.; Harnos, A.; Lang, Z.; Monostori, A.; Strain, S.; Fodor, I. The Impact of Paratuberculosis on Milk Production, Fertility, and Culling in Large Commercial Hungarian Dairy Herds. Front. Vet. Sci. 2020, 7, 778. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Vanleeuwen, J.A.; Dohoo, I.R.; Stryhn, H.; Keefe, G.P.; Haddad, J.P. Effects of seropositivity for bovine leukemia virus, bovine viral diarrhoea virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum on culling in dairy cattle in four Canadian provinces. Vet. Microbiol. 2005, 109, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Mato, I.; Pesqueira, N.; Factor, C.; Camino, F.; Hernán-Pérez, M.L.; Respaldiza, E.Y.; Diéguez, F.J. Effect of Mycobacterium avium subsp. paratuberculosis serostatus on carcass weight and conformation and fat cover scores. Span. J. Agric. Res. 2017, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Mato, I.; Pesqueira, N.; Factor, C.; Sanjuan, M.L.; Yus, E.; Fouz, R.; Arnaiz, I.; Camino, F.; Diéguez, F.J. Effect of Mycobacterium avium subsp. paratuberculosis infection status on culling and calving difficulty in dairy cattle. Livest. Sci. 2015, 177, 151–158. [Google Scholar]
- Smith, R.L.; Strawderman, R.L.; Schukken, Y.H.; Wells, S.J.; Pradhan, A.K.; Espejo, L.A.; Whitlock, R.H.; Van Kessel, J.S.; Smith, J.M.; Wolfgang, D.R.; et al. Effect of Johne’s disease status on reproduction and culling in dairy cattle. J. Dairy Sci. 2010, 93, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.S. Could Mycobacterium avium subspecies paratuberculosis cause Crohn’s disease, ulcerative colitis…and colorectal cancer? Infect. Agent Cancer 2018, 13, 1. [Google Scholar] [CrossRef]
- McAloon, C.G.; Doherty, M.L.; Whyte, P.; More, S.J.; O’Grady, L.; Citer, L.; Green, M.J. Relative importance of herd-level risk factors for probability of infection with paratuberculosis in Irish dairy herds. J. Dairy Sci. 2017, 100, 9245–9257. [Google Scholar] [CrossRef]
- Puerto-Parada, M.; Arango-Sabogal, J.C.; Paré, J.; Doré, E.; Côté, G.; Wellemans, V.; Buczinski, S.V.; Roy, J.P.; Labrecque, O.; Fecteau, G. Risk factors associated with Mycobacterium avium subsp. paratuberculosis herd status in Québec dairy herds. Prev. Vet. Med. 2018, 152, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Correa-Valencia, N.M.; Ramírez, N.F.; Arango-Sabogal, J.C.; Fecteau, G.; Fernández-Silva, J.A. Prevalence of Mycobacterium avium subsp. paratuberculosis infection in dairy herds in Northern Antioquia (Colombia) and associated risk factors using environmental sampling. Prev. Vet. Med. 2019, 170, 104739. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.A.; Whittington, R.J. Evidence for age susceptibility of cattle to Johne’s disease. Vet. J. 2010, 184, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Fecteau, M.E. Paratuberculosis in Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; Shalloo, L. Invited review: The economic impact and control of paratuberculosis in cattle. J. Dairy Sci. 2015, 98, 5019–5039. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente (MAPAMA). Panel Situación Sector Lácteo España. 2018. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/resultados_provisionales_may2019_bovino_webmapa_tcm30-514025.pdf (accessed on 2 December 2019).
- Vilar, A.L.; Santos, C.S.; Pimenta, C.L.; Freitas, T.D.; Brasil, A.W.; Clementino, I.J.; Alves, C.J.; Bezerra, C.S.; Riet-Correa, F.; Oliveira, T.S.; et al. Herd-level prevalence and associated risk factors for Mycobacterium avium subsp. paratuberculosis in cattle in the State of Paraíba, Northeastern Brazil. Prev. Vet. Med. 2015, 121, 49–55. [Google Scholar] [CrossRef]
- Wolf, R.; Barkema, H.W.; De Buck, J.; Orsel, K. Dairy farms testing positive for Mycobacterium avium ssp. paratuberculosis have poorer hygiene practices and are less cautious when purchasing cattle than test-negative herds. J. Dairy Sci. 2016, 99, 4526–4536. [Google Scholar] [CrossRef]
- Villaamil, F.J.; Arnaiz, I.; Allepuz, A.; Molins, M.; Lazaro, M.; Benavides, B.; Moya, S.J.; Casal, J.; Yus, E.; Dieguez, F.J. A survey of biosecurity measures and serological status for bovine viral diarrhoea virus and bovine herpesvirus 1 on dairy cattle farms in north-west and north-east Spain. Vet. Rec. Open 2020, 7, e000399. [Google Scholar] [CrossRef]
- OIE. Handbook on Import Risk Analysis for Animals and Animal Products, 1st ed.; World Organization for Animal Health (Office International des Epizooties): Paris, France, 2004; Volume 2. [Google Scholar]
- Dufour, B.; Ple´e, L.; Moutou, F.; Boisseleau, D.; Chartier, C.; Durand, B.; Ganière, J.P.; Guillotin, J.; Lancelot, R.; Saegerman, C.; et al. A qualitative risk assessment methodology for scientific expert panels. Rev. Sci. Tech. 2011, 30, 673–681. [Google Scholar] [CrossRef]
- Arnaiz, I.; Eiras, C.; Carnero, M.I.; Rubinos, V.; Calavia, P.M.; Cortón, M.E.; López, M.; Yus, E.; Diéguez, F.J.; Orejas, J.J.; et al. 15 años de programa de control voluntario frente al IBR en Galicia: Situación ante un programa de erradicación. In Proceedings of the XXIII Congreso Internacional de Medicina Bovina Anembe, Vigo, Spain, 6–8 June 2018. [Google Scholar]
- Khol, J.L.; Baunmgartner, W. Examples and suggestions for the control of paratuberculosis in European cattle. Jpn. J. Vet. Res. 2012, 60, S1–S7. [Google Scholar]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Santi, A. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorge, U.S.; Lissemore, K.; Godkin, A.; Jansen, J.; Hendrick, S.; Wells, S.; Kelton, D.F. Risk factors for herds to test positive for Mycobacterium avium ssp. paratuberculosis-antibodies with a commercial milk enzyme-linked immunosorbent assay (ELISA) in Ontario and western Canada. Can. Vet. J. 2012, 53, 963. [Google Scholar] [PubMed]
- AFRICOR. Memoria Africor Lugo. 2017. Available online: https://issuu.com/transmediacomunicacion/docs/memoria_africorlugo_2017 (accessed on 2 December 2019).
- Sergeant, E.S.G.; McAloon, C.G.; Tratalos, J.A.; Citer, L.R.; Graham, D.A.; More, S.J. Evaluation of national surveillance methods for detection of Irish dairy herds infected with Mycobacterium avium ssp. paratuberculosis. J. Dairy Sci. 2019, 102, 2525–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xunta de Galicia. Orden Ayudas ADSG. 2019. Available online: https://www.xunta.gal/dog/Publicados/2019/20190201/AnuncioG0426-080119-0002_es.html (accessed on 2 December 2019).
- Stevenson, K. Genetic diversity of Mycobacterium avium subspecies paratuberculosis and the influence of strain type on infection and pathogenesis: A review. Vet. Res. 2015, 46, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.V.; Singh, A.V.; Kumar, A.; Singh, P.K.; Deb, R.; Verma, A.K.; Kumar, A.; Tiwari, R.; Sandip, C.; Dhama, K. Survival mechanisms of Mycobacterium avium subspecies paratuberculosis within host species and in the environment—A review. Nat. Sci. 2013, 5, 710–723. [Google Scholar]
- Smith, R.L.; Schukken, Y.H.; Pradhan, A.K.; Smith, J.M.; Whitlock, R.H.; Van Kessel, J.S.; Wolfgang, D.R.; Grohn, Y.T. Environmental contamination with Mycobacterium avium subsp. paratuberculosis in endemically infected dairy herds. Prev. Vet. Med. 2011, 102, 1–9. [Google Scholar] [CrossRef]
- Eisenberg, S.W.F.; Nielen, M.; Hoeboer, J.; Rutten, V.; Heederik, D.; Koets, A.P. Environmental contamination with Mycobacterium avium subspecies paratuberculosis within and around a dairy barn under experimental conditions. J. Dairy Sci. 2012, 95, 6477–6482. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, A.K.; Kramer, A.J.; Mitchell, R.M.; Whitlock, R.H.; Smith, J.M.; Hovingh, E.; Van Kessel, J.S.; Karns, J.S.; Schukken, Y.H. Multilocus short sequence repeat analysis of Mycobacterium avium subsp. paratuberculosis isolates from dairy herds in Northeastern United States of a longitudinal study indicates low shedders are truly infected. In Proceedings of the 10th International Colloquium on Paratuberculosis, Minneapolis, MN, USA, 9–14 August 2009. [Google Scholar]
- Ayele, W.Y.; Svastova, P.; Roubal, P.; Bartos, M.; Pavlik, I. Mycobacterium avium subspecies paratuberculosis cultured from locally and commercially pasteurized cow’s milk in the Czech Republic. Appl. Environ. Microbiol. 2005, 71, 1210–1214. [Google Scholar] [CrossRef] [Green Version]
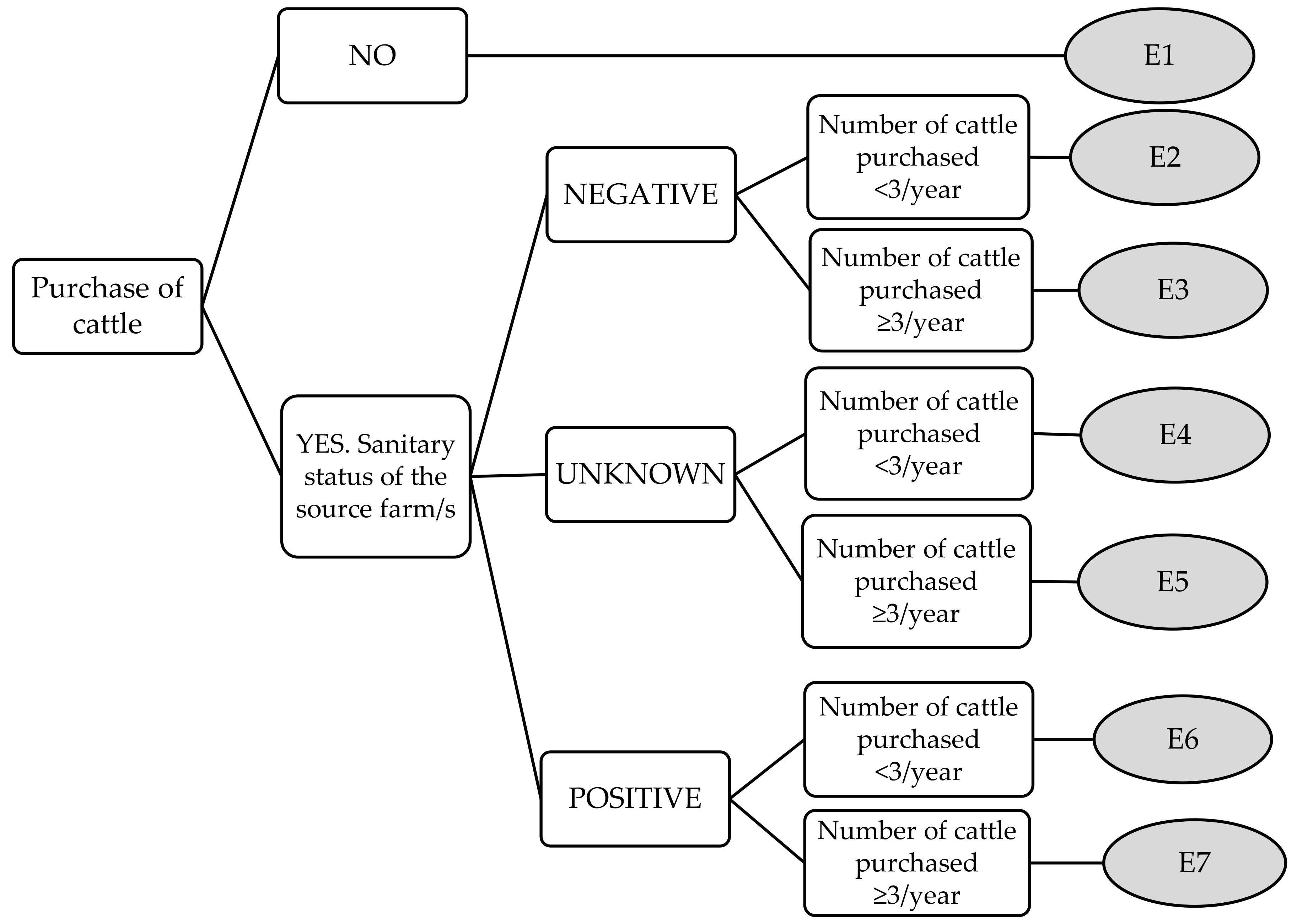
| Mean | Median | Maximum | Minimum | ||
|---|---|---|---|---|---|
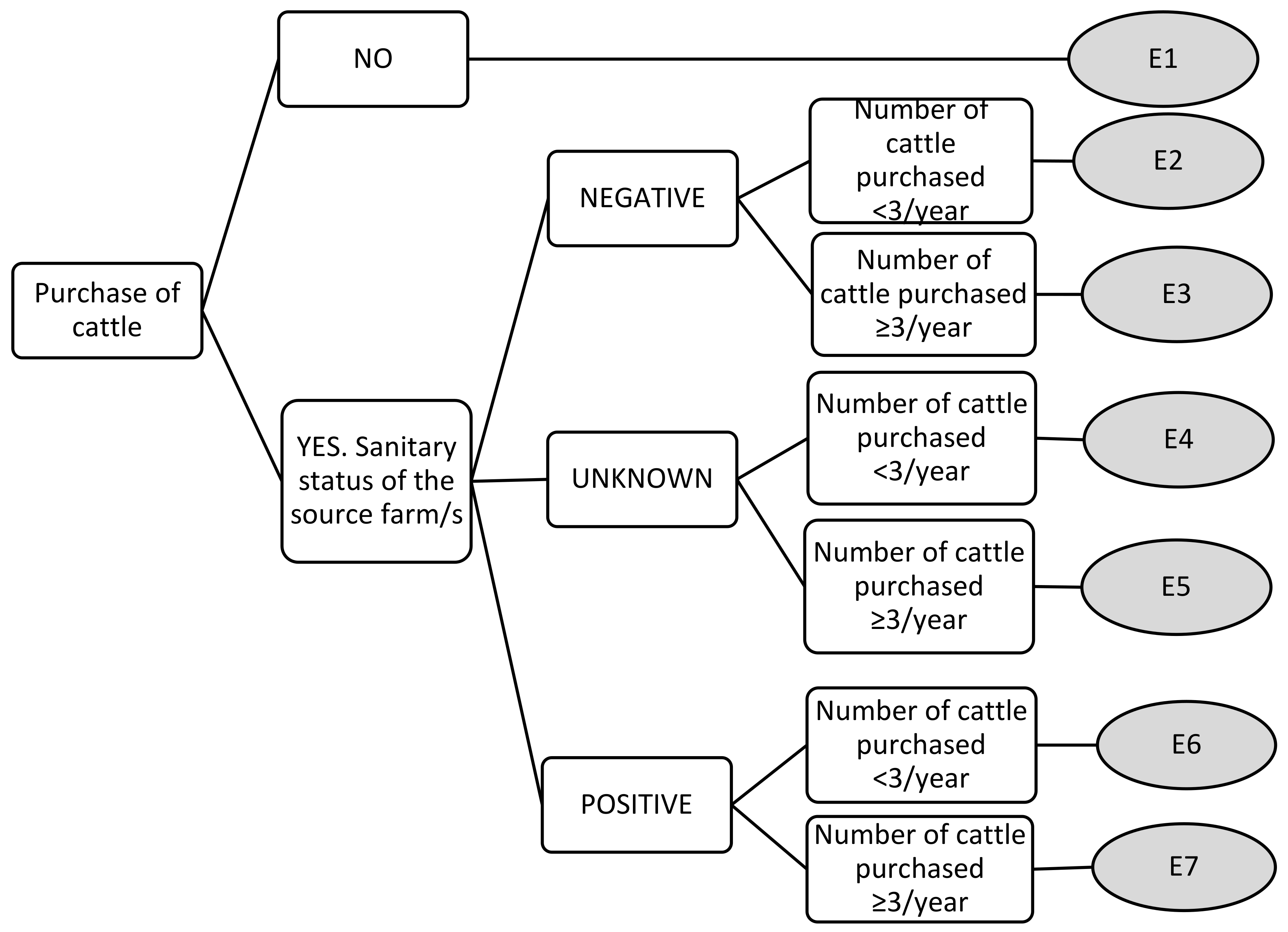
| Purchase of cattle | E1 | 0 | 0 | 0 | 0 |
| E2 | 2 | 2 | 3 | 1 | |
| E3 | 3.11 | 3 | 4 | 2 | |
| E4 | 6.22 | 6 | 8 | 5 | |
| E5 | 7.33 | 7 | 9 | 6 | |
| E6 | 8.11 | 8 | 9 | 7 | |
| E7 | 8.89 | 9 | 9 | 8 | |
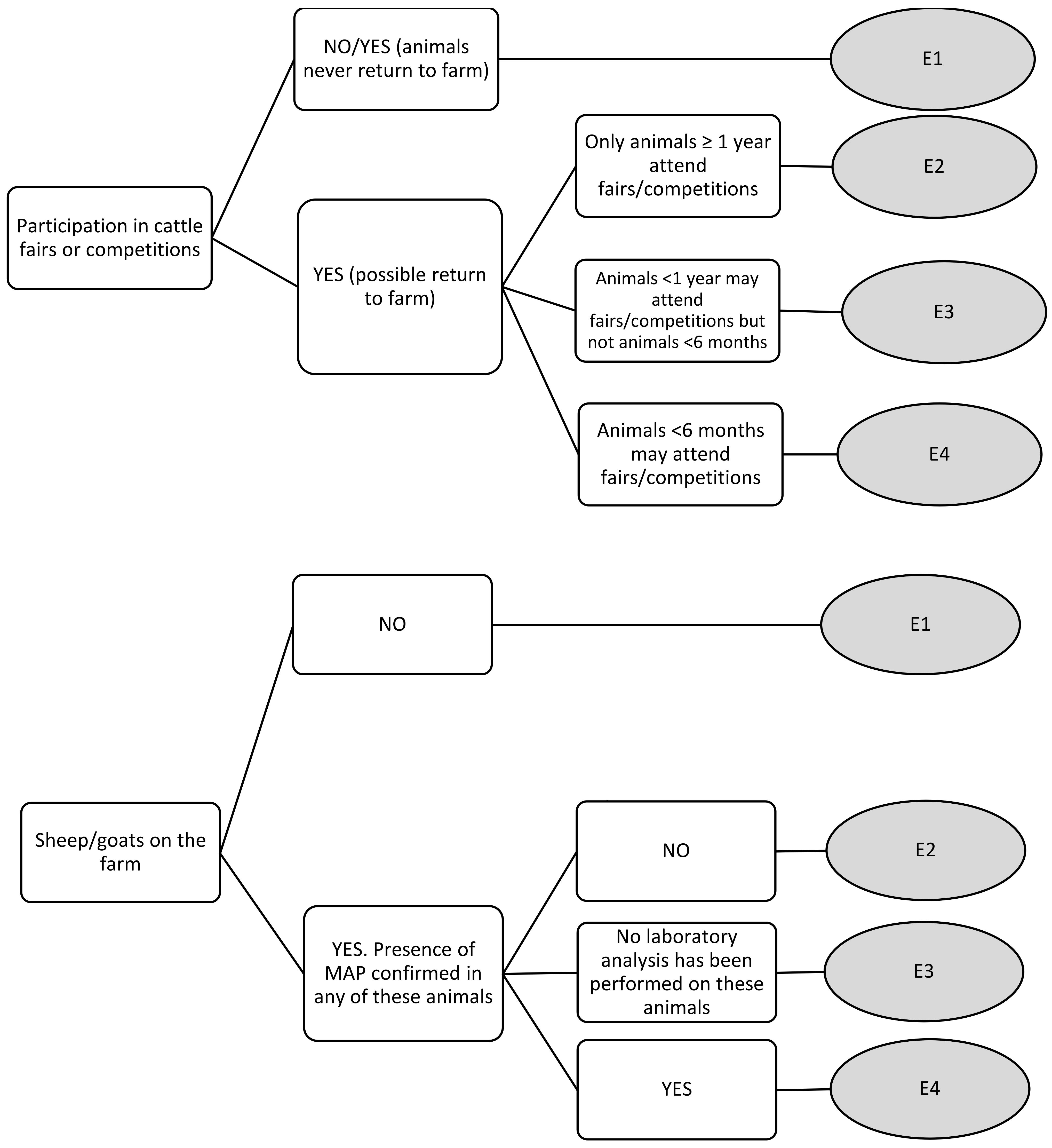
| Participation in cattle fairs or competitions | E1 | 0 | 0 | 0 | 0 |
| E2 | 3.44 | 3 | 5 | 1 | |
| E3 | 5.22 | 5 | 7 | 4 | |
| E4 | 7 | 7 | 9 | 5 | |
| Sheep/goats on the farm | E1 | 0 | 0 | 0 | 0 |
| E2 | 2.22 | 2 | 3 | 0 | |
| E3 | 5.44 | 5 | 7 | 4 | |
| E4 | 8 | 9 | 9 | 6 | |
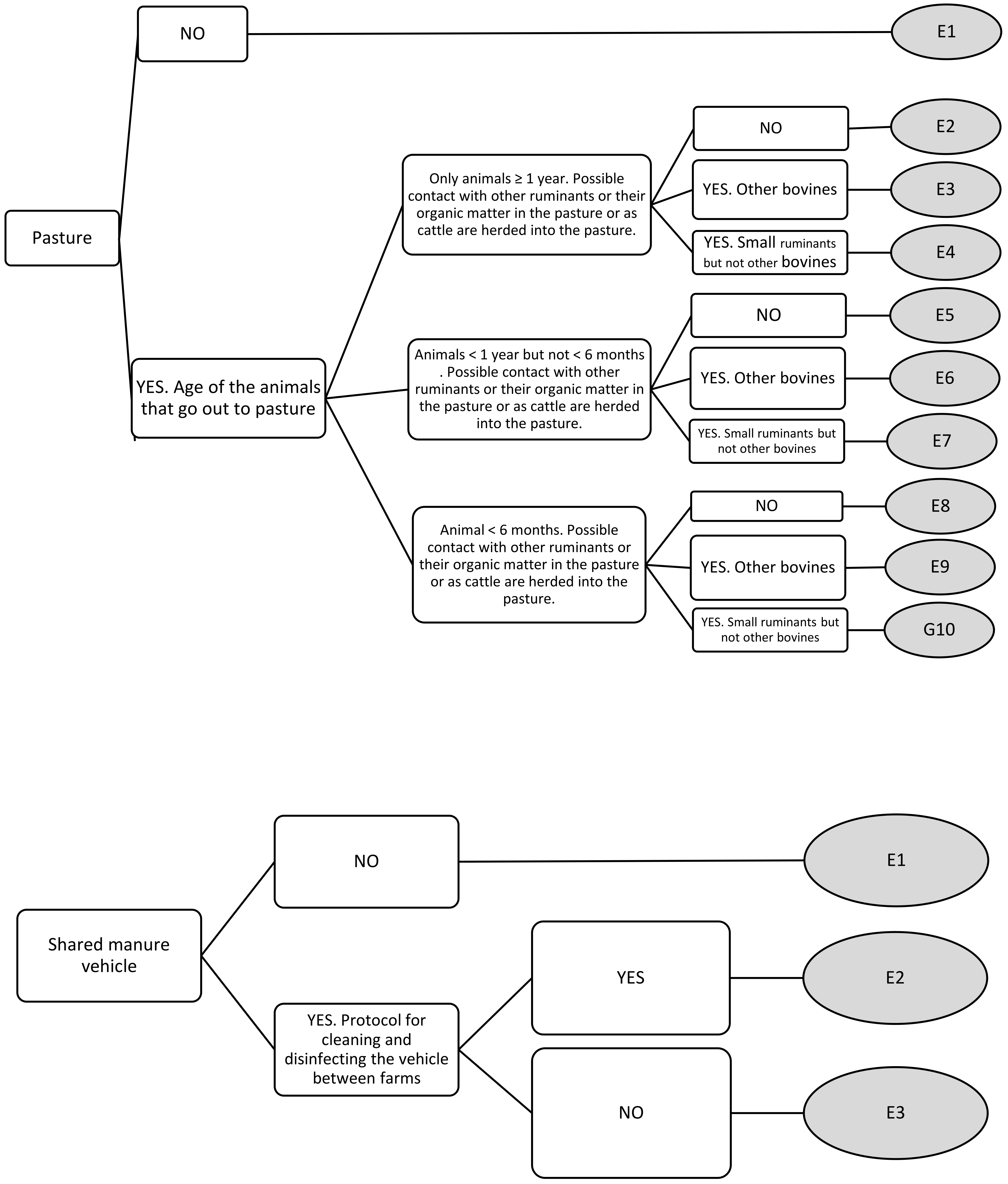
| Pasturing | E1 | 0 | 0 | 0 | 0 |
| E2 | 1.44 | 1 | 3 | 0 | |
| E3 | 4.44 | 5 | 5 | 3 | |
| E4 | 3.78 | 4 | 5 | 2 | |
| E5 | 1.33 | 1 | 3 | 0 | |
| E6 | 6.22 | 6 | 7 | 5 | |
| E7 | 5.55 | 6 | 7 | 3 | |
| E8 | 2.22 | 2 | 5 | 0 | |
| E9 | 7.67 | 7 | 9 | 6 | |
| E10 | 7 | 7 | 9 | 4 | |
| Shared manure vehicle | E1 | 0 | 0 | 0 | 0 |
| E2 | 3 | 3 | 5 | 1 | |
| E3 | 6.89 | 7 | 9 | 4 | |
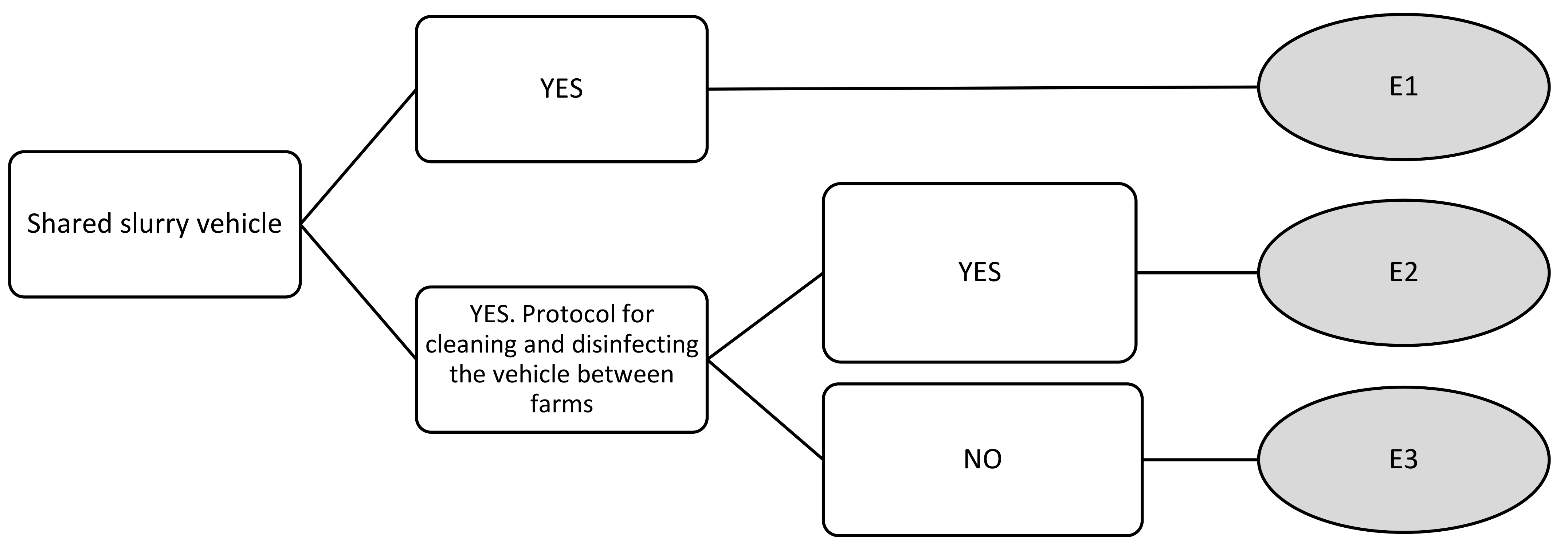
| Shared slurry vehicle | E1 | 0 | 0 | 0 | 0 |
| E2 | 2.89 | 2 | 5 | 1 | |
| E3 | 6.67 | 7 | 9 | 4 | |
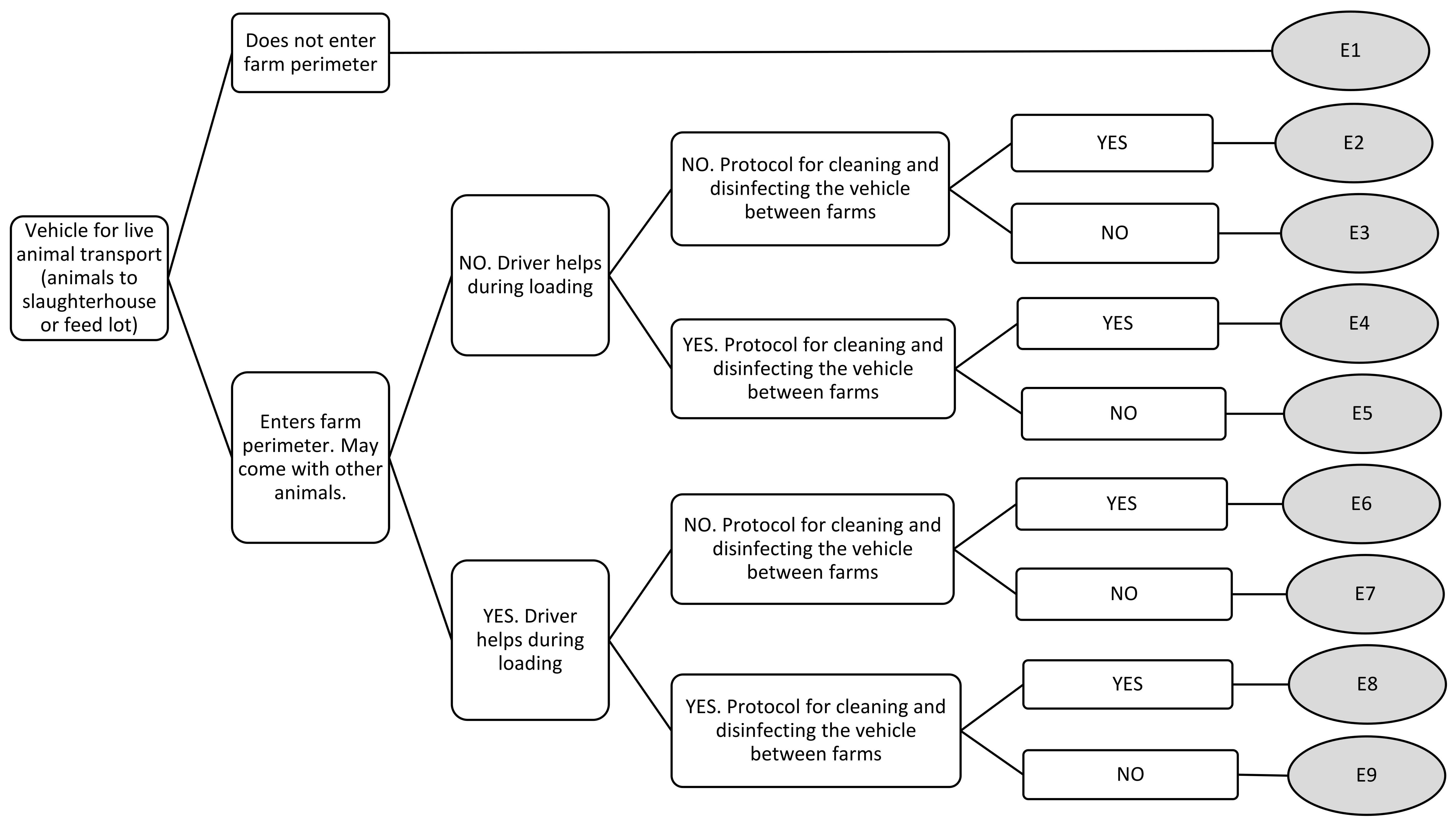
| Vehicle for live animal transport (animals to slaughterhouse or feed lot) | E1 | 0 | 0 | 0 | 0 |
| E2 | 1.11 | 1 | 2 | 0 | |
| E3 | 2.67 | 2 | 5 | 1 | |
| E4 | 2.11 | 2 | 4 | 1 | |
| E5 | 3.44 | 3 | 5 | 2 | |
| E6 | 3.22 | 3 | 5 | 2 | |
| E7 | 4.78 | 5 | 6 | 3 | |
| E8 | 4.44 | 4 | 7 | 2 | |
| E9 | 6.22 | 7 | 8 | 3 | |
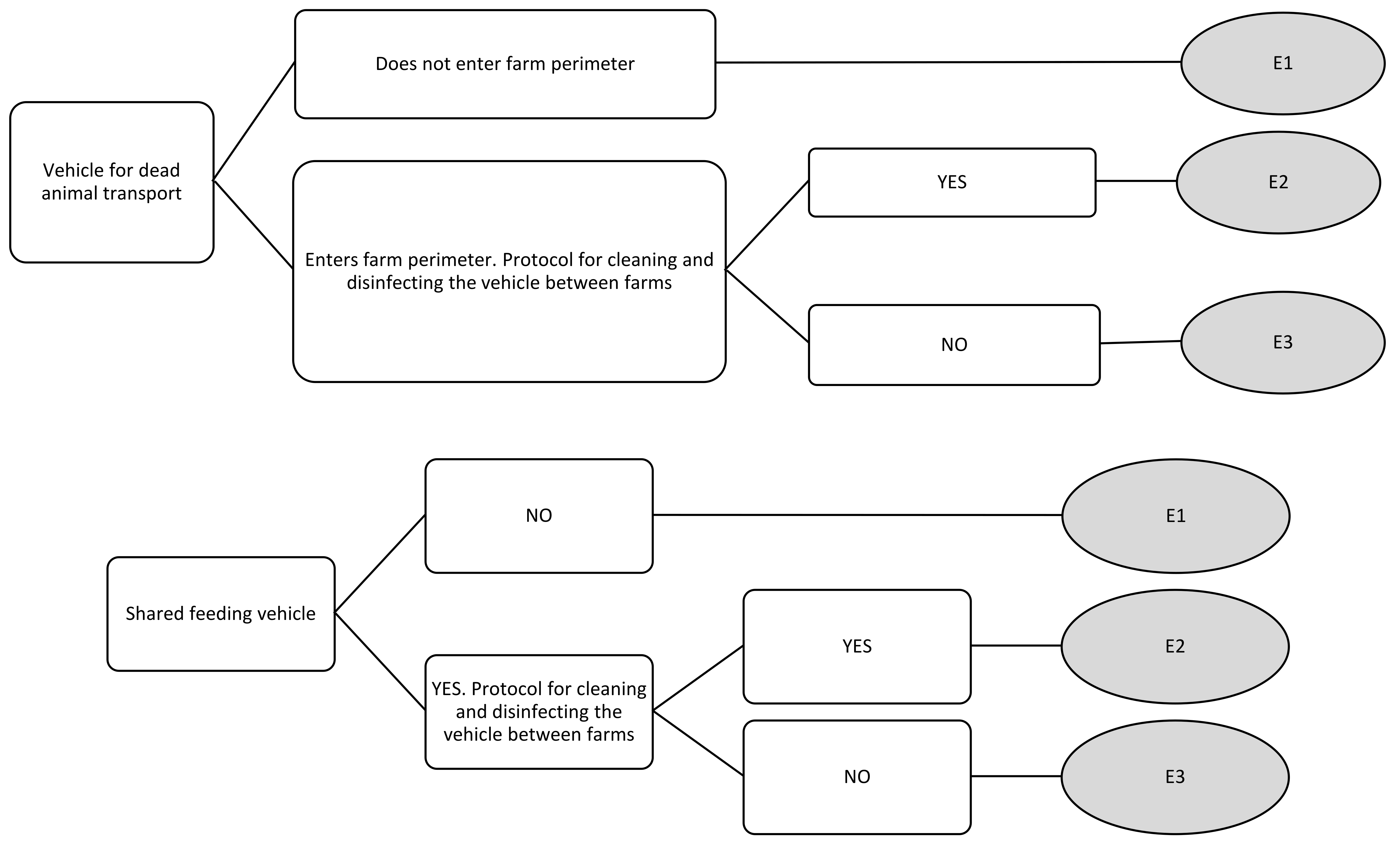
| Vehicle for dead animal transport | E1 | 0 | 0 | 0 | 0 |
| E2 | 2.11 | 2 | 4 | 1 | |
| E3 | 4.55 | 5 | 6 | 3 | |
| Shared feed vehicle | E1 | 0 | 0 | 0 | 0 |
| E2 | 2.55 | 2 | 4 | 2 | |
| E3 | 4.78 | 5 | 6 | 3 | |
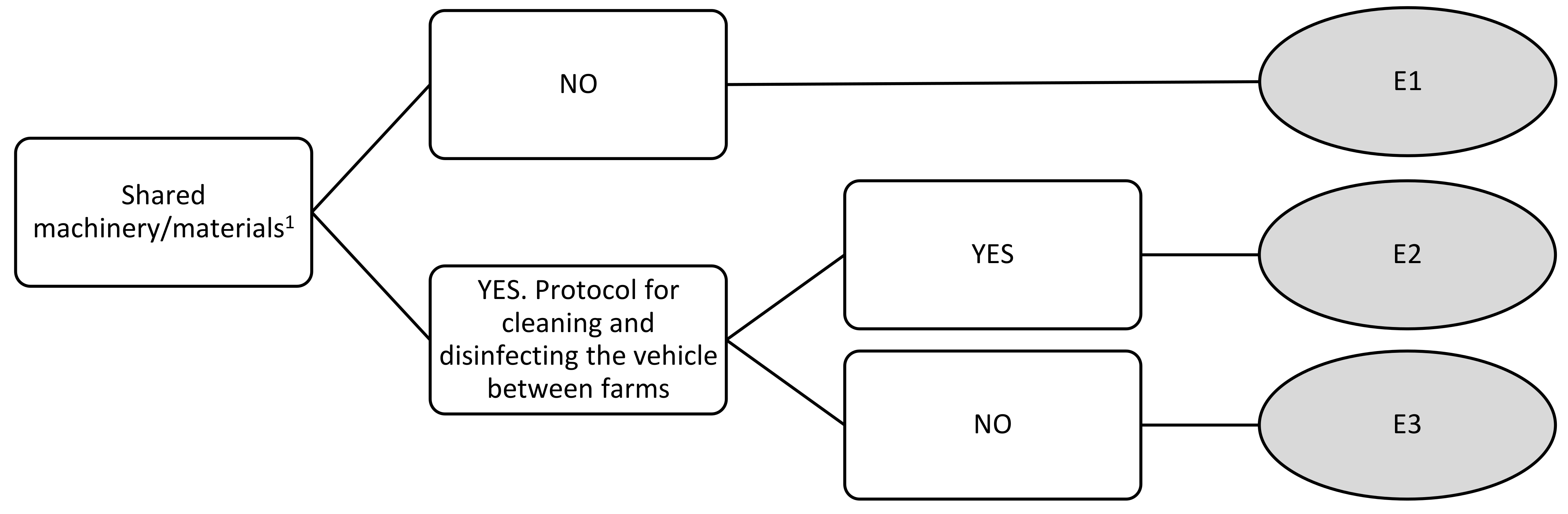
| Shared machinery/materials | E1 | 0 | 0 | 0 | 0 |
| E2 | 2.67 | 3 | 4 | 2 | |
| E3 | 5.33 | 5 | 7 | 4 | |
| (External) employees | E1 | 0 | 0 | 0 | 0 |
| E2 | 0.55 | 1 | 1 | 0 | |
| E3 | 1.78 | 2 | 3 | 0 | |
| E4 | 5.22 | 5 | 7 | 3 | |
| E5 | 2.22 | 3 | 4 | 0 | |
| E6 | 5.78 | 6 | 7 | 3 | |
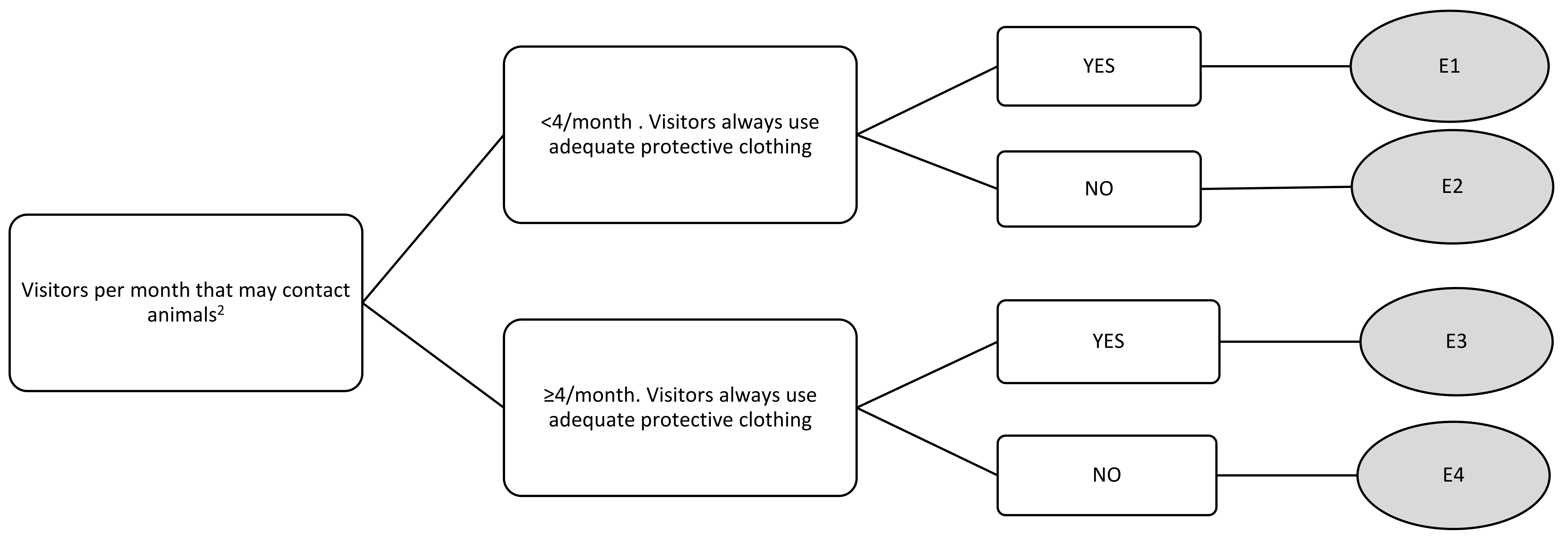
| Visitors per month that could contact animals | E1 | 2.11 | 2 | 3 | 0 |
| E2 | 4.55 | 5 | 5 | 4 | |
| E3 | 2.78 | 3 | 5 | 0 | |
| E4 | 6.11 | 7 | 7 | 4 |
| Farm MAP Status | Total | |||
|---|---|---|---|---|
| Negative | Positive | |||
| Total score | <25.94 | 46 | 5 | 51 |
| ≥25.94 | 21 | 11 | 32 | |
| Total | 67 | 16 | 83 | |
| Farm | Highest Scoring Event | Second-Highest Scoring Event |
|---|---|---|
| 1 | Shared manure vehicle and no cleaning and disinfection protocol between farms (6.89) | Shared slurry vehicle and no cleaning and disinfection protocol between farms (6.67) |
| 2 | Animals < 6 months go to pasture with possible contact with cattle from other farms (7.67) | Purchase ≥ 3 animals/year from farm with unknown sanitary status (7.33) |
| 3 | Purchase ≥ 3 animals/year from farm with unknown sanitary status (7.33) | Shared manure vehicle and no cleaning and disinfection protocol between farms (6.89) |
| 4 | Shared manure vehicle and no cleaning and disinfection protocol between farms (6.89) | Visitor/month > 4 without protective clothing (6.11) |
| 5 | Purchase ≥ 3 animals/year from farm with unknown sanitary status (7.33) | Shared manure vehicle and no cleaning and disinfection protocol between farms (6.89) |
| 6 | Purchase ≥ 3 animals/year from farm with unknown sanitary status (7.33) | Vehicle for live animal transport enters farm perimeter, with other animals, driver helps with loading and there are no cleaning and disinfection protocols between farms (6.22) |
| 7 | Vehicle for live animal transport enters farm perimeter, with other animals, driver helps with loading and there are no cleaning and disinfection protocols between farms (6.22) | Shared feeding vehicle and no cleaning and disinfection protocol between farms (4.78) |
| 8 | Small ruminants in the farm with unknown sanitary status (5.44) | Vehicle for live animal transport enters farm perimeter, with other animals, driver does not help with loading and there are no cleaning and disinfection protocols between farms (4.78) |
| 9 | Vehicle for live animal transport enters farm perimeter, with other animals, driver helps with loading and there are no cleaning and disinfection protocols between farms (6.22) | Visitors/month > 4 without protective clothing (6.11) |
| 10 | Vehicle for live animal transport enters farm perimeter, with other animals, driver helps with loading and there are no cleaning and disinfection protocols between farms (6.22)) | Visitors/month > 4 without protective clothing (6.11) |
| 11 | Purchase ≥ 3 animals/year from farm with unknown sanitary status (7.33) | Shared feeding vehicle and no cleaning and disinfection protocol between farms (4.78) |
| 12 | Purchase ≥ 3 animals/year from farm with unknown sanitary status (7.33) | Shared slurry vehicle and no cleaning and disinfection protocol between farms (6.67) |
| 13 | Visitors/month > 4 without protective clothing (6.11) | Shared feeding vehicle and no cleaning and disinfection protocol between farms (4.78) |
| 14 | Animals < 6 months go to pasture with possible contact with cattle from other farms (7.67) | Visitors/month > 4 without protective clothing (6.11) |
| 15 | Visitors/month > 4 without protective clothing (6.11) | Vehicle for live animal transport enters farm perimeter, with other animals, driver does not help with loading and there are no cleaning and disinfection protocols between farms (4.78) |
| 16 | Shared feeding vehicle and no cleaning and disinfection protocol between farms (4.78) | Visitors/month < 4 without protective clothing (4.55) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villaamil, F.J.; Yus, E.; Benavides, B.; Allepuz, A.; Moya, S.J.; Casal, J.; Ortega, C.; Diéguez, F.J. Factors Associated with the Introduction of Mycobacterium avium spp. Paratuberculosis (MAP) into Dairy Herds in Galicia (North-West Spain): The Perception of Experts. Animals 2021, 11, 166. https://doi.org/10.3390/ani11010166
Villaamil FJ, Yus E, Benavides B, Allepuz A, Moya SJ, Casal J, Ortega C, Diéguez FJ. Factors Associated with the Introduction of Mycobacterium avium spp. Paratuberculosis (MAP) into Dairy Herds in Galicia (North-West Spain): The Perception of Experts. Animals. 2021; 11(1):166. https://doi.org/10.3390/ani11010166
Chicago/Turabian StyleVillaamil, Francisco Javier, Eduardo Yus, Bibiana Benavides, Alberto Allepuz, Sebastián Jesús Moya, Jordi Casal, Carmelo Ortega, and Francisco Javier Diéguez. 2021. "Factors Associated with the Introduction of Mycobacterium avium spp. Paratuberculosis (MAP) into Dairy Herds in Galicia (North-West Spain): The Perception of Experts" Animals 11, no. 1: 166. https://doi.org/10.3390/ani11010166
APA StyleVillaamil, F. J., Yus, E., Benavides, B., Allepuz, A., Moya, S. J., Casal, J., Ortega, C., & Diéguez, F. J. (2021). Factors Associated with the Introduction of Mycobacterium avium spp. Paratuberculosis (MAP) into Dairy Herds in Galicia (North-West Spain): The Perception of Experts. Animals, 11(1), 166. https://doi.org/10.3390/ani11010166





