Abstract
Ca2+ signaling has been involved in controling critical cellular functions such as activation of proteases, cell death, and cell cycle control. The endoplasmatic reticulum plays a significant role in Ca2+ storage inside the cell, but mitochondria have long been recognized as a fundamental Ca2+ pool. Protozoan parasites such as Plasmodium falciparum, Toxoplasma gondii, and Trypanosoma cruzi display a Ca2+ signaling toolkit with similarities to higher eukaryotes, including the participation of mitochondria in Ca2+-dependent signaling events. This review summarizes the most recent knowledge in mitochondrial Ca2+ signaling in protozoan parasites, focusing on the mechanism involved in mitochondrial Ca2+ uptake by pathogenic protists.
1. Calcium Channels, Receptors, Compartmentalization, and Signaling in Animal Cells
Evolutionarily, Ca2+ ions have emerged as one of the most important second messengers that regulate different cellular processes, from muscle contractions and synapses to cell division and apoptosis [1,2]. Therefore, the precise regulation of this ion is a common feature among all forms of life. Cooperation between channels, transport pumps, and stock organelles promote homeostasis of Ca2+ ions, preventing cytotoxicity, and the consequent cell death caused by uncontrolled ion increase [3].
Apart from their role in energy metabolism, mitochondria also participate in the Ca2+ signaling inside the cell, reaching micromolar values of Ca2+. Ca2+ accumulation into the mitochondria stimulates ATP production by modulating enzymes of the tricarboxylic acid cycle (TCA cycle) such as pyruvate dehydrogenase, 2-oxoglutarate, and isocitrate-dehydrogenases. Additionally, a high concentration of Ca2+ inside the cell may trigger cell death by resulting in an excessive mitochondrial Ca2+ uptake and the release of apoptotic factors [4].
The transport of Ca2+ into and out of the mitochondria is tightly regulated by channels and transporters located in the outer and inner mitochondrial membrane. The increase in cytoplasmatic (Ca2+) occurs through two mechanisms in eukaryotes: the exit of Ca2+ from intracellular stores to the cytoplasm, and the entry of Ca2+ through the cell membrane [5]. Ca2+ permeable channels, such as voltage Ca2+ channels (VGCC) and P2X ionotropic receptors, participate in the increase of Ca2+ influx through the plasma membrane in excitable cells. In non-excitable cells, the mobilization of intracellular stores guarantees Ca2+ dependent cell signaling. In addition to membrane channels, ATP-dependent pumps and Na+/Ca2+ exchangers promote the cell’s Ca2+ efflux, maintaining the low intracellular concentration.
In excitable cells, changes in ion gradients (Ca2+, K+, Cl−, Na+) through the plasma membrane’s depolarization regulate essential activities that generate physiological responses, such as muscle contraction, neurotransmitter secretion, learning, and memory mechanisms. Voltage-operated Ca2+ channels are examples of proteins present on the plasma membrane’s surface that regulate ion concentration in the cytoplasm, generating signaling and cellular response [6,7,8]. These channels transform the electrical excitability from membrane depolarization into cell signaling mediated by an increase in cytoplasmatic (Ca2+).
Ionotropic receptors are ion channels also present in the cell membrane that, when activated by an agonist, allow the passage of ions to the cell’s cytosol. An example is the P2X family purinoceptors. These receptors are present in different excitable and non-excitable cells and when activated by ATP, have high permeability to monovalent cations, Ca2+, and other anions [9,10].
The transient receptor potential (TRP) ion channels are membrane proteins found in several tissues and cell types, permeable to mono- or divalent cations, and are involved in cellular responses such as the perception of stimuli (temperature, pheromones, pain, taste) and ion homeostasis [11,12,13]. The stimulation of TRP channels promotes cellular depolarization with the consequent activation of voltage-gated ion channels. In addition, Ca2+ permeable TRP channels regulate intracellular ion concentration, and therefore different cellular responses [11].
One of the most extensive stocks of Ca2+ in animal cells is the endoplasmic reticulum (ER). Two ion channels present in the ER membrane are responsible for the release of Ca2+ ions from the organelle to the cytosol: the inositol 1,4,5-triphosphate receptor (IP3R) and the ryanodine receptor (RyR). IP3R activation occurs through a signaling pathway mediated by second messengers that involve the activation of G protein-coupled receptors (GPCRs) [14,15]. Briefly, in response to the stimulation of GPCRs, phospholipase C (PLC) catalyzes the transformation of phosphoinositol-4,5-bisphosphate (PIP2) into IP3 and diacylglycerol (DAG). IP3 binds to its receptor, activating it, depleting Ca2+ from the ER, and increasing cytoplasmatic (Ca2+). Three types of IP3R are described in vertebrates, IP3R types 1–3, and differences in splicing and phosphorylation sites. In affinity for IP3 and associated molecules, they promote unique responses attributed to each different destination of the activated signaling pathways [16].
Another channel that regulates the output of Ca2+ to the cytosol is RyR. This receptor can be activated indirectly by stimulating the voltage modulated channel type-L Cav1.1–1.2, and by Ca2+, Mg2+ ions, protein kinase A (PKA), calmodulin (CaM), Ca2+ dependent protein kinase/calmodulin (CaMK), FK506 binding proteins, calsequestrin (CSQ), triadin, and junction [17]. In vertebrates, three isoforms of RyR are described and homologs have already been identified in Drosophila melanogaster, Caenorhabditis elegans, and Homarus americanus.
The decrease in (Ca2+) in the ER lumen stimulates a process of interaction between Stromal interaction molecules (STIM), anchored in the ER membrane, and the Calcium release-activated calcium channel protein (ORAI) Ca2+ permeable channels present in the cell’s plasma membrane. This process, called store-operated calcium entry (SOCE), first described by JW Putney Jr (1986) [18], has been widely described in several types of non-excitatory cells and is fundamental for the amplification of cell signaling or to fill the intracellular Ca2+ stocks. Due to the high concentration of Ca2+ in the ER, the N-terminal domain of the STIM protein, located in the ER membrane, is linked to these ions. After the depletion of Ca2+ stores mediated by IP3 and consequently uncoupling of Ca2+ from the STIM protein, the latter dimerizes and translocates to the junction region of the ER membrane with the plasma membrane, allowing the interaction with the ORAI channels. This channel opens, allowing the passage of Ca2+ into the cell’s cytoplasm [19].
Cytoplasmatic Ca2+ is sequestered into the ER for the maintenance of intracellular Ca2+ stocks through an ATP-dependent protein called SERCA-ATPase, present in all eukaryotic cells [20]. This pump guarantees the low concentration of cytosolic Ca2+, regulating the termination of signaling pathways and preventing cell intoxication by an excess of Ca2+.
Acidic organelles, such as endo-lysosomes and acidocalcisomes, also contribute to cellular Ca2+ oscillations. Two-pore channels (TPCs) found in the membrane of endo-lysosomes of animals and plants allow the passage of Ca2+ ions to the cell cytoplasm when activated [21,22,23]. Acidocalcisomes are organelles first described in the parasitic protozoa Trypanosoma brucei [24] that maintain high internal concentrations of Ca2+ and are rich in orthophosphate, pyrophosphate, and polyphosphate, being conserved from bacteria to humans [25,26,27]. The internal acidity is maintained by pumps that allow the passage of hydrogen ions into the organelle’s interior. Moreover, Ca2+ permeable channels’ presence participates in the cytosolic Ca2+ oscillations, and ATP-dependent exchanger pumps transport Ca2+ back into the organelle, contributing to homeostasis.
Golgi Apparatus (GA) is also considered an organelle that participates in oscillations and homeostasis of Ca2+. Ca2+ permeable channels, such as IP3R, RyR, and TRPs are present in the organelle membrane and contribute to the increase in the cytoplasmatic (Ca2+). On the other hand, the presence of SERCA and SPCAs (secretory-pathway ATPases) guarantees the sequestration of Ca2+ into the GA [28,29].
Once free in the cell’s cytoplasm, Ca2+ can bind to different molecules and regulate different cell signaling pathways. A wide range of proteins present in other tissues has a specific motif for Ca2+ binding: EF-hand. Calmodulin (CaM) is an abundant and conserved protein among eukaryotes. It has the EF-hand domains and, when bound to Ca2+, regulates the activation of calmodulin kinase (CaMK) [30,31], which stimulates transcription and is responsible for the expression of genes and regulation of cellular functions. CaM also interacts with Calcineurin, a serine/threonine phosphatase that participates in the regulation of various cellular processes in lower and upper eukaryotes [32].
2. The Role of Ca2+ Signaling in Protozoan Parasites
The life cycle of parasitic protozoa is complex, involving multiple hosts, several cell types, different tissues, and microenvironments. This great diversity implies a finely regulated cell signaling mechanism that allows the parasites to adapt to different stimuli. For example, the Trypanosoma cruzi life cycle begins when a vector insect bites and releases metacyclic trypomastigotes in its feces. These forms enter the injury site and invade nearby cells, and then differentiate into intracellular amastigotes. This form can divide by binary fission and differentiate into trypomastigotes, which are released into the bloodstream. Trypomastigotes in the bloodstream can invade multiple cells, resulting in new intracellular amastigotes, or they can remain in the extracellular medium to be ingested by the insect vector. Ingested trypomastigotes differentiate into epimastigotes in the midgut of the insect vector, multiply, and differentiate into metacyclic trypomastigotes in the hindgut, ready to be released and infect a new host and continue the cycle. In parallel, the Plasmodium falciparum cycle is more complex: it begins with the bite of an infected anopheles mosquito, which injects sporozoites into the host. Once in the bloodstream, sporozoites migrate to the liver and invade hepatocytes, where they can remain inactive or replicate asexually, forming a large number of merozoites in the host cell. The release of merozoites into the bloodstream marks the beginning of the erythrocytic stages. Merozoites invade erythrocytes and develop within the parasitophorous vacuole, undergoing various biochemical and morphological transformations, which can be identified by three stages called ring, trophozoite, and schizont. The erythrocyte rupture by schizonts releases new merozoites, continuing the intra-erythrocytic cycle. During the cycle, a small percentage of parasites differentiate in female or male gametocytes, capable of infecting the vector mosquito during blood ingestion. In the mosquito’s intestine, gametocytes mature in macrogametocyte (female) and exflagellated microgametocyte (male), which is followed by fertilization and zygote formation. The zygote migrates to the intestinal epithelium, where it develops into an oocyst. The rupture of the oocyst releases sporozoites, which migrate to the salivary gland and are injected into the human bloodstream during the mosquito’s feeding, completing the cycle.
Intracellular Ca2+ signaling is essential for cell mobility, invasion, and egress of the host cell and cell differentiation in some protozoa. Among them, the ciliates (Paramecium spp.), Trypanosomatids (Trypanosoma spp., Leishmania spp.), and apicomplexan Plasmodium spp., Toxoplasma spp., Cryptosporidium spp., Babesia spp.) [33,34]. Due to the significant evolutionary distance between some eukaryotic model organisms, such as Paramecium and Apicomplexa, little is known about the mechanisms that regulate calcium-mediated concentration and signaling in these organisms [35,36].
The free-living ciliated protozoan Paramecium spp. is a model organism that has been widely studied on the mechanisms of intracellular (Ca2+) regulation. A range of Ca2+ permeable channels have already been described in these ciliates. These include IP3R and RyR homologs present in different cell compartments, ATP-dependent channels (SERCA and PMCA), voltage-operated, and mechanosensitive channels. Ca2+ binding proteins, such as calmodulins, calcineurins, and protein kinases, have also been identified [34,37,38,39,40].
Trypanosomatids are a large group of protozoa that include two genera of great importance for human health: Trypanosoma and Leishmania. Several Ca2+ permeable channels have been described in Trypanosoma spp. and Leishmania spp. among them: Ca2+-ATPase channels SERCA homologs are present in the ER; Ca2+-ATPases (PMCA) present in the plasma membrane and acidocalcisomes; voltage-operated channels present in the plasma membrane; IP3R homolog channels present in acidocalcisomes; TRP channels present in acid organelles; and Ca2+ influx and efflux channels present in the mitochondria. Ca2+ binding proteins, such as CaM, CaM-like proteins, calcireticulin, and Ca2+ binding proteins present in the flagellum membrane were also identified [41,42,43].
In Plasmodium, Toxoplasma, and Cryptosporidium organisms, some Ca2+-ATPases proteins are known, such as SERCA in the ER, Ca2+ transporters in the GA membrane, Ca2+/H+ exchanger pumps, and components that regulate upstream signaling via Ca2+ [33,36,44,45,46]. In Toxoplasma gondii, TPC and TRPs homologous Ca2+ channels were identified, but none of the three organisms shows a canonic mitochondrial calcium uniporter (MCU)-type channel [43,47]. Pharmacological evidence in Plasmodium and Toxoplasma indicate that the mobilization of Ca2+ from intracellular stocks occurs via the PLC-IP3 pathway [34,48] and by cyclic ADP ribose [49], respectively. In addition, P. falciparum parasites display four GPCR-like proteins [50] and one of them, PfSR25, is enrolled in Ca2+ and PLC signaling [51]. However, there is no confirmation of the presence of IP3R or RyR in Apicomplexa. The genomic analysis identified Ca2+ binding proteins in apicomplexan parasites, such as calmodulins and most notably calcium-dependent protein kinases (CDPKs) [33,36,44,52].
In Plasmodium, ER is one of the main intracellular stores of Ca2+, in addition to the mitochondria and an acidocalcisome [52,53,54]. Pereira et al. (2020) [55] recently reported a newly generated transgenic line of P. falciparum (PfGCaMP3) that expresses the genetically encoded Ca2+ indicator GCaMP3. The authors showed the dynamics of Ca2+ release and influx elicited by inhibitors of the SERCA pumps, cyclopiazonic acid (CPA), and Thapsigargin (Thg) [55]. Only one canonical sequence of the SERCA Ca2+-ATPase (PfATP6) transporter was identified in the P. falciparum genome [33].
Oscillations in the cytoplasmatic (Ca2+) are essential for the hepatic stage of the malaria parasite, thus activating motility, regulating the secretion of adhesion and invasion proteins sporozoites. Carey et al. (2014) demonstrated that sporozoites treated with a Ca2+ chelating agent, PLC inhibitor, or IP3R inhibitor harmed motility and adhesin secretion, highlighting the importance of the PLC-IP3 pathway during the parasite’s liver cycle [56]. By using a protein knockdown system, Philip and Waters (2015) [57] induced depletion of calcineurin. They observed a reduction in P. berghei sporozoites’ invasion in HepG2 cells and the development throughout the liver cycle [57].
The egress process in Plasmodium exposes the parasite to a change in the microenvironment. The parasite is exposed to K+ concentration, variation from 5 mM in the bloodstream to 140 mM in the host cell cytoplasm. By using schizonts marked with the exogenous Ca2+ indicator, Fluo-4/AM, Singh et al. (2010) [58] demonstrated that the transfer of P. falciparum merozoites from a high (K+) (140 mM) to a low (K+) (5 mM) buffer results in cytoplasmatic (Ca2+) rise. Moreover, a shift in K+ concentration leads to an increase in the expression of the microneme (secretory organelles of parasitic apicomplexans) proteins EBA175 (erythrocyte-binding antigen-175) and AMA-1 (apical membrane antigen-1) on the surface of the parasites [58]. These events are induced in the presence of a Ca2+ A23187 ionophore and inhibited after treatment with BAPTA-AM. Even in the absence of extracellular Ca2+, the change in (K+) induced the same effect, but treatment with PLC inhibitor U73122 abolished the response. The authors proposed that the contact of merozoites with the extracellular environment induces changes in cytoplasmatic (Ca2+) via PLC-IP3 and secretion of apical proteins responsible for interaction with the host cell.
Plasmodium protein kinase G (PKG) is involved in parasite motility and invasion during the hepatic phase through the activation of CDPK4 (Ca2+ dependent protein kinase). Using P. berghei parasites mutants for PKG or that carried deletions in the gene encoding CDPK4, Govindasamy et al. (2016) [59] demonstrated that sporozoites from these strains showed inefficiency in the invasion of HepG2 cells. In addition, the treatment of sporozoites with PKG and CDPK4 inhibitors inhibited parasite motility [59]. Protein kinase A is also involved in parasite’s development and can be activated by melatonin in a Ca2+ dependent signaling process [60].
In the Anopheles mosquito’s intestine, Plasmodium parasites encounter low temperatures, pH differences, and xanthurenic acid. The last is capable of inducing an increase in cytoplasmatic (Ca2+) via PLC-IP3 in gametocytes, and the formation of cGMP, regulating Ca2+ oscillations through the production of IP3 [61]. Using genetically modified parasites and specific PKG inhibitors, McRobert et al. (2013) [62] demonstrated that in the absence of xanthurenic acid, the increase in cGMP formation by inhibiting phosphodiesterase activates the complete maturation of male and female gametocytes of P. falciparum. On the other hand, both in the presence of xanthurenic acid and high cGMP concentrations, treatment with BAPTA-AM inhibits the maturation of the male gametocytes [62]. In another study, P. berghei gametocytes resistant to PKG inhibitor treatment showed inhibition of Ca2+ oscillation in the presence of xanthurenic acid [63].
P. falciparum phosphoproteome revealed that PfCDPK1 may be downstream to GMPc/PKG signaling cascade [63,64] and apical proteins responsible for the invasion and egress of the host cell are PfCDPK1 substrates [65,66]. A recent study showed that PfCDPK1 knockout P. falciparum parasites showed deficiency during intra-erythrocytic development, with low growth compared to wild-type parasites, and changes in the expression of AP2-G and GDV1 genes in gametocytes [67]. Furthermore, the PfCDPK1-KO parasites were unable to complete gametogenesis and infect mosquitoes.
In P. berghei, the cpdk3 gene’s deletion results in ookinetes with impaired motility and access to the mosquito’s intestinal epithelium, which decreases the transmissibility of the parasites [68,69]. Sporozoites with deletion of the cdpk3 gene were shown to be viable, which demonstrates that the activity of PbCDPK3 is probably limited to ookinetes [69].
Like CDPK1 and CDPK3, CDKP4 plays an important role during the infection of parasites in mosquitoes. Through the use of inhibitors and the generation of genetically modified parasites, it was possible to determine that, both in P. berghei and in P. falciparum, after the infection of mosquitoes, CPDK4 participates in the regulation of the parasites’ cell cycle, in the replication of genetic material, as well as in the gametogenesis process [70,71]. In P. falciparum, the induction of PfCDPK5 deficiency in schizonts results in merozoites unable to egress, even with the apical complex protein secretion [72,73].
3. Mitochondrial Calcium Dynamics and Signaling in Apicomplexan Parasites
Considered the energetic matrix of the cell, the mitochondria are also fundamental in cellular Ca2+ homeostasis. After the release of Ca2+ through the IP3R and RyR channels present in the ER membrane, these ions can be quickly incorporated by the mitochondria through regions of interaction between the two organelles observed in fungi and different mammalian cells, and where membranes associated with the mitochondria (MAM) interact with the ER network [74,75]. In addition, the evidence points to the existence of a large number of molecules that mediate communication between the ER and the mitochondria for controlling several intracellular signals induced by Ca2+ oscillations, for example, during ER stress and control over the generation of reactive oxygen species (ROS) [75,76,77].
Mitochondrial calcium uniporter (MCU) is an essential protein for the transport of calcium across the mitochondrial membrane and has a fundamental role in the regulation of Ca2+ signaling, in apoptosis, and in aerobic respiration (for review, see [78]). The first MCU described among less complex life forms, such as plants, invertebrates, insects, and yeasts, was in Trypanosoma cruzi [79]. Similar properties to those found in mammalian MCU were observed, such as low sensitivity to Ca2+, sensitivity to ruthenium red, and electrogenic transport. The knowledge that Trypanosoma and Leishmania had a protein that played the role of the MCU was fundamental to the work carried out by De Stefani et al. (2011), who used comparative in silico analysis of conserved sequences to determine the sequence corresponding to the MCU [80].
Several proteins located at the inner and outer mitochondrial membrane play a central role in regulating the absorption and release of Ca2+ [81]. The voltage-dependent anion channel (VDAC1), present in the mitochondrial outer membrane, allows the influx of Ca2+ into the space between membranes, being fundamental for the decrease of cytoplasmatic (Ca2+), and on the other hand, also transports the Ca2+ back to the cytoplasm [82]. Free Ca2+ ions in the intermembrane space are transported to the mitochondrial matrix via the mitochondrial calcium uniporter (MCU), present in the inner mitochondrial membrane [80]. Na+/Ca2+ exchange pumps found in the inner membrane allow the Ca2+ to go from the mitochondrial matrix to the intermembrane space [81].
In addition to participating in the reduction of the cytosolic concentration of Ca2+, the uptake of this ion by the mitochondria is also fundamental for the ATP synthesis, activating Ca2+-dependent enzymes, oxidative phosphorylation, metabolic carriers, and reactive oxygen species (ROS). Ca2+ overload of the mitochondrial matrix compromises the functioning of this subcellular compartment. This event results in a decrease in ATP production, an increase in ROS concentration, and induction of the cell death process [75,83,84]. Likewise, in other eukaryotes, P. chabaudi and P. falciparum’s mitochondria can sequester cytoplasmatic Ca2+ during the increase in the concentration of this ion within the cells. This increase may be due to an ER discharge caused by Thg and CPA, or stimulation with an agonist that results in a signaling event and culminates in the release of Ca2+ [83] (Figure 1). Of note, the transport of Ca2+ into the mitochondria is membrane potential-dependent since the pretreatment with electron transport chain uncoupler Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) prevents this process. The action of melatonin on the mitochondrial dynamics of P. falciparum has also been reported. This hormone can activate the expression of mitochondrial fission-related genes in a stage-specific manner [84]. Results reported by Rotmann et al. (2010) suggest that a Ca2+/H+ exchange protein (PfCHA, PF3D7_0603500) may be responsible for the mitochondrial Ca2+ efflux in P. falciparum [85].
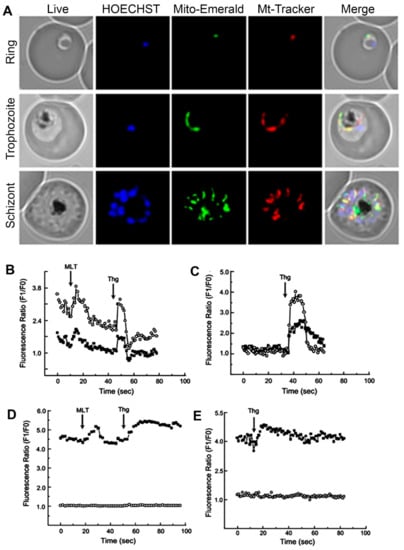
Figure 1.
Fluorescent microscopy Plasmodium falciparum ring, trophozoites, and schizonts stages using a mitochondrial green fluorescent protein (GFP) construction: the parasite nucleus was stained by HOECHST33342 (blue) and mitochondria with MitoTracker Red CMX Ros (red) to demonstrate co-localization with Mito-Emerald-GFP (green) (A). P. chabaudi parasites were stained with Fluo4 (cytoplasmatic calcium indicator) and Rhod2 (mitochondrial calcium indicator): effect of melatonin (MLT) and thapsigargin (Thg) on Ca2+ (B); addition of thapsigargin (C); effect of melatonin and thapsigargin on Ca2+ fluorescence in the presence of FCCP (D); addition of thapsigargin in the presence of FCCP (E). Traces represent fluorescence intensity ratio of Ca2+ probes Rhod-2 AM-mitochondria (open circles) and Fluo-3 AM-cytosol (fill squares). The images were retrieved with the author’s consent from the following references: [84] (A) and [83] (B–E).
The addition of Ca2+ to a buffer containing digitonin permeated P. berghei trophozoites causes stimulation of mitochondrial respiration, proportionally to Ca2+ concentration in the medium, altering stage 4 of cellular respiration. Oximetry experiments revealed a decrease in mitochondrial membrane potential, proportional to Ca2+ concentration added to the buffer, which is compatible with Ca2+ influx into the matrix. The addition of succinate to a medium containing digitonin permeabilized trophozoites and 3.5 µM Ca2+ led to a drop in this concentration to 0.6 µM. The subsequent addition of FCCP caused a massive increase in free Ca2+ in the medium, indicating an efficient mitochondrial calcium transport mechanism is operative [86].
Ca2+/H+ antiporter (PfCHA) has been described as a mitochondrial transport of divalent ions responsible for exchanging H+ for Ca2+ or Mn2+, with kinetics supporting the role of mitochondria as a dynamic Ca2+ stock. Although a homologous protein in humans could not be found, PfCHA has the function of transporting the excess of these ions back to the cytoplasm, helping to maintain mitochondrial concentrations. The low affinity for Ca2+ (Tm of 2.2 mM) supports the hypothesis that this transporter would only act in conditions where the mitochondria is loaded with Ca2+ [85].
Knowledge of the role of mitochondrial Ca2+ in trypanosomes is limited when compared to mammalian cells because Ca2+ regulated dehydrogenases are not present or have not been well studied so far. Pyruvate dehydrogenase E1, which is sensitive to Ca2+, had its gene identified in T. cruzi and has phosphorylation sites to regulate the activity, but there is no evidence that this enzyme works in the same way as in mammals [87]. Mitochondrial isocitrate dehydrogenase is dependent on NADP, unlike the same mammalian enzyme, whose activity depends on NAD and is regulated by Ca2+ [88]. There is no evidence of expression of FAD-glycerol phosphate dehydrogenase, which is activated by Ca2+ in animals, in trypanosomes [89]. Moreover, the aspartate-glutamate and ATP-Mg-Pi carriers, which in mammals are regulated by Ca2+, are present in trypanosomes but do not have the EF-hand domain for binding to Ca2+ and are probably insensitive to this ion [90].
Similar to mammals, Trypanosomatids have a well-characterized MCU-mediated Ca2+ influx mechanism, although some proteins are absent [91,92]. Interference in MCU expression using siRNA or conditional knockdown causes a deficiency in the influx of mitochondrial Ca2+. It directly reflects in the metabolism, resulting in abnormal ATP concentrations, growth defects, and autophagy. These effects become more prominent when oxidative phosphorylation is essential, such as in low-glucose media found in the insect vector. On the other hand, MCU overexpression results in a pro-apoptotic state with Ca2+ overloaded mitochondria [91]. Other studies with T. cruzi supported these findings: using a CRISPR/Cas9 knockout system, there is a deficiency in calcium influx without changing the mitochondrial membrane potential. Although the parasites remain viable, the growth defects are notable: low cellular respiration, increased autophagy, and low infectivity [93,94].
As observed in Plasmodium, trypanosome mitochondria are also capable of acting as a Ca2+ buffer. The uptake of cytoplasmic Ca2+ into the mitochondria can occur in both molar and micromolar concentrations, depending on the membrane potential [95]. This phenomenon suggests an approximation of mitochondria with microdomains of high Ca2+ concentration, such as plasma membrane, acidocalcisomes, or endoplasmic reticulum [96]. As IP3R has not been identified, and SERCA has low sensitivity to Thg, a link between the transport of calcium from the endoplasmic reticulum to the mitochondria has not yet been established [97]. Despite this, it is expected that the MCU will act as a modulator of the cytoplasmic Ca2+ space-temporal fluctuations resulting from various cellular processes [98,99]. Another important role that mitochondrial Ca2+ is its involvement with apoptosis. This process in trypanosomes is well studied, although some important effector and regulatory molecules have not yet been described, such as caspases and TNF receptors [100,101]. In T. brucei, the production of reactive oxygen species blocks the mitochondrial transport of Ca2+, which results in its accumulation in the nucleus and causes cell death [102]. In T. cruzi, calcium overload-related apoptosis is dependent on the formation of superoxide ions [103].
Elmahallawy et al. (2014) investigated the harmful effects of melatonin in Leishmania infantum promastigotes. Using concentrations between 25 and 50 nM, significant inhibition of complexes I, III, and IV of the electron transport chain was noted, and eventually, the death of the parasite [104]. In addition, the authors identified that melatonin caused changes in Ca2+ homeostasis by altering the functioning of the mitochondrial permeability transition pore, controlling the capacity of mitochondrial Ca2+ retention and release, which can also be associated with cell death [105].
Apicomplexa organisms have peculiar and unusual mitochondria, with primitive characteristics that place the organisms of this group as strong candidates to the first existing eukaryotes. Among these characteristics, the presence of a single mitochondria during most of the life cycle, a small and highly fragmented mitochondrial genome, simple protein import machinery, and low activity of the electron transport chain stand out, all of this in organisms with a need to adapt to several different microenvironments. In addition to the obvious public health need to study these organisms in order to identify new therapeutic targets, the study of parasites’ biology can help to understand molecular aspects still unknown in more complex organisms, such as mammals.
Funding
This work was supported by a grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) to CRSG (Process 17/08684-7 and 18/07177-7).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Dupont, G.; Combettes, L.; Bird, G.S.; Putney, J.W. Calcium oscillations. Cold Spring Harb. Perspect. Biol. 2011, 3, a004226. [Google Scholar] [CrossRef] [PubMed]
- Plattner, H.; Verkhratsky, A. Ca2+ signalling early in evolution--all but primitive. J. Cell Sci. 2013, 126, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.B.; Putney, J.W. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef]
- Zamponi, G.W. A Crash Course in Calcium Channels. ACS Chem. Neurosci. 2017, 8, 2583–2585. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Egan, T.M.; Khakh, B.S. Contribution of Calcium Ions to P2X Channel Responses. J. Neurosci. 2004, 24, 3413–3420. [Google Scholar] [CrossRef]
- Gees, M.; Colsoul, B.; Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a003962. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Miyawaki, A.; Furuichi, T.; Mikoshiba, K. Inositol 1,4,5-trisphosphate receptors and calcium signaling. Crit. Rev. Neurobiol. 1996, 10, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Mikoshiba, K. Role of IP3 receptor signaling in cell functions and diseases. Adv. Biol. Regul. 2015, 57, 217–227. [Google Scholar] [CrossRef]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef]
- Putney, J.W. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-operated calcium channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.L.E.; Young, H.S. The sarcoendoplasmic reticulum calcium ATPase. In Subcellular Biochemistry; Springer: Singapore, 2018; Volume 87, pp. 229–258. [Google Scholar]
- Patel, S. Two-pore channels open up. Nature 2018, 556, 38–40. [Google Scholar] [CrossRef]
- Patel, S. Function and dysfunction of two-pore channels. Sci. Signal. 2015, 8, re7. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zeng, W.; Jiang, Y. Tuning the ion selectivity of two-pore channels. Proc. Natl. Acad. Sci. USA 2017, 114, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Vercesi, A.E.; Moreno, S.N.J.; Docampo, R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem. J. 1994, 304, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Moreno, S.N.J. Acidocalcisomes. Cell Calcium 2011, 50, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R. The origin and evolution of the acidocalcisome and its interactions with other organelles. Mol. Biochem. Parasitol. 2016, 209, 3–9. [Google Scholar] [CrossRef]
- Lander, N.; Cordeiro, C.; Huang, G.; Docampo, R. Polyphosphate and acidocalcisomes. Biochem. Soc. Trans. 2016, 44, 1–6. [Google Scholar] [CrossRef]
- Missiaen, L.; Dode, L.; Vanoevelen, J.; Raeymaekers, L.; Wuytack, F. Calcium in the Golgi apparatus. Cell Calcium 2007, 41, 405–416. [Google Scholar] [CrossRef]
- Micaroni, M. Calcium around the Golgi apparatus: Implications for intracellular membrane trafficking. Adv. Exp. Med. Biol. 2012, 740, 439–460. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Swulius, M.T.; Waxham, M.N. Ca2+/calmodulin-dependent protein kinases. Cell. Mol. Life Sci. 2008, 47, 10587–10599. [Google Scholar] [CrossRef]
- Rusnak, F.; Mertz, P. Calcineurin: Form and function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef] [PubMed]
- Lourido, S.; Moreno, S.N.J. The calcium signaling toolkit of the Apicomplexan parasites Toxoplasma gondii and Plasmodium spp. Cell Calcium 2015, 57, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.S.; Alves, E.; Pereira, P.H.S.; Bartlett, P.J.; Thomas, A.P.; Mikoshiba, K.; Plattner, H.; Sibley, L.D. InsP3 Signaling in Apicomplexan Parasites. Curr. Top. Med. Chem. 2017, 17, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Baldauf, S.L. The deep roots of eukaryotes. Science 2003, 300, 1703–1706. [Google Scholar] [CrossRef]
- Nagamune, K.; Moreno, S.N.; Chini, E.N.; Sibley, L.D. Calcium regulation and signaling in apicomplexan parasites. Subcell. Biochem. 2008, 47, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Plattner, H. Calcium regulation in the protozoan model, Paramecium tetraurelia. J. Eukaryot. Microbiol. 2014, 61, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Plattner, H.; Sehring, I.M.; Mohamed, I.K.; Miranda, K.; De Souza, W.; Billington, R.; Genazzani, A.; Ladenburger, E.M. Calcium signaling in closely related protozoan groups (Alveolata): Non-parasitic ciliates (Paramecium, Tetrahymena) vs. parasitic Apicomplexa (Plasmodium, Toxoplasma). Cell Calcium 2012, 51, 351–382. [Google Scholar] [CrossRef]
- Ladenburger, E.M.; Plattner, H. Calcium-release channels in Paramecium. genomic expansion, differential positioning and partial transcriptional elimination. PLoS ONE 2011, 6, e27111. [Google Scholar] [CrossRef]
- Plattner, H.; Verkhratsky, A. The ancient roots of calcium signalling evolutionary tree. Cell Calcium 2015, 57, 123–132. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Docampo, R. Membrane proteins in trypanosomatids involved in Ca2+ homeostasis and signaling. Genes 2018, 9, 304. [Google Scholar] [CrossRef]
- Docampo, R.; Huang, G. Calcium signaling in Trypanosomatid parasites. Cell Calcium 2015, 57, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Moreno, S.N.J.; Plattner, H. Intracellular calcium channels in protozoa. Eur. J. Pharmacol. 2014, 57, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.N.J.; Ayong, L.; Pace, D.A. Calcium storage and function in apicomplexan parasites. Essays Biochem. 2011, 51, 97–110. [Google Scholar] [CrossRef]
- Pecenin, M.F.; Borges-Pereira, L.; Levano-Garcia, J.; Budu, A.; Alves, E.; Mikoshiba, K.; Thomas, A.; Garcia, C.R.S. Blocking IP3 signal transduction pathways inhibits melatonin-induced Ca2+ signals and impairs P. falciparum development and proliferation in erythrocytes. Cell Calcium 2018, 72, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Borges-Pereira, L.; Budu, A.; McKnight, C.A.; Moore, C.A.; Vella, S.A.; Hortua Triana, M.A.; Liu, J.; Garcia, C.R.S.; Pace, D.A.; Moreno, S.N.J. Calcium Signaling throughout the Toxoplasma gondii Lytic Cycle. J. Biol. Chem. 2015, 290, 26914–26926. [Google Scholar] [CrossRef]
- Prole, D.L.; Taylor, C.W. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS ONE 2011, 6, e26218. [Google Scholar] [CrossRef]
- Alves, E.; Bartlett, P.J.; Garcia, C.R.S.; Thomas, A.P. Melatonin and IP3-induced Ca2+ release from intracellular stores in the malaria parasite Plasmodium falciparum within infected red blood cells. J. Biol. Chem. 2011, 286, 5905–5912. [Google Scholar] [CrossRef]
- Chini, E.N.; Nagamune, K.; Wetzel, D.M.; Sibley, L.D. Evidence that the cADPR signalling pathway controls calcium-mediated microneme secretion in Toxoplasma gondii. Biochem. J. 2005, 389, 269–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madeira, L.; Galante, P.A.F.; Budu, A.; Azevedo, M.F.; Malnic, B.; Garcia, C.R.S. Genome-wide detection of serpentine receptor-like proteins in malaria parasites. PLoS ONE 2008, 3, e1889. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.S.; Budu, A.; Singh, M.K.; Borges-Pereira, L.; Levano-Garcia, J.; Currà, C.; Picci, L.; Pace, T.; Ponzi, M.; Pozzan, T.; et al. Plasmodium falciparum GPCR-like receptor SR25 mediates extracellular K+ sensing coupled to Ca2+ signaling and stress survival. Sci. Rep. 2017, 7, 9545. [Google Scholar] [CrossRef] [PubMed]
- Biagini, G.A.; Bray, P.G.; Spiller, D.G.; White, M.R.H.; Ward, S.A. The digestive food vacuole of the malaria parasite is a dynamic intracellular Ca2+ store. J. Biol. Chem. 2003, 278, 27910–27915. [Google Scholar] [CrossRef]
- Garcia, C.R.S.; Dluzewski, A.R.; Catalani, L.H.; Burting, R.; Hoyland, J.; Mason, W.T. Calcium homeostasis in intraerythrocytic malaria parasites. Eur. J. Cell Biol. 1996, 71, 409–413. [Google Scholar] [PubMed]
- Varotti, F.P.; Beraldo, F.H.; Gazarini, M.L.; Garcia, C.R.S. Plasmodium falciparum malaria parasites display a THG-sensitive Ca2+ pool. Cell Calcium 2003, 33, 137–144. [Google Scholar] [CrossRef]
- Pereira, L.B.; Thomas, S.J.; Silva, A.L.A.; Bartlett, P.J.; Thomas, A.P.; Garcia, C.R.S. The genetic Ca2+ sensor GCaMP3 reveals multiple Ca2+ stores differentially coupled to Ca2+ entry in the human malaria parasite Plasmodium falciparum. J. Biol. Chem. 2020, 295, 14998–15012. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.F.; Singer, M.; Bargieri, D.; Thiberge, S.; Frischknecht, F.; Ménard, R.; Amino, R. Calcium dynamics of Plasmodium berghei sporozoite motility. Cell. Microbiol. 2014, 16, 768–783. [Google Scholar] [CrossRef]
- Philip, N.; Waters, A.P. Conditional degradation of Plasmodium calcineurin reveals functions in parasite colonization of both host and vector. Cell Host Microbe 2015, 18, 122–131. [Google Scholar] [CrossRef]
- Singh, S.; Alam, M.M.; Pal-Bhowmick, I.; Brzostowski, J.A.; Chitnis, C.E. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010, 6, e1000746. [Google Scholar] [CrossRef]
- Govindasamy, K.; Jebiwott, S.; Jaijyan, D.K.; Davidow, A.; Ojo, K.K.; Van Voorhis, W.C.; Brochet, M.; Billker, O.; Bhanot, P. Invasion of hepatocytes by Plasmodium sporozoites requires cGMP-dependent protein kinase and calcium dependent protein kinase 4. Mol. Microbiol. 2016, 102, 349–363. [Google Scholar] [CrossRef]
- Gazarini, M.L.; Beraldo, F.H.; Almeida, F.M.; Bootman, M.; Da Silva, A.M.; Garcia, C.R.S. Melatonin triggers PKA activation in the rodent malaria parasite Plasmodium chabaudi. J. Pineal Res. 2011, 50, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Brochet, M.; Billker, O. Calcium signalling in malaria parasites. Mol. Microbiol. 2016, 100, 397–408. [Google Scholar] [CrossRef] [PubMed]
- McRobert, L.; Taylor, C.J.; Deng, W.; Fivelman, Q.L.; Cummings, R.M.; Polley, S.D.; Billker, O.; Baker, D.A. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008, 6, e139. [Google Scholar] [CrossRef]
- Brochet, M.; Collins, M.O.; Smith, T.K.; Thompson, E.; Sebastian, S.; Volkmann, K.; Schwach, F.; Chappell, L.; Gomes, A.R.; Berriman, M.; et al. Phosphoinositide Metabolism Links cGMP-Dependent Protein Kinase G to Essential Ca2+ Signals at Key Decision Points in the Life Cycle of Malaria Parasites. PLoS Biol. 2014, 12, e1001806. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Solyakov, L.; Bottrill, A.R.; Flueck, C.; Siddiqui, F.A.; Singh, S.; Mistry, S.; Viskaduraki, M.; Lee, K.; Hopp, C.S.; et al. Phosphoproteomics reveals malaria parasite Protein Kinase G as a signalling hub regulating egress and invasion. Nat. Commun. 2015, 6, 7285. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, M.; Ekka, R.; Dvorin, J.D.; Paul, A.S.; Madugundu, A.K.; Gilberger, T.; Gowda, H.; Duraisingh, M.T.; Keshava Prasad, T.S.; et al. PfCDPK1 mediated signaling in erythrocytic stages of Plasmodium falciparum. Nat. Commun. 2017, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Singh, S.; More, K.R.; Hans, D.; Nangalia, K.; Yogavel, M.; Sharma, A.; Chitnis, C.E. Characterization of Plasmodium falciparum calcium-dependent protein kinase 1 (PFCDPK1) and its role in microneme secretion during erythrocyte invasion. J. Biol. Chem. 2013, 288, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Molina-Cruz, A.; Brzostowski, J.; Liu, P.; Luo, Y.; Gunalan, K.; Li, Y.; Ribeiro, J.M.C.; Miller, L.H. Pf CDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proc. Natl. Acad. Sci. USA 2018, 115, 774–779. [Google Scholar] [CrossRef]
- Ishino, T.; Orito, Y.; Chinzei, Y.; Yuda, M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol. Microbiol. 2006, 59, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Siden-Kiamos, I.; Ecker, A.; Nybäck, S.; Louis, C.; Sinden, R.E.; Billker, O. Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Mol. Microbiol. 2006, 60, 1355–1363. [Google Scholar] [CrossRef]
- Fang, P.; Han, H.; Wang, J.; Chen, K.; Chen, X.; Guo, M. Structural Basis for Specific Inhibition of tRNA Synthetase by an ATP Competitive Inhibitor. Chem. Biol. 2015, 22, 734–744. [Google Scholar] [CrossRef]
- Ojo, K.K.; Eastman, R.T.; Vidadala, R.; Zhang, Z.; Rivas, K.L.; Choi, R.; Lutz, J.D.; Reid, M.C.; Fox, A.M.W.; Hulverson, M.A.; et al. A specific inhibitor of pfcdpk4 blocks malaria transmission: Chemical-genetic validation. J. Infect. Dis. 2014, 209, 275–284. [Google Scholar] [CrossRef]
- Dvorin, J.D.; Martyn, D.C.; Patel, S.D.; Grimley, J.S.; Collins, C.R.; Hopp, C.S.; Bright, A.T.; Westenberger, S.; Winzeler, E.; Blackman, M.J.; et al. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science 2010, 328, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Absalon, S.; Blomqvist, K.; Rudlaff, R.M.; DeLano, T.J.; Pollastri, M.P.; Dvorin, J.D. Calcium-dependent protein kinase 5 is required for release of egress-specific organelles in Plasmodium falciparum. MBio 2018, 9, e00130-18. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.; Vicencio, J.M.; Parra, V.; Troncoso, R.; Munoz, J.P.; Bui, M.; Quiroga, C.; Rodriguez, A.E.; Verdejo, H.E.; Ferreira, J.; et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J. Cell Sci. 2011, 124, 2143–2152. [Google Scholar] [CrossRef]
- Booth, D.M.; Enyedi, B.; Geiszt, M.; Várnai, P.; Hajnóczky, G. Redox Nanodomains Are Induced by and Control Calcium Signaling at the ER-Mitochondrial Interface. Mol. Cell 2016, 63, 240–248. [Google Scholar] [CrossRef]
- De Stefani, D.; Patron, M.; Rizzuto, R. Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1853, 2006–2011. [Google Scholar] [CrossRef]
- Docampo, R.; Vercesi, A.E. Characteristics of Ca2+ transport by Trypanosoma cruzi mitochondria in situ. Arch. Biochem. Biophys. 1989, 272, 122–129. [Google Scholar] [CrossRef]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabó, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De, S. Mitochondrial VDAC, the Na+/Ca2+ exchanger, and the Ca2+ uniporter in Ca2+ dynamics and signaling. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; Volume 981. [Google Scholar]
- Shoshan-Barmatz, V.; Krelin, Y.; Shteinfer-Kuzmine, A. VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease. Cell Calcium 2018, 69, 81–100. [Google Scholar] [CrossRef]
- Gazarini, M.L.; Garcia, C.R.S. The malaria parasite mitochondrion senses cytosolic Ca2+ fluctuations. Biochem. Biophys. Res. Commun. 2004, 321, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, P.; Almeida, G.T.; Vicoso, K.L.; Lima, W.R.; Pereira, L.B.; Meissner, K.A.; Wrenger, C.; Rafaello, A.; Rizzuto, R.; Pozzan, T.; et al. Melatonin activate FIS1, DYN1 and DYN2 Plasmodium falciparum related-genes for mitochondria fission: Mitoemerald-GFP as a tool to visualize mitochondria structure. J. Pineal Res. 2019, 66, e12484. [Google Scholar] [CrossRef] [PubMed]
- Rotmann, A.; Sanchez, C.; Guiguemde, A.; Rohrbach, P.; Dave, A.; Bakouh, N.; Planelles, G.; Lanzer, M. PfCHA is a mitochondrial divalent cation/H+ antiporter in Plasmodium falciparum. Mol. Microbiol. 2010, 76, 1591–1606. [Google Scholar] [CrossRef] [PubMed]
- Uyemura, S.A.; Luo, S.; Moreno, S.N.J.; Docampo, R. Oxidative phosphorylation, Ca2+ transport, and fatty acid-induced uncoupling in malaria parasites mitochondria. J. Biol. Chem. 2000, 275, 9709–9715. [Google Scholar] [CrossRef] [PubMed]
- Buscaglia, C.A.; Pollevick, G.D.; Veloso, C.; Lorca, M.; Frasch, A.C.C.; Sánchez, D.O. A putative pyruvate dehydrogenase α subunit gene from Trypanosoma cruzi. Biochim. Biophys. Acta Gene Struct. Expr. 1996, 1309, 53–57. [Google Scholar] [CrossRef]
- Leroux, A.E.; Maugeri, D.A.; Cazzulo, J.J.; Nowicki, C. Functional characterization of NADP-dependent isocitrate dehydrogenase isozymes from Trypanosoma cruzi. Mol. Biochem. Parasitol. 2011, 177, 61–64. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Docampo, R.; Moreno, S.N.J. Energization-dependent Ca2+ accumulation in Trypanosoma brucei bloodstream and procyclic trypomastigotes mitochondria. Mol. Biochem. Parasitol. 1992, 56, 251–257. [Google Scholar] [CrossRef]
- Docampo, R.; Lukeš, J. Trypanosomes and the solution to a 50-year mitochondrial calcium mystery. Trends Parasitol. 2012, 28, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Vercesi, A.E.; Docampo, R. Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat. Commun. 2013, 4, 2865. [Google Scholar] [CrossRef]
- Lander, N.; Chiurillo, M.A.; Bertolini, M.S.; Docampo, R.; Vercesi, A.E. The mitochondrial calcium uniporter complex in trypanosomes. Cell Biol. Int. 2018, 42, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Chiurillo, M.A.; Lander, N.; Bertolini, M.S.; Storey, M.; Vercesi, A.E.; Docampo, R. Different roles of mitochondrial calcium uniporter complex subunits in growth and infectivity of Trypanosoma cruzi. MBio 2017, 8, e00574-17. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.S.; Chiurillo, M.A.; Lander, N.; Vercesi, A.E.; Docampo, R. MICU1 and MICU2 play an essential role in mitochondrial Ca2+ uptake, growth, and infectivity of the human pathogen Trypanosoma cruzi. MBio 2019, 10, e00348-19. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.H.; Ruben, L. Trypanosoma brucei: The dynamics of calcium movement between the cytosol, nucleus, and mitochondrion of intact cells. Exp. Parasitol. 1998, 88, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.H.; Ridgley, E.L.; Enis, D.; Olness, F.; Ruben, L. Selective transfer of calcium from an acidic compartment to the mitochondrion of Trypanosoma brucei: Measurements with targeted aequorins. J. Biol. Chem. 1997, 272, 31022–31028. [Google Scholar] [CrossRef] [PubMed]
- Singha, U.K.; Sharma, S.; Chaudhuri, M. Downregulation of mitochondrial porin inhibits cell growth and alters respiratory phenotype in Trypanosoma brucei. Eukaryot. Cell 2009, 8, 1418–1428. [Google Scholar] [CrossRef]
- Hajnóczky, G.; Hager, R.; Thomas, A.P. Mitochondria suppress local feedback activation of inositol 1,4,5- trisphosphate receptors by Ca2+. J. Biol. Chem. 1999, 274, 14157–14162. [Google Scholar] [CrossRef] [PubMed]
- Boitier, E.; Rea, R.; Duchen, M.R. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J. Cell Biol. 1999, 145, 795–808. [Google Scholar] [CrossRef]
- Smirlis, D.; Duszenko, M.; Ruiz, A.J.; Scoulica, E.; Bastien, P.; Fasel, N.; Soteriadou, K. Targeting essential pathways in trypanosomatids gives insights into protozoan mechanisms of cell death. Parasites Vectors 2010, 3, 107. [Google Scholar] [CrossRef]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasites Vectors 2011, 4, 44. [Google Scholar] [CrossRef]
- Ridgley, E.L.; Xiong, Z.H.; Ruben, L. Reactive oxygen species activate a Ca2+-dependent cell death pathway in the unicellular organism Trypanosoma brucei brucei. Biochem. J. 1999, 340, 33–40. [Google Scholar] [CrossRef]
- Irigoín, F.; Inada, N.M.; Fernandes, M.P.; Piacenza, L.; Gadelha, F.R.; Vercesi, A.E.; Radi, R. Mitochondrial calcium overload triggers complement-dependent superoxide-mediated programmed cell death in Trypanosoma cruzi. Biochem. J. 2009, 418, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, E.K.; Jiménez-Aranda, A.; Martínez, A.S.; Rodriguez-Granger, J.; Navarro-Alarcón, M.; Gutiérrez-Fernández, J.; Agil, A. Activity of melatonin against Leishmania infantum promastigotes by mitochondrial dependent pathway. Chem. Biol. Interact. 2014, 220, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).