The Association between Hepatic Encephalopathy and Diabetic Encephalopathy: The Brain-Liver Axis
Abstract
1. Introduction
2. Hepatic Encephalopathy
3. Diabetic Encephalopathy
4. Common Risk Factors in Hepatic Encephalopathy and Diabetic Encephalopathy
4.1. Brain Structural Abnormalities Both in Hepatic Encephalopathy and Diabetic Encephalopathy
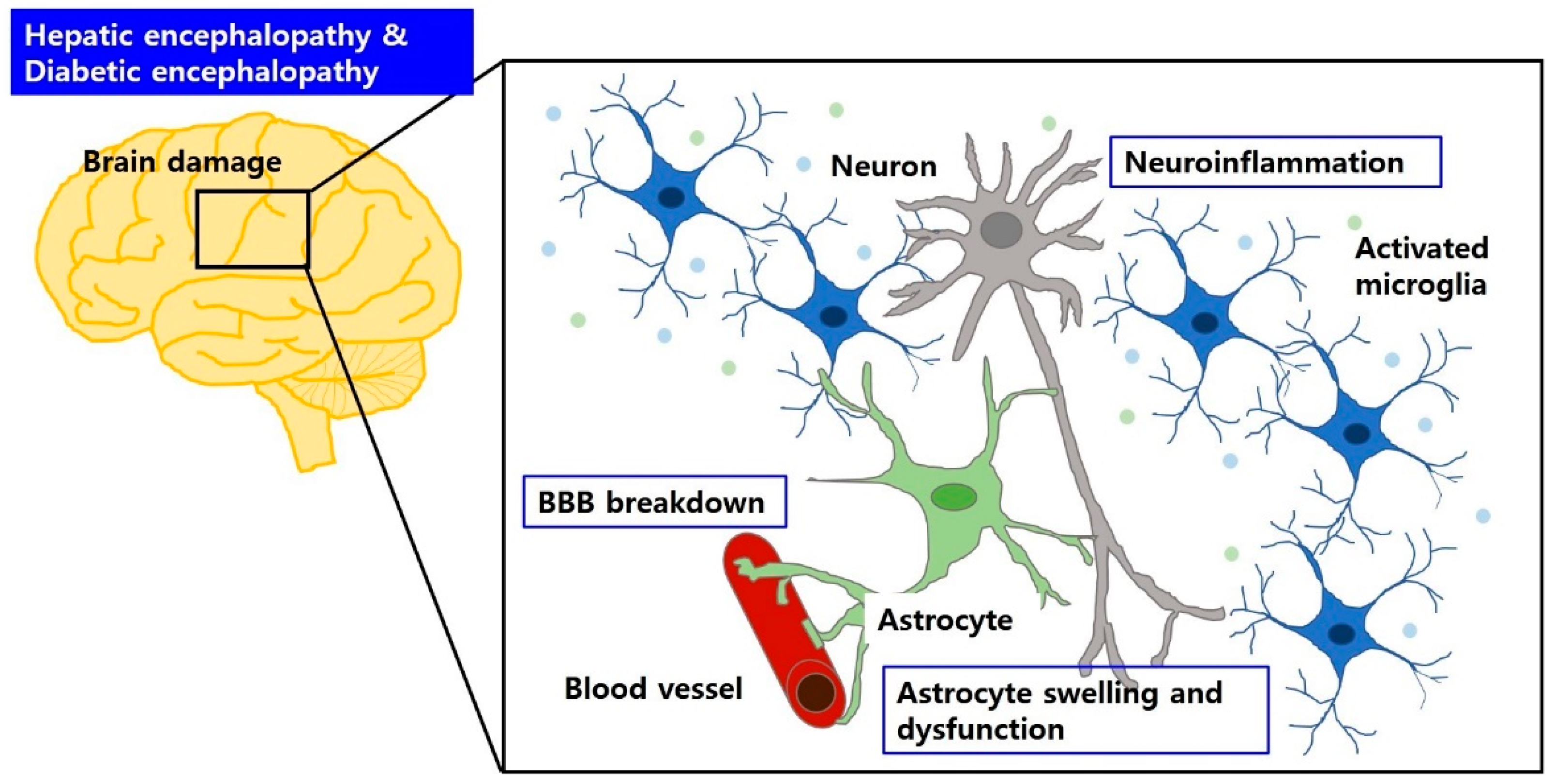
4.2. Aberrant Glia and Neuroinflammation Both in Hepatic Encephalopathy and Diabetic Encephalopathy
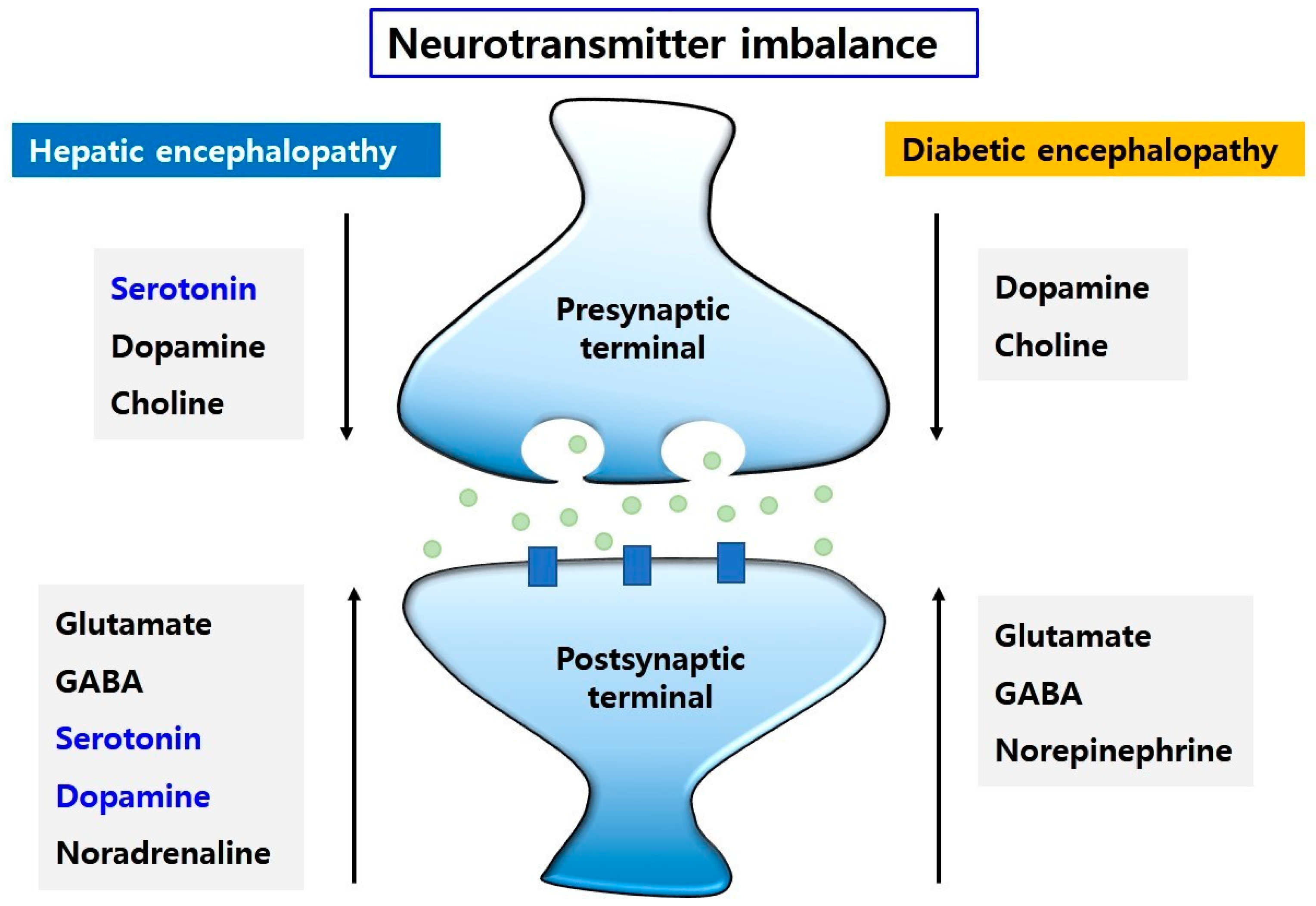
4.3. Imbalance of Neurotransmission Both in Hepatic Encephalopathy and Diabetic Encephalopathy
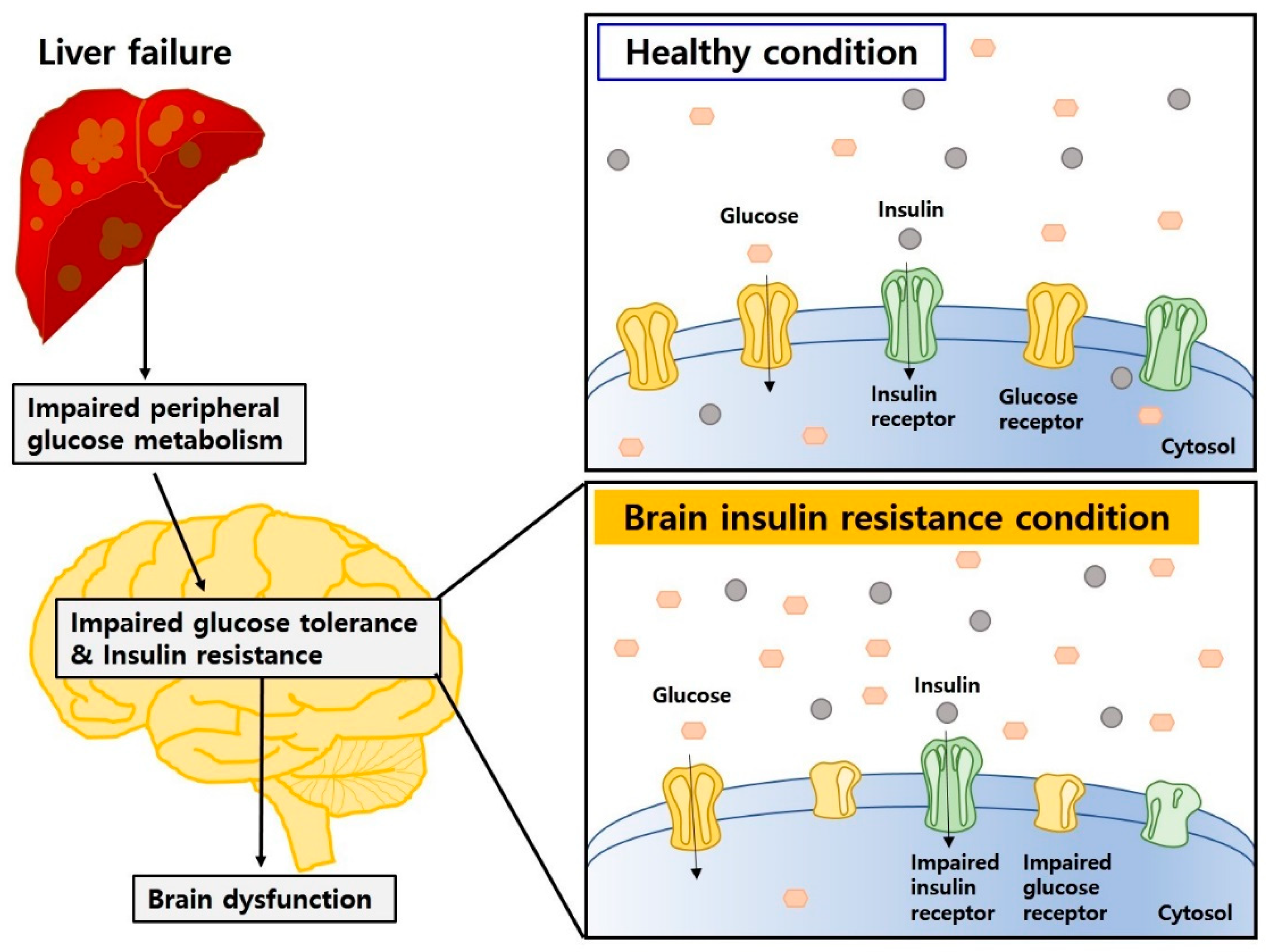
4.4. Insulin Resistance and Impaired Glucose Metabolism Both in Hepatic Encephalopathy and Diabetic Encephalopathy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
List of Abbreviations
References
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Nardelli, S.; Allampati, S.; Riggio, O.; Mullen, K.D.; Prakash, R.; Gioia, S.; Unser, A.; White, M.B.; Fagan, A.C.; Wade, J.B.; et al. Hepatic Encephalopathy Is Associated with Persistent Learning Impairments Despite Adequate Medical Treatment: A Multicenter, International Study. Dig. Dis. Sci. 2017, 62, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Weissenborn, K. Neurology and the liver. J. Neurol. Neurosurg. Psychiatry 1997, 63, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F.; Norenberg, M.D.; Felipo, V.; Ferenci, P.; Albrecht, J.; Blei, A.T.; Members of the ISHEN Commission on Experimental Models of HE. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009, 29, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.E.; Fernandez-Lorente, J.; Romero-Vives, M.; Barcena, R.; Gaztelu, J.M. Brain oscillatory activity during sleep shows unknown dysfunctions in early encephalopathy. J. Physiol. Biochem. 2014, 70, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, K.; Bokemeyer, M.; Krause, J.; Ennen, J.; Ahl, B. Neurological and neuropsychiatric syndromes associated with liver disease. AIDS 2005, 19 (Suppl. S3), S93–S98. [Google Scholar] [CrossRef]
- Nardone, R.; Taylor, A.C.; Holler, Y.; Brigo, F.; Lochner, P.; Trinka, E. Minimal hepatic encephalopathy: A review. Neurosci. Res. 2016, 111, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Schroeter, A.; Klocker, N. Synaptic plasticity in hepatic encephalopathy—A molecular perspective. Arch. Biochem. Biophys. 2013, 536, 183–188. [Google Scholar] [CrossRef]
- Weiss, N.; Barbier Saint Hilaire, P.; Colsch, B.; Isnard, F.; Attala, S.; Schaefer, A.; Amador, M.D.; Rudler, M.; Lamari, F.; Sedel, F.; et al. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J. Hepatol. 2016, 65, 1120–1130. [Google Scholar] [CrossRef]
- Jaeger, V.; DeMorrow, S.; McMillin, M. The Direct Contribution of Astrocytes and Microglia to the Pathogenesis of Hepatic Encephalopathy. J. Clin. Transl. Hepatol. 2019, 7, 352–361. [Google Scholar] [CrossRef]
- Shawcross, D.L.; Shabbir, S.S.; Taylor, N.J.; Hughes, R.D. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2010, 51, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N. Ammonia and Alzheimer’s disease. Neurochem. Int. 2002, 41, 189–207. [Google Scholar] [CrossRef]
- Agarwal, A.N.; Mais, D.D. Sensitivity and Specificity of Alzheimer Type II Astrocytes in Hepatic Encephalopathy. Arch. Pathol. Lab. Med. 2019, 143, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gerevini, G.T.; Dain, A.; Pasqualini, M.E.; Lopez, C.B.; Eynard, A.R.; Repossi, G. Diabetic encephalopathy: Beneficial effects of supplementation with fatty acids omega3 and nordihydroguaiaretic acid in a spontaneous diabetes rat model. Lipids Health Dis. 2019, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Mijnhout, G.S.; Scheltens, P.; Diamant, M.; Biessels, G.J.; Wessels, A.M.; Simsek, S.; Snoek, F.J.; Heine, R.J. Diabetic encephalopathy: A concept in need of a definition. Diabetologia 2006, 49, 1447–1448. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Zhao, S.; Huang, L. Diabetic encephalopathy causes the imbalance of neural activities between hippocampal glutamatergic neurons and GABAergic neurons in mice. Brain Res. 2020, 1742, 146863. [Google Scholar] [CrossRef]
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 2013, 4, 640–650. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef]
- Biessels, G.J.; Strachan, M.W.; Visseren, F.L.; Kappelle, L.J.; Whitmer, R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. Lancet Diabetes Endocrinol. 2014, 2, 246–255. [Google Scholar] [CrossRef]
- Kroner, Z. The relationship between Alzheimer’s disease and diabetes: Type 3 diabetes? Altern. Med. Rev. 2009, 14, 373–379. [Google Scholar]
- Hanyu, H. Diabetes-Related Dementia. Adv. Exp. Med. Biol. 2019, 1128, 147–160. [Google Scholar] [CrossRef]
- Takechi, R.; Lam, V.; Brook, E.; Giles, C.; Fimognari, N.; Mooranian, A.; Al-Salami, H.; Coulson, S.H.; Nesbit, M.; Mamo, J.C.L. Blood-Brain Barrier Dysfunction Precedes Cognitive Decline and Neurodegeneration in Diabetic Insulin Resistant Mouse Model: An Implication for Causal Link. Front. Aging Neurosci. 2017, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Kong, X.; Lu, G.M.; Zhang, L.J. Diabetes Mellitus is Associated with More Severe Brain Spontaneous Activity Impairment and Gray Matter Loss in Patients with Cirrhosis. Sci. Rep. 2017, 7, 7775. [Google Scholar] [CrossRef]
- Garcia-Compean, D.; Jaquez-Quintana, J.O.; Gonzalez-Gonzalez, J.A.; Maldonado-Garza, H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J. Gastroenterol. 2009, 15, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.; Watson, H.; Andersen, P.K.; Vilstrup, H. Diabetes as a risk factor for hepatic encephalopathy in cirrhosis patients. J. Hepatol. 2015, 63, 1133–1138. [Google Scholar] [CrossRef]
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar] [CrossRef]
- Elkrief, L.; Rautou, P.E.; Sarin, S.; Valla, D.; Paradis, V.; Moreau, R. Diabetes mellitus in patients with cirrhosis: Clinical implications and management. Liver Int. 2016, 36, 936–948. [Google Scholar] [CrossRef]
- De Silva, N.M.G.; Borges, M.C.; Hingorani, A.D.; Engmann, J.; Shah, T.; Zhang, X.; Luan, J.; Langenberg, C.; Wong, A.; Kuh, D.; et al. Liver Function and Risk of Type 2 Diabetes: Bidirectional Mendelian Randomization Study. Diabetes 2019, 68, 1681–1691. [Google Scholar] [CrossRef]
- Sookoian, S.; Castano, G.O.; Scian, R.; Fernandez Gianotti, T.; Dopazo, H.; Rohr, C.; Gaj, G.; San Martino, J.; Sevic, I.; Flichman, D.; et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am. J. Clin. Nutr. 2016, 103, 422–434. [Google Scholar] [CrossRef]
- Ampuero, J.; Ranchal, I.; Nunez, D.; Diaz-Herrero Mdel, M.; Maraver, M.; del Campo, J.A.; Rojas, A.; Camacho, I.; Figueruela, B.; Bautista, J.D.; et al. Metformin inhibits glutaminase activity and protects against hepatic encephalopathy. PLoS ONE 2012, 7, e49279. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, W.; Ming, R.; Deng, Q.; Zuo, A.; Zhang, S.; Ying, Y.; Zhao, Y.; Ma, J. Noninvasive 40-Hz Light Flicker Rescues Circadian Behavior and Abnormal Lipid Metabolism Induced by Acute Ethanol Exposure via Improving SIRT1 and the Circadian Clock in the Liver-Brain Axis. Front. Pharmacol. 2020, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yaginuma, R.; Ikejima, K.; Miyazaki, A. Liver diseases and metabolic syndrome. J. Gastroenterol. 2008, 43, 509–518. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Khungar, V.; Poordad, F. Hepatic encephalopathy. Clin. Liver Dis. 2012, 16, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, D.; Sundaram, V.; Saab, S. Evaluation and Management of Hepatic Encephalopathy: Current Status and Future Directions. Gut Liver 2016, 10, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ahluwalia, V.; Kang, J.D.; Ghosh, S.S.; Zhou, H.; Li, Y.; Zhao, D.; Gurley, E.; Li, X.; White, M.B.; et al. Effect of Increasing Age on Brain Dysfunction in Cirrhosis. Hepatol. Commun. 2019, 3, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Management options for minimal hepatic encephalopathy. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 785–790. [Google Scholar] [CrossRef]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef]
- Lemberg, A.; Fernandez, M.A. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann. Hepatol. 2009, 8, 95–102. [Google Scholar] [CrossRef]
- Hambuchen, M.D.; Berquist, M.D.; Simecka, C.M.; McGill, M.R.; Gunnell, M.G.; Hendrickson, H.P.; Owens, S.M. Effect of Bile Duct Ligation-induced Liver Dysfunction on Methamphetamine Pharmacokinetics and Locomotor Activity in Rats. J. Pharm. Pharm. Sci. 2019, 22, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure. J. Clin. Exp. Hepatol. 2015, 5, S96–S103. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M. Update on cerebral uptake of blood ammonia. Metab. Brain Dis. 2013, 28, 155–159. [Google Scholar] [CrossRef]
- Sinke, A.P.; Jayakumar, A.R.; Panickar, K.S.; Moriyama, M.; Reddy, P.V.; Norenberg, M.D. NFkappaB in the mechanism of ammonia-induced astrocyte swelling in culture. J. Neurochem. 2008, 106, 2302–2311. [Google Scholar] [CrossRef]
- Rodrigo, R.; Cauli, O.; Gomez-Pinedo, U.; Agusti, A.; Hernandez-Rabaza, V.; Garcia-Verdugo, J.M.; Felipo, V. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 2010, 139, 675–684. [Google Scholar] [CrossRef]
- Vaquero, J.; Butterworth, R.F. Mechanisms of brain edema in acute liver failure and impact of novel therapeutic interventions. Neurol. Res. 2007, 29, 683–690. [Google Scholar] [CrossRef]
- Savlan, I.; Liakina, V.; Valantinas, J. Concise review of current concepts on nomenclature and pathophysiology of hepatic encephalopathy. Medicina (Kaunas) 2014, 50, 75–81. [Google Scholar] [CrossRef]
- Jalan, R.; Hayes, P.C. Hepatic encephalopathy and ascites. Lancet 1997, 350, 1309–1315. [Google Scholar] [CrossRef]
- Saczynski, J.S.; Siggurdsson, S.; Jonsson, P.V.; Eiriksdottir, G.; Olafsdottir, E.; Kjartansson, O.; Harris, T.B.; van Buchem, M.A.; Gudnason, V.; Launer, L.J. Glycemic status and brain injury in older individuals: The age gene/environment susceptibility-Reykjavik study. Diabetes Care 2009, 32, 1608–1613. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability. Diabetes Obes. Metab. 2009, 11, 480–490. [Google Scholar] [CrossRef]
- van Gemert, T.; Wolwer, W.; Weber, K.S.; Hoyer, A.; Strassburger, K.; Bohnau, N.T.; Bruggen, M.A.; Ovelgonne, K.; Gossmann, E.M.; Burkart, V.; et al. Cognitive Function Is Impaired in Patients with Recently Diagnosed Type 2 Diabetes, but Not Type 1 Diabetes. J. Diabetes Res. 2018, 2018, 1470476. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Horiuchi, M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ. J. 2011, 75, 1042–1048. [Google Scholar] [CrossRef]
- Gispen, W.H.; Biessels, G.J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000, 23, 542–549. [Google Scholar] [CrossRef]
- Hwang, I.K.; Choi, J.H.; Nam, S.M.; Park, O.K.; Yoo, D.Y.; Kim, W.; Yi, S.S.; Won, M.H.; Seong, J.K.; Yoon, Y.S. Activation of microglia and induction of pro-inflammatory cytokines in the hippocampus of type 2 diabetic rats. Neurol. Res. 2014, 36, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Bernacki, J.; Dobrowolska, A.; Nierwinska, K.; Malecki, A. Physiology and pharmacological role of the blood-brain barrier. Pharmacol. Rep. 2008, 60, 600–622. [Google Scholar]
- Winterdahl, M.; Abbas, Z.; Noer, O.; Thomsen, K.L.; Gras, V.; Nahimi, A.; Vilstrup, H.; Shah, N.J.; Dam, G. Cerebral water content mapping in cirrhosis patients with and without manifest HE. Metab. Brain Dis. 2019, 34, 1071–1076. [Google Scholar] [CrossRef]
- Wright, G.; Soper, R.; Brooks, H.F.; Stadlbauer, V.; Vairappan, B.; Davies, N.A.; Andreola, F.; Hodges, S.; Moss, R.F.; Davies, D.C.; et al. Role of aquaporin-4 in the development of brain oedema in liver failure. J. Hepatol. 2010, 53, 91–97. [Google Scholar] [CrossRef]
- Thumburu, K.K.; Dhiman, R.K.; Vasishta, R.K.; Chakraborti, A.; Butterworth, R.F.; Beauchesne, E.; Desjardins, P.; Goyal, S.; Sharma, N.; Duseja, A.; et al. Expression of astrocytic genes coding for proteins implicated in neural excitation and brain edema is altered after acute liver failure. J. Neurochem. 2014, 128, 617–627. [Google Scholar] [CrossRef]
- Dhanda, S.; Sandhir, R. Blood-Brain Barrier Permeability Is Exacerbated in Experimental Model of Hepatic Encephalopathy via MMP-9 Activation and Downregulation of Tight Junction Proteins. Mol. Neurobiol. 2018, 55, 3642–3659. [Google Scholar] [CrossRef]
- Gonzalez-Regueiro, J.A.; Moreno-Castaneda, L.; Uribe, M.; Chavez-Tapia, N.C. The Role of Bile Acids in Glucose Metabolism and Their Relation with Diabetes. Ann. Hepatol. 2017, 16, 16–21. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Jiang, R.; Zhao, A.; Yan, J.; Zheng, X.; Huang, F.; Liu, X.; Panee, J.; Rajani, C.; et al. Dysregulated bile acid signaling contributes to the neurological impairment in murine models of acute and chronic liver failure. EBioMedicine 2018, 37, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Haussinger, D.; Kircheis, G.; Fischer, R.; Schliess, F.; vom Dahl, S. Hepatic encephalopathy in chronic liver disease: A clinical manifestation of astrocyte swelling and low-grade cerebral edema? J. Hepatol. 2000, 32, 1035–1038. [Google Scholar] [CrossRef]
- Acharya, N.K.; Levin, E.C.; Clifford, P.M.; Han, M.; Tourtellotte, R.; Chamberlain, D.; Pollaro, M.; Coretti, N.J.; Kosciuk, M.C.; Nagele, E.P.; et al. Diabetes and hypercholesterolemia increase blood-brain barrier permeability and brain amyloid deposition: Beneficial effects of the LpPLA2 inhibitor darapladib. J. Alzheimers Dis. 2013, 35, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wang, L.; Zhang, L.; Qin, C.; Song, Y.; Ma, Y.; Chen, Y.; Chen, S.; Wang, Y.; Zhang, Z.; et al. Blood-Brain Barrier Disruption Induced Cognitive Impairment Is Associated With Increase of Inflammatory Cytokine. Front. Aging Neurosci. 2018, 10, 129. [Google Scholar] [CrossRef]
- Muresanu, D.F.; Sharma, A.; Sharma, H.S. Diabetes aggravates heat stress-induced blood-brain barrier breakdown, reduction in cerebral blood flow, edema formation, and brain pathology: Possible neuroprotection with growth hormone. Ann. N. Y. Acad. Sci. 2010, 1199, 15–26. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, F.; Yu, X.; Zhao, Y.; Li, D.; Shi, H.; Sun, Y. Expression of aquaporin 4 and breakdown of the blood-brain barrier after hypoglycemia-induced brain edema in rats. PLoS ONE 2014, 9, e107022. [Google Scholar] [CrossRef]
- Nicchia, G.P.; Frigeri, A.; Liuzzi, G.M.; Svelto, M. Inhibition of aquaporin-4 expression in astrocytes by RNAi determines alteration in cell morphology, growth, and water transport and induces changes in ischemia-related genes. FASEB J. 2003, 17, 1508–1510. [Google Scholar] [CrossRef]
- Belanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V. Hepatic encephalopathy: Effects of liver failure on brain function. Nat. Rev. Neurosci. 2013, 14, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Bosoi, C.R.; Zwingmann, C.; Marin, H.; Parent-Robitaille, C.; Huynh, J.; Tremblay, M.; Rose, C.F. Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J. Hepatol. 2014, 60, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.; Sargsyan, S.; Monk, P.N.; Shaw, P.J. Astrocyte function and role in motor neuron disease: A future therapeutic target? Glia 2009, 57, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.; Judd, R.; Hoe, L.; Dennis, J.; Posner, P. Effects of diabetes mellitus on astrocyte GFAP and glutamate transporters in the CNS. Glia 2004, 48, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Sepehrinezhad, A.; Zarifkar, A.; Namvar, G.; Shahbazi, A.; Williams, R. Astrocyte swelling in hepatic encephalopathy: Molecular perspective of cytotoxic edema. Metab. Brain Dis. 2020, 35, 559–578. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Hakvoort, T.B.; He, Y.; Kulik, W.; Vermeulen, J.L.; Duijst, S.; Ruijter, J.M.; Runge, J.H.; Deutz, N.E.; Koehler, S.E.; Lamers, W.H. Pivotal role of glutamine synthetase in ammonia detoxification. Hepatology 2017, 65, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Suarez, I.; Bodega, G.; Fernandez, B. Glutamine synthetase in brain: Effect of ammonia. Neurochem. Int. 2002, 41, 123–142. [Google Scholar] [CrossRef]
- Tofteng, F.; Hauerberg, J.; Hansen, B.A.; Pedersen, C.B.; Jorgensen, L.; Larsen, F.S. Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J. Cereb. Blood Flow Metab. 2006, 26, 21–27. [Google Scholar] [CrossRef]
- Hourani, B.T.; Hamlin, E.M.; Reynolds, T.B. Cerebrospinal fluid glutamine as a measure of hepatic encephalopathy. Arch. Intern. Med. 1971, 127, 1033–1036. [Google Scholar] [CrossRef]
- Girard, G.; Giguere, J.F.; Butterworth, R.F. Region-selective reductions in activities of glutamine synthetase in rat brain following portacaval anastomosis. Metab. Brain Dis. 1993, 8, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wiegers, E.C.; Rooijackers, H.M.; van Asten, J.J.A.; Tack, C.J.; Heerschap, A.; de Galan, B.E.; van der Graaf, M. Elevated brain glutamate levels in type 1 diabetes: Correlations with glycaemic control and age of disease onset but not with hypoglycaemia awareness status. Diabetologia 2019, 62, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Roy Choudhury, G.; Winters, A.; Prah, J.; Lin, W.; Liu, R.; Yang, S.H. Hyperglycemia Alters Astrocyte Metabolism and Inhibits Astrocyte Proliferation. Aging Dis. 2018, 9, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Azhari, H.; Swain, M.G. Role of Peripheral Inflammation in Hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2018, 8, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Akrivos, J.; Ravona-Springer, R.; Schmeidler, J.; LeRoith, D.; Heymann, A.; Preiss, R.; Hoffman, H.; Koifman, K.; Silverman, J.M.; Schnaider Beeri, M. Glycemic control, inflammation, and cognitive function in older patients with type 2 diabetes. Int. J. Geriatr. Psychiatry 2015, 30, 1093–1100. [Google Scholar] [CrossRef]
- Kabba, J.A.; Xu, Y.; Christian, H.; Ruan, W.; Chenai, K.; Xiang, Y.; Zhang, L.; Saavedra, J.M.; Pang, T. Microglia: Housekeeper of the Central Nervous System. Cell Mol. Neurobiol. 2018, 38, 53–71. [Google Scholar] [CrossRef]
- Wright, G.A.; Sharifi, Y.; Newman, T.A.; Davies, N.; Vairappan, B.; Perry, H.V.; Jalan, R. Characterisation of temporal microglia and astrocyte immune responses in bile duct-ligated rat models of cirrhosis. Liver Int. 2014, 34, 1184–1191. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Wright, G.; Swain, M.; Annane, D.; Saliba, F.; Samuel, D.; Arroyo, V.; DeMorrow, S.; Witt, A. Neuroinflammation in liver disease: Sessional talks from ISHEN. Metab. Brain Dis. 2016, 31, 1339–1354. [Google Scholar] [CrossRef]
- Bampi, S.R.; Casaril, A.M.; Domingues, M.; de Andrade Lourenco, D.; Pesarico, A.P.; Vieira, B.; Begnini, K.R.; Seixas, F.K.; Collares, T.V.; Lenardao, E.J.; et al. Depression-like behavior, hyperglycemia, oxidative stress, and neuroinflammation presented in diabetic mice are reversed by the administration of 1-methyl-3-(phenylselanyl)-1H-indole. J. Psychiatr. Res. 2020, 120, 91–102. [Google Scholar] [CrossRef]
- Felipo, V.; Urios, A.; Montesinos, E.; Molina, I.; Garcia-Torres, M.L.; Civera, M.; Olmo, J.A.; Ortega, J.; Martinez-Valls, J.; Serra, M.A.; et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab. Brain Dis. 2012, 27, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Hepatic encephalopathy: A central neuroinflammatory disorder? Hepatology 2011, 53, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Deans, K.A.; Sattar, N. “Anti-inflammatory” drugs and their effects on type 2 diabetes. Diabetes Technol. Ther. 2006, 8, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Miyagi, K.; Obata, T.; Morimoto, Y.; Nakamoto, K.; Kim, K.I.; Kim, S.K.; Kim, S.R.; Tokuyama, S. Influence of hyperglycemia on liver inflammatory conditions in the early phase of non-alcoholic fatty liver disease in mice. J. Pharm. Pharmacol. 2017, 69, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Palomero-Gallagher, N.; Zilles, K. Neurotransmitter receptor alterations in hepatic encephalopathy: A review. Arch. Biochem. Biophys. 2013, 536, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Cauli, O.; Rodrigo, R.; Llansola, M.; Montoliu, C.; Monfort, P.; Piedrafita, B.; El Mlili, N.; Boix, J.; Agusti, A.; Felipo, V. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab. Brain Dis. 2009, 24, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Jung, S.; Kim, J.Y.; Goo, Y.M.; Cho, K.M.; Lee, D.H.; Roh, G.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; et al. Type 1 diabetes alters astrocytic properties related with neurotransmitter supply, causing abnormal neuronal activities. Brain Res. 2015, 1602, 32–43. [Google Scholar] [CrossRef]
- Monfort, P.; Munoz, M.D.; Felipo, V. Chronic hyperammonemia in vivo impairs long-term potentiation in hippocampus by altering activation of cyclic GMP-dependent-protein kinase and of phosphodiesterase 5. J. Neurochem. 2005, 94, 934–942. [Google Scholar] [CrossRef]
- Malone, J.I.; Hanna, S.; Saporta, S.; Mervis, R.F.; Park, C.R.; Chong, L.; Diamond, D.M. Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatr. Diabetes 2008, 9, 531–539. [Google Scholar] [CrossRef]
- Hernandez-Rabaza, V.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Gonzalez-Usano, A.; Agusti, A.; Balzano, T.; Llansola, M.; Felipo, V. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J. Neuroinflamm. 2016, 13, 83. [Google Scholar] [CrossRef]
- Cauli, O.; Llansola, M.; Erceg, S.; Felipo, V. Hypolocomotion in rats with chronic liver failure is due to increased glutamate and activation of metabotropic glutamate receptors in substantia nigra. J. Hepatol. 2006, 45, 654–661. [Google Scholar] [CrossRef] [PubMed]
- van Bussel, F.C.; Backes, W.H.; Hofman, P.A.; Puts, N.A.; Edden, R.A.; van Boxtel, M.P.; Schram, M.T.; Stehouwer, C.D.; Wildberger, J.E.; Jansen, J.F. Increased GABA concentrations in type 2 diabetes mellitus are related to lower cognitive functioning. Medicine (Baltimore) 2016, 95, e4803. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Walecka-Kapica, E.; Stepien, A.; Pawlowicz, M.; Wachowska-Kelly, P.; Chojnacki, J. Serum and ascitic fluid serotonin levels and 5-hydroxyindoleacetic acid urine excretion in the liver of cirrhotic patients with encephalopathy. Adv. Med. Sci. 2013, 58, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Borg, J.; Warter, J.M.; Schlienger, J.L.; Imler, M.; Marescaux, C.; Mack, G. Neurotransmitter modifications in human cerebrospinal fluid and serum during hepatic encephalopathy. J. Neurol. Sci. 1982, 57, 343–356. [Google Scholar] [CrossRef]
- Zhuge, W.; Wen, F.; Ni, Z.; Zheng, Z.; Zhu, X.; Lin, J.; Wang, J.; Zhuge, Q.; Ding, S. Dopamine Burden Triggers Cholesterol Overload Following Disruption of Synaptogenesis in Minimal Hepatic Encephalopathy. Neuroscience 2019, 410, 1–15. [Google Scholar] [CrossRef]
- Dhanda, S.; Sandhir, R. Role of dopaminergic and serotonergic neurotransmitters in behavioral alterations observed in rodent model of hepatic encephalopathy. Behav. Brain Res. 2015, 286, 222–235. [Google Scholar] [CrossRef]
- Parashar, A.; Mehta, V.; Malairaman, U. Type 2 Diabetes Mellitus Is Associated with Social Recognition Memory Deficit and Altered Dopaminergic Neurotransmission in the Amygdala. Ann. Neurosci. 2018, 24, 212–220. [Google Scholar] [CrossRef]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast 2017, 2017, 6031478. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Ishibashi, T.; Kudo, N.; Kawashima, Y.; Mitsumoto, A. Behavioral and biochemical characterization of rats treated chronically with thioacetamide: Proposal of an animal model for hepatic encephalopathy associated with cirrhosis. J. Toxicol. Sci. 2012, 37, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Garris, D.R. Age- and diabetes-associated alterations in regional brain norepinephrine concentrations and adrenergic receptor populations in C57BL/KsJ mice. Brain Res. Dev. Brain Res. 1990, 51, 161–166. [Google Scholar] [CrossRef]
- Rahn, K.A.; Slusher, B.S.; Kaplin, A.I. Glutamate in CNS neurodegeneration and cognition and its regulation by GCPII inhibition. Curr. Med. Chem. 2012, 19, 1335–1345. [Google Scholar] [CrossRef]
- Monfort, P.; Erceg, S.; Piedrafita, B.; Llansola, M.; Felipo, V. Chronic liver failure in rats impairs glutamatergic synaptic transmission and long-term potentiation in hippocampus and learning ability. Eur. J. Neurosci. 2007, 25, 2103–2111. [Google Scholar] [CrossRef]
- Llansola, M.; Montoliu, C.; Cauli, O.; Hernandez-Rabaza, V.; Agusti, A.; Cabrera-Pastor, A.; Gimenez-Garzo, C.; Gonzalez-Usano, A.; Felipo, V. Chronic hyperammonemia, glutamatergic neurotransmission and neurological alterations. Metab. Brain Dis. 2013, 28, 151–154. [Google Scholar] [CrossRef]
- Lyoo, I.K.; Yoon, S.J.; Musen, G.; Simonson, D.C.; Weinger, K.; Bolo, N.; Ryan, C.M.; Kim, J.E.; Renshaw, P.F.; Jacobson, A.M. Altered prefrontal glutamate-glutamine-gamma-aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Arch. Gen. Psychiatry 2009, 66, 878–887. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Mooradian, A.D. Blood-brain barrier choline transport is reduced in diabetic rats. Diabetes 1987, 36, 1094–1097. [Google Scholar] [CrossRef]
- Binesh, N.; Huda, A.; Thomas, M.A.; Wyckoff, N.; Bugbee, M.; Han, S.; Rasgon, N.; Davanzo, P.; Sayre, J.; Guze, B.; et al. Hepatic encephalopathy: A neurochemical, neuroanatomical, and neuropsychological study. J. Appl. Clin. Med. Phys. 2006, 7, 86–96. [Google Scholar] [CrossRef]
- Romero-Gomez, M.; Jover, M.; Del Campo, J.A.; Royo, J.L.; Hoyas, E.; Galan, J.J.; Montoliu, C.; Baccaro, E.; Guevara, M.; Cordoba, J.; et al. Variations in the promoter region of the glutaminase gene and the development of hepatic encephalopathy in patients with cirrhosis: A cohort study. Ann. Intern. Med. 2010, 153, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Anstee, Q.M.; Valenti, L. Genetic predisposition in NAFLD and NASH: Impact on severity of liver disease and response to treatment. Curr. Pharm. Des. 2013, 19, 5219–5238. [Google Scholar] [CrossRef] [PubMed]
- Sirdah, M.M.; Reading, N.S. Genetic predisposition in type 2 diabetes: A promising approach toward a personalized management of diabetes. Clin. Genet. 2020, 98, 525–547. [Google Scholar] [CrossRef]
- Smith, E.M.; Watford, M. Molecular cloning of a cDNA for rat hepatic glutaminase. Sequence similarity to kidney-type glutaminase. J. Biol. Chem. 1990, 265, 10631–10636. [Google Scholar] [PubMed]
- De la Monte, S.M.; Tong, M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Seaquist, E.R.; Damberg, G.S.; Tkac, I.; Gruetter, R. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes 2001, 50, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.M.; Ferreira-Neto, H.C.; Antunes, V.R. Subdiaphragmatic vagus nerve activity and hepatic venous glucose are differentially regulated by the central actions of insulin in Wistar and SHR. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.; Belgardt, B.F.; Bruning, J.C. Central insulin action in energy and glucose homeostasis. J. Clin. Investig. 2006, 116, 1761–1766. [Google Scholar] [CrossRef]
- Cholerton, B.; Baker, L.D.; Craft, S. Insulin, cognition, and dementia. Eur. J. Pharmacol. 2013, 719, 170–179. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Vidoni, E.D.; Honea, R.A.; Burns, J.M.; Alzheimer’s Disease Neuroimaging Initiative. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol. Aging 2014, 35, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef] [PubMed]
- David-Silva, A.; Esteves, J.V.; Morais, M.; Freitas, H.S.; Zorn, T.M.; Correa-Giannella, M.L.; Machado, U.F. Dual SGLT1/SGLT2 Inhibitor Phlorizin Ameliorates Non-Alcoholic Fatty Liver Disease and Hepatic Glucose Production in Type 2 Diabetic Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiao, Y.; Xing, Y.; Gao, P. Diabetes Mellitus and Risk of Hepatic Fibrosis/Cirrhosis. BioMed Res. Int. 2019, 2019, 5308308. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Triguero, D.; Farrell, C.R. Downregulation of blood-brain barrier glucose transporter in experimental diabetes. Diabetes 1990, 39, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, S.J.; Gibbs, E.M.; Simpson, I.A. Glucose utilization and glucose transporter proteins GLUT-1 and GLUT-3 in brains of diabetic (db/db) mice. Am. J. Physiol. 1997, 272, E267–E274. [Google Scholar] [CrossRef]
- Belanger, M.; Desjardins, P.; Chatauret, N.; Butterworth, R.F. Selectively increased expression of the astrocytic/endothelial glucose transporter protein GLUT1 in acute liver failure. Glia 2006, 53, 557–562. [Google Scholar] [CrossRef]
- Wanner, C.; Marx, N. SGLT2 inhibitors: The future for treatment of type 2 diabetes mellitus and other chronic diseases. Diabetologia 2018, 61, 2134–2139. [Google Scholar] [CrossRef]
- Zhang, W.; Ning, N.; Li, X.; Li, M.; Duan, X.; Guo, Y.; Dang, Y.; Li, Y.; Gao, J.; Ye, J.; et al. Impaired brain glucose metabolism in cirrhosis without overt hepatic encephalopathy: A retrospective 18F-FDG PET/CT study. Neuroreport 2019, 30, 776–782. [Google Scholar] [CrossRef]
- Poh, Z.; Chang, P.E. A current review of the diagnostic and treatment strategies of hepatic encephalopathy. Int. J. Hepatol. 2012, 2012, 480309. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Jessy, J.; Mans, A.M.; Chedid, A.; DeJoseph, M.R. Neomycin reduces the intestinal production of ammonia from glutamine. Adv. Exp. Med. Biol. 1994, 368, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ndraha, S.; Hasan, I.; Simadibrata, M. The effect of L-ornithine L-aspartate and branch chain amino acids on encephalopathy and nutritional status in liver cirrhosis with malnutrition. Acta Med. Indones 2011, 43, 18–22. [Google Scholar] [PubMed]
- Hopp, A.E.; Dirks, M.; Petrusch, C.; Goldbecker, A.; Tryc, A.B.; Barg-Hock, H.; Strassburg, C.; Klempnauer, J.; Weissenborn, K.; Pflugrad, H. Hepatic Encephalopathy Is Reversible in the Long Term After Liver Transplantation. Liver Transpl. 2019, 25, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
| Similarities between Hepatic Encephalopathy and Diabetic Encephalopathy | Differences between Hepatic Encephalopathy and Diabetic Encephalopathy | |
|---|---|---|
| Brain vasculature |
|
|
| Glial cells |
|
|
| Neurotransmitters |
|
|
| Glucose metabolism |
|
|
| Cognitive function |
|
|
| etc. |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, S.Y.; Song, J. The Association between Hepatic Encephalopathy and Diabetic Encephalopathy: The Brain-Liver Axis. Int. J. Mol. Sci. 2021, 22, 463. https://doi.org/10.3390/ijms22010463
Cheon SY, Song J. The Association between Hepatic Encephalopathy and Diabetic Encephalopathy: The Brain-Liver Axis. International Journal of Molecular Sciences. 2021; 22(1):463. https://doi.org/10.3390/ijms22010463
Chicago/Turabian StyleCheon, So Yeong, and Juhyun Song. 2021. "The Association between Hepatic Encephalopathy and Diabetic Encephalopathy: The Brain-Liver Axis" International Journal of Molecular Sciences 22, no. 1: 463. https://doi.org/10.3390/ijms22010463
APA StyleCheon, S. Y., & Song, J. (2021). The Association between Hepatic Encephalopathy and Diabetic Encephalopathy: The Brain-Liver Axis. International Journal of Molecular Sciences, 22(1), 463. https://doi.org/10.3390/ijms22010463





