Concentration and Recovery of Valuable Heavy Minerals from Dredged Fine Aggregate Waste
Abstract
:1. Introduction
2. Materials and Methods
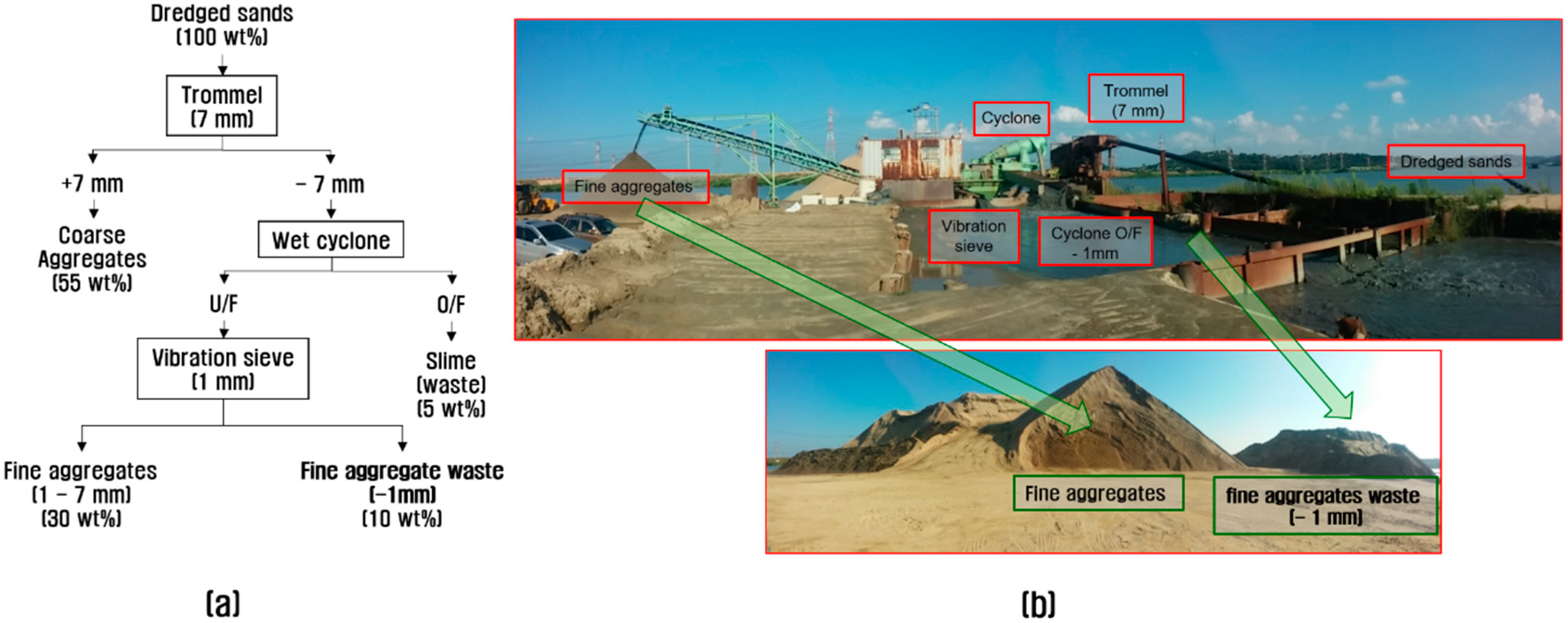
2.1. Background (Study Area)
2.2. Characterization of the Fine Aggregate Waste
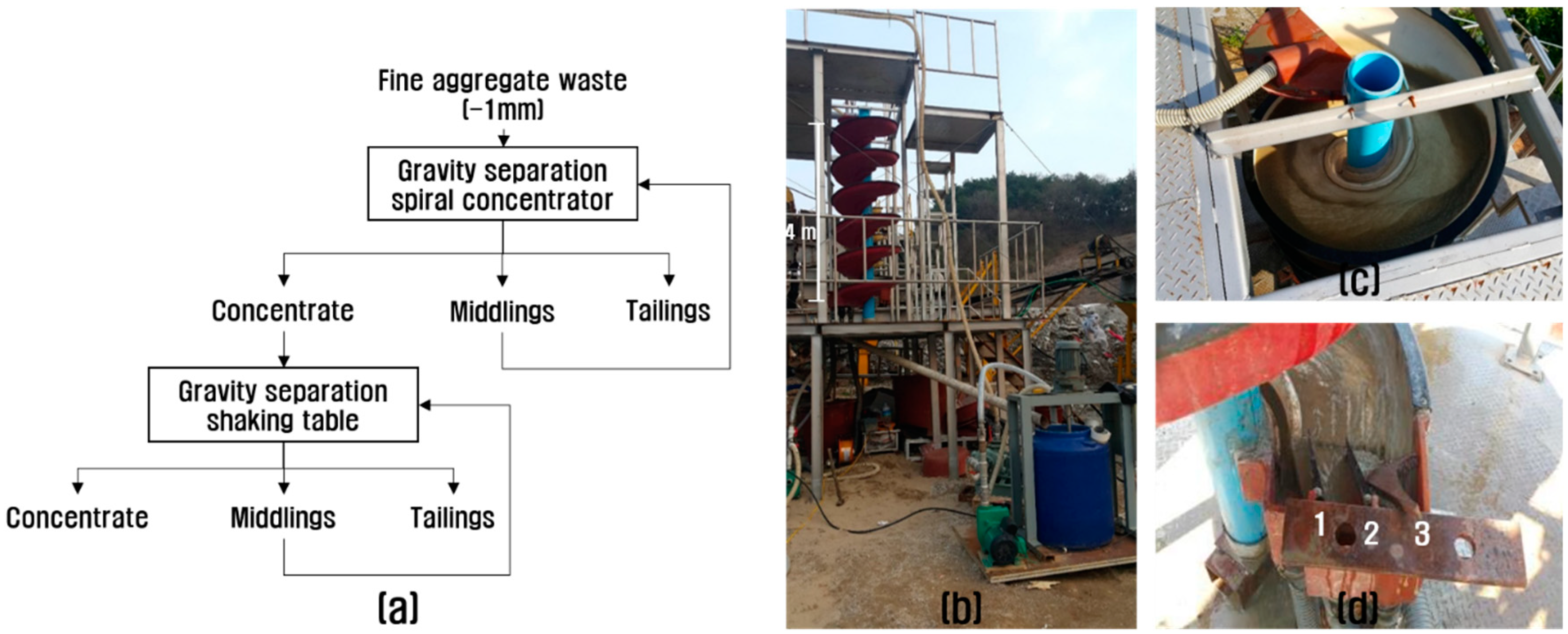
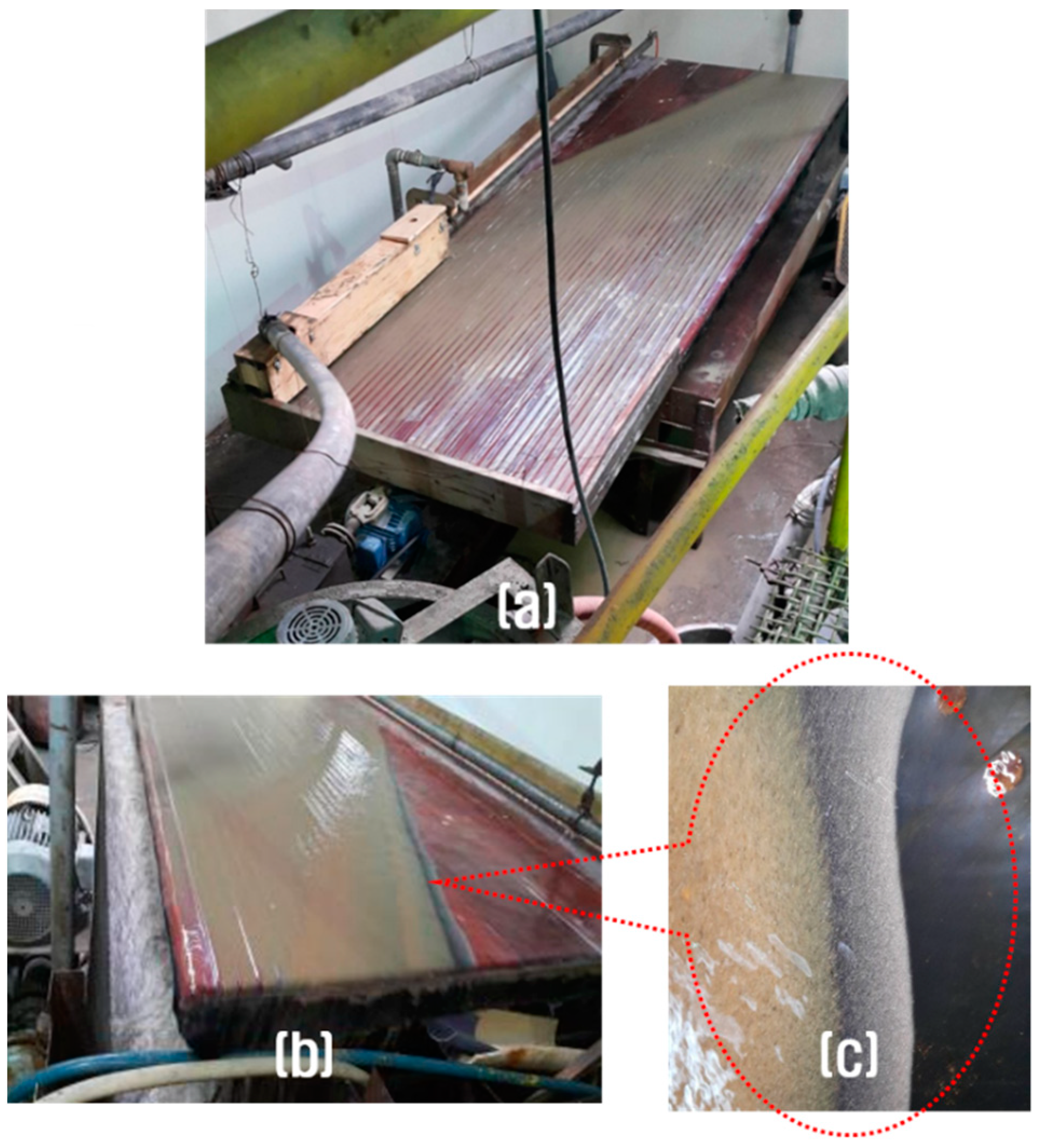
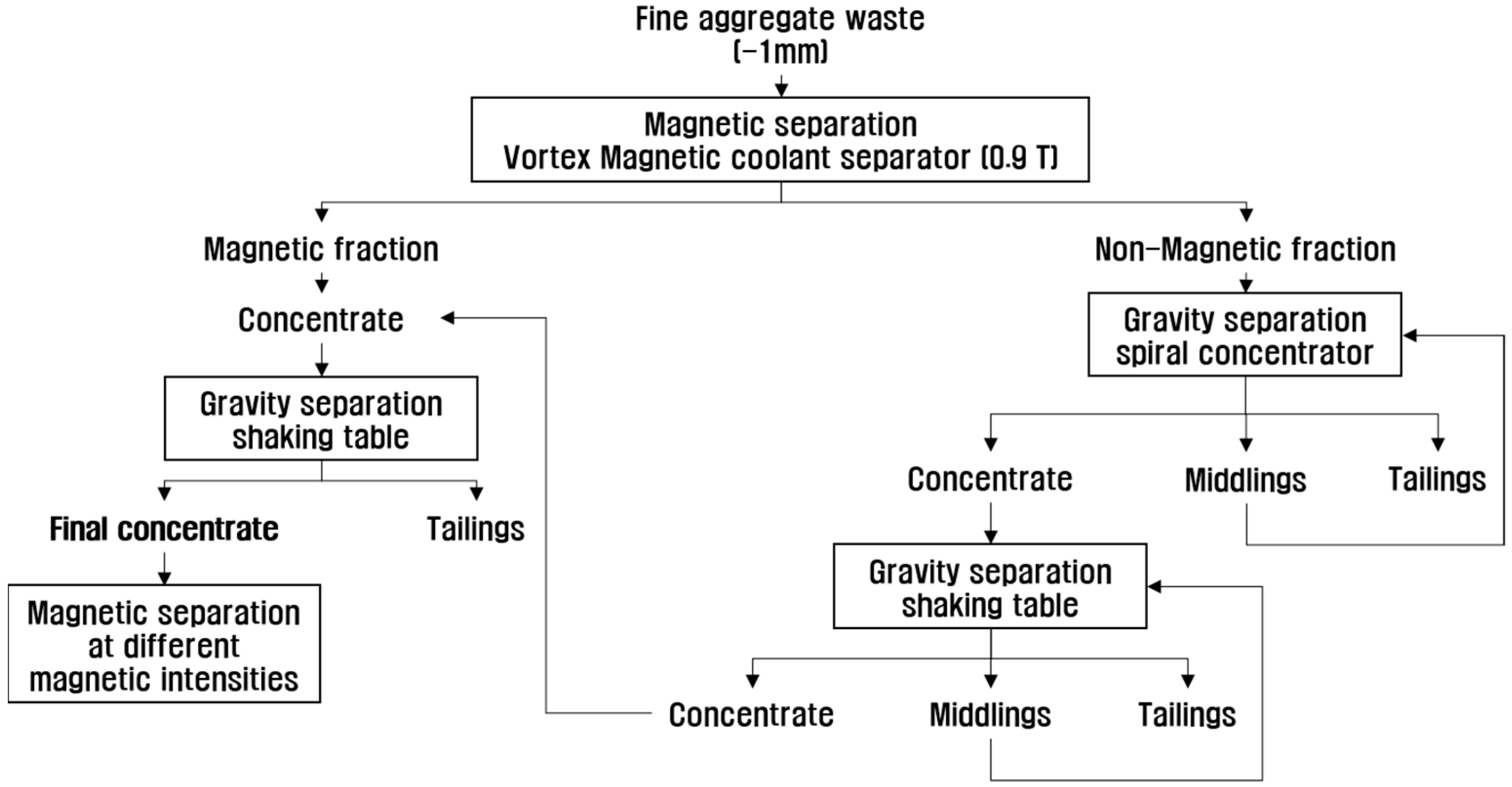
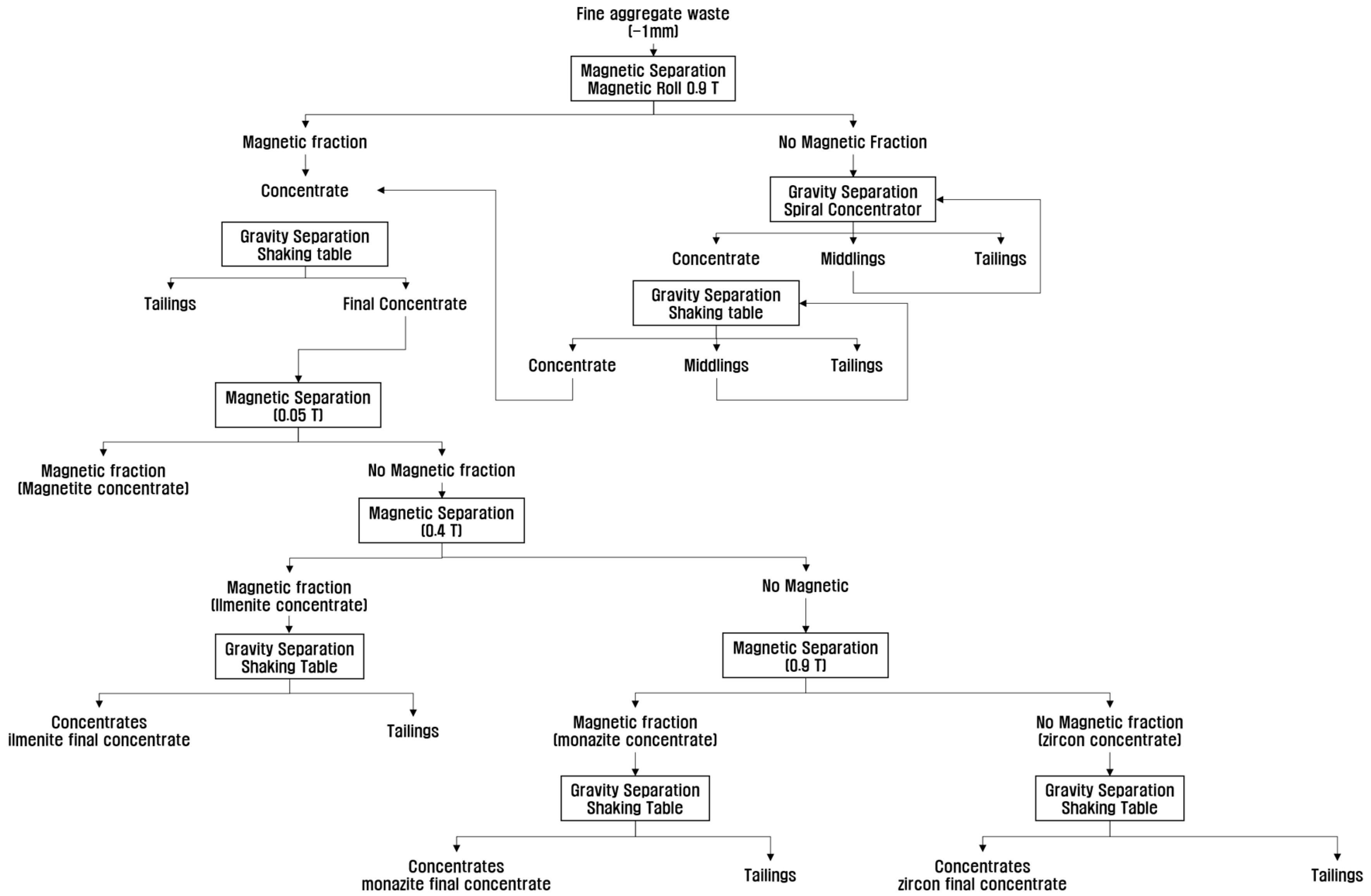
2.3. Concentration of the Valuable Heavy Minerals by Gravity Separation and Magnetic Separation
2.4. Separation of Each Valuable Heavy Mineral by Magnetic Separation at Different Magnetic Intensities
3. Results and Discussion
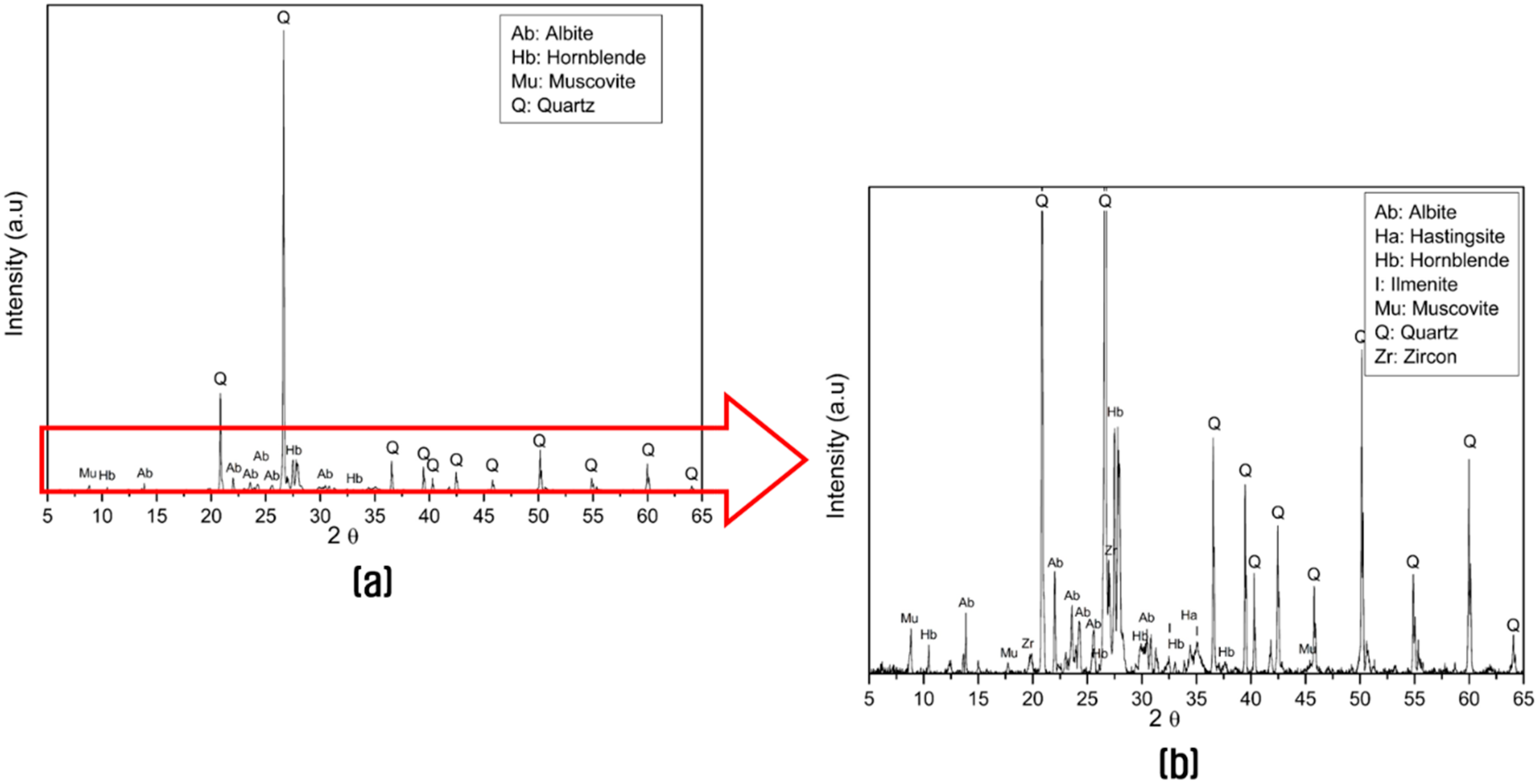
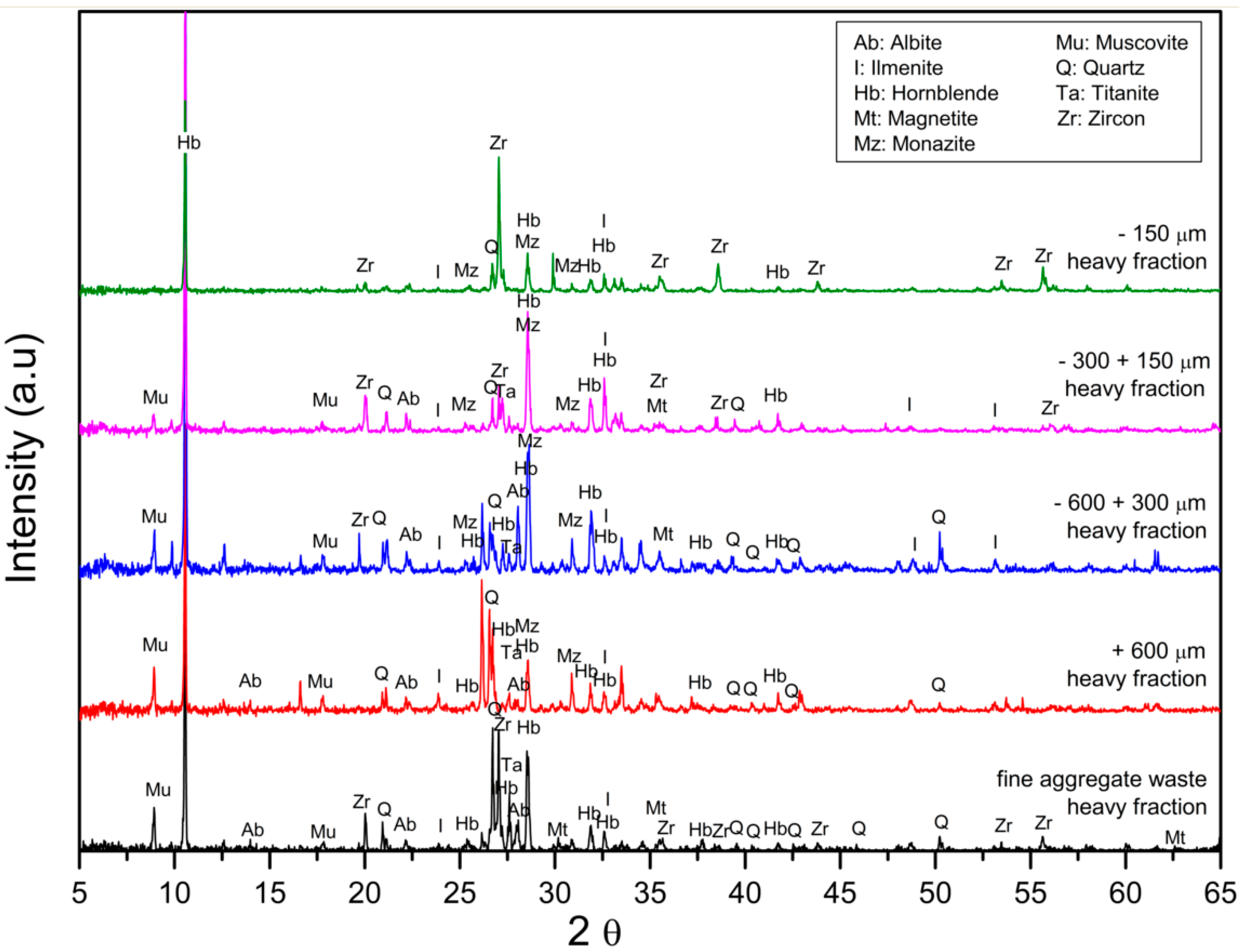
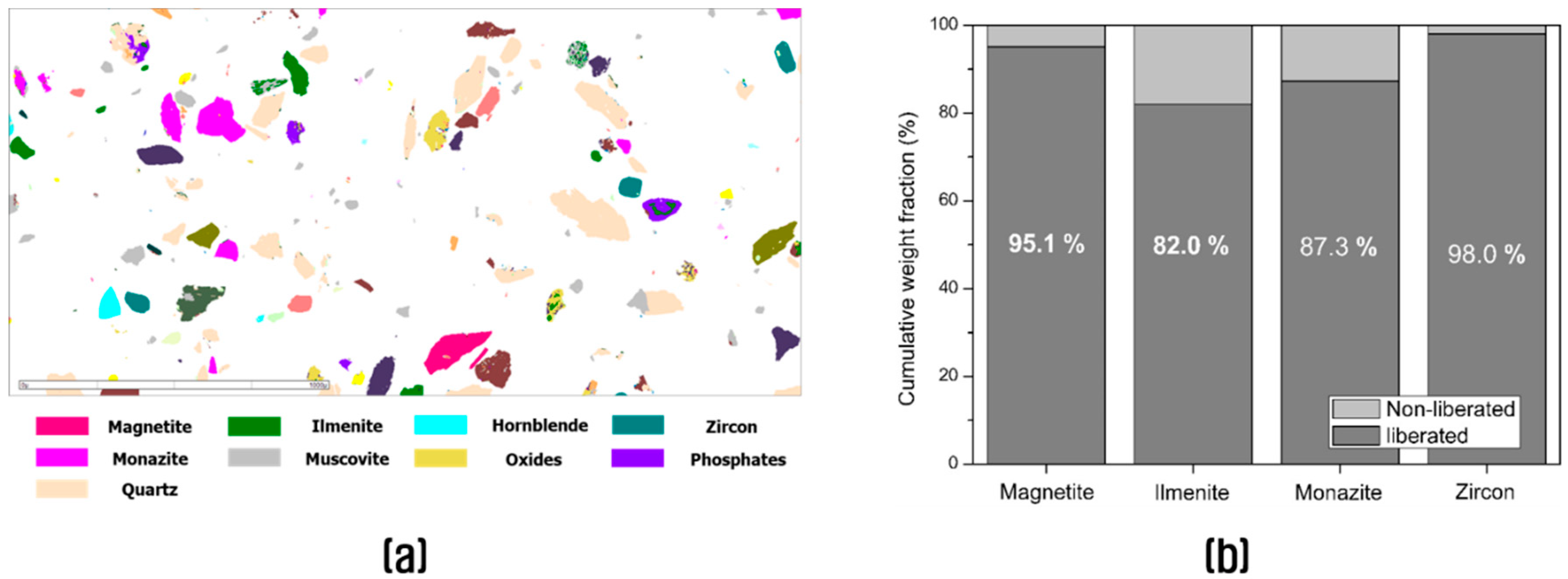
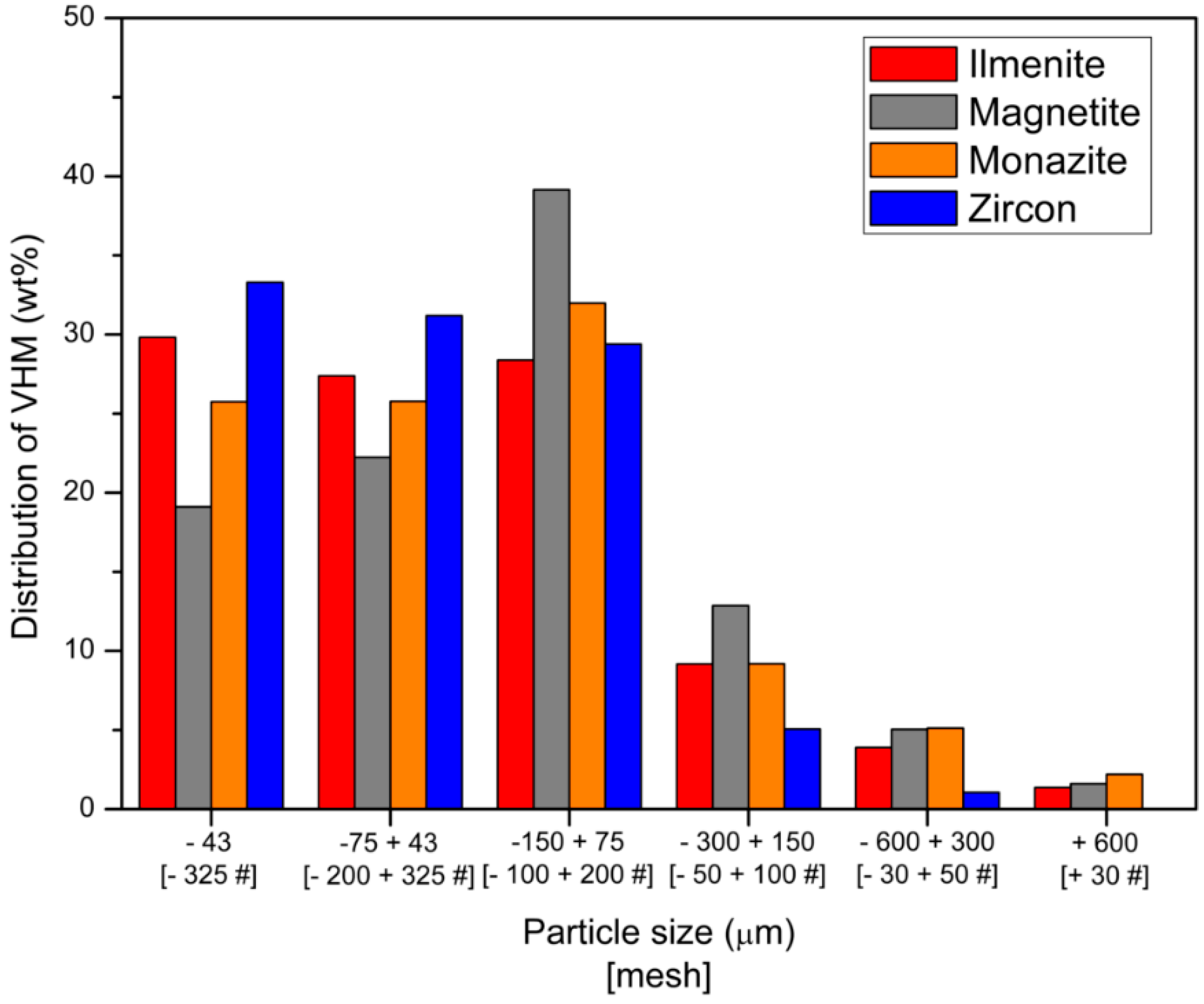
3.1. Characterization of the Fine Aggregate Waste
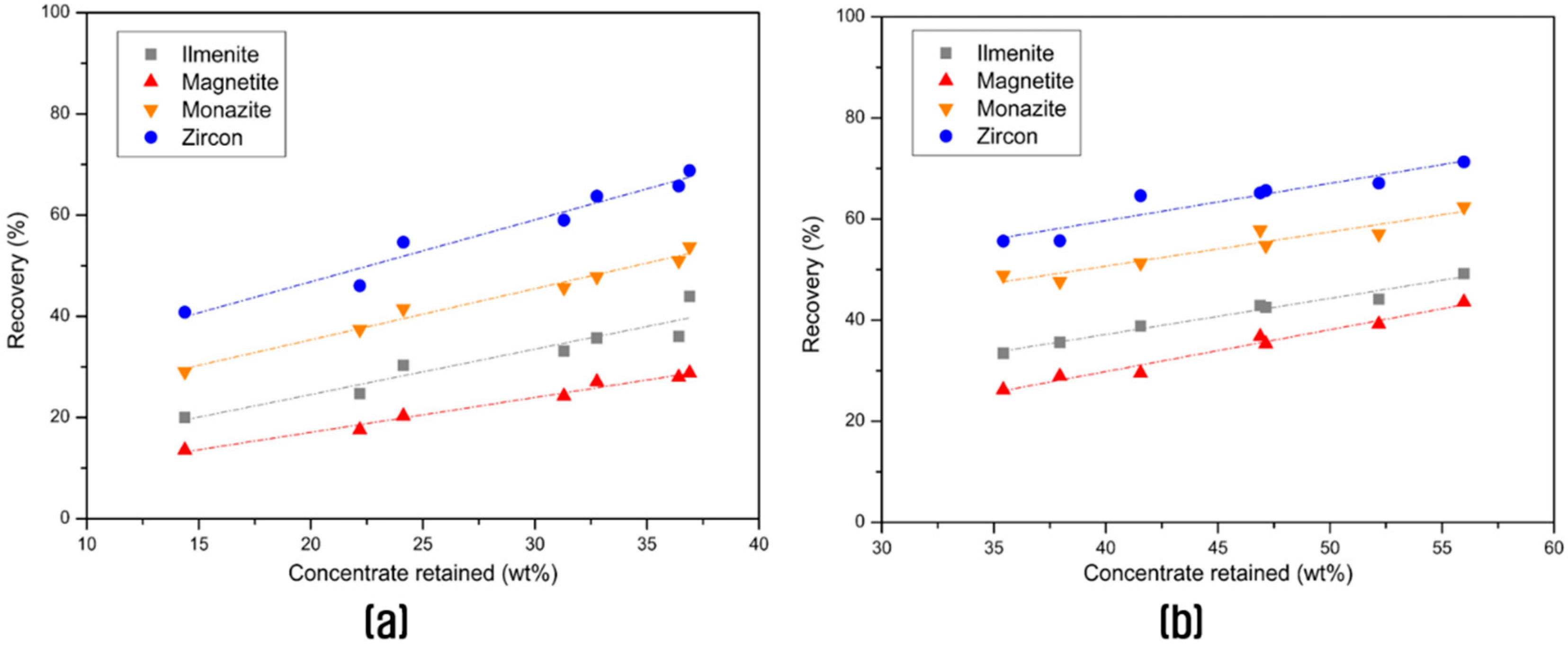
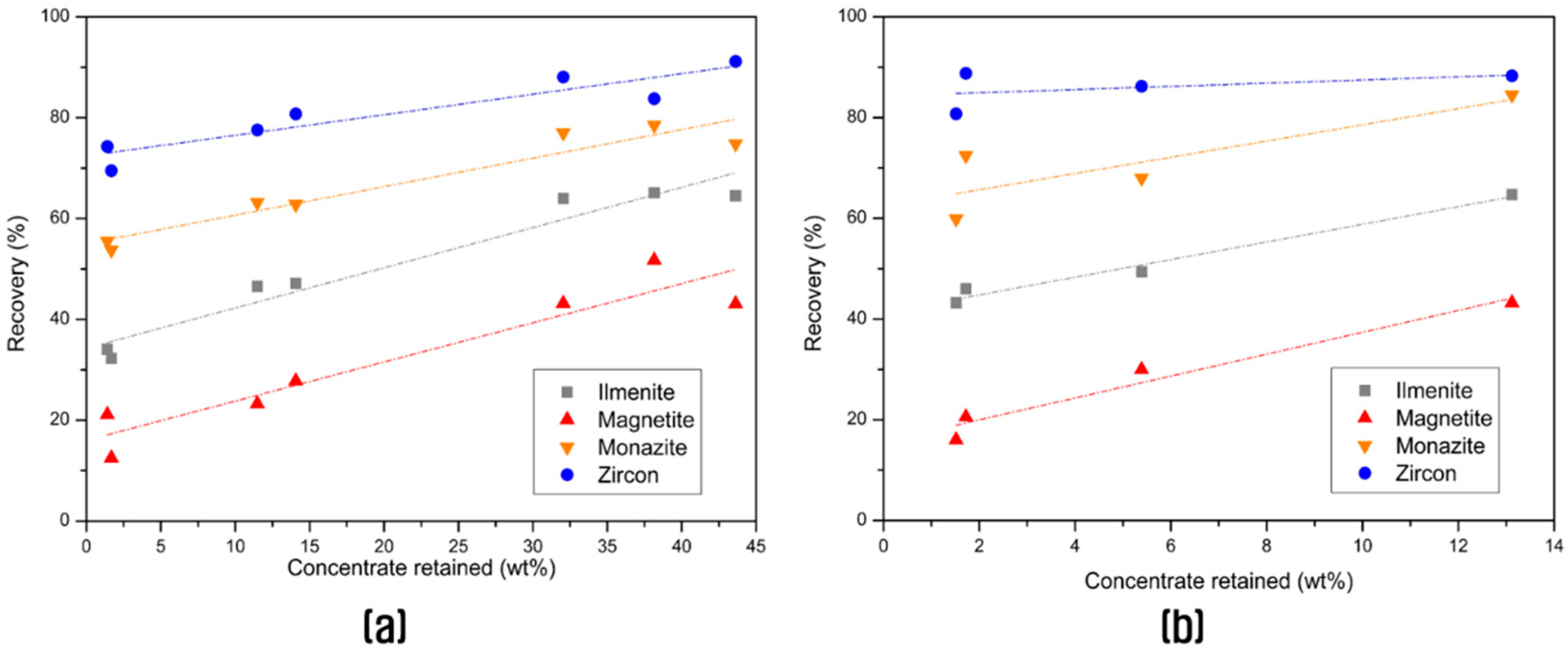
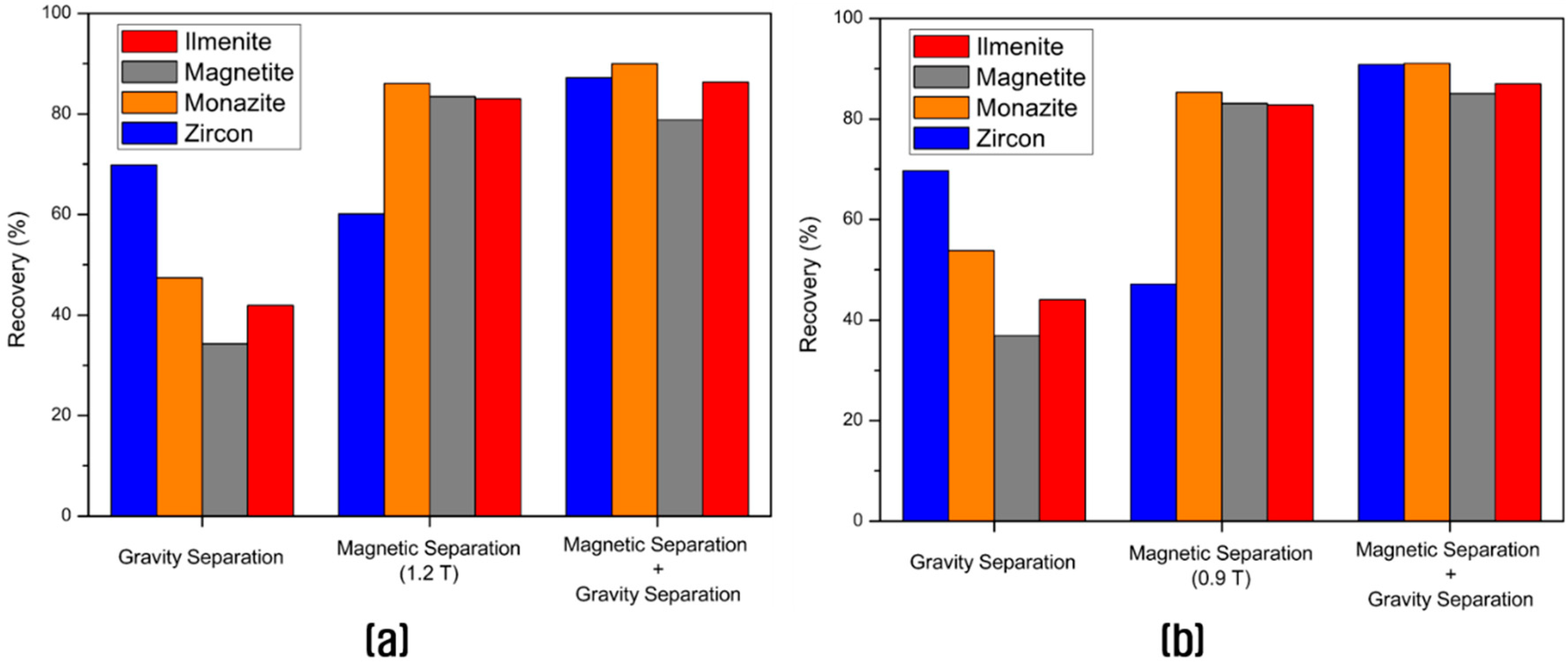
3.2. Concentration of the Valuable Heavy Minerals by Gravity Separation and Magnetic Separation
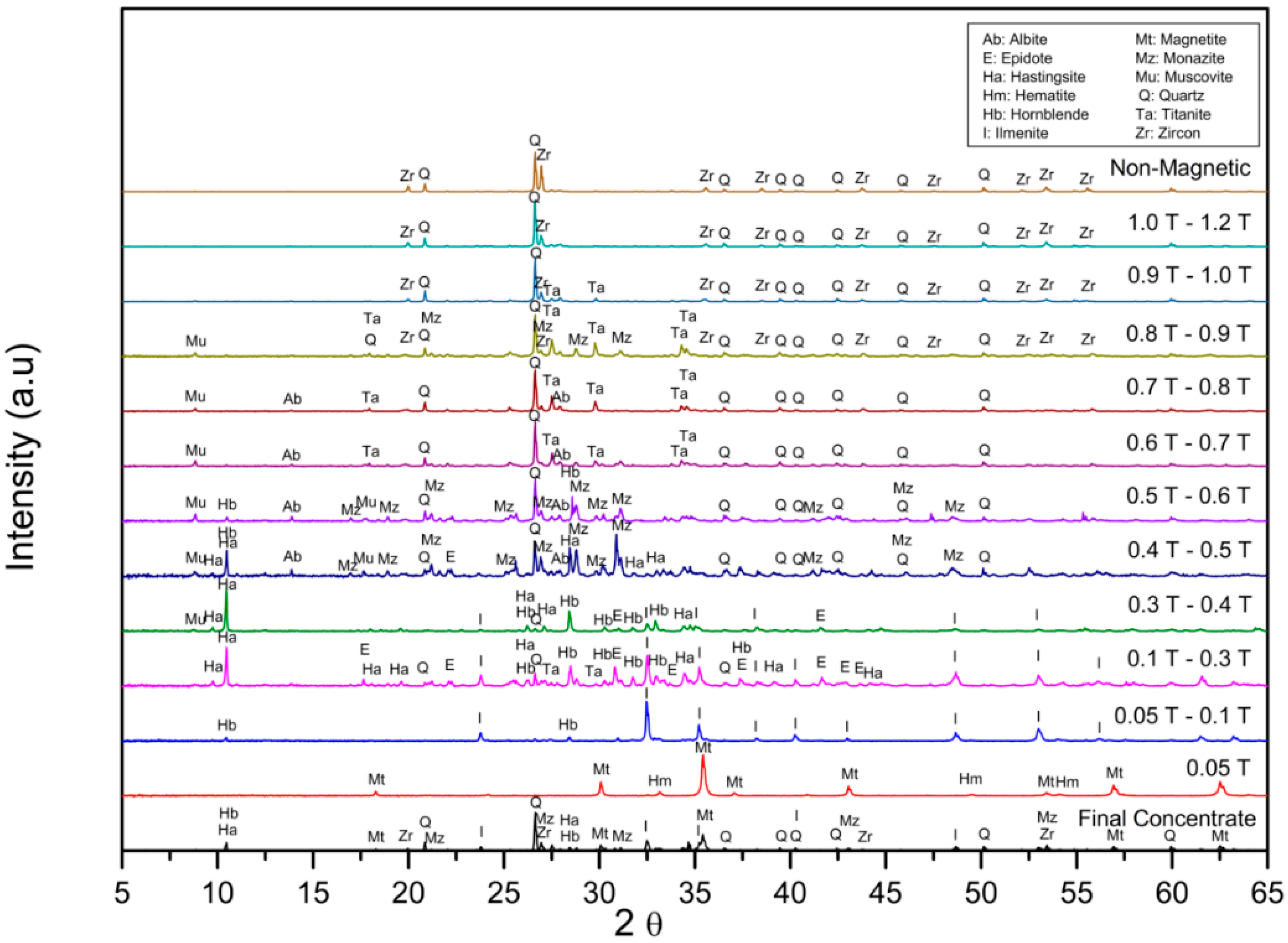
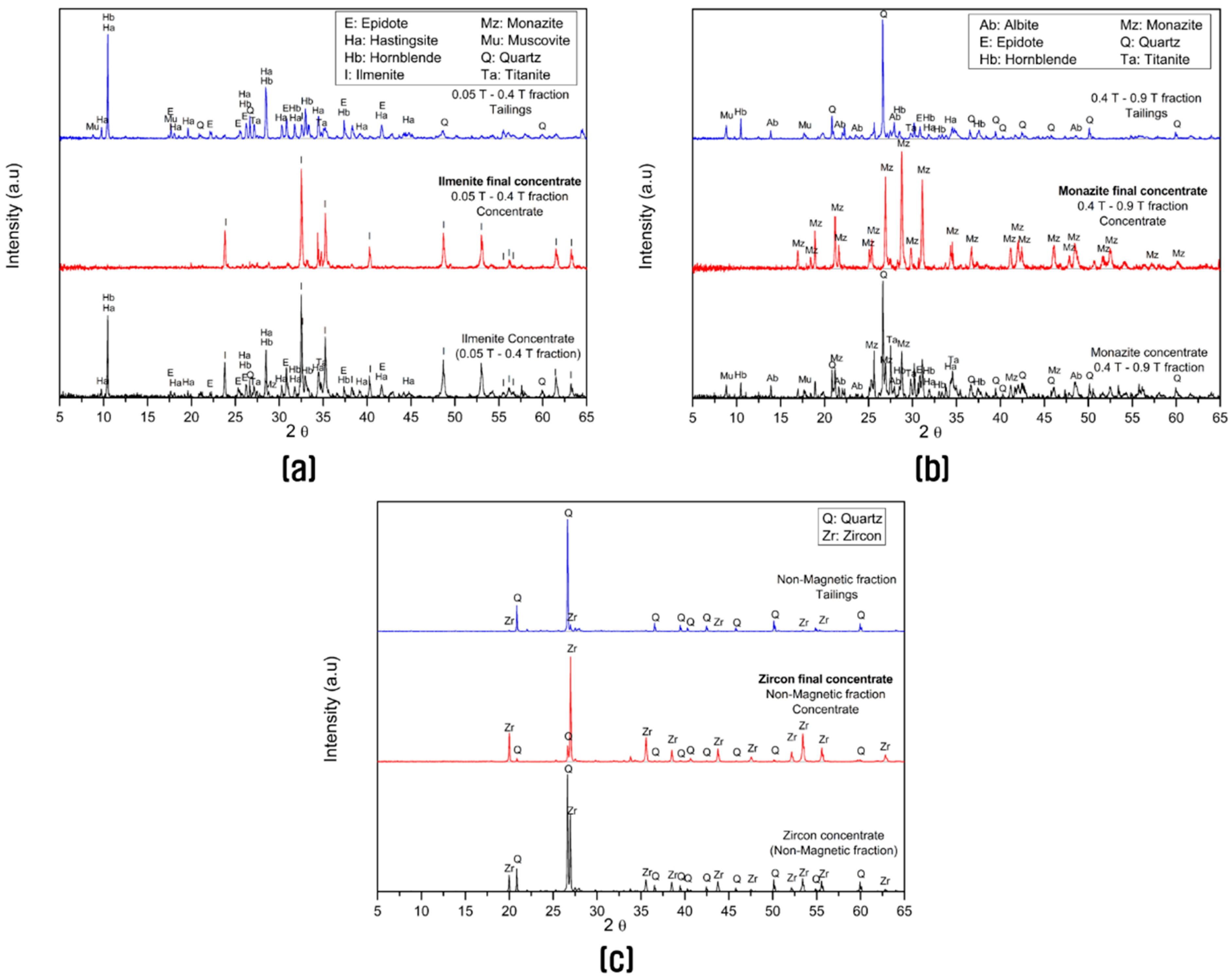
3.3. Separation of Each Valuable Heavy Mineral by Magnetic Separation at Different Magnetic Intensities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dehaine, Q.; Filippov, L.O.; Joussmet, R. Rare earths (La, Ce, Nd) and rare metals (Sn, Nb, W) as by-products of kaolin production—Part 2: Gravity processing of micaceous residues. Miner. Eng. 2017, 100, 200–210. [Google Scholar] [CrossRef]
- Schmid, M. Rare Earths in the Trade Dispute between the US and China: A Déjà Vu. Available online: https://www.intereconomics.eu/contents/year/2019/number/6/article/rare-earths-in-the-trade-dispute-between-the-us-and-china-a-deja-vu.html#footnote-032 (accessed on 17 December 2020).
- Bohlsen, M. The U.S. Rare Earth Saga Continues…. Available online: https://investorintel.com/markets/technology-metals/technology-metals-intel/the-rare-earths-state-of-the-market-july-2019/ (accessed on 17 December 2020).
- European Commission. Study on the Review of the List of Critical Raw Materials; European Commission: Brussels, Belgium, 2017. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van-Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Bank of Korea. 2019 Economic Statistics Yearbook; Bank of Korea: Seoul, Korea, 2019. [Google Scholar]
- Route One Communications Ltd. South Korea’s Infrastructure Development Is Key to Growth, Aggregates Business International. 2016. Available online: http://www.aggbusiness.com/sections/market-reports/features/south-koreas-infrastructure-development-is-key-to-growth/ (accessed on 10 January 2018).
- ASTM International. ASTM C 33-03: Standard specification for Concrete Aggregates. In Annual Book of ASTM Standards 2010; ASTM International: Philadelphia, PA, USA, 2010; pp. 1–9. [Google Scholar]
- Kosmatka, S.; Wilson, M. Chapter 6 Aggregates for concrete. In Design and Control of Concrete Mixtures, 15th ed.; Portland Cement Association: Skokie-Illinois, IL, USA, 2011; pp. 95–102. [Google Scholar]
- Rahman, M.A.; Pownceby, M.I.; Haque, N.; Bruckard, W.J.; Zaman, M.N. Valuable heavy minerals from Brahmaputra River sands if Northern Bangladesh. Appl. Earth Sci. 2016, 125, 174–188. [Google Scholar] [CrossRef]
- Abdel-Karim, A.A.; Barakat, M.G. Separation, upgrading, and mineralogy of placer magnetite in the black sands, northern coast of Egypt. Arab. J. Geosci. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Philander, C.; Rozendaal, A. A process mineralogy approach model refinement for the Namakwa Sands heavy minerals operations, west coast of South Africa. Miner. Eng. 2014, 65, 9–16. [Google Scholar] [CrossRef]
- Rejith, R.G.; Sundararajan, M. Combined magnetic, electrostatic, and gravity separation techniques for recovering strategic heavy minerals from beach sands. Mar. Georesources. Geotechnol. 2018, 35, 959–965. [Google Scholar] [CrossRef]
- Frihy, O.; Deabes, E.; Moufaddal, W.; El-Shahat, A. Recycling of coastal dredged sediments from the Northern Nile Delta, Egypt, for Heavy Minerals Exploitation. Mar. Georesources Geotechnol. 2015, 33, 408–418. [Google Scholar] [CrossRef]
- Babu, N.; Vasumathi, N.; Rao, R.B. Recovery of Ilmenite and Other Heavy Minerals from Teri Sands (Red Sands) of Tamil Nadu, India. JMMCE J. Miner. Mater. Charact. Eng. 2009, 8, 149–159. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, S. Separation of Monazite from Placer Deposit by Magnetic Separation. Minerals 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Jordens, A.; Sheridan, R.S.; Rowson, N.A.; Waters, K.R. Processing a rare earth mineral deposit using gravity and magnetic separation. Miner. Eng. 2014, 38, 9–18. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Langlois, R.; Grammatikopoulos, T.; Sheridan, R.S.; Teng, C.; Demers, H.; Gauvin, R.; Rowson, N.A.; Waters, K.E. Beneficiation of the Nechalacho rare earth deposit. Part 2: Characterisation of products from gravity and magnetic separation. Miner. Eng. 2016, 99, 96–110. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Makkonen, H.T.; Pakkanen, L. Rare Earth Occurrences in Streams of Processing a Phosphate Ore. Minerals 2019, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Zhai, J.; Chen, P.; Wang, H.; Hu, Y.; Sun, W. Floatability improvement of Ilmenite Using Attrition-Scrubbing as a Pretreatment method. Minerals 2017, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Iranmanesh, M.; Hulliger, J. Magnetic separation: Its application in mining, waste purification, medicine, biochemistry and chemistry. Chem. Soc. Rev. 2017, 46, 5925–5934. [Google Scholar] [CrossRef]
- Laxmi, T.; Behera, J.R.; Rao, R.B. Beneficiation Studies on Recovery of Ilmenite from Red Sediments of Badlands Topography, Andhra Pradesh. Adv. Sci. Lett. 2016, 22, 344–348. [Google Scholar] [CrossRef]
- Sullivan, G.V.; Browning, J.S. Recovery of heavy minerals from Alabama sand and gravel operations. In Bureau of Mines Nonmetallic Minerals Program Technical Progress Report-22: U.S. Department of the Interior; National Technical Information Service: Springfield, VA, USA, 1970. Available online: https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB190997.xhtml (accessed on 18 December 2020).
- ILUKA Resources Limited. Mineral Sands Industry Information. 2015, Updated 2019. Available online: https://www.iluka.com/CMSPages/GetFile.aspx?guid=bd24ecdc-5b71-4681-9340-87c85555cca5 (accessed on 18 December 2020).
- Wills, B.A.; Napier-Munn, T.J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 7th ed.; Elsevier Science & Technology Books: London, UK, 2006; ISBN 0750644508. [Google Scholar]
- Rosenblum, S.; Brownfield, I.K. Magnetic Susceptibilities of Minerals; US Geological Survey Open-File Report 99-529; US Department of the Interior: Washington, DC, USA, 2000. Available online: http://pubs.usgs.gov/of/1999/ofr-99-0529/ (accessed on 28 November 2020).
- Arvidson, B.R. Magnetic Separator Assembly for Use in Material Separator Equipment. U.S. Patent 5 101 980, 11 October 1990. Available online: https://www.google.ch/patents/US5101980 (accessed on 15 August 2020).
- Lee, Y.; Cho, M.; Yi, K. In situ U-Pb and Lu-Hf isotopic studies of zircon from the Sancheong-Hadong AMCG suite, Yeongnam Massig, Korea: Implications for the petrogenesis of ~1.86 Ga massif-type anorthosite. J. Asian Earth Sci. 2017, 138, 629–646. [Google Scholar] [CrossRef]
- Grammatikopoulos, W.M.; Gunning, C. Mineralogical characterization using QEMSCAM of the Nechalacho heavy rare earth metal deposit, Northwest Territories, Canada. Can. Metall. Q. 2013, 52, 265–277. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Langlois, R.; Grammatikopoulos, T.; Rowson, N.A.; Waters, K.E. Beneficiation of the Nechalacho rare earth deposit. Part 1: Gravity and magnetic separation. Miner. Eng. 2016, 99, 111–122. [Google Scholar] [CrossRef]
- Kato, Y. Mineralogical study of weathering products of granodiorite at Shinshiro City (III). J. Soil Sci. Plant Nutr. 1965, 11, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Korea Mineral Resources Information Services (KOMIS). The Prime, Precious Rare Important and Industrial metal Elements, Monthly Report, October 2017. Available online: https://www.kores.net/common/pdfPreview.do?fid=trendFile&file_seq=2690 (accessed on 10 October 2020).
- Kropáček, V.; Krs, M.; Janák, F. Magnetism of natural pyrrhotite, haematite and ilmenite. Stud. Geophys. Geod. 1971, 15, 161–172. [Google Scholar] [CrossRef]
- Patruni, B.M.; King, P.; Rao, J. Ilmenite Recovery from Beach sand minerals rejects using Dry Magnetic Separator (RED). IJESC 2016, 6, 7678–7692. [Google Scholar] [CrossRef]
- Abaka-Wood, G.; Addai-Mensah, J.; Skinner, W. Magnetic separation of monazite from mixed minerals. In Proceedings of the CHEMECA Conference, Adelaide, Australia, 25–28 September 2016; Engineers Australia: Melbourne, Australia, 2016; pp. 596–604. ISBN 9781922107831. [Google Scholar]


| Particle Size Distribution (µm) | Mass Fraction (wt%) | Total Heavy Mineral Content * (wt%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | MnO | P2O5 | ZrO2 | LOI ** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1000 + 600 | 8.92 | 0.06 | 88.67 | 4.99 | 0.44 | 0.27 | 0.13 | 3.41 | 0.86 | 0.07 | 0.01 | 0.02 | 0.00 | 0.77 |
| −600 + 300 | 10.62 | 0.24 | 85.74 | 6.68 | 1.14 | 0.45 | 0.28 | 3.19 | 1.15 | 0.17 | 0.02 | 0.04 | 0.01 | 0.95 |
| −300 + 150 | 12.06 | 1.26 | 77.17 | 11.19 | 2.54 | 0.91 | 0.63 | 3.42 | 1.79 | 0.36 | 0.04 | 0.07 | 0.04 | 1.60 |
| −150 + 75 | 34.19 | 4.48 | 76.13 | 11.57 | 2.74 | 1.04 | 0.76 | 3.33 | 2.05 | 0.40 | 0.04 | 0.08 | 0.08 | 1.59 |
| −75 + 43 | 19.93 | 6.60 | 75.41 | 11.57 | 2.96 | 1.30 | 0.90 | 2.87 | 2.21 | 0.65 | 0.05 | 0.11 | 0.15 | 1.61 |
| −43 | 14.29 | 1.51 | 73.13 | 12.05 | 3.75 | 1.42 | 1.03 | 2.59 | 2.18 | 0.99 | 0.07 | 0.15 | 0.22 | 2.36 |
| TOTAL (raw) | 100.0 | 3.25 | 77.82 | 10.49 | 2.53 | 1.00 | 0.70 | 3.14 | 1.87 | 0.48 | 0.04 | 0.09 | 0.09 | 1.56 |
| Mineral | Fraction (wt%) | Mineral | Fraction (wt%) |
|---|---|---|---|
| Ilmenite | 30.2 | Magnetite | 24.6 |
| Monazite | 1.3 | Hornblende | 21.7 |
| Zircon | 5.3 | Oxide | 5.1 |
| Muscovite | 0.2 | Phosphates | 1.4 |
| Quartz | 10.1 | Others | 0.2 |
| Mass Concentrate (wt%) | Grade (wt%) | Recovery (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ilmenite | Magnetite | Monazite * | Zircon | Ilmenite | Magnetite | Monazite | Zircon | ||
| Fine Aggregate Waste | 0.98 | 0.80 | 618.4 | 0.18 | |||||
| Humphrey spiral | 35.4 | 0.99 | 0.60 | 852.3 | 0.29 | 35.76 | 26.55 | 48.79 | 57.03 |
| Spiral + Shaking table | 11.5 | 1.85 | 0.80 | 2224.8 | 0.77 | 21.71 | 11.50 | 41.37 | 49.19 |
| Spiral (recirculation of middlings) | 36.9 | 1.17 | 0.80 | 901.0 | 0.34 | 44.05 | 36.90 | 53.76 | 69.70 |
| Spiral + Shaking table (recirculation of middlings) | 13.1 | 2.13 | 0.97 | 2142.9 | 0.83 | 28.47 | 15.88 | 45.39 | 60.41 |
| Magnetic Separation | 20.7 | 3.92 | 3.21 | 2548.6 | 0.41 | 82.80 | 83.06 | 85.31 | 47.15 |
| Magnetic Separation + Gravity Separation (see Figure 5) | 26.8 | 3.18 | 2.54 | 2101.2 | 0.61 | 86.96 | 85.09 | 91.06 | 90.82 |
| Magnetic Fraction | Fraction (wt%) | SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | MnO | P2O5 | ZrO2 | REO * |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 T | 17.86 | 1.7 | 0.38 | 88.54 | 0.36 | 3.31 | 0.00 | 0.13 | 0.13 | 0.00 |
| 0.05 T to 0.4 T | 40.71 | 22.85 | 9.64 | 29.19 | 6.22 | 26.28 | 1.24 | 0.60 | 0.11 | 1.43 |
| 0.4 T to 0.9 T | 10.00 | 35.41 | 13.10 | 4.44 | 7.71 | 5.37 | 0.11 | 6.71 | 0.63 | 19.50 |
| Non-magnetic | 31.43 | 58.00 | 7.02 | 0.88 | 2.68 | 3.37 | 0.04 | 1.00 | 23.92 | 3.63 |
| Final concentrate | 100.00 | 31.38 | 7.51 | 28.42 | 4.21 | 12.89 | 0.53 | 1.25 | 7.65 | 3.67 |
| Concentrate Fraction | Quartz | Hornblende | Ilmenite | Magnetite | REO * | Zircon |
|---|---|---|---|---|---|---|
| Final concentrate | 20.04 | 6.71 | 26.88 | 19.27 | 3.11 | 9.43 |
| Magnetite | 1.7 | 0.87 | 2.1 | 95.1 | 0 | 0 |
| Ilmenite | 7.59 | 3.58 | 84.21 | 0 | 2.03 | 0.36 |
| Monazite | 15.96 | 3.44 | 8.21 | 0 | 45.24 | 2.21 |
| Zircon | 4.3 | 1.86 | 1.05 | 0 | 1.88 | 91.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscoso-Pinto, F.; Kim, H.-S. Concentration and Recovery of Valuable Heavy Minerals from Dredged Fine Aggregate Waste. Minerals 2021, 11, 49. https://doi.org/10.3390/min11010049
Moscoso-Pinto F, Kim H-S. Concentration and Recovery of Valuable Heavy Minerals from Dredged Fine Aggregate Waste. Minerals. 2021; 11(1):49. https://doi.org/10.3390/min11010049
Chicago/Turabian StyleMoscoso-Pinto, Fausto, and Hyung-Seok Kim. 2021. "Concentration and Recovery of Valuable Heavy Minerals from Dredged Fine Aggregate Waste" Minerals 11, no. 1: 49. https://doi.org/10.3390/min11010049





