The Effect of Mechanical Vibration during Transport under Model Conditions on the Shelf-Life, Quality and Physico-Chemical Parameters of Four Apple Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Growth Conditions
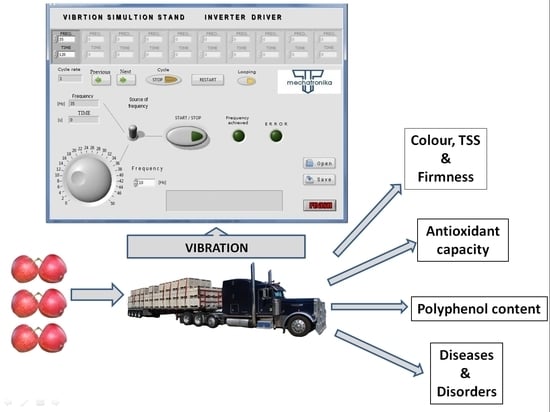
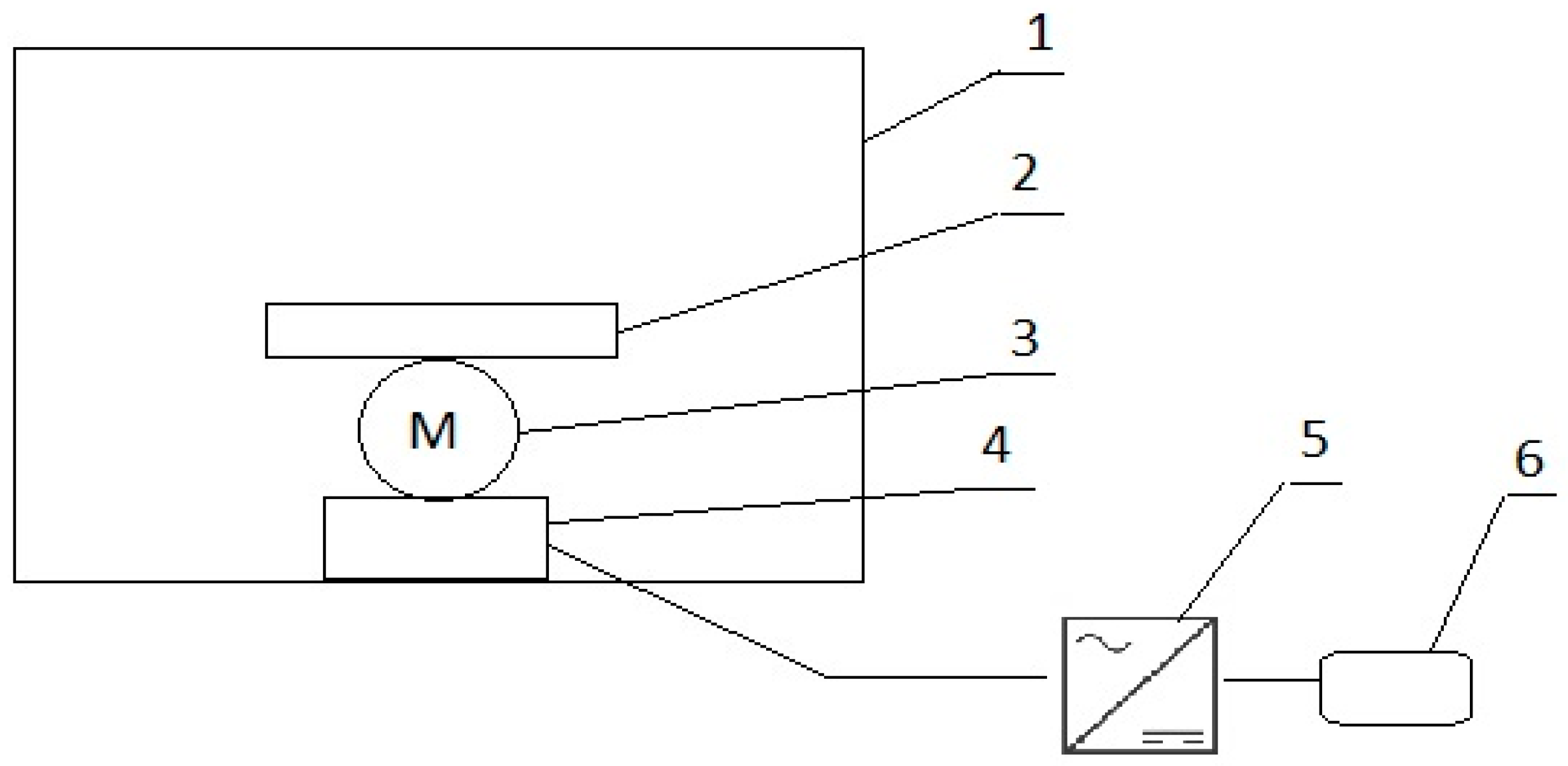
2.2. Test Stand
2.3. The Model System of Experiments
2.4. Analytical Methods
2.4.1. Colour Measurement
2.4.2. Firmness Measurement
2.4.3. Measurement of Total Soluble Solids
2.4.4. Measurement of Dry Matter Content
2.4.5. Measurement of pH Value
2.4.6. Measurement of Titratable Acidity
2.4.7. Measurement of Total Polyphenol Content
2.4.8. Measurement of Antioxidant Capacity
2.4.9. Physiological Assessment
2.4.10. Statistical Analysis
3. Results and Discussion
3.1. Colour
3.2. Firmness
3.3. Total Soluble Solids, Dry Matter, Titratable Acidity and pH Value
3.4. Polyphenol Content and Antioxidant Capacity
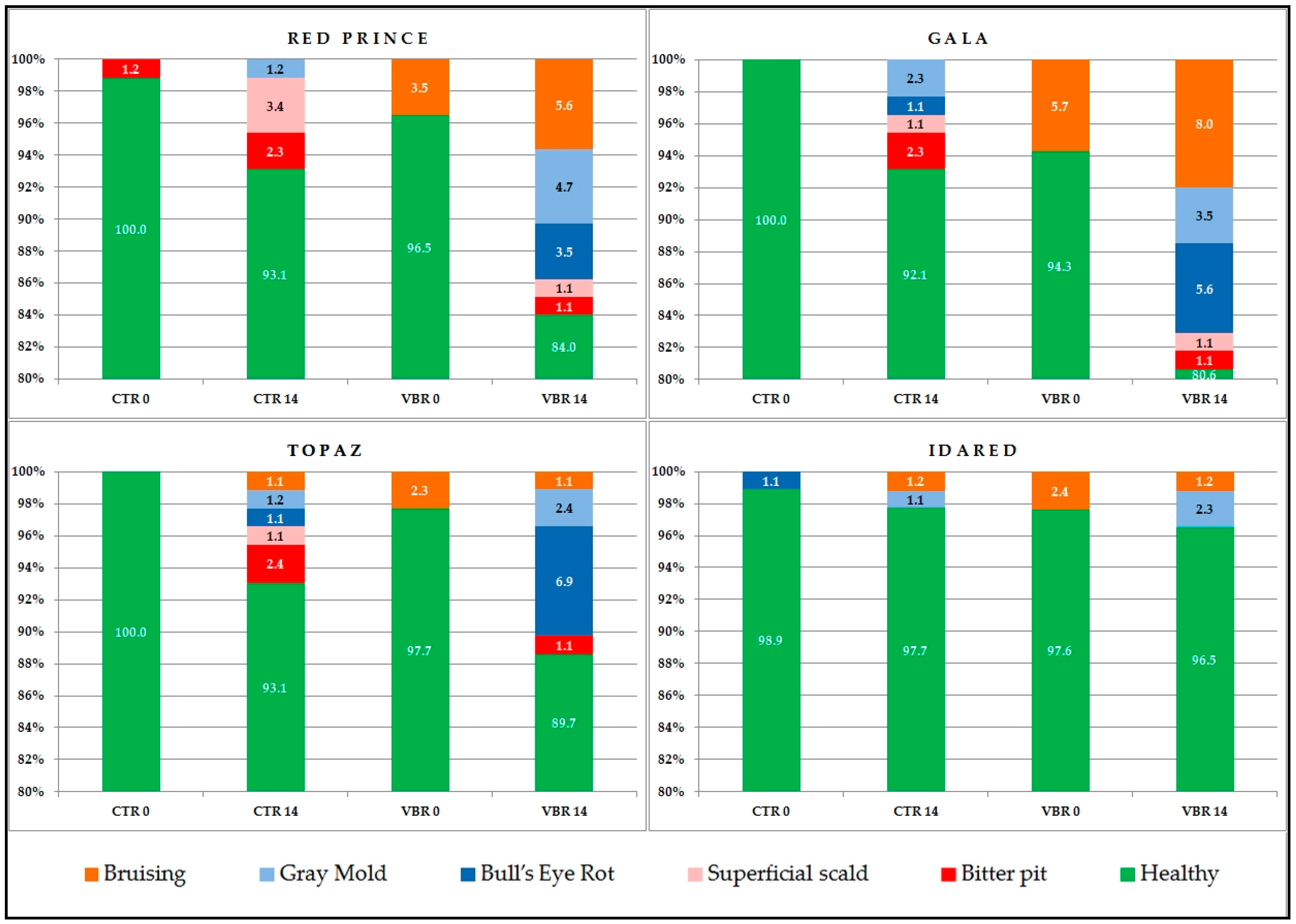
3.5. Changes Caused by Diseases and Disorders
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization. FAOSTAT Crops. Production Quantity. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 November 2020).
- Zhou, R.; Wang, X.; Hu, Y.; Zhang, G.; Yang, P.; Huang, B. Reduction in Hami melon (Cucumis melo var. saccharinus) softening caused by transport vibration by using hot water and shellac coating. Postharvest Biol. Technol. 2015, 110, 214–223. [Google Scholar] [CrossRef]
- USDA Fresh Apples, Grapes, and Pears: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/fruit.pdf (accessed on 13 November 2020).
- Łysiak, G. The influence of harvest maturity and basic macroelement content in fruit on the incidence of diseases and disorders after storage of the ‘Ligol’apple cultivar. Folia Hortic. 2013, 25, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Łysiak, G. The determination of harvest index of “Šampion” apples intended for long storage. Acta Sci. Pol. Hortorum Cultus 2011, 10, 273–282. [Google Scholar]
- Huang, S. Global Trade Patterns in Fruit and Vegetables. SSRN Electron. J. 2004. [Google Scholar] [CrossRef] [Green Version]
- Paternoster, A.; Vanlanduit, S.; Springael, J.; Braet, J. Vibration and shock analysis of specific events during truck and train transport of food products. Food Packag. Shelf Life 2018, 15, 95–104. [Google Scholar] [CrossRef]
- Jung, H.M.; Lee, S.; Lee, W.-H.; Cho, B.-K.; Lee, S.H. Effect of vibration stress on quality of packaged grapes during transportation. Eng. Agric. Environ. Food 2018, 11, 79–83. [Google Scholar] [CrossRef]
- Zhou, R.; Su, S.; Yan, L.; Li, Y. Effect of transport vibration levels on mechanical damage and physiological responses of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua). Postharvest Biol. Technol. 2007, 46, 20–28. [Google Scholar] [CrossRef]
- Paternoster, A.; Jaskula-Goiris, B.; De Causmaecker, B.; Vanlanduit, S.; Springael, J.; Braet, J.; De Rouck, G.; Cooman, L. The interaction effect between vibrations (50 Hz, 15m/s2) and temperature (5 °C, 30 °C, 45 °C), simulating truck transport, on the flavor stability of beer. J. Sci. Food Agric. 2018, 99. [Google Scholar] [CrossRef]
- Paternoster, A.; Springael, J.; Braet, J. Reducing postharvest losses of apples: Optimal transport routing (while minimizing total costs). Comput. Electron. Agric. 2018, 146. [Google Scholar] [CrossRef]
- Pason, N.L.; Timm, E.J.; Brown, G.; Marshall, D.; Burton, C. Apple Damage Assessment during Intrastate Transportation. Appl. Eng. Agric. 1990, 6, 753–758. [Google Scholar] [CrossRef]
- Timm, E.; Brown, G.; Armstrong, P. Apple Damage in Bulk Bins during Semi-trailer Transport. Appl. Eng. Agric. 1996, 12, 369–377. [Google Scholar] [CrossRef]
- Vursavuş, K.; Ozguven, F. Determining the Effects of Vibration Parameters and Packaging Method on Mechanical Damage in Golden Delicious Apples. Turk. J. Agric. For. 2004, 28, 311–320. [Google Scholar]
- Soleimani, B.; Ahmadi, E. Measurement and analysis of truck vibration levels as a function of packages locations in truck bed and suspension. Comput. Electron. Agric. 2014, 109, 141–147. [Google Scholar] [CrossRef]
- Zeebroeck, M.; Van Linden, V.; Ramon, H.; De Baerdemaeker, J.; Nicolaï, B.; Tijskens, E. Impact damage of apples during transport and handling. Postharvest Biol. Technol. 2007, 45, 157–167. [Google Scholar] [CrossRef]
- Kupferman, E. Minimizing bruising in apples. Postharvest Inf. Netw. Washingt. State Univ. 2006. Available online: https://postharvest.tfree.wsu.edu/EMK2006B.pdf (accessed on 3 September 2020).
- Studman, C.J.; Brown, G.K.; Timm, E.J.; Schulte, N.L.; Vreede, M.J. Bruising on blush and non-blush sides in apple-to-apple impacts. Trans. ASAE 1997, 40, 1655–1663. [Google Scholar] [CrossRef]
- Pang, W.; Studman, C.J.; Ward, G.T. Bruising damage in apple-to-apple impact. J. Agric. Eng. Res. 1992, 52, 229–240. [Google Scholar] [CrossRef]
- Singh, S.; Xu, M. Bruising in Apples as a Function of Truck Vibration and Packaging. Appl. Eng. Agric. 1993, 9, 455–460. [Google Scholar] [CrossRef]
- Kellerhals, M.; Szalatnay, D.; Hunziker, K.; Duffy, B.; Nybom, H.; Ahmadi-Afzadi, M.; Höfer, M.; Richter, K.; Lateur, M. European pome fruit genetic resources evaluated for disease resistance. Trees 2012, 26, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Łysiak, G.P.; Michalska-Ciechanowska, A.; Wojdyło, A. Postharvest changes in phenolic compounds and antioxidant capacity of apples cv. Jonagold growing in different locations in Europe. Food Chem. 2020, 310, 125912. [Google Scholar] [CrossRef] [PubMed]
- Peršić, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT Food Sci. Technol. 2017, 82. [Google Scholar] [CrossRef]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a Source of Dietary Phytonutrients: Bioavailability and Evidence of Protective Effects against Human Cardiovascular Disease. Food Nutr. Sci. 2014, 5, 1234–1246. [Google Scholar] [CrossRef] [Green Version]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.; Fei, J.; Stanley, R.; Enshaei, H. Measurement and evaluation of the effect of vibration on fruits in transit—Review. Packag. Technol. Sci. 2018, 31, 723–738. [Google Scholar] [CrossRef]
- Ctifl (Centre Technique Interprofessionnel des Fruits et Légumes) Code Amidon Pomme (Starch Conversion Chart for Apples). Available online: http://www.ctifl.fr/Pages/Kiosque/DetailsOuvrage.aspx?idouvrage=819 (accessed on 13 November 2020).
- Łysiak, G. The sum of active temperatures as a method of determining the optimum harvest date of “Šampion” and “Ligol” apple cultivars. Acta Sci. Pol. Hortorum Cultus 2012, 11, 3–13. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). International Standards for Fruits and Vegetables, APPLES. Available online: https://read.oecd-ilibrary.org/agriculture-and-food/apples_9789264088795-en-fr#page11 (accessed on 5 December 2020).
- Walkowiak-Tomczak, D.; Idaszewska, N.; Bieńczak, K.; Kómoch, W. The Effect of Mechanical Actions Occurring during Transport on Physicochemical Changes in Agaricus bisporus Mushrooms. Sustainability 2020, 12, 4993. [Google Scholar] [CrossRef]
- Idaszewska, N.; Szymański, G.M. Identification of characteristic vibration signal parameters during transport of fruit and vegetable. Vib. Phys. Syst. 2020, 31, 2020111-1–2020111-10. [Google Scholar]
- CIE Recommendations on Uniform Color Spaces, Color-Difference Equations, and Metric Color Terms. Color Res. Appl. 1976, 2, 5–6. [CrossRef]
- Mieszkalska, A.; Piotrowski, D. Wykorzystanie modeli barwy do oceny suszonych surowców roślinnych. Postępy Tech. Przetwórstwa Spożywczego 2014, 2, 105–111. [Google Scholar]
- Wang, Y.; Zhao, H.; Deng, H.; Song, X.; Zhang, W.; Wu, S.; Wang, J. Influence of Pretreatments on Microwave Vacuum Drying Kinetics, Physicochemical Properties and Sensory Quality of Apple Slices. Pol. J. Food Nutr. Sci. 2019, 69, 297–306. [Google Scholar] [CrossRef]
- International Organization for Standardization. Fruit and Vegetable Products—Determination of Dry Matter Content by Drying under Reduced Pressure and of Water Content by Azeotropic Distillation; ISO: Geneva, Switzerland, 1982. [Google Scholar]
- EN 12147:1996. Fruit and Vegetable Juices—Determination of Titrable Acidity; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- Fang, Z.; Zhang, M.; Sun, Y.; Sun, J. How To Improve Bayberry (Myrica rubra Sieb. et Zucc.) Juice Color Quality: Effect of Juice Processing on Bayberry Anthocyanins and Polyphenolics. J. Agric. Food Chem. 2006, 54, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Łysiak, G.; Kurlus, R.; Zydlik, Z.; Walkowiak-Tomczak, D. Apple skin colour changes during harvest as an indicator of maturity. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–83. [Google Scholar]
- Sypuła, M.; Pietron, A.; Klonowski, J.; Strużyk, A. Changes in qualitative characteristics of apples stored in modified atmosphere packaging. In Proceedings of the Conference: 17th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 23–25 May 2018. [Google Scholar]
- Kupferman, E. Controlled atmosphere storage regimes for apples and pears. Acta Hortic. 2003, 600, 729–735. [Google Scholar] [CrossRef]
- Lafer, G. Storability and fruit quality of “Golden Delicious” as affected by harvest date, AVG and 1-MCP treatments. J. Fruit Ornam. Plant Res. 2006, 14, 203–212. [Google Scholar]
- Jan, I.; Rab, A.; Bhutta, M.; Ali, A.; Jan, C. Response Response of apple cultivars to different storage durations. Sarhad J. Agric. Pak. 2012, 28, 219–225. [Google Scholar]
- Amer Eissa, A.; Albaloushi, N.; Azam, M. Vibration analysis influence during crisis transport of the quality of fresh fruit on food security. Agric. Eng. Int. CIGR J. 2013, 15, 181–189. [Google Scholar]
- Madalina, M.; Butac, M.; Popescu, G.C.; Costinel, B.; Cosmina, S. Influence of storage duration on apple fruit quality. Fruit Grow. Res. 2016, XXXII, 86–92. [Google Scholar]
- Chaiwong, S.; Bishop, C. Effect of vibration damage on the storage quality of ‘Elsanta’ strawberry. Aust. J. Crop Sci. 2015, 9, 859–864. [Google Scholar]
- Kojima, T.; Liu, J.Y.; Fujita, S.; Inaba, S. Analysis of vibration and its effects on strawberries during highway transport. J. Soc. Agric. Struct. 2001, 80, 23–29. [Google Scholar]
- Rop, O.; Jurikova, T.; Sochor, J.; Mlcek, J.; Kramarova, D. Antioxidant capacity, scavenging radical activity and selected chemical composition of native apple cultivars from Central Europe. J. Food Qual. 2011, 34, 187–194. [Google Scholar] [CrossRef]
- Fischer, D.; Craig, W.L.; Watada, A.E.; Douglas, W.; Ashby, B.A. Simulated In-Transit Vibration Damage to Packaged Fresh Market Grapes and Strawberries. Appl. Eng. Agric. 1992, 8, 363–366. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhu, C.; Han, Z. Effects of aqueous chlorine dioxide treatment on nutritional components and shelf-life of mulberry fruit (Morus alba L.). J. Biosci. Bioeng. 2011, 111, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Cascone, A.; Graziani, G.; Ferracane, R.; Scalfi, L.; Di Vaio, C.; Ritieni, A.; Fogliano, V. Influence of Variety and Storage on the Polyphenol Composition of Apple Flesh. J. Agric. Food Chem. 2004, 52, 6526–6531. [Google Scholar] [CrossRef]
- Łysiak, G. Measurement of ethylene production as a method for determining the optimum harvest date of ‘Jonagored’ apples. Folia Hortic. 2014, 26, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Salvador, A.; Arnal, L.; Monterde, A.; Cuquerella, J. Reduction of chilling injury symptoms in persimmon fruit cv. ‘Rojo Brillante’ by 1-MCP. Postharvest Biol. Technol. 2004, 33, 285–291. [Google Scholar] [CrossRef]
- ZhiGuo, L.; Thomas, C. Quantitative evaluation of mechanical damage to fresh fruits. Trends Food Sci. Technol. 2014, 35, 138–150. [Google Scholar] [CrossRef]
- Matthes, A.; Schmitz-Eiberger, M. Polyphenol content and antioxidant capacity of apple fruit: Effect of cultivar and storage conditions. J. Appl. Bot. Food Qual. Bot. 2009, 82, 152–157. [Google Scholar]
- Radenkovs, V.; Juhnevica-Radenkova, K. Effect of storage technology on the chemical composition of apples of the cultivar “Auksis”. Zemdirbyste Agricult. 2017, 104, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Gastélum, J.A.; Alvarez-Parrilla, E.; De la Rosa, L.; Martinez-Ruiz, N.; Aguilar, G.; Rodrigo-Garcia, J. Effect of harvest date and storage duration on chemical composition, sugar and phenolic profile of ‘Golden Delicious’ apples from northwest Mexico. N. Z. J. Crop Hortic. Sci. 2015, 43. [Google Scholar] [CrossRef]
- Duda-Chodak, A.A.D.; Tarko, T.; Tuszyński, T. Antioxidant activity of apples—An impact of maturity stage and fruit part. Acta Sci. Pol. Technol. Aliment. 2011, 10, 443–454. [Google Scholar] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef] [PubMed]
- María Ruiz-Rodríguez, B.; de Ancos, B.; Sánchez-Moreno, C.; Fernández-Ruiz, V.; de Cortes Sánchez-Mata, M.; Cámara, M.; Tardío, J. Wild blackthorn (Prunus spinosa L.) and hawthorn (Crataegus monogyna Jacq.) fruits as valuable sources of antioxidants. Fruits 2014, 69, 61–73. [Google Scholar] [CrossRef]
- Stanger, M.C.; Steffens, C.A.; Soethe, C.; Moreira, M.A.; do Amarante, C.V.T.; Both, V.; Brackmann, A. Phenolic compounds content and antioxidant activity of ‘Galaxy’ apples stored in dynamic controlled atmosphere and ultralow oxygen conditions. Postharvest Biol. Technol. 2018, 144, 70–76. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef] [Green Version]
- Korićanac, A.; Miletić, N.; Popović, B.; Mitrović, O.; Lukić, M.; Pešaković, M.; Tomić, J. The Effect of ULO and NA Storage on Changes in the Quality of Apple Fruit (Malus domestica Borkh.) during Shelf Life. Agronomy 2019, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Bryk, H.; Rutkowski, K.P. Efficacy of alternative methods in controlling of bull’s eye rot (Pezicula spp.). Prog. Plant Prot. 2012, 52, 727–732. [Google Scholar] [CrossRef]
- Stow, J. Storage of ‘Jonagold’ apples. Sci. Hortic. 1987, 31, 245–251. [Google Scholar] [CrossRef]

| Cultivar | Treatment | L* | a* | b* | C* | h | ΔE |
|---|---|---|---|---|---|---|---|
| ‘Gala’ | Ctr 0 1 | 68.5 ± 4.13 ab | 7.55 ± 6.18 b | 33.1 ± 1.83 b | 34.5 ± 1.27 b | 77.2 ± 10.56 b | |
| Vbr 0 2 | 69.2 ± 1.62 ab | 4.39 ± 2.78 a | 41.3 ± 2.22 c | 31.4 ± 2.09 a | 78.5 ± 4.15 b | 8.82 | |
| Ctr 14 3 | 66.9 ± 4.38 a | 7.86 ± 8.11 b | 28.0 ± 5.32 a | 30.4 ± 3.15 a | 73.1 ± 17.49 a | 5.37 | |
| Vbr 14 4 | 69.8 ± 5.46 ab | 5.31 ± 6.53 a | 29.2 ± 4.11 a | 30.1 ± 3.36 a | 79.0 ± 13.83 b | 4.63 | |
| ‘Idared’ | Ctr 0 | 73.5 ± 3.35 b | −5.25 ± 4.17 a | 34.9 ± 2.60 b | 35.5 ± 2.77 b | 98.2 ± 6.88 b | |
| Vbr 0 | 69.4 ± 3.65 a | 2.91 ± 5.52 b | 30.3 ± 2.28 a | 31.0 ± 1.38 a | 84.1 ± 10.99 a | 10.22 | |
| Ctr 14 | 71.8 ± 5.56 ab | 1.01 ± 5.53 b | 30.3 ± 2.33 a | 30.9 ± 1.28 a | 87.7 ± 11.30 a | 7.93 | |
| Vbr 14 | 71.6 ± 3.10 ab | −2.79 ± 2.49 a | 36.9 ± 1.84 c | 37.1 ± 1.91 c | 94.2 ± 3.83 b | 3.69 | |
| ‘Topaz’ | Ctr 0 | 65.4 ± 2.94 a | 13.86 ± 5.11 b | 35.2 ± 3.47 a | 38.3 ± 1.68 a | 68.2 ± 8.93 a | |
| Vbr 0 | 65.4 ± 5.66 a | 13.22 ± 1.12 ab | 36.7 ± 5.24 ab | 40.5 ± 1.75 b | 69.7 ± 16.16 ab | 1.64 | |
| Ctr 14 | 66.5 ± 2.72 a | 12.32 ± 4.49 ab | 37.2 ± 3.34 ab | 39.5 ± 1.95 ab | 71.4 ± 7.65 ab | 2.76 | |
| Vbr 14 | 66.9 ± 3.12 a | 8.98 ± 4.62 a | 38.5 ± 4.51 b | 40.0 ± 3.10 b | 76.4 ± 8.48 b | 6.10 | |
| ‘Red Prince’ | Ctr 0 | 62.1 ± 7.47 a | 15.22 ± 11.74 a | 29.4 ± 6.05 a | 35.4 ± 2.85 b | 62.9 ± 20.79 a | |
| Vbr 0 | 60.6 ± 2..90 a | 12.73 ± 7.49 a | 26.1 ± 2.91 a | 30.0 ± 2.88 a | 64.7 ± 14.36 a | 4.34 | |
| Ctr 14 | 62.5 ± 7.77 a | 12.82 ± 12.57 a | 28.6 ± 5.39 a | 34.0 ± 2.64 b | 66.4 ± 22.87 a | 2.57 | |
| Vbr 14 | 61.6 ± 5.21 a | 9.30 ± 5.38 a | 28.6 ± 4.22 a | 30.6 ± 3.84 a | 71.9 ± 10.84 a | 5.99 |
| Cultivar | Treatment | L* | a* | b* | C* | h | ΔE |
|---|---|---|---|---|---|---|---|
| ‘Gala’ | Ctr 0 | 77.1 ± 1.13 a | −2.33 ± 0.55 b | 23.1 ± 1.65 bc | 23.3 ± 1.67 bc | 95.7 ± 1.22 a | |
| Vbr 0 | 77.1 ± 0.47 a | −2.82 ± 0.86 ab | 22.2 ± 1.17 a | 22.4 ± 1.09 a | 97.3 ± 2.49 bc | 1.11 | |
| Ctr 14 | 78.0 ± 0.87 b | −3.01 ± 0.86 a | 22.3 ± 0.81 ab | 22.5 ± 0.84 ab | 97.6 ± 2.12 c | 1.42 | |
| Vbr 14 | 76.9 ± 2.05 a | −2.51 ± 1.16 ab | 23.8 ± 1.45 c | 23.9 ± 1.40 c | 96.1 ± 2.81 ab | 0.73 | |
| ‘Idared’ | Ctr 0 | 79.3 ± 1.04 a | −3.07 ± 0.25 ab | 15.7 ± 0.72 b | 16.1 ± 0.75 b | 101.2 ± 0.51 b | |
| Vbr 0 | 79.5 ± 1.64 a | −2.79 ± 0.27 c | 14.2 ± 1.49 a | 14.4 ± 1.50 a | 101.2 ± 0.71 b | 1.62 | |
| Ctr 14 | 79.0 ± 0.65 a | −2.95 ± 0.50 bc | 15.8 ± 1.39 b | 16.0 ± 1.45 b | 100.5 ± 1.01 a | 0.30 | |
| Vbr 14 | 79.2 ± 0.55 a | −3.21 ± 0.21 a | 16.6 ± 0.92 c | 16.9 ± 0.94 c | 101.0 ± 0.35 ab | 0.83 | |
| ‘Topaz’ | Ctr 0 | 74.9 ± 1.62 ab | −0.06 ± 0.58 b | 25.5 ± 1.44 a | 25.5 ± 1.45 a | 88.7 ± 1.26 a | |
| Vbr 0 | 74.5 ± 1.47 a | −1.12 ± 0.35 a | 24.9 ± 1.77 a | 24.9 ± 1.77 a | 92.6 ± 0.87 c | 1.27 | |
| Ctr 14 | 75.4 ± 0.87 b | −0.09 ± 0.63 b | 25.2 ± 1.40 a | 25.2 ± 1.40 a | 90.2 ± 1.43 b | 0.57 | |
| Vbr 14 | 75.5 ± 0.64 b | −0.12 ± 0.30 b | 25.4 ± 1.13 a | 25.4 ± 1.13 a | 90.3 ± 0.68 b | 0.60 | |
| ‘Red Prince’ | Ctr 0 | 75.0 ± 1.19 a | −1.89 ± 0.51 b | 24.1 ± 1.77 a | 24.2 ± 1.74 a | 94.6 ± 1.41 a | |
| Vbr 0 | 75.8 ± 0.68 b | −1.75 ± 0.54 b | 24.4 ± 1.54 a | 24.4 ± 1.57 a | 94.1 ± 1.06 a | 0.84 | |
| Ctr 14 | 75.7 ± 0.68 b | −2.43 ± 0.46 a | 23.6 ± 1.47 a | 23.7 ± 1.44 a | 95.9 ± 1.38 b | 1.03 | |
| Vbr 14 | 76.1 ± 1.09 b | −2.11 ± 0.79 a | 25.8 ± 2.02 b | 26.0 ± 2.04 b | 96.9 ± 2.27 b | 2.02 |
| Cultivar | Firmness [N] | |||
|---|---|---|---|---|
| Control 0 | Vibration 0 | Control 14 | Vibration 14 | |
| ‘Gala’ | 58.6 ± 2.90 b | 53.0 ± 2.70 a | 52.3 ± 3.72 a | 48.4 ± 5.34 a |
| ‘Idared’ | 45.3 ± 5.06 b | 41.5 ± 2.24 a,b | 41.7 ± 2,41 a,b | 39.5 ± 2,81 a |
| ‘Topaz’ | 52.8 ± 1.80 b | 50.1 ± 2.50 a,b | 49.6 ± 3.64 a,b | 47.6 ± 2.20 a |
| ‘Red Prince’ | 53.2 ± 1.63 a | 52.9 ± 1.72 a | 51.4 ± 1.97 a | 49.5 ± 6.10 a |
| Storage Time | Sample | Cultivar | ||||
|---|---|---|---|---|---|---|
| Gala | Idared | Topaz | Red Prince | |||
| TSS [%] | 0 | control | 13.41 ± 0.57 a | 12.81 ± 0.84 a | 15.89 ± 0.70 b | 14.32 ± 0.42 a |
| vibration | 14.40 ± 0.69 bc | 13.32 ± 0.44 a | 14.49 ± 1.02 a | 14.40 ± 0.36 a | ||
| 14 | control | 14.72 ± 0.35 c | 13.11 ± 0.37 a | 15.53 ± 0.52 ab | 14.14 ± 0.60 a | |
| vibration | 13.81 ± 0.52 ab | 13.29 ± 0.48 a | 14.88 ± 0.47 a | 14.49 ± 0.22 a | ||
| Dry matter [%] | 0 | control | 13.85 ± 0.74 a | 13.65 ± 1.10 a | 15.96 ± 0.46 a | 14.93 ± 0.91 a |
| vibration | 14.41 ± 0.66 a | 14.15 ± 0.73 a | 15.22 ± 0.66 a | 15.04 ± 0.55 a | ||
| 14 | control | 15.48 ± 0.51 a | 14.10 ± 0.42 a | 16.37 ± 0.46 a | 14.13 ± 1.41 a | |
| vibration | 15.37 ± 1.19 a | 14.49 ± 0.78 a | 15.51 ± 0.84 a | 15.05 ± 0.20 a | ||
| pH value | 0 | control | 4.10 ± 0.11 b | 3.78 ± 0.14 c | 3.54 ± 0.12 b | 3.80 ± 0.05 bc |
| vibration | 3.92 ± 0.05 a | 3.39 ± 0.05 a | 3.40 ± 0.06 b | 3.94 ± 0.08 c | ||
| 14 | control | 3.87 ± 0.06 a | 3.59 ± 0.06 b | 3.04 ± 0.07 a | 3.79 ± 0.11 b | |
| vibration | 4.06 ± 0.06 b | 3.72 ± 0.05 bc | 3.46 ± 0.09 b | 3.59 ± 0.06 a | ||
| TA [g/L] | 0 | control | 2.59 ± 0.15 ab | 3.13 ± 0.20 a | 6.34 ± 0.08 ab | 3.17 ± 0.28 a |
| vibration | 2.72 ± 0.08 b | 4.33 ± 0.08 c | 6.61 ± 0.08 b | 3.08 ± 0.13 a | ||
| 14 | control | 2.77 ± 0.15 b | 3.71 ± 0.08 b | 6.81 ± 0.20 b | 3.39 ± 0.08 a | |
| vibration | 2.37 ± 0.08 a | 3.84 ± 0.08 b | 6.21 ± 0.08 a | 4.24 ± 0.08 b | ||
| Storage Time | Sample | Cultivar | ||||
|---|---|---|---|---|---|---|
| Gala | Idared | Topaz | Red Prince | |||
| TPC [mg/100 g d.m] | 0 | control | 479 ± 56.0 a | 517.1 ± 33.4 a | 763.8 ± 39.3 a | 596.6 ± 67.8 a |
| vibration | 486 ± 9.7 ab | 549.8 ± 21.9 b | 824.7 ± 85.0 b | 620.7 ± 56.4 ab | ||
| 14 | control | 517 ± 11.7 bc | 563.1 ± 36.6 b | 802.5 ± 30.2 ab | 664.9 ± 21.5 bc | |
| vibration | 540 ± 36.8 c | 564.2 ± 20.8 b | 840.3 ± 23.2 b | 683.5 ± 20.1 c | ||
| Storage Time | Sample | Cultivar | ||||
|---|---|---|---|---|---|---|
| Gala | Idared | Topaz | Red Prince | |||
| Antioxidant capacity [µmol Trolox/g d.m.] | 0 | control | 32.6 ± 3.8 a | 35.2 ± 2.3 a | 51.9 ± 2.7 a | 40.6 ± 4.6 a |
| vibration | 33.4 ± 0.7 a | 37.7 ± 1.5 bc | 56.6 ± 5.8 b | 42.6 ± 3.8 ab | ||
| 14 | control | 34.2 ± 0.8 a | 37.2 ± 2.4 b | 52.9 ± 2.0 a | 43.9 ± 1.4 b | |
| vibration | 37.7 ± 2.6 b | 39.4 ± 1.45 c | 58.7 ± 1.6 b | 47.7 ± 1.4 c | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkowiak-Tomczak, D.; Idaszewska, N.; Łysiak, G.P.; Bieńczak, K. The Effect of Mechanical Vibration during Transport under Model Conditions on the Shelf-Life, Quality and Physico-Chemical Parameters of Four Apple Cultivars. Agronomy 2021, 11, 81. https://doi.org/10.3390/agronomy11010081
Walkowiak-Tomczak D, Idaszewska N, Łysiak GP, Bieńczak K. The Effect of Mechanical Vibration during Transport under Model Conditions on the Shelf-Life, Quality and Physico-Chemical Parameters of Four Apple Cultivars. Agronomy. 2021; 11(1):81. https://doi.org/10.3390/agronomy11010081
Chicago/Turabian StyleWalkowiak-Tomczak, Dorota, Natalia Idaszewska, Grzegorz P. Łysiak, and Krzysztof Bieńczak. 2021. "The Effect of Mechanical Vibration during Transport under Model Conditions on the Shelf-Life, Quality and Physico-Chemical Parameters of Four Apple Cultivars" Agronomy 11, no. 1: 81. https://doi.org/10.3390/agronomy11010081
APA StyleWalkowiak-Tomczak, D., Idaszewska, N., Łysiak, G. P., & Bieńczak, K. (2021). The Effect of Mechanical Vibration during Transport under Model Conditions on the Shelf-Life, Quality and Physico-Chemical Parameters of Four Apple Cultivars. Agronomy, 11(1), 81. https://doi.org/10.3390/agronomy11010081