Abstract
The present study was designed to investigate the in vitro antiproliferative activity against ten human cancer cell lines of a series of galloyl derivatives bearing substituted-1,3,4-oxadiazole and carbohydrazide moieties. The compounds were also assessed in an in silico study of the absorption, distribution, metabolism and excretion (ADME) in the human body using Lipinski’s parameters, the topological polar surface area (TPSA) and percentage of absorption (%ABS). In general, the introduction of N'-(substituted)-arylidene galloyl hydrazides 4–8 showed a moderate antitumor activity, while the 2-methylthio- and 2-thioxo-1,3,4-oxadiazol-5-yl derivatives 9 and 10 led to increased inhibition of cancer cell proliferation. The precursor compound methyl gallate 2 and the intermediary galloyl hydrazide 3 showed greater antiproliferative activity with GI50 values < 5.54 µM against all human tumor cell lines tested. A higher inhibition effect against ovarian cancer (OVCAR-3) (GI50 = 0.05–5.98 µM) was also shown, with compounds 2, 3, 9 and 10 with GI50 ≤ 0.89 µM standing out in this respect. The in silico study revealed that the compounds showed good intestinal absorption.
1. Introduction
Gallic acid (3,4,5-trihydroxybenzoic acid) is a polyphenol that possesses a wide spectrum of important pharmacological properties. In particular, gallic acid affects several pharmacological and biochemical pathways and has strong antioxidant [1,2], anti-inflammatory [3], antimutagenic and anticancer properties [4,5,6], showing selective cytotoxicity against a variety of tumor cells and much less toxicity against normal cells [7,8,9,10,11,12,13,14,15].
Studies with a variety of synthetic galloyl derivatives have demonstrated the influence of substituents on cytotoxic activity, e.g., digalloylresveratrol shows induction of apoptosis and necrosis in HT-29 colon cancer cells and HL-60 human promyelocytic leukemia cells, and gallic acid esters show inhibition of cancer cell proliferation [14,16,17,18,19,20,21,22,23]. Appropriate substituents in the galloyl group positions could lead to more potent compounds.
The isolation of methyl gallate from the methanol extract of Schinus terebinthifolius leaves by our research group (data not shown) and the biological potential of the galloyl group directed us to develop derivatives as antitumor agents. Mannich bases of oxadiazoles and carbohydrazides possess important activities, including anticancer functions [24,25,26,27] and our prior studies showed that introducing 1,3,4-oxadiazole and carbohydrazide units at the C-3 position of the β-carboline derivatives led to significant antitumor action [28,29,30]. As such, we proposed the synthesis and in vitro antiproliferative activity evaluation of a series of galloyl derivatives bearing the substituted-1,3,4-oxadiazole and carbohydrazide moiety at position C-1, expecting that the incorporation of these substituent may improve the antitumor activities of the galloyl group. Herein, the synthesis of 2-methylthio- and 2-thioxo-1,3,4-oxadiazol-5-yl derivatives is reported for the first time. Additionally, an in silico study of the absorption, distribution, metabolism, and excretion (ADME) properties of the compounds was performed by investigating their match of Lipinski’s rules, topological polar surface area (TPSA) and percentage of absorption (%ABS). In silico ADME is currently used widely to determine whether it is possible for a drug candidate to reach its site of action.
2. Results and Discussion
2.1. Chemistry
The synthetic route for the preparation of the galloyl derivatives is presented in Scheme 1. Methyl gallate (2) was prepared by esterification of the corresponding carboxylic acid 1 with methanol and sulfuric acid [31]. The reaction of methyl gallate with hydrazine hydrate yielded the galloyl hydrazide 3. Condensation of the hydrazide 3 with aromatic aldehydes (benzaldehyde, 4-dimethylaminobenzaldehyde, 4-nitrobenzaldehyde, 4-methoxybenzaldehyde and 4-hydroxybenzaldehyde), in ethanol heated at reflux, yielded the N'-(substituted)-arylidenegalloyl hydrazides 4–8, respectively. The IR and 1H-NMR spectroscopic data of these compounds were consistent with the related literature and the experimental conditions and yields for the reaction were satisfactory [32].
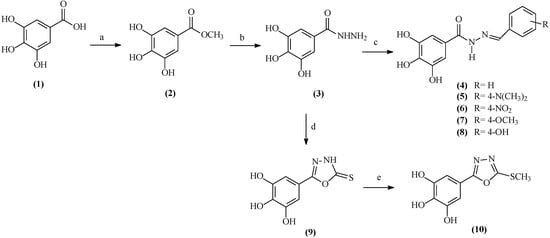
Scheme 1.
Synthetic route for the preparation of the galloyl derivatives.
For preparation of new derivatives of galloyl-2-thioxo-1,3,4-oxadiazole (9), the key intermediate 3 was subjected to a reaction with carbon disulfide in the presence of KOH and ethanol heated at reflux. The 1,3,4-oxadiazolyl 9 was subsequently S-methylated with methyl iodide in the presence of K2CO3 at room temperature to yield afford the galloyl-2-methylthio-1,3,4-oxadiazole 10.
The chemical structures of all compounds were confirmed by spectral data (1H- and 13C-NMR, IR, and MS) (see Section 3). The 1H-NMR spectra of N'-(substituted)-arylidene galloyl hydrazides 4–8 displayed signals at δH 8.40 ppm integrating for one proton and δH 6.70–9.00 ppm, corresponding to the imine and aromatic hydrogens, respectively, of the (substituted-benzylidene) galloyl hydrazide group. The presence of this group was confirmed by the signals at δC 147–150 (C=N), 158–162 (C=O), and 110–135 ppm (aromatic carbons of R group) in the 13C-NMR spectra.
The galloyl-2-thioxo-1,3,4-oxadiazole 9 may exist in a thione-thiol tautomeric structure, as indicated by the –NH and –SH proton signals at 13.7–14.1 ppm in the 1H-NMR spectrum, as a broad singlet integrating for one hydrogen each. In the solid state, these compounds are present in the thione (C=S) form, as indicated in their IR spectrum by the absence of the νs(S–H) absorption band at 2500 cm−1 and the presence of two ν(C=S) absorption bands at 1230–1339 cm−1, which is characteristic for this class of compounds. The S-methylated derivative 10 was characterized by the presence of an addition signal at δH/δC 2.84/14.7 ppm, corresponding to the S-methyl group attached to the oxadiazole nucleus.
2.2. Antiproliferative Activity
Three response parameters (GI50, TGI, and LC50) were calculated for each compound and cell line, and the results are summarized in Table 1 and Table 2.

Table 1.
GI50 values (µM) for compounds 2–10 in ten tumor cell lines.
| Cancer Cell Lines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| U251 | MCF-7 | NCI/ADR-RES | 786-0 | NCI-H460 | PC-3 | OVCAR-3 | HT-29 | K-562 | HaCaT | |
| 2 | 0.16 | 0.16 | 0.12 | 0.65 | 0.13 | 0.57 | 0.05 | 0.49 | 0.31 | 0.26 |
| 3 | 1.11 | 0.85 | 0.91 | 1.56 | 5.54 | 3.53 | 0.88 | 1.21 | 1.17 | 2.50 |
| 4 | 7.80 | 5.55 | 7.16 | 9.06 | 11.20 | 5.07 | 1.24 | 7.53 | 9.96 | 8.29 |
| 5 | 24.10 | 8.61 | 4.68 | 8.93 | 21.64 | - | 2.93 | 16.24 | 2.05 | 4.05 |
| 6 | 5.12 | - | 8.46 | 7.60 | - | 13.99 | 5.91 | - | - | 31.55 |
| 7 | 9.14 | 15.66 | 11.34 | 18.85 | 41.05 | 19.64 | 5.98 | 25.12 | 8.93 | 18.08 |
| 8 | 6.60 | - | - | 26.80 | 9.38 | - | 1.35 | - | 9.17 | 8.72 |
| 9 | 4.20 | 1.08 | 2.83 | 8.90 | 8.77 | 6.05 | 0.89 | 2.61 | 1.72 | 2.25 |
| 10 | 5.20 | 0.34 | 0.17 | 9.20 | 9.44 | 7.43 | 0.14 | 2.77 | 0.60 | 2.50 |
| Dox | 0.02 | 0.01 | 0.20 | 0.04 | 0.01 | 0.18 | 0.02 | 0.09 | 0.03 | 0.01 |
GI50: (growth inhibitory activity) the drug concentration that reduces cellular growth by 50%; Dox: Doxorubicin; (-): GI50 value not presented.

Table 2.
TGI and LC50 (values in parentheses) in µg/mL for compounds 2–10 in ten tumor cell lines.
| Cancer Cell Lines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| U251 | MCF-7 | NCI/ADR-RES | 786-0 | NCI-H460 | PC-3 | OVCAR-3 | HT-29 | K-562 | HaCaT | |
| 2 | 4.10 | 5.12 | 3.62 | 8.62 | 7.97 | 13.10 | 1.59 | 8.52 | 5.67 | 16.74 |
| (29.19) | (-) | (30.26) | (31.36) | (34.92) | (-) | (33.69) | (30.60) | (-) | (-) | |
| 3 | 6.13 | 2.36 | 2.56 | 17.98 | 17.35 | 18.31 | 9.77 | 3.27 | - | 29.02 |
| (31.36) | (-) | (-) | (-) | (-) | (-) | (-) | (26.21) | - | >100 | |
| 4 | - | 23.54 | - | - | - | - | 29.61 | - | - | - |
| (-) | (-) | |||||||||
| 5 | - | 20.26 | 83.07 | - | - | - | 41.52 | 48.50 | 8.98 | 18.41 |
| (47.93) | (-) | (-) | (-) | (-) | (55.32) | |||||
| 6 | - | - | - | - | - | 84.67 | - | - | - | - |
| (-) | ||||||||||
| 7 | 64.22 | - | 66.56 | - | - | - | - | - | 41.76 | - |
| (-) | (-) | (-) | ||||||||
| 8 | 26.07 | - | - | - | - | - | - | - | - | - |
| (-) | ||||||||||
| 9 | 14.58 | 5.06 | 15.86 | 24.68 | 19.43 | 18.09 | 13.62 | 13.74 | 11.35 | 16.53 |
| (41.26) | (-) | (-) | (-) | (42.67) | (56.99) | (58.99) | (58.99) | (58.99) | (-) | |
| 10 | 16.40 | 3.20 | 2.33 | 29.40 | 29.52 | 28.45 | 2.38 | 17.66 | 3.70 | 18.00 |
| (30.43) | (20.24) | (19.65) | (-) | (65.78) | (69.30) | (17.34) | (60.32) | (20.20) | (-) | |
| Dox | 2.68 | 2.52 | 22.42 | 0.51 | 3.73 | 1.03 | 1.47 | 26.03 | 2.60 | 0.77 |
| (-) | (-) | (-) | (17.82) | (-) | (7.95) | (-) | (-) | (20.09) | (-) | |
TGI: the drug concentration required for total growth inhibition; LC50: the drug concentration required for killing 50% of cells; Dox: Doxorubicin; (-): not presented.
The GI50 values (growth inhibitory activity) (Table 1) refer to the drug concentration that produces a 50% reduction of cellular growth compared with the untreated control cells. The TGI (cytostatic activity) and LC50 (cytotoxic activity) values (Table 2) refer to the drug concentration required for total growth inhibition and killing 50% of the cells, respectively. GI50 values were used to classify a compound’s activity as follows: inactive, >100 µM; moderate, between >10 and <100 µM; and active, <10 µM.
The N'-(substituted)-arylidene galloyl hydrazide 4, which has a phenyl group attached to the galloyl hydrazide moiety, possesses antiproliferative activity with GI50 values ranging from 1.24 to 9.96 µM for nine human tumor cell lines. In particular, the compound displayed activity against the ovarian cell line OVCAR-3 with a GI50 value of 1.24 µM (Table 1). Derivative 5, which has a dimethylaminophenyl group attached to the galloyl hydrazide moiety, displayed cytotoxic efficacy against OVCAR-3 (GI50 = 2.93 µM; TGI = 41.52 µM) and leukemia K-562 (GI50 = 2.05 µM; TGI = 8.98 µM) cell lines (Table 1 and Table 2). Compounds 6 and 7 did not demonstrate cytotoxic efficacy in these cell lines. The activity against the OVCAR-3 cell line was also observed for compound 8 (GI50 = 1.35 µM), containing the p-hydroxyphenyl group to the galloyl hydrazide moiety.
The introduction of the 2-thioxo-1,3,4-oxadiazol-5-yl group 9 at C-1 of the galloyl group led to significant cytotoxicity, with GI50 less than 8.90 µM towards all cell lines, and displayed significant activity against OVCAR-3 (GI50 = 0.89 µM), MCF-7 (GI50 = 1.08 µM), K-562 (GI50 = 1.72 µM), HaCaT (GI50 = 2.25 µM), HT-29 (GI50 = 2.61 µM), and NCI/ADR/RES (GI50 = 2.83 µM) cell lines. The 2-methylthio analogue 10 showed an increase in antiproliferative activity, with particular effectiveness against OVCAR-3 (GI50 = 0.14 µM, TGI = 2.38 µM, LC50 = 17.34 µM), MCF-7 (GI50 = 0.34 µM, TGI = 3.20 µM, LC50 = 20.24 µM), K-562 (GI50 = 0.60 µM, TGI = 3.70 µM, LC50 = 20.20 µM) and NCI/ADR/RES (GI50 = 0.17 µM, TGI = 2.33 µM, LC50 = 19.65 µM) cell line (Table 1 and Table 2).
In addition, the precursor compound methyl gallate 2 exhibited potent, broad-spectrum antitumor activity with GI50 values < 1.0 µM against all human tumor cell lines tested, with GI50 (TGI) values ranging from 0.05 to 0.65 µM (1.59–16.74 µM) (Table 1 and Table 2). The intermediary, galloyl hydrazide 3, also showed significant activity, with GI50 values in the range of 0.85–5.54 µM for all cell lines tested, and displayed cytotoxic efficacy against MCF-7 breast (GI50 = 0.85 µM, TGI = 2.36 µM), NCI/ADR-RES multiple drug resistant breast (GI50 = 0.91 µM, TGI = 2.56 µM) and OVCAR-3 ovarian (GI50 = 0.88 µM, TGI = 9.77 µM) cells (Table 1 and Table 2). Methyl gallate is a derivative of gallic acid that has been extensively investigated for important biological properties such as antioxidant, anti-inflammatory, antimicrobial and antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cell and glioma cells [19,33,34,35,36,37]. However, the antitumor activity of this compound has not been extensively examined in other cancer cells. We examined the effect of methyl gallate in ten human tumor cell lines and observed selective cytotoxicity in all cell lines tested.
An analysis of the relationship between the GI50 data and the effects of the substitutions in the aromatic ring at the N'-(substituted)-arylidene galloyl hydrazide with electron-withdrawing and electron-donating substituents 5–8 did not display enhanced activity compared with the phenyl substituent 4, which demonstrated a pronounced effect on cell line proliferation. These results suggest that the electronic features of the various substituents affect how easily the drug can interact with biological molecules. Regarding the relationship between the 1,3,4-oxadiazolyl derivatives, compound 10 displayed the highest antiproliferative activity compared to the corresponding precursor 9, exhibiting selectivity for four cell lines. Substitution by ester, hydrazine and 1,3,4-oxadiazolyl moiety side chains in compounds 2, 3, 9 and 10 resulted in a reduction in the percentage of growth inhibition, thereby indicating the importance of these side chain groups.
The anthracyclins doxorubicin (DOX) and daunorubicin (DNR) are among the most useful antibiotic, antitumor natural isolates from a species of the bacteria Streptomyces for the treatment of breast cancer, solid tumors and leukemia [38,39,40,41,42]. The mechanism of action is complex. Briefly, doxorubicin interferes with DNA replication by intercalation into DNA molecules, thus inhibiting the biosynthesis of DNA, RNA and protein as well as the induction of the free radicals inside the cell, leading to senescence and cell death by apoptosis or necrosis [43,44]. The compounds synthesized have functional groups similar to DOX, which suggests that they may act in a similar manner. Thus, the development of new molecular targets for antitumor therapies is directly correlated to many tumors that are resistant to therapeutic strategies and side effects. The main side effect of DOX is cardiac insufficiency, which is caused by the production of free radicals in the myocardium. In clinical research, chemotherapy resistance has been identified as a serious problem when the concentrations of chemotherapy drugs reach toxic and harmful doses for killing tumors.
2.3. Lipinski’s Rule of Five
The present study focused on the antiproliferative activity and synthesis of structurally modified galloyl derivatives, but we also explored the bioavailability of the synthesized derivatives, which is of importance for further development of drugs based upon these substances and their analogues. Drug-likeness is a promising paradigm to identify a balance that influences the pharmacodynamic and pharmacokinetic properties of a compound that ultimately optimizes its absorption, distribution, metabolism and excretion (ADME) in the human body [45].
These parameters were tentatively assessed using theoretical calculations following Lipinski’s rule of five, which establishes that the absorption or permeation of an orally administered compound is more likely to be efficient if the drug satisfies the established criteria: molecular weight (MW) ≤ 500 Da, log P ≤ 5, H-bond donors (HBD) ≤ 5 and H-bond acceptors (HBA).
Our results (Table 3) revealed that derivatives 2, 4–10, presented lipophilicities less than 5, with values between 0.34 and 1.94. Galloyl hydrazide (3) showed lipophilicity, Log P = −0.94, which is not in violation of Lipinski’s rules. To further evaluate the drug-likeness, the rules have spawned many extensions, such as the partition coefficient, Log P in −0.4 to +5.6 ranges [46]. All derivatives have a number of hydrogen bond acceptors (HBA) (n-ON = 5–9), and their molecular weights were smaller than 500 (184.15 > MW < 317.26), which is in agreement with Lipinski’s rules. With the exception of the hydrazide 3, they all have a number of hydrogen bond donors (HBD) (n-OHNH = 6), violating one of the Lipinski’s rules. The calculated percent absorption (%ABS) of all derivatives ranged between 57.95% and 78.98%, indicating that these compounds have good permeability in the cellular membrane. Other rules include the number of rotatable bonds, indicating the flexibility of the molecule, the volume and the polar surface area. The topological polar surface area (TPSA) is recognized as a good indicator of drug absorption in the intestine (TPSA less than 140 Angstroms squared [Å2]) and blood-brain barrier penetration (TPSA less than 60 Å2). The compounds exhibit computational TPSA values between 86.99 and 122.37 Å2 and have good intestinal absorption except 6 (147.97 Å2). However, the derivatives do not have adequate blood-brain barrier penetration, as the TPSA values are more than 60 Å2.
The empirical conditions to satisfy Lipinski’s rule and to manifest a good oral bioavailability involve a balance between the solubility of a compound and its ability to diffuse passively through the different biological barriers. Compounds with high solubility are more easily metabolized and eliminated from the organism, thus leading to a lower probability of adverse effects and bioaccumulation. The compound 2 (Log S = −0.87) presented good solubility, thus encouraging its use as a precursor to produce new drugs that have better absorption.

Table 3.
Lipinski’s rule and %ABS, TPSA, Log S for compounds 2–10.
| Lipinski’s Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comp. | %ABS | TPSA a (Å2) | nHBA a (nON) | nHBD a (nOHNH) | Log P a | MW a | n violations a | Log S b |
| 2 | 78.98 | 86.99 | 5 | 3 | 0.85 | 184.15 | 0 | −0.87 |
| 3 | 69.04 | 115.80 | 6 | 6 | −0.94 | 184.15 | 1 | −1.09 |
| 4 | 73.75 | 102.15 | 6 | 4 | 1.84 | 272.26 | 0 | −2.83 |
| 5 | 72.64 | 105.38 | 7 | 4 | 1.94 | 315.33 | 0 | −2.87 |
| 6 | 57.95 | 147.97 | 9 | 4 | 1.80 | 317.26 | 0 | −3.47 |
| 7 | 70.57 | 111.38 | 7 | 4 | 1.89 | 302.29 | 0 | −2.85 |
| 8 | 66.78 | 122.37 | 7 | 5 | 1.36 | 288.26 | 0 | −2.54 |
| 9 | 73.63 | 102.51 | 6 | 4 | 0.34 | 226.21 | 0 | −2.10 |
| 10 | 74.63 | 99.61 | 6 | 3 | 1.27 | 240.24 | 0 | −3.07 |
a www.molinspiration.com [47]; b www.organic-chemistry.org/prog/peo [48]; %ABS = 109 − 0.345 × TPSA; Number hydrogen bond acceptor (NO) = nHBA ≤ 10; Number hydrogen bond donors (OHNH) = nHBD ≤ 5; MW ≤ 500; Octanol-water partition coefficient = Log P < 5; Solubility = Log S between −1 and −5.
3. Experimental Section
3.1. Chemistry
3.1.1. General
1H- and 13C-NMR spectra were recorded at 300 MHz and 75 MHz, respectively, using a model Mercury plus BB spectrometer (Varian, Palo Alto, CA, USA). Mass spectra (MS) were recorded in a Focus-DSQ II spectrometer (Thermoelectron Corporation, Austin, TX, USA). IR spectra were recorded on a model MB-100 spectrometer (BOMEM, Quebec, CA, USA). For TLC, precoated plates (silica gel 60 G254, Macherey-Nagel, Duren, GER) were used. All reagents were purchased from commercial suppliers.
3.1.2. Galloyl hydrazide (3)
Briefly, 80% hydrazine hydrate (1.8 mL, 48.2 mmol) was added to a solution of methyl gallate (2) (2.5 g, 2.97 mmol) that was previously prepared by esterification of commercial gallic acid (1) in ethanol (30 mL). This mixture was heated at reflux for 60 h, when TLC analysis indicated the end of the reaction. Then, the media was poured on ice, and the resulting precipitate was filtered, affording the title compound 3 (1.85 g, 74%) as pale white powder, mp 164–167 °C (MeOH); mp 167 °C [32]; IR (KBr) νmax (cm−1): 3365–3284 (O-H, N-H), 1623 (C=O), 1509–1303 (C=C), 1260 (C-O); 1H-NMR (DMSO-d6): δH 6.77 (s, 2H); 8.59 (NH); 9.32 (OH). 13C-NMR (DMSO-d6): δC 106.5, 123.9, 136.2, 145.4, 166.4. MS m/z (%): 185 (MH+, 12), 153 (100), 126 (42).
3.1.3. General Procedure for the Preparation of N'-(Substituted)-arylidene galloyl hydrazides 4–8
A solution of galloyl hydrazide (3) (0.28 g, 1 mmol) in water (10 mL) containing two drops of conc. H2SO4 was heated at reflux for 0.5 h until complete dissolution. Then, aromatic aldehydes (benzaldehyde, 4-N-dimethylaminobenzaldehyde, 4-nitrobenzaldehyde, 4-methoxybenzaldehyde and 4-hydroxybenzaldehyde, 1.5 mmol) in EtOH (3 mL) were added to the solution, which was then heated at reflux for 48 h. The mixtures were poured into cold water and neutralized with 10% aqueous NaHCO3. The precipitates were collected by filtration, recrystallized and dried, yielding the compounds 4–8 in 74%–83% yields.
N'-(Benzylidene)galloyl hydrazide (4). White powder (0.20 g, 71%), mp 170–173 °C (MeOH); mp 170–172 °C [32]; IR (KBr) νmax (cm−1): 3362–3270 (O-H, N-H), 1630 (C=O), 1518 (C=C), 1264 (C-O); 1H-NMR (CD3OD): δH 7.30–7.68 (5H, m), 7.70 (2H, s), 8.65 (1H, s, N'=CH). 13C-NMR (CD3OD): δC 115.4, 126.3, 128.7, 128.8, 131.0, 133.9, 140.4, 145.2, 148.7, 162.5. MS m/z (%): 272 (MH+, 8), 153 (100), 126 (30).
N'-(4-Dimethylaminobenzylidene)galloyl hydrazide (5). White powder, (0.22 g, 78%) mp 174–178 °C (MeOH); mp 175–178 °C [32]; IR (KBr) νmax (cm−1): 3325 (O-H, N-H), 1661 (C=O), 1520 (C=C), 1264 (C-O); 1H-NMR (CD3OD): δH 3.03 (6H, s), 6.93 (2H, d, J = 8.7 Hz), 7.68 (2H, s), 7.78 (2H, d, J = 8.7 Hz), 8.36 (1H, s, N'=CH). 13C-NMR (CD3OD): δC 40.3, 111.9, 114.1, 125.9, 129.8, 130.4, 141.4, 146.2, 149.4, 151.2, 162.6. MS m/z (%): 315 (MH+, 12), 153 (100), 126 (33).
N'-(4-Nitrobenzylidene)galloyl hydrazide (6). Pale yellow powder (0.24 g, 83%); mp 171–174 °C (MeOH); mp 171–172 °C [32]; IR (KBr) νmax (cm−1): 3422–3280 (O-H, N-H), 1665 (C=O), 1520 (C=C), 1264 (C-O); 1H-NMR (CD3OD): δH 6.98 (2H, s), 7.94 (2H, d, J = 8.5 Hz), 7.28 (2H, d, J = 8.5 Hz), 8.49 (1H, s, N'=CH), 9.19 (OH, NH). 13C-NMR (CD3OD): δC 116.3, 122.8, 124.1, 127.8, 137.3, 141.0, 143.9, 145.6, 147.6, 163.4; MS m/z (%): 317 (MH+, 10), 153 (100), 126 (21).
N'-(4-Methoxybenzylidene)galloyl hydrazide (7). Pale white powder (0,23 g, 80%), mp 170–172 °C (MeOH); mp 171–173 °C [32]; IR (KBr) νmax (cm−1): 3410–3260 (O-H, N-H), 1668 (C=O), 1520 (C=C), 1262 (C-O); 1H-NMR (CD3OD): δH 3.87 (3H, s, OCH3), 7.00 (2H, s), 7.70 (2H, d, J = 8.3 Hz), 6.80 (2H, d, J = 8.3 Hz), 8.58 (1H, s, N'=CH). 13C-NMR (CD3OD): δC 55.6, 110.2, 114.4, 126.5, 128.6, 130.0, 136.8, 145.5, 146.6, 160.5, 161.6; MS m/z (%): 302 (MH+, 31), 153 (100), 126 (17).
N'-(4-Hydroxybenzylidene)galloyl hydrazide (8). White powder (0,20 g, 78%), mp 170–173 °C (MeOH); mp 172–174 °C [32]; IR (KBr) νmax (cm−1): 3418–3266 (O-H, N-H), 1668 (C=O), 1571 (C=C), 1260 (C-O); 1H-NMR (CD3OD): δH 7.12 (2H, s), 7.34 (2H, d, J = 7.8 Hz), 6.60 (2H, d, J = 7.8 Hz), 8.66 (1H, s, N'=CH). 13C-NMR (CD3OD): δC 115.6, 114.8, 125.5, 128.7, 132.0, 136.8, 147.5, 147.6, 158.5, 160.6; MS m/z (%): 288 (MH+, 18), 153 (100), 126 (44).
3.1.4. Galloyl-2-thioxo-1,3,4-oxadiazole (9)
Carbon disulfide (0.8 mL, 5 mmol) and potassium hydroxide (0,213 g, 1 mmol) were added to a solution of galloyl hydrazide (3) (0.456 g, 1 mmol) in EtOH (10 mL) at 0 °C. The resulting solution was heated at reflux for 48 h. The solvent was evaporated, and the residue was dissolved in water and acidified with a dilute solution of HCl. The solid obtained was filtered and recrystallized to give the title compound 9 (0.36 g, 80%) as a white powder, mp 168–170 °C (MeOH); IR (KBr) νmax (cm−1): 3370–3280 (O-H), 1595–1430 (C=C), 1245 (C-S), 1168 (C-O-C); 1H-NMR (CD3OD): δH 6.82 (2H, s), 9.05 (OH, NH); 13C-NMR (CD3OD): δC 105.0, 112.0, 137.6, 146.5, 161.1, 176.9. MS m/z (%): 226 (MH+, 12), 150 (100), 77 (28).
3.1.5. Galloyl-2-methylthio-1,3,4-oxadiazole (10)
To a solution of galloyl-2-thioxo-1,3,4-oxadiazole (9) (0.20 g, 0.6 mmol) in anhydrous THF (10 mL) was to added K2CO3 (0.14 g, 0.6 mmol). The resulting solution was stirred at room temperature for 1 h. Methyl iodide (0.19 mL, 0.8 mmol) was then added, and the mixture was stirred for an additional 48 h. The solvent was removed, and the residue obtained was crystallized to give the title compound 10 (0.1192 g, 60%), as a brown powder, mp 166–168 °C (MeOH); IR (KBr) νmax (cm−1): 3376 (O-H, N-H), 1668 (C=O), 1565–1430 (C=C), 1170 (C-O-C), 720 (C-S-C); 1H-NMR (CD3OD): δH 2.80 (3H, s, SCH3); 7.00 (2H, s); 9.62 (OH). 13C-NMR (CD3OD): δC 16.2, 110.2, 112.4, 136.6, 146.8, 162.2, 160.4; MS m/z (%): 240 (MH+, 8), 150 (100), 91 (28).
3.2. Anticancer Assay in Vitro
The synthesized compounds were assessed in the following ten human tumor cell lines from various tissues kindly provide by the National Cancer Institute (Frederick, MD, USA): U251 (glioma, CNS), MCF-7 (breast), NCI-ADR/RES (breast expressing the multiple drug resistance phenotype), 786-0 (renal), NCI-H460 (lung, non-small cells), PC-3 (prostate), OVCAR-3 (ovarian), HT-29 (colon), K-562 (leukemia) and HaCaT (keratinocytes). Stock cultures were grown in media containing 5 mL of RPMI 1640 (Gibco BRL) supplemented with 5% fetal bovine serum. Gentamicin (50 mg/mL) was added to the experimental cultures. Cells in 96-well plates (100 µL cells/well) were exposed to sample concentrations in DMSO/RPMI (0.25, 2.5, 25, and 250 µg/mL) at 37 °C and 5% CO2 for 48 h. The final DMSO concentration did not affect cell viability. Next, cells were fixed with 50% trichloroacetic acid, and cell proliferation was determined by spectrophotometric quantification (540 nm) of cellular protein content employing the sulforhodamine B assay [49]. Doxorubicin (0.025–25 µg/mL) was used as a positive control. Three measurements were obtained: first at time point zero (T0, at the beginning of incubation) and then 48 h post-incubation for both the compound-free (C) and tested (T) cells. Cell proliferation was determined using the equation 100 × [(T − T0)/C − T0]. A cytostatic effect was observed when T ≥ T0, while a cytocidal effect occurred when T < T0. The experiments were performed in triplicate.
3.3. In Silico Study
An in silico computational study of the synthesized compounds 2–10 was performed to determine Lipinski’s rules, topological polar surface area (TPSA) and percentage of absorption (%ABS). Calculations were performed using Molinspiration online property calculation toolkit software [47] and OSIRIS property explorer software [48]. The percentage of absorption was estimated using the following equation: %ABS = 109 − [0.345 × TPSA]. Lipinski’s rule states that an orally active drug generally has no more than one violation of the following criteria [50]:
- (I)
- hydrogen bond donors ≤ 5 (OH and NH groups);
- (II)
- hydrogen bond acceptors ≤ 10 (N and O atoms);
- (III)
- molecular weight < 500;
- (IV)
- calculated logP < 5.
4. Conclusions
The results suggest that synthetic derivatives of N'-(substituted)-arylidenegalloyl hydrazide do not have a very strong synergistic effect in the inhibition of cancer cell proliferation compared with methyl gallate (2), galloyl hydrazide (3) and 1,3,4-oxadiazolyl derivatives 9 and 10. These compounds showed higher inhibition effect against ovarian (OVCAR-3) cells. Computational drug-likeness and TPSA calculations revealed that the compounds show good intestinal absorption. Finally, the in silico study identified these derivatives as potential new drug candidates. More detailed studies with other models, such in vivo assays, are essential for the characterization of these derivatives as anticancer agents.
Acknowledgments
We are grateful to Fundect and CAPES fellowships for financial support (ASNF and MMS). The authors are grateful Maria Helena Sarragiotto (Departamento de Química—UEM, Maringá-PR) for technical support. Maria do Carmo Vieira in acquisition materials.
Author Contributions
ASNF, MMS, TSD and MC designed the study, synthesis of compounds and in silico study. MAF and JEC participated in the antiproliferative assay. MCV helped conduct the literature review and in implementation of the study.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Golumbic, C.; Mattill, H.A. The antioxidant properties of gallic acid and allied compounds. J. Am. Chem. Soc. 1942, 19, 144–145. [Google Scholar]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Kroes, B.H.; van den Berg, A.J.J.; Quarles van Ufford, H.C.; van Dijk, H.; Labadie, R.P. Anti-inflammatory activity of gallic acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Gichner, T. Two types of antimutagenic effects of gallic and tannic acids towards N-nitroso-compounds-induced mutagenicity in the Ames Salmonella assay. Folia Microbiol. 1987, 32, 55–62. [Google Scholar] [CrossRef]
- Mirvish, S.S.; Cardesa, A.; Wallcave, L.; Shubik, P. Induction of mouse lung adenomas by amines or ureas plus nitrite and by N-nitroso compounds: Effect of ascorbate, gallic acid, thiocyanate and caffeine. J. Natl. Cancer Inst. 1975, 55, 633–666. [Google Scholar] [PubMed]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.; Chen, Y. Role of reactive oxygen species in gallic acid-induced apoptosis. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Kataoka, T.; Hayashi, T.; Hasegawa, M.; Ishi, Y.; Hibasami, H. Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO205 cell lines. Oncol. Rep. 2000, 6, 1221–1223. [Google Scholar]
- Hsu, C.; Lo, W.; Yen, G. Gallic acid induces apoptosis in 3T3-L1 pre-adipocytes via a Fas- and mitochondrial-mediated pathway. J. Agric. Food Chem. 2007, 55, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic acid, an active constituent of grape seed extract, exhibits antiproliferative, pro apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Park, W.H. Gallic acid induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol. In Vitro 2010, 24, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Moon, H.J.; Han, Y.H.; Park, W.H. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem. Toxicol. 2010, 48, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, F.; Jiang, H.; Wu, K.; Zheng, X.; Cai, Y.; Katakowski, M.; Chopp, M.; To, S.T. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur. J. Pharmacol. 2010, 641, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Madlener, S.; Illmer, C.; Horvath, Z.; Saiko, P.; Losert, A.; Herbacek, I.; Grusch, M.; Elford, H.L.; Krupitza, G.; Bernhaus, A.; et al. Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Lett. 2007, 245, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Bernhaus, A.; Fritzer-Szekeres, M.; Grusch, M.; Saiko, P.; Krupitza, G.; Venkateswarlu, G.; Trimurtulu, G.; Jaeger, W.; Szekeres, T. Digalloylresveratrol, a new phenolic acid derivative induces apoptosis and cell cycle arrest in human HT-29 colon cancer cells. Cancer Lett. 2009, 274, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.C.; Rajbanshi, R.; Calhoun, C.; Chiu, R.H. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules 2010, 15, 8377–8389. [Google Scholar] [CrossRef] [PubMed]
- Horvath, Z.; Saiko, P.; Illmer, C.; Madlener, S.; Hoechtl, T.; Bauer, W.; Erker, T.; Jaeger, W.; Fritzer-Szekeres, M.; Szekeres, T. Synergistic action of resveratrol, an ingredient of wine, with Ara-C and tiazofurin in HL-60 human promyelocytic leukemia cells. Exp. Hematol. 2005, 33, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.C.; Tsai, S.; Chen, L.; Lin-Shiau, L.; Lin, J. Resveratrol-induced G2 arrest through the inhibition of CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells. Biochem. Pharmacol. 2003, 65, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y. Development of Gallic Acid-Conjugated Linoleic Acid Ester as Novel Functional Materials and Its Biological Activity. Ph.D. Thesis, Chonbuk National University, Jeonju, Korea, 2007. [Google Scholar]
- Lee, H.; Kwon, Y.; Lee, J.H.; Kim, J.; Shin, M.K.; Kim, S.H.; Bae, H. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells. J. Immunol. 2010, 185, 6698–6705. [Google Scholar] [CrossRef] [PubMed]
- Wacher, V.J. Use of Gallic Acid Esters to Increase Bioavailability of Orally Administered Pharmaceutical Compounds. Ph.D. Thesis, University of California, San Francisco, CA, USA, 2001. [Google Scholar]
- Spickenreither, M.; Braun, S.; Bernhardt, G.; Dove, S.; Buschauer, A. Novel 6-O-acylated vitamin C derivatives as hyaluronidase inhibitors with selectivity for bacterial lyases. Bioorg. Med. Chem. Lett. 2006, 16, 5313–5316. [Google Scholar] [CrossRef] [PubMed]
- Isoyama, T.; Thwaites, D.; Selzer, M.G.; Carey, R.I.; Barbucci, R.; Lokeshwar, V.B. Differential selectivity of hyaluronidase inhibitors toward acidic and basic hyaluronidases. Glycobiology 2006, 16, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reaction, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.A.; Shaharyar, M. Synthesis, Characterization, and In Vitro Anticancer Evaluation of Novel 2,5-Disubstituted 1,3,4-Oxadiazole Analogue. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Kothayer, H.; Elshanawani, A.A.; Abu Kull, M.E.; El-Sabbagh, O.I.; Shekhar, M.P.; Brancale, A.; Jones, A.T.; Westwell, A.D. Design, synthesis and in vitro anticancer evaluation of 4,6-diamino-1,3,5-triazine-2-carbohydrazides and -carboxamides. Bioorg. Med. Chem. Lett. 2013, 23, 6886–6889. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.R.; Charris, J.; Camacho, J.; Barazarte, A.; Gamboa, N.; Antunes, F. Cytotoxic effects of N'-formyl-2-(5-nitrothiophen-2-yl)benzothiazole-6-carbohydrazide in human breast tumor cells by induction of oxidative stress. Anticancer Res. 2012, 32, 2721–2726. [Google Scholar] [PubMed]
- Ali, M.A.; Shaharyar, M. Oxadiazole mannich bases: Synthesis and antimycobacterial activity. Bioorg. Med. Chem. Lett. 2007, 17, 3314–3316. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.N.; Tonin, L.T.D.; Foglio, M.A.; Madjarof, C.; de Carvalho, J.E.; da Costa, W.F.; Cardoso, F.P.; Sarragiotto, M.H. Synthesis and antitumoral activity of novel 3-(2-substituted-1,3,4-oxadiazol-5-yl) and 3-(5-substituted-1,2,4-triazol-3-yl) β-carboline derivatives. Bioorg. Med. Chem. Lett. 2008, 16, 9660–9667. [Google Scholar] [CrossRef]
- Savariz, F.C.; Formagio, A.S.N.; Barbosa, V.A.; Foglio, M.A.; de Carvalho, J.E.; Duarte, M.C.T.; Filho, B.P.D.; Sarragiotto, M.H. Synthesis, Antitumor and Antimicrobial Activity of Novel 1-Substituted Phenyl-3-[3-alkylamino(methyl)-2-thioxo-1,3,4-oxadiazol-5-yl] β-Carboline Derivatives. J. Braz. Chem. Soc. 2010, 21, 288–298. [Google Scholar] [CrossRef]
- Barbosa, V.A.; Formagio, A.S.N.; Savariz, F.C.; Foglio, M.A.; Spindola, H.M.; Carvalho, J.E.; Meyer, E.; Sarragiotto, M.H. Synthesis and antitumor activity of β-carboline 3-(substituted-carbohydrazide) derivatives. Bioorg. Med. Chem. 2011, 19, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.I. A Textbook of Practical Organic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1957; pp. 538–539. [Google Scholar]
- Ilango, K.; Arunkumar, S. Synthesis, antimicrobial and antitubercular activities of some novel trihydroxy benzamido azetidin-2-one derivatives. Trop. J. Pharm. Res. 2011, 10, 219–229. [Google Scholar] [CrossRef]
- Hsieh, T.J.; Liu, T.Z.; Chi, Y.C.; Chern, C.L.; Lu, F.J.; Chuang, M.C.; Mau, S.Y.; Chen, S.H.; Syu, Y.H.; Chen, C.H. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem. Toxicol. 2004, 42, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kang, O.H.; Lee, Y.S.; Oh, Y.C.; Chae, H.S.; Jang, H.J.; Shin, D.W.; Kwon, D.Y. Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules 2009, 14, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Jang, H.S.; Oh, J.S.; Yang, K.H.; Choi, N.K.; Lim, H.S.; Kim, S.M. Effects of methyl gallate and gallic acid on the production of inflammatory mediators interleukin-6 and interleukin-8 by oral epithelial cells stimulated with Fusobacterium nucleatum. J. Microbiol. 2009, 47, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Oh, J.S.; Kang, I.C.; Hong, S.J.; Choi, C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.K.; Kim, D.W.; Hwang, H.S.; Eum, W.S.; Park, J.; Han, K.H.; Oh, J.S.; Choi, S.Y. Antitumor activity of methyl gallate by inhibition of focal adhesion formation and Akt phosphorylation in glioma cells. Biochim. Biophys. Acta 2013, 1830, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Tsuruo, T.; Naito, M.; Tomida, A.; Fujita, N.; Mashima, T.; Sakamoto, H.; Haga, N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003, 94, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hui, R.C.; Francis, R.E.; Guest, S.K.; Costa, J.R.; Gomes, A.R.; Myatt, S.S.; Brosens, J.J.; Lam, E.W.F. Doxorubicinactivates FOXO3a to induce the expression of multidrug resistance gene ABCB1(MDR1) in K562 leukemic cells. Mol. Cancer Ther. 2008, 7, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Harisi, R.; Dudas, J.; Nagy-Olah, J.; Timar, F.; Szendroi, M.; Jeney, A. Extracellular matrixinduces doxorubicin-resistance in human osteosarcoma cells by suppression of p53 function. Cancer Biol. Ther. 2007, 6, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Duarte, N.; Gyemant, N.; Radics, R.; Cherepnev, G.; Varga, A.; Molnár, J. Inter-action between doxorubicin and the resistance modifier stilbene on multidrugresistant mouse lymphoma and human breast cancer cells. Anticancer Res. 2006, 26, 3541–3546. [Google Scholar] [PubMed]
- Song, X.; Liu, X.; Chi, W.; Liu, Y.; Wei, L.; Wang, X.; Jinming, Y. Hypoxia-induced resistance tocisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencingof HIF-1alpha gene. Cancer Chemother. Pharmacol. 2006, 58, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Biing, J.T.; Yang, Y.F.; Liu, H.S.; Ye, C.T.; Chao, C.F. The induction of multidrug resistancein human cervical carcinoma cell lines by estrogenic hormones. Proc. Natl. Sci. Counc. Repub. China Part B: Life Sci. 1994, 18, 64–70. [Google Scholar]
- Turner, J.G.; Sullivan, D.M. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr. Med. Chem. 2008, 15, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Pedretti, A.; Testa, B. Assessing drug-likeness e what are we missing? Drug Discov. Today 2008, 13, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P. Calculation of Molecular Properties and Bioactivity Score. Available online: http://www.molinspiration.com (accessed on 2 November 2014).
- Sander, T. Molecular Property Explorer. Available online: http://www.organic-chemistry.org/prog/peo (accessed on 2 November 2014).
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langlet, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bi, L.; Wang, W.; Wang, C.; Baudy-Floc’h, M.; Ju, J.; Peng, S. Synthesis and cytotoxic activities of β-carboline amino acid ester conjugates. Bioorg. Med. Chem. 2006, 14, 6998–7010. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 2–10 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).