Physico-Chemical, Thermal, and Electrochemical Analysis of Solid Polymer Electrolyte from Vegetable Oil-Based Polyurethane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyurethane Polymer Electrolytes Matrix
2.3. Characterizations
3. Results and Discussion
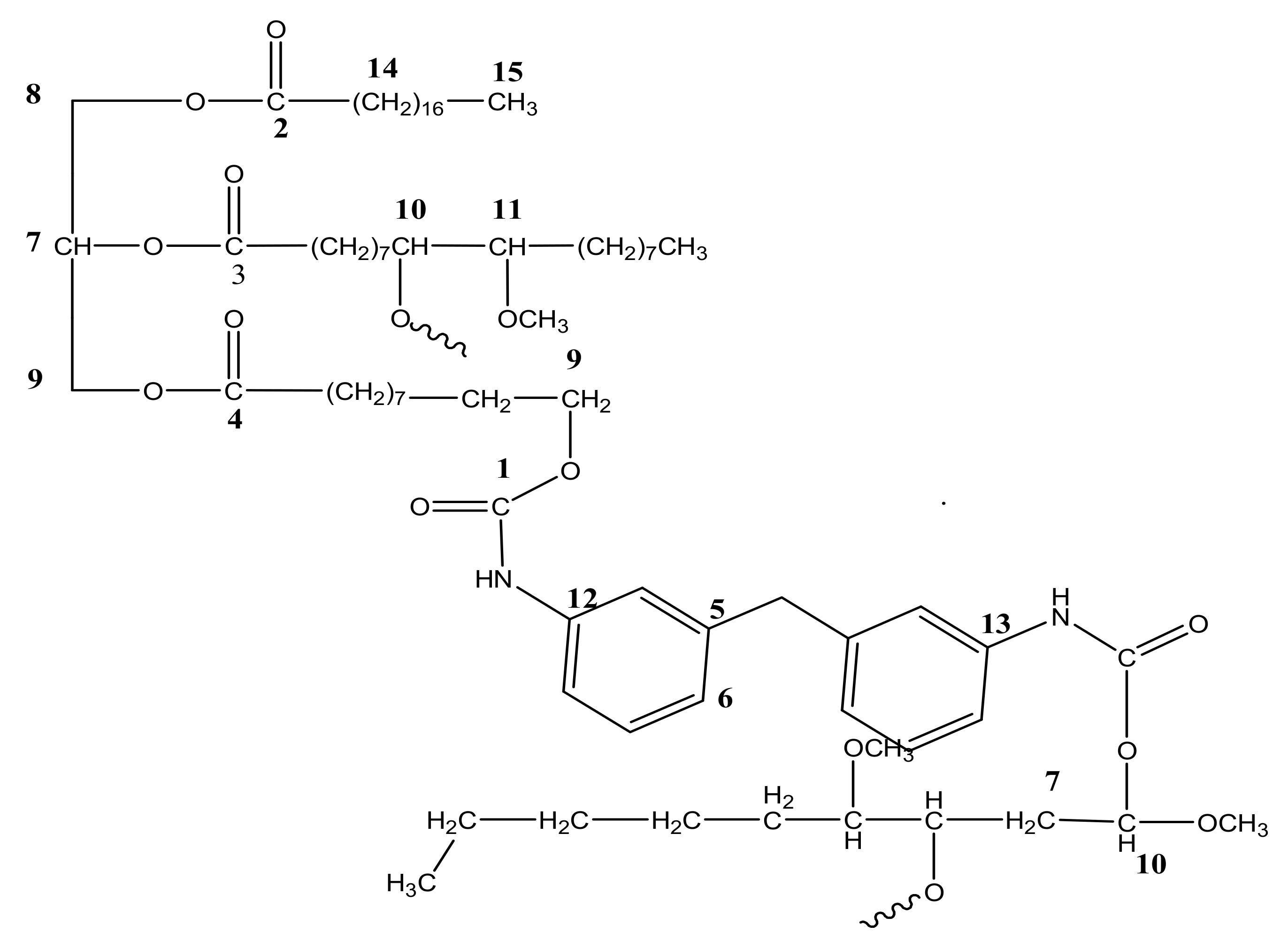
3.1. NMR Analysis of EJO, Polyol, and PU
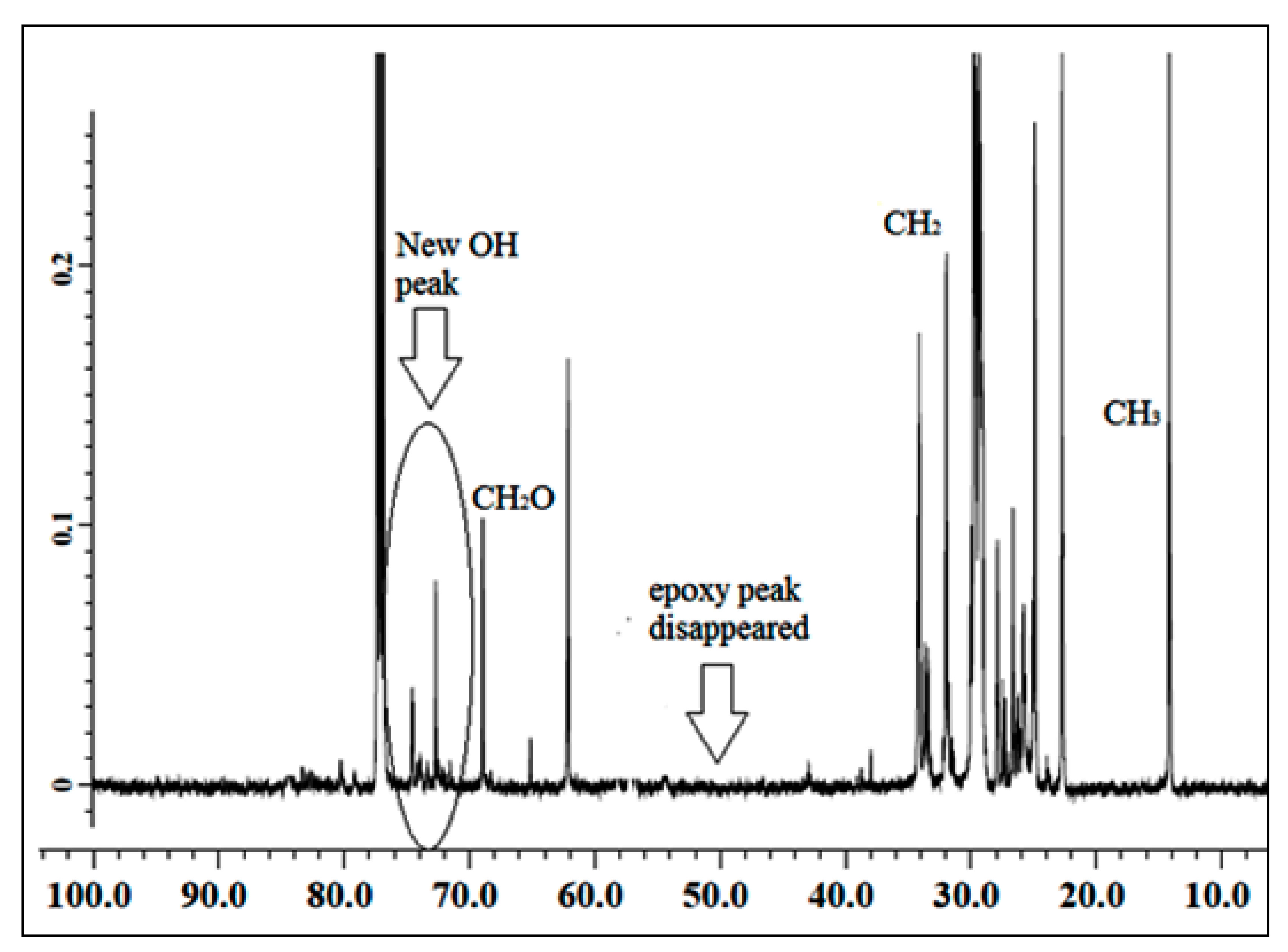
3.1.1. 13C NMR of Jatropha Oil, Epoxidized Jatropha Oil, and Polyol
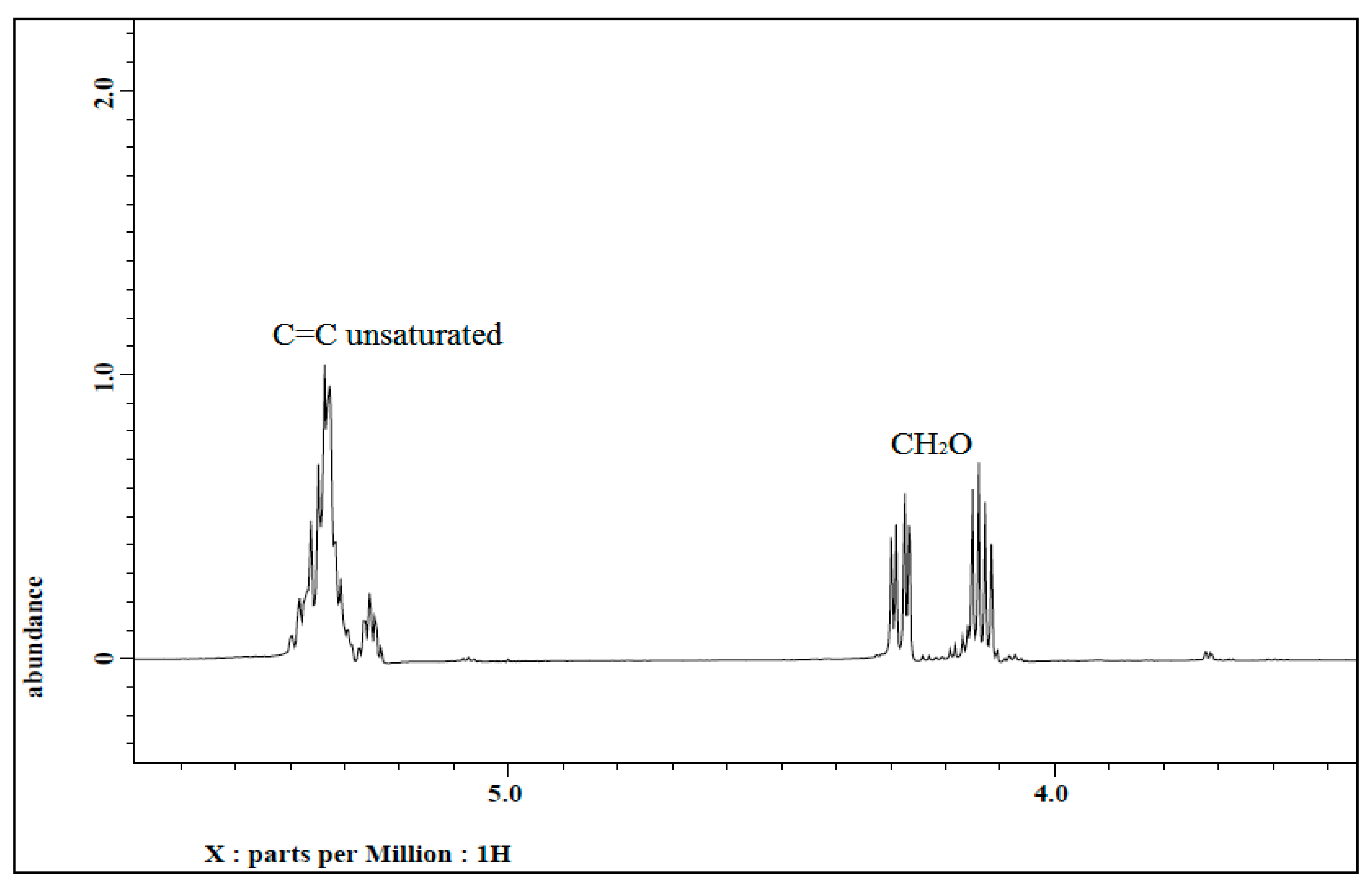
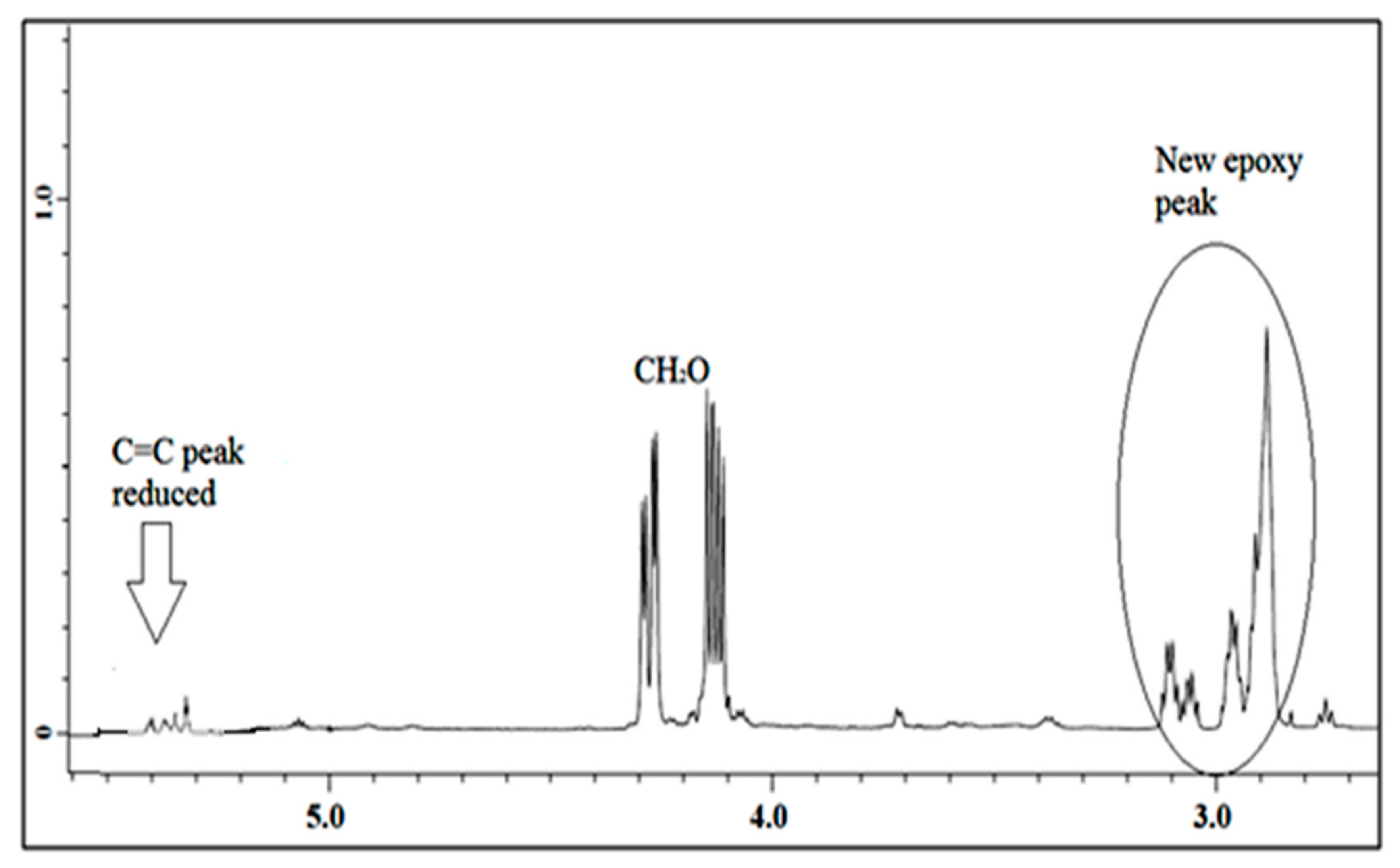
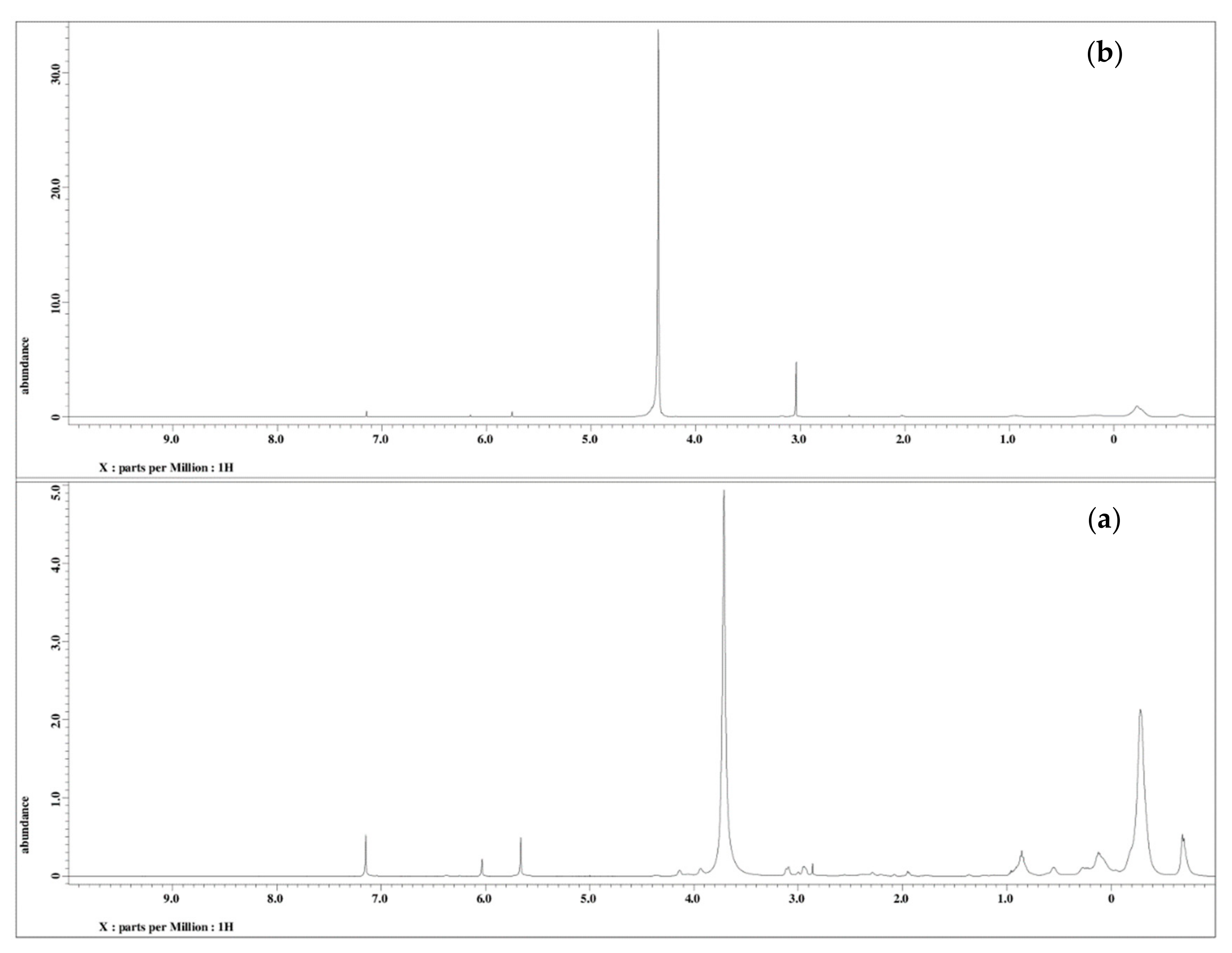
3.1.2. 1H NMR of Jatropha Oil, Epoxidized Jatropha Oil, and Polyol
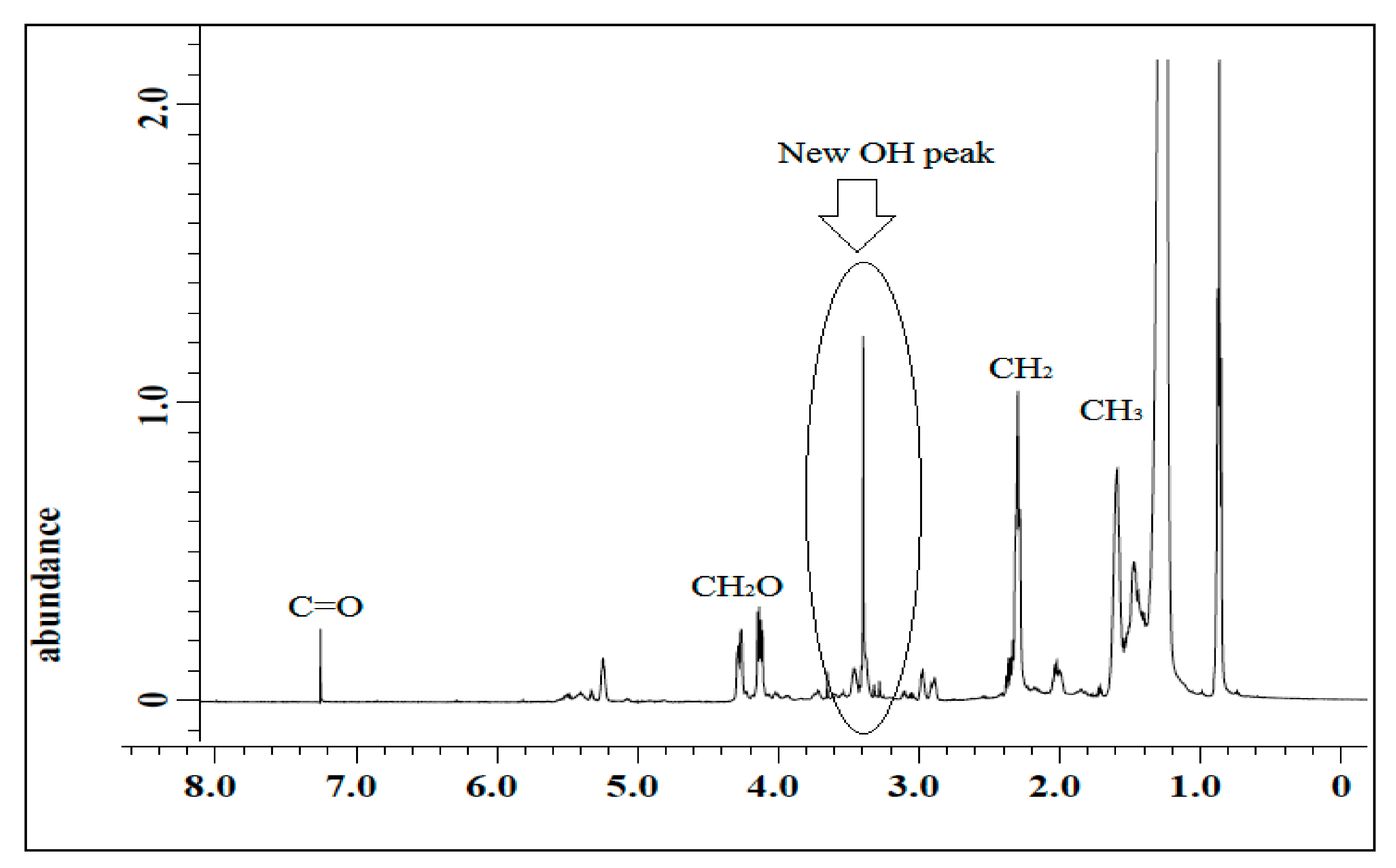
3.1.3. 1H NMR of PU and PU-25LiClO4
3.2. Observation of PU with Different NCO/OH Ratios
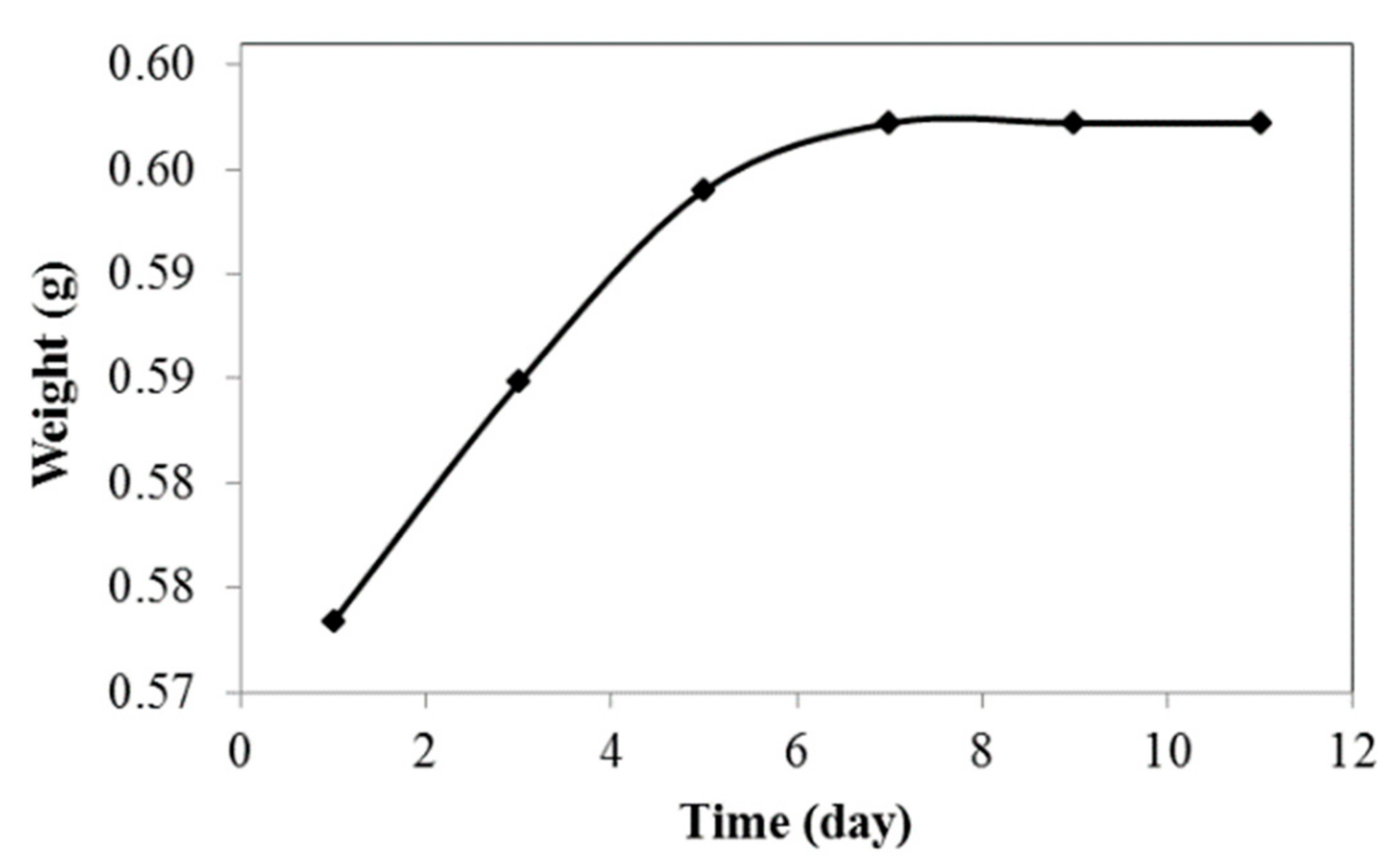
3.3. Water Absorption Test
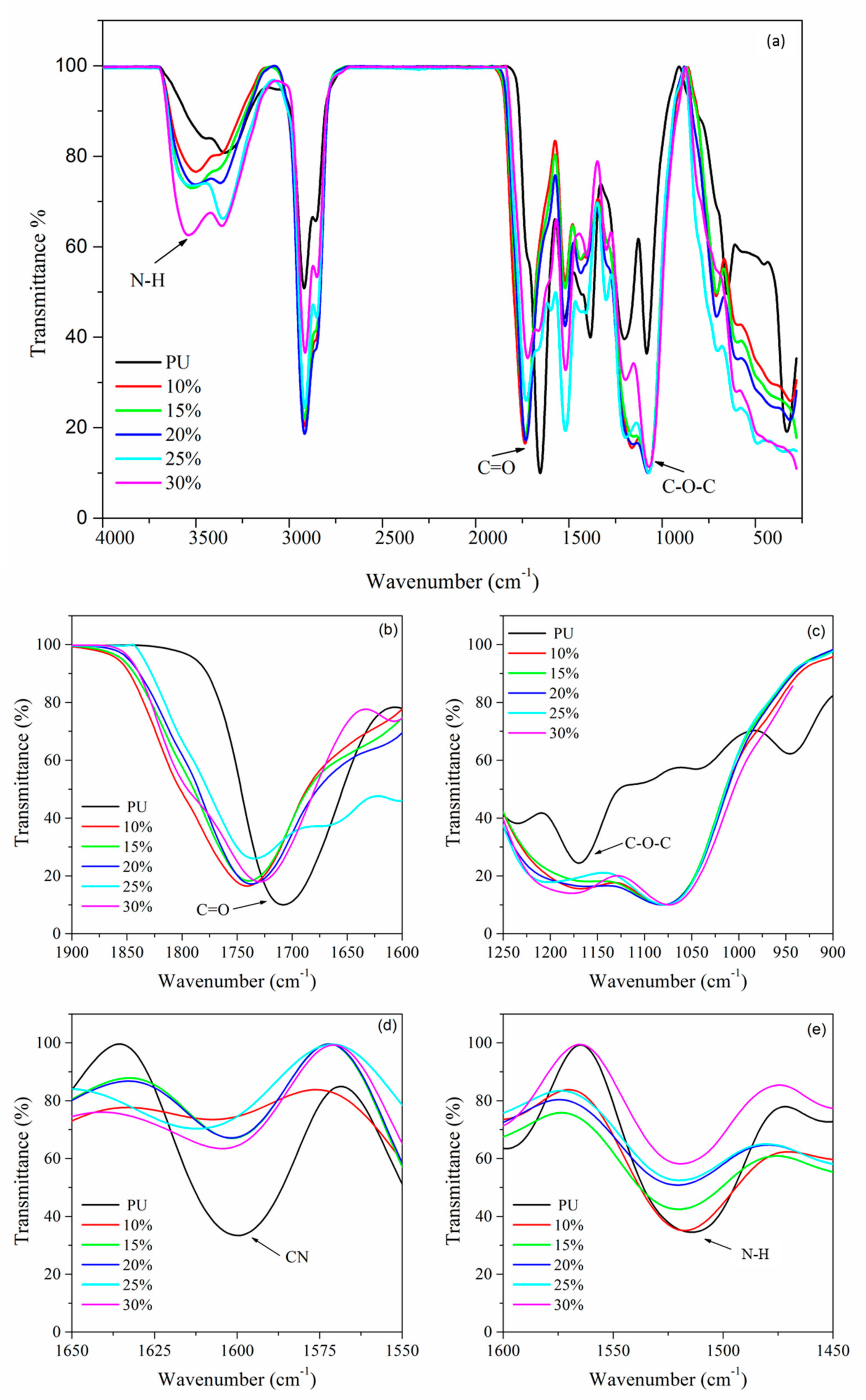
3.4. FTIR Analysis
3.5. EIS Analysis
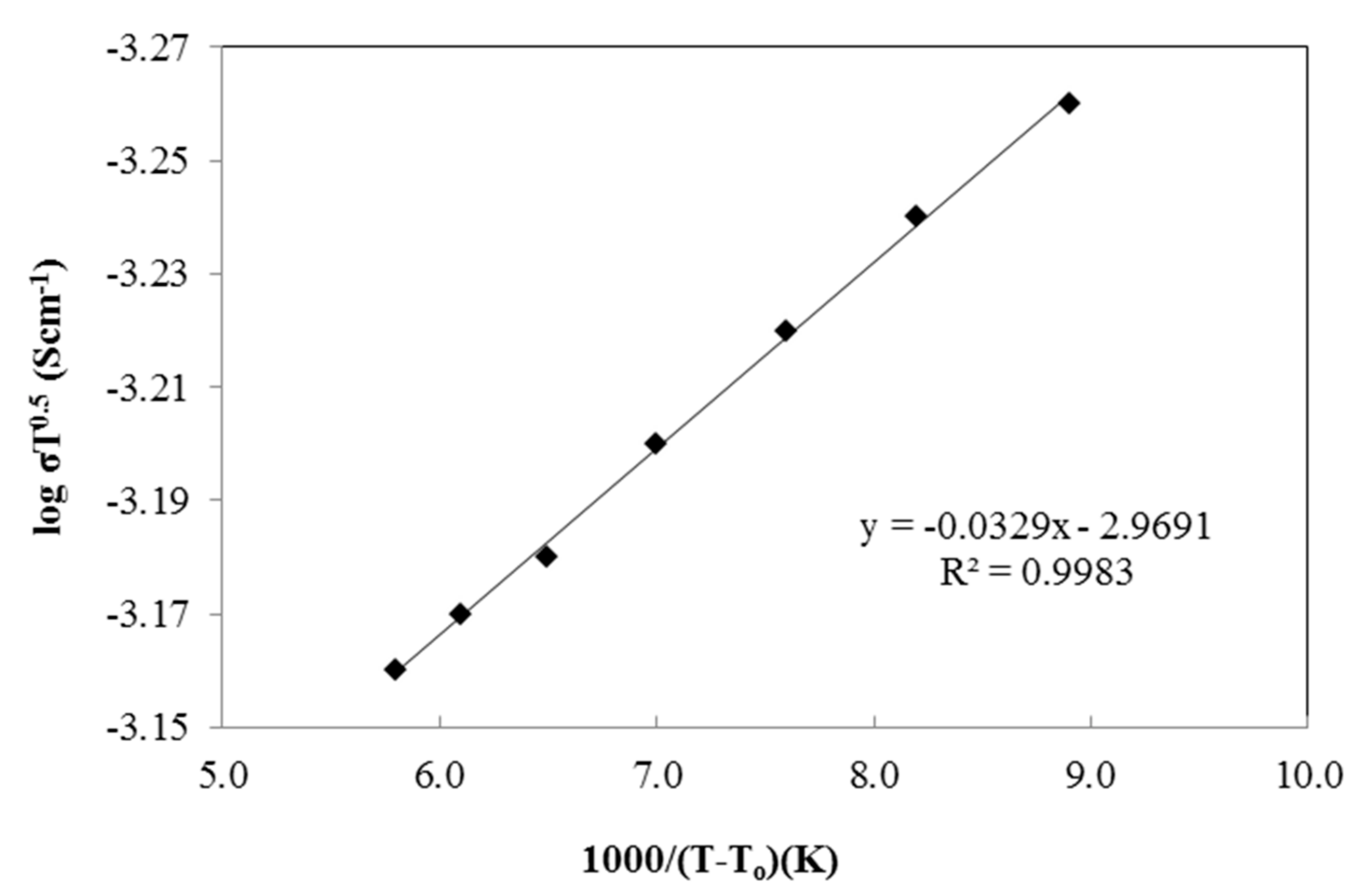
3.6. Temperature Dependence Analysis
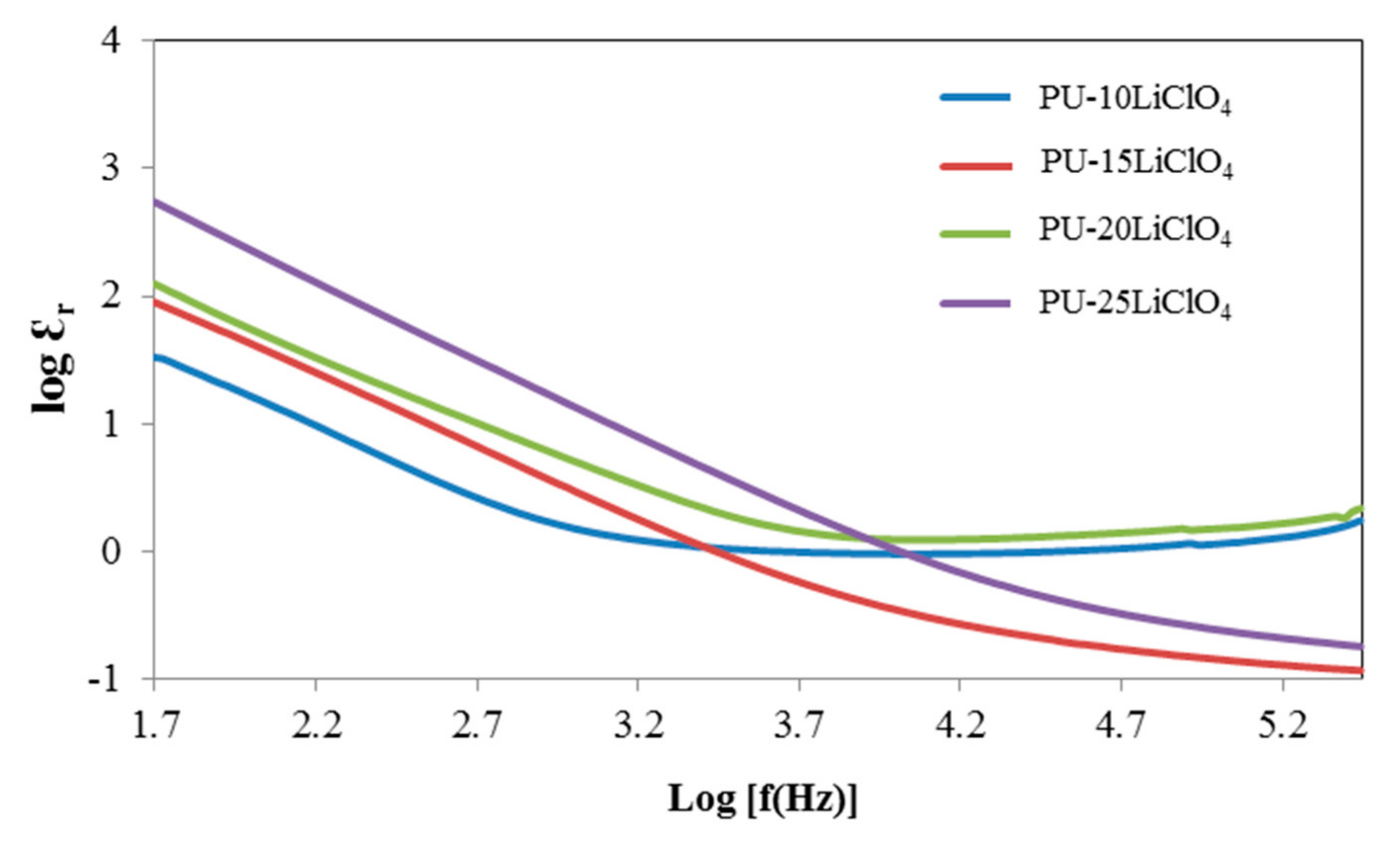
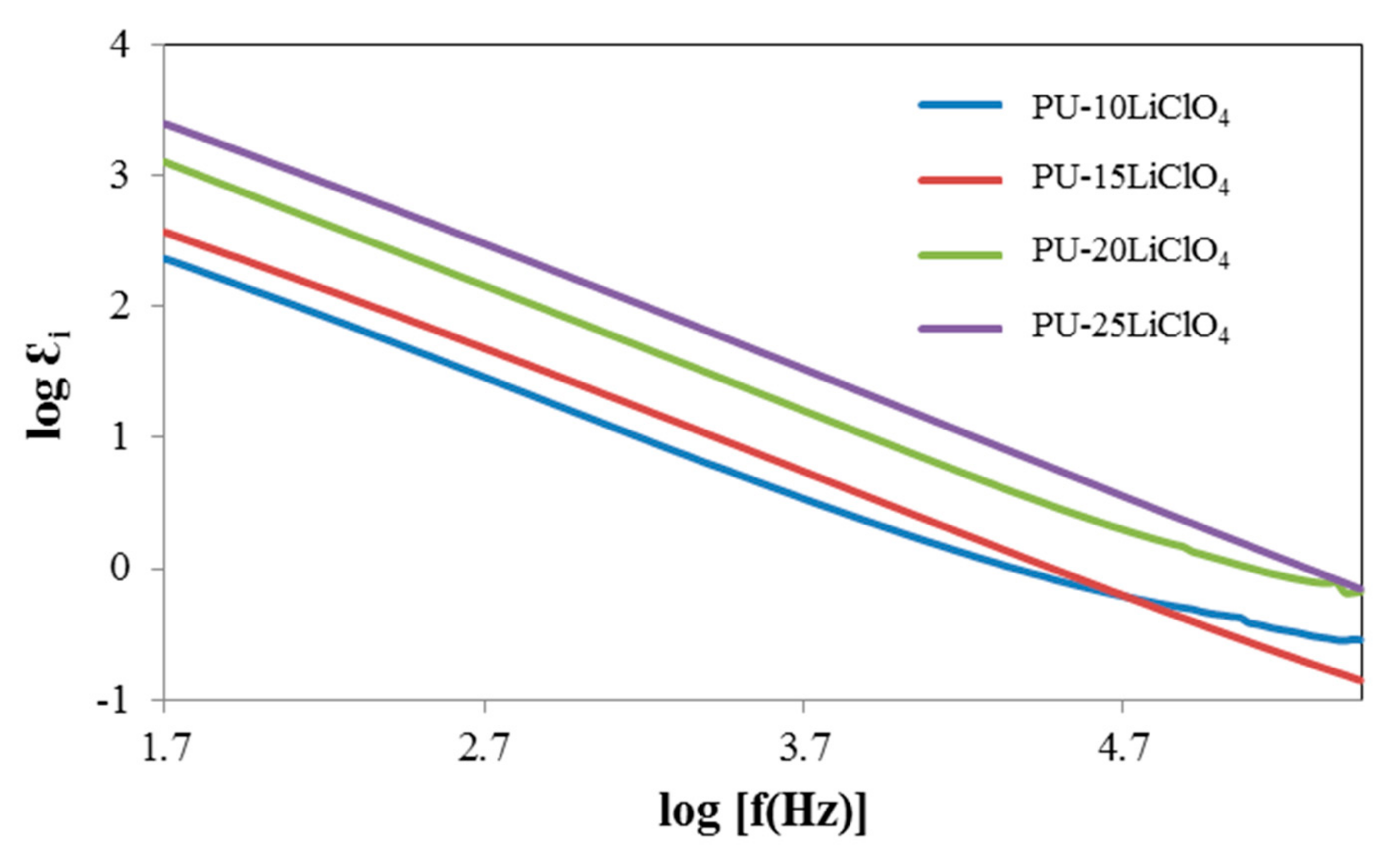
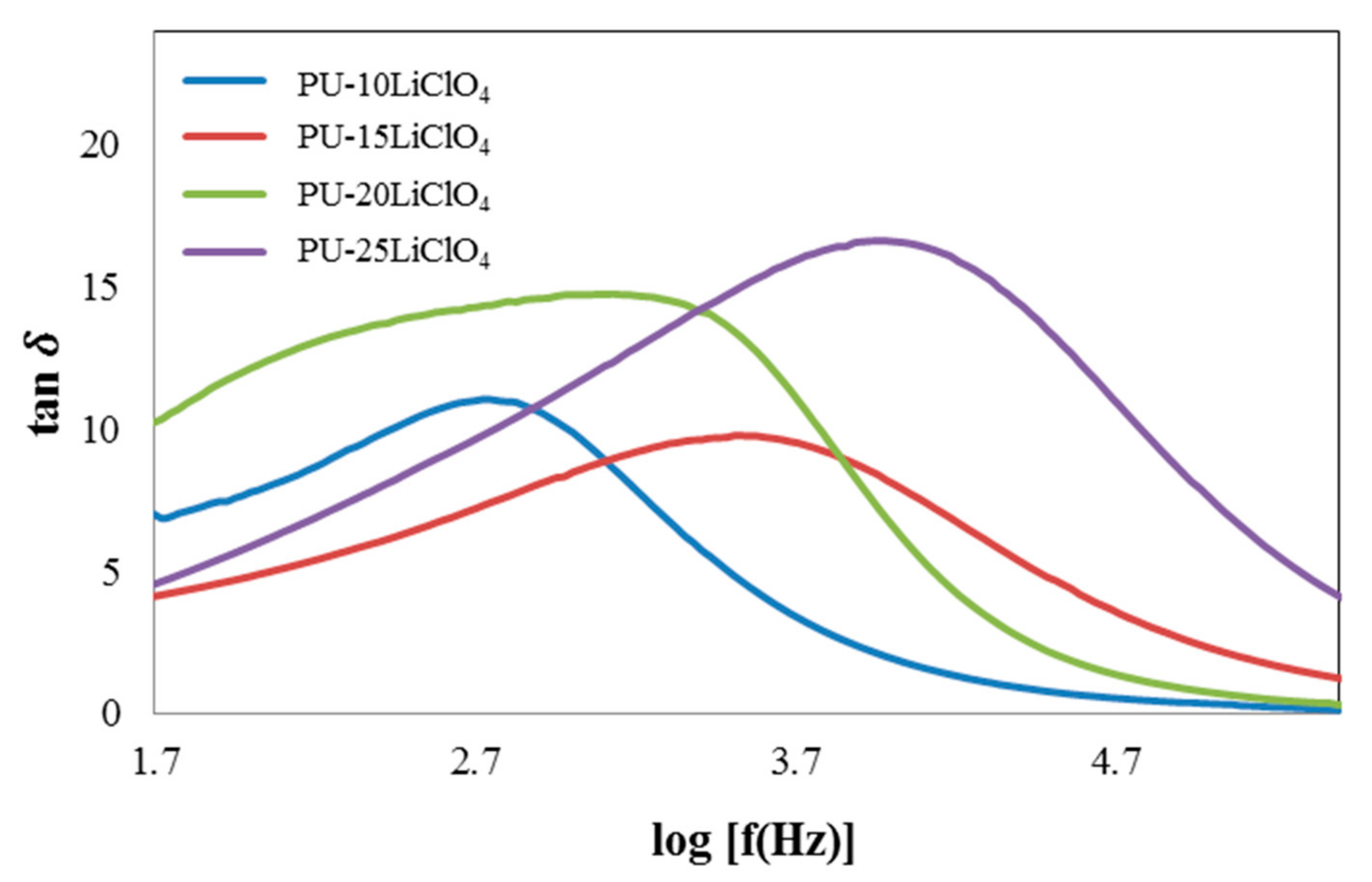
3.7. Dielectric Properties
3.8. DSC Analysis
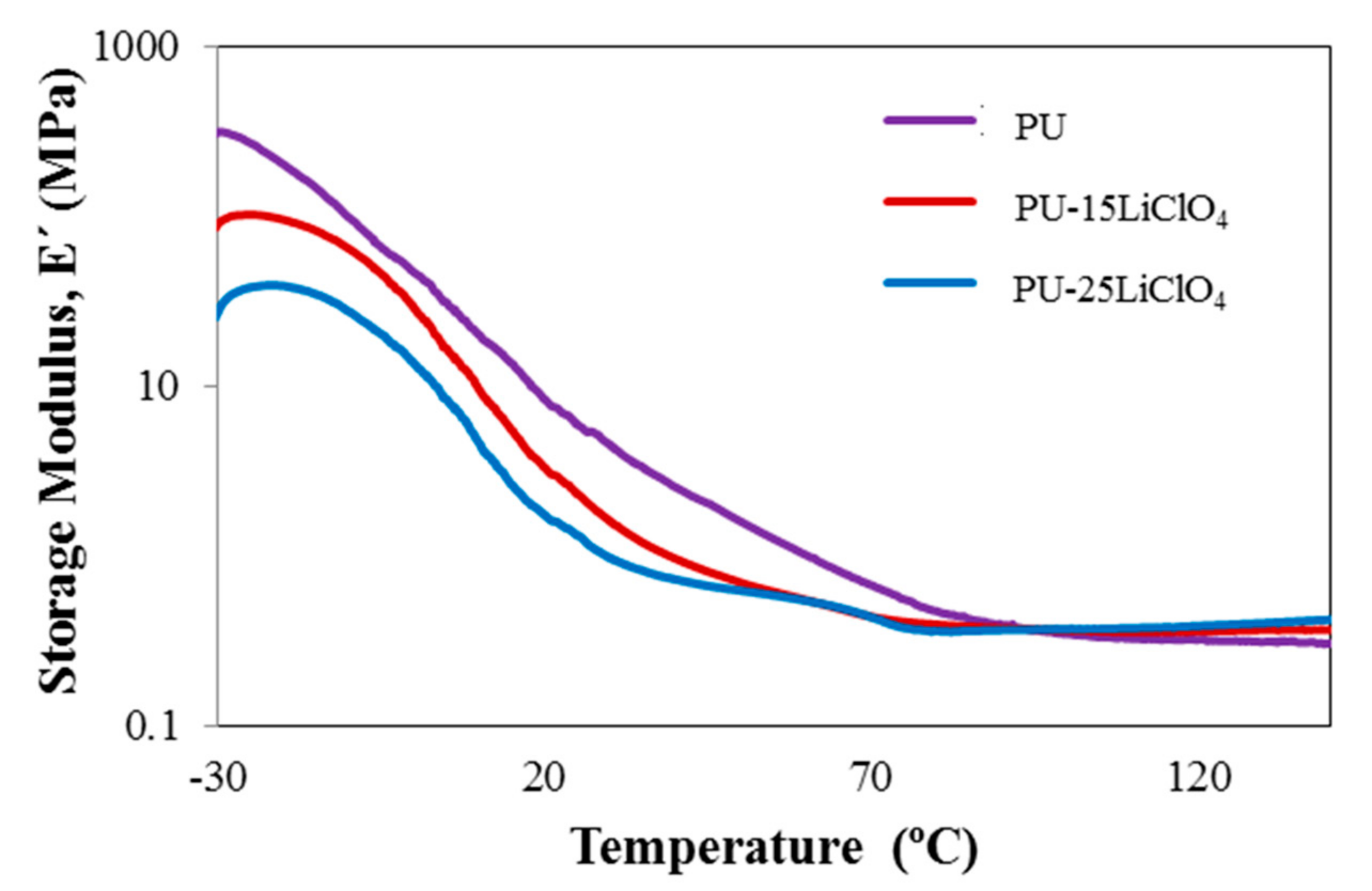
3.9. Dynamic Mechanical Analysis (DMA)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Basri, M.; Taib, E.R.J.; Mamat, S. Physicochemical properties of jatropha oil-based polyol produced by a two steps method. Molecules 2017, 22, 551. [Google Scholar] [CrossRef]
- Taib, E.R.J.; Abdullah, L.C.; Aung, M.M.; Basri, M.; Salleh, M.Z.; Saalah, S.; Mamat, S.; Chee, C.Y.; Wong, J.L. Physico-chemical characterisation of epoxy acrylate resin from jatropha seed oil. Pigment Resin Technol. 2017, 46, 485–495. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Hu, S. Polyols and polyurethanes from vegetable oils and their derivatives. In Bio-Based Polyols and Polyurethanes; SpringerBriefs in Molecular Science; Springer: Cham, Switzerland, 2015; pp. 15–43. [Google Scholar]
- Zhang, J.; Tang, J.J.; Zhang, J.X. Polyols prepared from ring-opening epoxidized soybean oil by a castor oil-based fatty diol. Int. J. Polym Sci. 2015, 1–8. [Google Scholar] [CrossRef]
- Hazmi, A.S.A.; Nurfatmah, N.; Nik, P.; Zulina, P.; Maurad, A.; Abdullah, L.C.; Aung, M.M.; Ahmad, A.; Salleh, M.Z.; Tajau, R.; et al. Understanding intrinsic plasticizer in vegetable oil-based polyurethane elastomer as enhanced biomaterial. J. Therm. Anal. Calorim. 2017, 130, 919–933. [Google Scholar] [CrossRef]
- Wong, C.S.; Badri, K. Chemical analyses of palm kernel oil-based polyurethane prepolymer. Mater. Sci. Appl. 2012, 3, 78–86. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Novel polyurethane produced from canola oil based poly(ether ester) polyols: Synthesis, characterization and properties. Eur. Polym. J. 2012, 48, 2097–2106. [Google Scholar] [CrossRef]
- Shen, Y.; He, J.; Xie, Z.; Zhou, X.; Fang, C.; Zhang, C. Synthesis and characterization of vegetable oil based polyurethanes with tunable thermomechanical performance. Ind. Crops Prod. 2019, 140, 111711. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wei, T. Castor oil-based polyurethane resin for low-density composites with bamboo charcoal. Polymers 2018, 10, 1100. [Google Scholar] [CrossRef]
- Sugita, P.; Alim, M.Z. Synthesis and application of jatropha oil based polyurethane as paint coating material. Makara J. Sci. 2012, 16, 134–140. [Google Scholar]
- Saalah, S.; Chuah, L.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Basri, M.; Taib, E.R.J. Waterborne polyurethane dispersions synthesized from jatropha oil. Ind. Crop. Prod. 2015, 64, 194–200. [Google Scholar] [CrossRef]
- Naiwi, T.S.R.T.; Aung, M.M.; Ahmad, A.; Rayung, M.; Su’ait, M.S.; Yusof, N.; Lae, K.Z.W. Enhancement of plasticizing effect on bio-based polyurethane acrylate solid polymer electrolyte and its properties. Polymers 2018, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Mudri, N.H.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Rayung, M. Comparative study of aromatic and cycloaliphatic isocyanate effects on physico-chemical properties of bio-based polyurethane acrylate coatings. Polymers 2020, 12, 1494. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, M.M.; Aung, M.M.; Chuah, L.A.; Salleh, M.Z. Assessment of corrosion protection and performance of bio-based polyurethane acrylate incorporated with nano zinc oxide coating. Polymer Testing 2020, 89, 106526. [Google Scholar] [CrossRef]
- Rayung, M.; Aung, M.M.; Ahmad, A.; Su’ait, M.S.; Chuah, L.A.; Jamil, N.A.M. Characteristics of ionically conducting jatropha oil-based polyurethane acrylate gel electrolyte doped with potassium iodide. Mater. Chem Phys. 2019, 222, 110–117. [Google Scholar] [CrossRef]
- Islam, M.A.; Ehtisham Khan, M.; Hossain, M.M.; Hasan, M. Electrical conductivity retention and electrochemical activity of CSA doped graphene/gold nanoparticle@ polyaniline composites. Prog. Nat. Sci. Mater. Int. 2016, 26, 341–346. [Google Scholar] [CrossRef]
- Ahmad, Z.; Isa, M.I.N. Ionics conduction via correlated barrier hoping mechanism in CMC-SA solid biopolymer electrolytes. Int. J. Latest Res. Sci. Technol. 2012, 1, 70–75. [Google Scholar]
- Su’ait, M.S.; Jumaah, F.N.; Faizzi, H.M.; Mamat, S.; Ludin, N.A.; Farhan, W.A.; Haron, A.; Atifah, N.; Latif, M.N.; Badri, K.H.; et al. Palm-based polyurethane-ionic liquid gel polymer electrolyte for quasi-solid state dye sensitized solar cell. Ind. Crops Prod. 2018, 113, 406–413. [Google Scholar] [CrossRef]
- Lee, C.S.; Ooi, T.L.; Chuah, C.H.; Ahmad, S. Rigid polyurethane foam production from palm oil-based epoxidized diethanolamides. J. Am. Oil Chem. Soc. 2007, 84, 1161–1167. [Google Scholar] [CrossRef]
- Bao, J.; Shi, G.; Tao, C.; Wang, C.; Zhu, C.; Cheng, L.; Qian, G.; Chen, C. Polycarbonate-based polyurethane as a polymer electrolyte matrix for all-solid-state lithium batteries. J. Power Sources 2018, 389, 84–92. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S. Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr. Polym. 2015, 124, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.Y.A.; Ahmad, A.; Lee, T.K.; Farina, Y.; Dahlan, H.M. LiClO4 salt concentration effect on the properties of PVC-modified low molecular weight LENR50-based solid polymer electrolyte. Polym. Polym. Compos. 2012, 124, 2227–2233. [Google Scholar] [CrossRef]
- Salleh, W.N.F.W.; Tahir, S.M.; Mohamed, N.S. Synthesis of waste cooking oil-based polyurethane for solid polymer electrolyte. Polym. Bull. 2018, 75, 109–120. [Google Scholar] [CrossRef]
- Gray, F.M. Polymer Electrolytes; The Royal Society of Chemistry: Cambridge, UK, 1997; p. 175. [Google Scholar]
- Ehtisham Khan, M.; Mansoob Khan, M.; Moo, H.C. Defected graphene nano-platelets for enhanced hydrophilic nature and visible light-induced photoelectrochemical performances. J. Phys. Chem. Solids 2017, 104, 233–242. [Google Scholar] [CrossRef]
- Hazmi, A.S.A.; Aung, M.M.; Abdullah, L.C.; Salleh, M.Z.; Mahmood, M.H. Producing jatropha oil-based polyol via epoxidation and ring opening. Ind. Crops Prod. 2013, 50, 563–567. [Google Scholar] [CrossRef]
- Mustapa, S.R.; Aung, M.M.; Ahmad, A.; Mansor, A.; Lee, T.K. Preparation and characterization of jatropha oil-based polyurethane as non-aqueous solid polymer electrolyte for electrochemical devices. Electrochim. Acta 2016, 222, 293–302. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F.; Jamil, A.; Bhatti, H.N. Jatropha curcas seed oil as a viable source for biodiesel. Pakintan J. Bot. 2009, 42, 575–582. [Google Scholar]
- Wong, J.L.; Aung, M.M.; Lim, H.N.; Md Jamil, S.N.A. Spectroscopic analysis of epoxidised jatropha oil (EJO) and acrylated epoxidised jatropha oil (AEJO). Trop. Agric. Sci. 2017, 40, 435–448. [Google Scholar]
- Baskaran, R.; Selvasekarapandian, S.; Kuwata, N.; Yoshiki, I.; Kawamura, J.; Hattori, T. 1H NMR, thermal, and conductivity studies on PVAc based gel polymer electrolytes. J. Appl. Polym. Sci. 2008, 110, 1945–1954. [Google Scholar] [CrossRef]
- Kypros, E. Synthesis and Characterization of a Polyurethane Prepolymer for the Development of a Novel Acrylate-Based Polymer Foam. Master’s Thesis of Science, Budapest University of Technology and Economics (BME), Budapest, Hungary, 2014. [Google Scholar]
- Dabagh, M.; Abdekhodaie, M.J.; Khorasani, M.T. Effects of polydimethylsiloxane grafting on the calcification, physical properties, and biocompatibility of polyurethane in a heart valve. J. Appl. Polym. Sci. 2005, 98, 758–766. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Ahmad, A.; Badri, K.H.; Mohamed, N.S.; Rahman, M.Y.A.; Ricardo, C.L.A.; Scardi, P. The potential of polyurethane bio-based solid polymer electrolyte for photoelectrochemical cell application. Int. J. Hydrog. Energy 2014, 39, 3005–3017. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, A.; Mohamed, N.S. Characterization of novel castor oil-based polyurethane polymer electrolytes. Polymers 2015, 7, 747–759. [Google Scholar] [CrossRef]
- Daud, F.N.; Ahmad, A.; Badri, K.H. An Investigation on the properties of palm-based polyurethane solid polymer electrolyte. Int. J. Polym. Sci. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Woo, H.J.; Majid, S.R.; Arof, A.K. Dielectric properties and morphology of polymer electrolyte based on poly(ε-caprolactone) and ammonium thiocyanate. Mater. Chem. Phys. 2012, 134, 755–761. [Google Scholar] [CrossRef]
- Prajakta, J.; Dinesh, K.; Poonam, S.; Gondaliya, N. Effect of nano-filler on electrical properties of PVA-PEO blend polymer electrolyte. Indian J. Pure Appl. Phys. 2013, 51, 350–353. [Google Scholar]
- Kumar, M.; Tiwari, T.; Srivastava, N. Electrical transport behaviour of bio-polymer electrolyte system: Potato starch + ammonium iodide. Carbohydr. Polym. 2012, 88, 54–60. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Choudhary, R.N.P.; Samantaray, B.K. Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int. J. Electrochem. Sci. 2008, 3, 597–608. [Google Scholar]
- Khiar, A.S.A.; Puteh, R.; Arof, A.K. Conductivity studies of a chitosan-based polymer electrolyte. Phys. B Condens. Matter. 2006, 373, 23–27. [Google Scholar] [CrossRef]
- Jimenez, G.; Nobuo, O.; Hidekazu, K.; Takashi, O. Structure and thermal/mechanical properties of poly(ϵ-Caprolactone)-clay blend. J. Appl. Polym. Sci. 1997, 64, 2211–2220. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Choudhary, R.N.P.; Samantaray, B.K.; Karan, N.K.; Katiyar, R.S. Effect of plasticizer on structural and electrical properties of polymer nanocompsoite electrolytes. Int. J. Electrochem. Sci. 2007, 3, 597–608. [Google Scholar]





| Sample | NCO/OH | Observations |
|---|---|---|
| PU/MDI1 | 1:2.5 | Not flexible, too rigid, difficult to remove from the mold |
| PU/MDI2 | 1:3.5 | Not flexible, rigid, difficult to remove from the mold |
| PU/MDI3 | 1:4.5 | Flexible, stable, not sticky, easy to remove from the mold |
| PU/MDI4 | 1:6.5 | Soft, easy to remove from the mold |
| PU/MDI5 | 1:8.5 | Too soft, a bit sticky, difficult to remove from the mold |
| PU/TDI1 | 1:6.0 | Not flexible, too rigid, difficult to remove from the mold |
| PU/TDI2 | 1:12.0 | Flexible, stable, not sticky, easy to remove from the mold |
| PU/TDI3 | 1:15.0 | Soft, easy to remove from the mold |
| Functional Groups | Wavenumber (cm−1) | |||||
|---|---|---|---|---|---|---|
| PU | 10 wt.% | 15 wt.% | 20 wt.% | 25 wt.% | 30 wt.% | |
| C=O | 1740.92 | 1739.97 | 1731.63 | 1729.32 | 1726.74 | 1727.00 |
| C‒N | 1607.36 | 1604.50 | 1601.77 | 1601.56 | 1600.00 | - |
| N‒H | 1528.93 | 1526.62 | 1524.75 | 1523.55 | 1520.27 | 1520.00 |
| C‒O‒C | 1076.00 | 1073.08 | 1072.36 | 1071.64 | 1071.33 | 1070.80 |
| Sample | Conductivity, σ (Scm−1) |
|---|---|
| PU | 3.00 × 10−9 |
| PU-10LiClO4 | 2.23 × 10−8 |
| PU-15LiClO4 | 2.90 × 10−7 |
| PU-20LiClO4 | 2.30 × 10−6 |
| PU-25LiClO4 | 1.29 × 10−4 |
| PU-30LiClO4 | 2.92 × 10−7 |
| Sample | τ (×10−4) s |
|---|---|
| PU–10LiClO4 | 14.00 |
| PU–15LiClO4 | 2.61 |
| PU–20LiClO4 | 4.17 |
| PU–25LiClO4 | 1.04 |
| Sample | Tg | Tm (°C) |
|---|---|---|
| PU | −5.0 | 198.0 |
| PU–15LiClO4 | −12.7 | 196.0 |
| PU–25LiClO4 | −22.1 | 150.3 |
| Sample | Tg by DMA (tan δ Peak), °C | Tg by DSC, °C |
|---|---|---|
| PU | 21.5 | −5.0 |
| PU–15LiClO4 | 13.5 | −12.7 |
| PU–25LiClO4 | 6.2 | −22.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapa, S.R.; Aung, M.M.; Rayung, M. Physico-Chemical, Thermal, and Electrochemical Analysis of Solid Polymer Electrolyte from Vegetable Oil-Based Polyurethane. Polymers 2021, 13, 132. https://doi.org/10.3390/polym13010132
Mustapa SR, Aung MM, Rayung M. Physico-Chemical, Thermal, and Electrochemical Analysis of Solid Polymer Electrolyte from Vegetable Oil-Based Polyurethane. Polymers. 2021; 13(1):132. https://doi.org/10.3390/polym13010132
Chicago/Turabian StyleMustapa, Siti Rosnah, Min Min Aung, and Marwah Rayung. 2021. "Physico-Chemical, Thermal, and Electrochemical Analysis of Solid Polymer Electrolyte from Vegetable Oil-Based Polyurethane" Polymers 13, no. 1: 132. https://doi.org/10.3390/polym13010132
APA StyleMustapa, S. R., Aung, M. M., & Rayung, M. (2021). Physico-Chemical, Thermal, and Electrochemical Analysis of Solid Polymer Electrolyte from Vegetable Oil-Based Polyurethane. Polymers, 13(1), 132. https://doi.org/10.3390/polym13010132





