The Effects of Physicochemical Parameters on Analysed Soil Enzyme Activity from Alice Landfill Site
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.1.1. Collection and Preparation of Soil Sample
2.1.2. Determination of the Physicochemical Properties of Soil
Soil Moisture Content
Soil pH
Soil Electrical Conductivity (Salinity)
Soil Organic Carbon
2.2. Enzyme Activity Assays
2.2.1. Invertase Enzyme
2.2.2. Urease Activity
2.2.3. Phosphatase Activity
2.2.4. Catalase Activity
2.3. Statistical Analysis
3. Results
3.1. Soil Physical Properties
3.2. Soil Enzyme Activity Results
3.3. Correlation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| ANOVA | |||||
| Activity | |||||
| Sum of Squares | df | Mean Square | F | Sig. | |
| Between Groups | 55.406 | 3 | 18.469 | 76.002 | 0.000 |
| Within Groups | 37.908 | 156 | 0.243 | ||
| Total | 93.314 | 159 | |||
| Multiple Comparisons | ||||||
| Dependent Variable: Activity | ||||||
| (I) Groups | (J) Groups | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | |
| Lower Bound | ||||||
| Tukey HSD | URE | INV | −1.386878 * | 0.110227 | 0.000 | −1.67313 |
| CAT | −0.279596 | 0.110227 | 0.058 | −0.56585 | ||
| PHO | −1.197627 * | 0.110227 | 0.000 | −1.48388 | ||
| INV | URE | 1.386878 * | 0.110227 | 0.000 | 1.10062 | |
| CAT | 1.107281 * | 0.110227 | 0.000 | 0.82103 | ||
| PHO | 0.189251 | 0.110227 | 0.318 | −0.09700 | ||
| CAT | URE | 0.279596 | 0.110227 | 0.058 | −0.00666 | |
| INV | −1.107281* | 0.110227 | 0.000 | −1.39354 | ||
| PHO | −0.918030 * | 0.110227 | 0.000 | −1.20428 | ||
| PHO | URE | 1.197627 * | 0.110227 | 0.000 | 0.91137 | |
| INV | −0.189251 | 0.110227 | 0.318 | −0.47550 | ||
| CAT | 0.918030 * | 0.110227 | 0.000 | 0.63178 | ||
| * The mean difference is significant at the 0.05 level. | ||||||
References
- Oishimaya, S.N. What Are the Harmful Effects of Littering? World Atlas-Environment. Available online: www.worldatlas.com (accessed on 8 September 2020).
- Bartkowiak, A.; Lemanowicz, J.; Siwik-Ziomek, A. Assessment of selected heavy metals and enzymes in soil within the range of impact of illegal dumping sites. Int. J. Environ. Res. 2016, 10, 245–254. [Google Scholar]
- Utobo, E.B.; Tewari, L. Soil enzymes as bioindicators of soil ecosystem status. Appl. Ecol. Environ. Res. 2015, 13, 147–169. [Google Scholar]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties, enzyme activity and land use. For. Res. Pap. 2017, 78, 39–44. [Google Scholar] [CrossRef]
- Tan, X.; Xie, B.; Wang, J.; He, W.; Wang, X.; Wei, G. County-Scale Spatial Distribution of Soil Enzyme Activities and Enzyme Activity Indices in Agricultural Land: Implications for Soil Quality Assessment. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Makoi, J.H.; Ndakidemi, P.A. Selected soil enzymes: Examples of their potential roles in the ecosystem. Afr. J. Biotechnol. 2008, 7, 181–191. [Google Scholar]
- Das, S.K.; Varma, A. Role of enzymes in maintaining soil health. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 22–42. [Google Scholar]
- Kujur, M.; Patel, A.K. Kinetics of soil enzyme activities under different ecosystems: An index of soil quality. Chil. J. Agric. Res. 2014, 74, 96–104. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Lee, S.S.; Niazi, N.K.; Lee, Y.H.; Kim, K.-H.; Park, J.-H.; Moon, D.H.; Ok, Y.S. Assessment of Soil Health in Urban Agriculture: Soil Enzymes and Microbial Properties. Sustainability 2017, 9, 310. [Google Scholar] [CrossRef]
- Rao, M.A.; Scelza, R.; Scotti, R.; Gianfreda, L. Role of enzymes in the remediation of polluted environments. J. Soil Sci. Plant Nutr. 2010, 10, 333–353. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Zhang, J.; Chen, Y.; Yang, L.; Li, H.; Wang, L. Factors influencing soil enzyme activity in China’s forest ecosystems. Plant Ecol. 2018, 219, 31–44. [Google Scholar] [CrossRef]
- Steinweg, J.M.; Dukes, J.S.; Paul, E.A.; Wallenstein, M.D. Microbial responses to multi-factor climate change: Effects on soil enzymes. Front. Microbiol. 2013, 4, 146. [Google Scholar] [CrossRef]
- Micuți, M.M.; Bădulescu, L.; Israel-Roming, F. A review on the enzymatic indicators for monitoring soil quality. Sci. Bull. Ser. F Biotechnol. 2017, 21, 223–228. [Google Scholar]
- Iheme, C.I.; Ukairo, D.I.; Ibegbulem, C.O.; Okorom, O.O.; Chibundu, K. Analysis of Enzymes Activities on Domestic Waste Dump Sites. J. Bioremed. Biodegrad. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil enzyme activities as biological indicators of soil health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P.B.; Duffy, S. Evaluation of soil organic carbon and soil moisture content from agricultural fields in Mississippi. Open J. Soil Sci. 2013, 3, 81. [Google Scholar] [CrossRef]
- Li, T.; Meng, L.; Herman, U.; Lu, Z.; Crittenden, J. A survey of soil enzyme activities along major roads in Beijing: The implications for traffic corridor green space management. Int. J. Environ. Res. Public Health 2015, 12, 12475–12488. [Google Scholar] [CrossRef]
- Ukpong, E.C.; Antigha, R.E.; Moses, E.O. Assessment of heavy metals content in soil and plants around waste dumpsites in Uyo Metropolis, Akwa Ibom State. Int. J. Eng. Sci. 2013, 2, 75–86. [Google Scholar]
- Chen, J.; Chen, D.; Xu, Q.; Fuhrmann, J.J.; Li, L.; Pan, G.; Li, Y.; Qin, H.; Liang, C.; Sun, X. Organic carbon quality, composition of main microbial groups, enzyme activities, and temperature sensitivity of soil respiration of an acid paddy soil treated with biochar. Biol. Fertil. Soils 2019, 55, 185–197. [Google Scholar] [CrossRef]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; Volume 19, pp. 101–122. [Google Scholar]
- Walworth, J.L. Review on Soil Sampling and Analysis: College of Agriculture and Life Sciences; The University of Arizona: Tucson, AZ, USA, 2006; pp. 1–5. [Google Scholar]
- Oloyede-Kosoko, S.O.A.; Idowu, A.O.; Ayoni, O.O. Evaluation of water quality with the World Health Organization and Nigeria Industrial Standards using geographic information system. World Sci. News 2015, 24, 18–42. [Google Scholar]
- Datta, R.; Anand, S.; Moulick, A.; Baraniya, D.; Pathan, S.I.; Rejsek, K.; Vranova, V.; Sharma, M.; Sharma, D.; Kelkar, A.; et al. How enzymes are adsorbed on soil solid phase and factors limiting its activity: A Review. Int. Agrophysics 2017, 31, 287. [Google Scholar] [CrossRef]
- Landesman, W.J.; Dighton, J. Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol. Biochem. 2010, 42, 1751–1758. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Soil moisture as a factor affecting the microbiological and biochemical activity of soil. Plant Soil Environ. 2016, 62, 250–255. [Google Scholar] [CrossRef]
- Fekete, I.; Varga, C.; Kotroczó, Z.; Krakomperger, Z.; Tóth, J.A. The effect of temperature and moisture on enzyme activity in Síkfőkút Site. Cereal Res. Commun. 2007, 35, 381–384. [Google Scholar] [CrossRef]
- Addis, W.; Abebaw, A. Analysis of selected physicochemical parameters of soils used for the cultivation of garlic (Allium sativum L.). Sci. Technol. Arts Res. J. 2015, 3, 29–35. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Antonkiewicz, J. Enzymatic Activity of Loess Soil in Organic and Conventional Farming Systems. Agriculture 2020, 10, 135. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Ren, D.; Li, Q.; Zhang, S.; Feng, T. Effect of direct-current electric field on enzymatic activity and the concentration of laccase. Indian J. Microbiol. 2015, 55, 278–284. [Google Scholar] [CrossRef]
- Visconti, F.; de Paz, J.M. Electrical conductivity measurements in agriculture: The assessment of soil salinity. New Trends Dev. Metrol. 2016, 1, 99–126. [Google Scholar]
- Lemanowicz, J.; Siwik-Ziomek, A.; Koper, J. Enzymatic variation of soils exposed to the impact of the soda plant in terms of biochemical parameters. Int. J. Environ. Sci. Technol. 2019, 16, 3309–3316. [Google Scholar] [CrossRef]
- Kühn, J.; Brenning, A.; Wehrhan, M.; Koszinski, S.; Sommer, M. Interpretation of electrical conductivity patterns by soil properties and geological maps for precision agriculture. Precis. Agric. 2009, 10, 490–507. [Google Scholar] [CrossRef]
- Deb, S.; Bhadoria, P.B.S.; Mandal, B.; Rakshit, A.; Singh, H.B. Soil organic carbon: Towards better soil health, productivity, and climate change mitigation. Clim. Chang. Environ. Sustain. 2015, 3, 26–34. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, J.; Li, Z.; Yang, W.; Duan, Y. The influence of soil Physico-chemical properties and enzyme activities on soil quality of saline-alkali agroecosystems in western Jilin Province, China. Sustainability 2018, 10, 1529. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Liang, G.; Qiu, Q.; Liu, J.; Zhou, G.; Yan, J. Effects of precipitation on soil organic carbon fractions in three subtropical forests in southern China. J. Plant Ecol. 2016, 9, 10–19. [Google Scholar] [CrossRef]
- Maharana, J.K.; Patel, A.K. Characterization of Physico-chemical Properties and their Impact on Enzyme Activities in a Chronosequence Coal Mine Overburden Spoil as Biomarker of Reclamation Process. J. Bacteriol. Parasitol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Chineyre, G.C.; Obisike, E.S.; Ugbogu, A.E.; Osuocha, K.U. Studies on municipal solid wastes dumping on soil anions, cations, and selected enzyme activities at Njoku sawmill waste dumpsite, Owerri Municipal, Imo State, Nigeria. Ethiop. J. Environ. Stud. Manag. 2013, 6, 774–783. [Google Scholar] [CrossRef]
- Kumari, J.A.; Rao, P.C.; Padmaja, G.; Madhavi, M. Effect of Physico-chemical properties on soil enzyme urease activity in some soils of Ranga reddy district of Telangana State, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1708–1714. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Kekane, S.S.; Chavan, R.P.; Shinde, D.N.; Patil, C.L.; Sagar, S.S. A review on the physicochemical properties of soil. Int. J. Chem. Stud. 2015, 3, 29–32. [Google Scholar]
- Wang, W.; Page-Dumroese, D.; Lv, R.; Xiao, C.; Li, G.; Liu, Y. Soil enzyme activities in pinus tabuliformis (Carriére) plantations in Northern China. Forests 2016, 7, 112. [Google Scholar] [CrossRef]
- Mondal, N.K.; Pal, K.C.; Dey, M.; Ghosh, S.; Das, C.; Datta, J.K. Seasonal variation of soil enzymes in areas of fluoride stress in Birbhum District, West Bengal, India. J. Taibah Univ. Sci. 2015, 9, 133–142. [Google Scholar] [CrossRef]
- Shi, Z.J.; Lu, Y.; Xu, Z.G.; Fu, S.L. Enzyme activities of urban soils under different land use in Shenzhen city, China. Plant Soil Environ. 2008, 54, 341–346. [Google Scholar] [CrossRef]
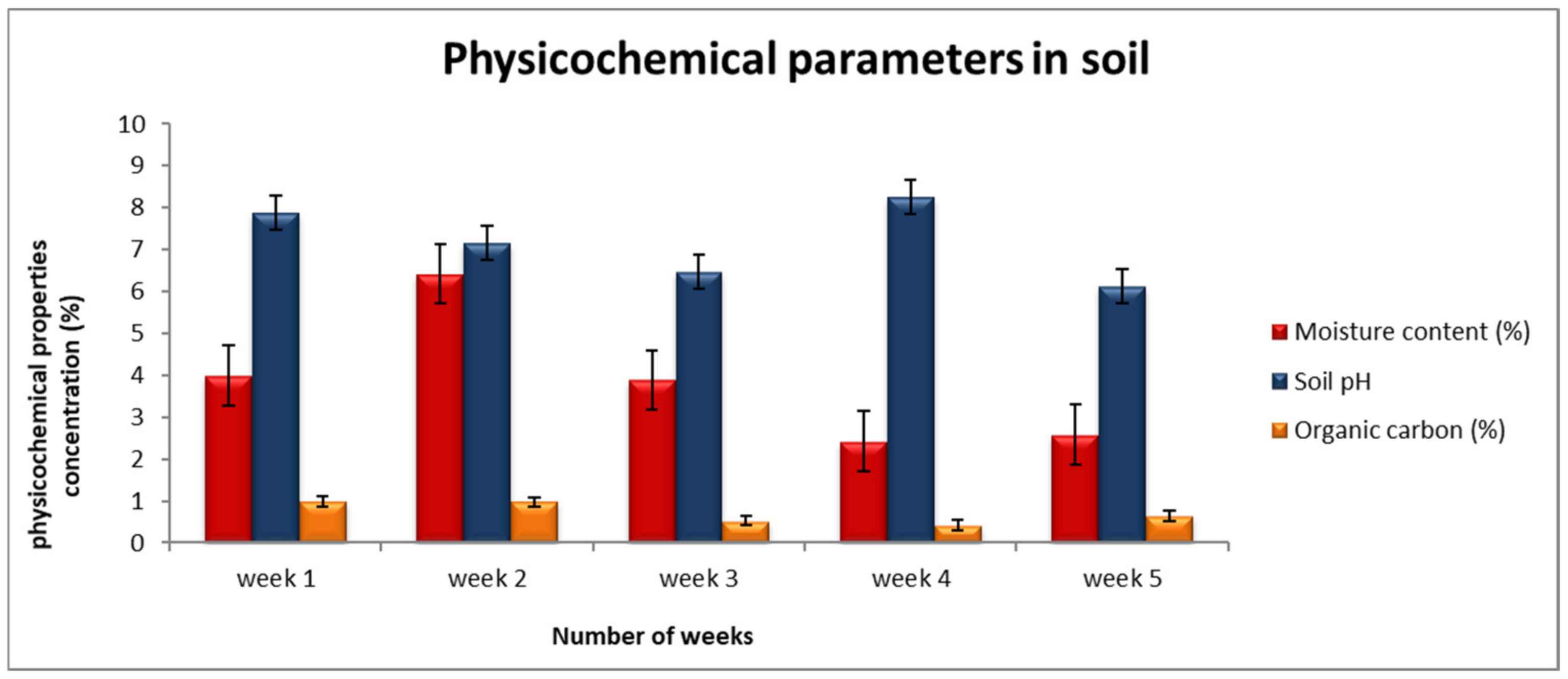
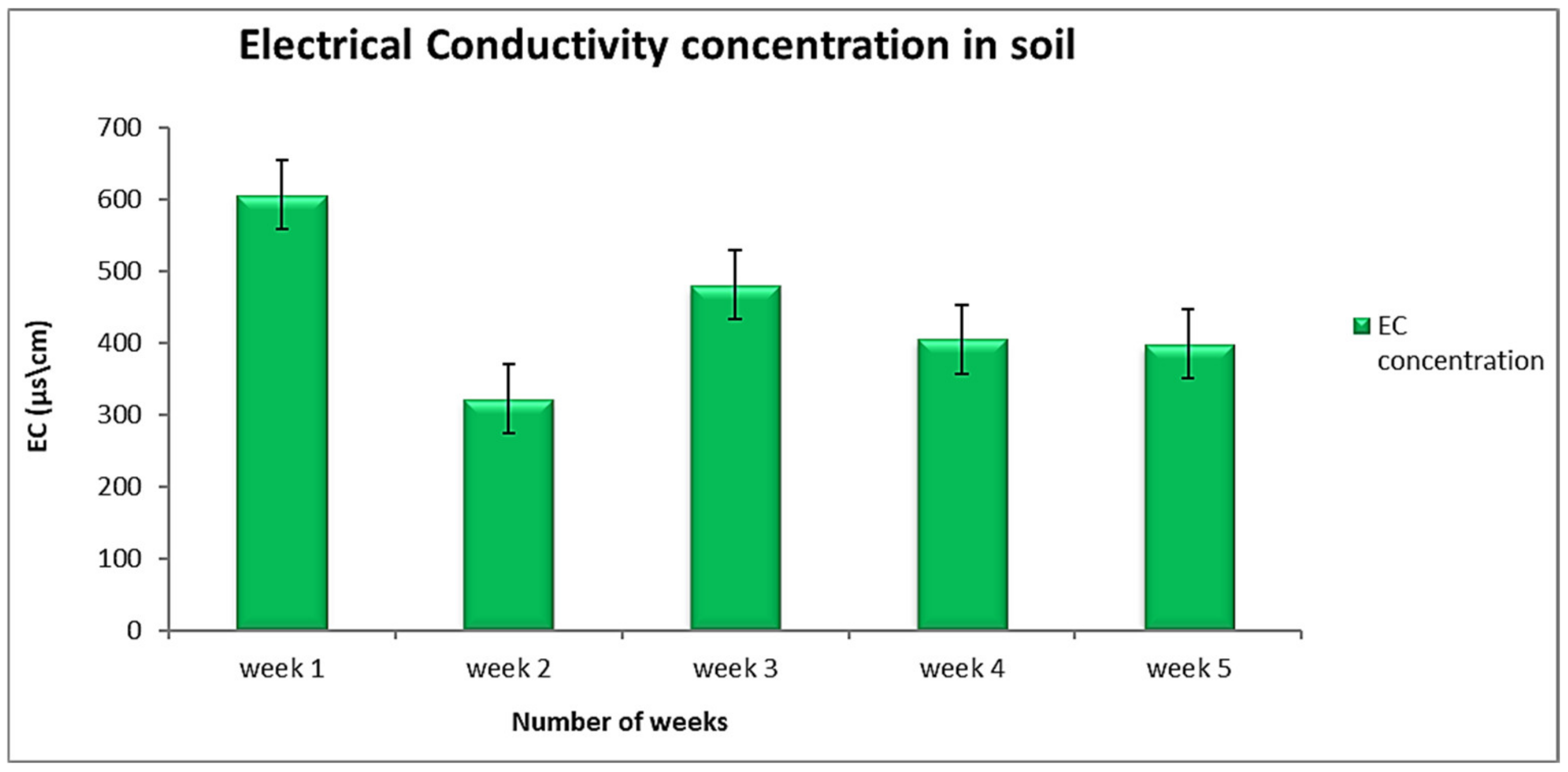

| Moisture Content (%) | pH | Electrical Conductivity (μs/cm) | Organic Carbon (%) | ||
|---|---|---|---|---|---|
| Week 1 | Site 1A | 2.24 ± 1.29 a | 7.65 ± 4.42 a | 1540 ± 889.12 a | 1.13 ± 0.65 a |
| Site 1B | 4.29 ± 2.48 b | 7.67 ± 4.43 a | 2.06 ± 1.19 b | 0.58 ± 0.33 b | |
| Site 1C | 5.64 ± 3.26 ab | 7.86 ± 4.54 a | 735.33 ± 424.54 ab | 1.54 ± 0.89 a | |
| Site 2 | 3.79 ± 2.19 c | 8.29 ± 4.79 b | 149.40 ± 86.26 c | 0.68 ± 0.39 b | |
| Week 2 | Site 1A | 9.09 ± 5.25 bc | 7.11 ±4.10 ab | 567 ± 327.36 cd | 0.35 ±0.20 ab |
| Site 1B | 4.30 ± 2.48 b | 6.89 ± 3.98 c | 391 ± 225.74 d | 1.43 ± 0.83 a | |
| Site 1C | 8.79 ± 5.07 bc | 7.22 ±4.17 ab | 295 ± 170.32 d | 0.48 ±0.28 ab | |
| Site 2 | 3.50 ± 2.02 c | 7.36 ±4.25 ab | 35.4 ± 20.44 b | 0.55 ± 0.32 b | |
| Week 3 | Site 1A | 4.80 ± 2.77 b | 6.92 ± 3.99 c | 327 ± 188.79 d | 0.74 ±0.43 b |
| Site 1B | 3.40 ± 1.96 c | 7.20 ±4.16 ab | 86.3 ± 49.82 b | 0.46 ±0.27 ab | |
| Site 1C | 3.81 ± 2.20 c | 5.61 ±3.24 bc | 1445 ± 834.27 a | 0.65 ± 0.37 b | |
| Site 2 | 3.54 ± 2.04 c | 6.18 ±3.57 bc | 67.5 ± 38.97 b | 0.26 ±0.15 ab | |
| Week 4 | Site 1A | 4.02 ± 2.32 b | 7.95 ± 4.59 a | 691 ± 398.95 ab | 0.44± 0.25 ab |
| Site 1B | 1.18 ± 0.68 d | 8.51 ±4.91 b | 306 ± 176.67 d | 0.48± 0.28 ab | |
| Site 1C | 2.81 ± 1.62 a | 8.40 ± 4.85 b | 77.1 ± 44.51 b | 0.60 ± 0.35 b | |
| Site 2 | 1.69 ± 0.98 d | 8.22 ± 4.75 b | 549 ± 316.96 cd | 0.15 ± 0.09 c | |
| Week 5 | Site 1A | 3.95 ± 2.28 c | 6.07± 3.50 bc | 741 ± 427.82 ab | 1.35 ± 0.78 a |
| Site 1B | 1.36 ± 0.78 d | 5.8 ± 3.36 bc | 296 ± 170.90 d | 0.53 ± 0.31 b | |
| Site 1C | 4.91 ± 2.83 b | 6.35± 3.67 bc | 463 ± 267.31 cd | 0.60 ±0.35 b | |
| Site 2 | 0.12 ± 0.07 cd | 6.21± 3.58 bc | 94.8 ± 54.73 b | 0.08 ±0.05 c |
| Urease (μg NH4- N g−1 Soil h−1) | Invertase (μg Glucose g−1 Soil h−1) | Catalase (mL KMnO4 g−1 Soil h−1) | Phosphatase (μg Phenol g−1 Soil h−1) | ||
|---|---|---|---|---|---|
| Week 1 | Site 1A | 0.50 ± 0.29 a | 1.35 ± 0.78 a | 1.22 ± 0.70 a | 2.85 ± 1.64 a |
| Site 1B | 1.96 ± 1.13 b | 1.00 ± 0.58 a | 0.55 ± 0.32 b | 1.72 ± 0.99 b | |
| Site 1C | 0.82 ± 0.47 a | 0.18 ± 0.11 b | 1.01 ± 0.58 a | 3.49 ± 2.01 ab | |
| Site 2 | 3.21 ± 1.85 ab | 1.08 ± 0.62 a | 0.65 ± 0.37 b | 2.22 ± 1.28 c | |
| Week 2 | Site 1A | 0.15 ± 0.08 c | 0.60 ± 0.34 ab | 0.51 ± 0.29 b | 0.55 ± 0.32 cd |
| Site 1B | 0.08 ± 0.04 c | 1.31 ± 0.76 a | 0.27 ± 0.16 ab | 1.07 ± 0.62 d | |
| Site 1C | 0.10 ± 0.05 c | 2.28 ± 1.31 c | 0.35 ± 0.20 ab | 0.67 ± 0.38 cd | |
| Site 2 | 0.06 ± 0.03 c | 0.79 ± 0.46 ab | 0.15 ± 0.09 c | 0.78 ± 0.45 cd | |
| Week 3 | Site 1A | 0.20 ± 0.12 cd | 1.85 ± 1.07 cd | 0.21 ± 0.12 ab | 1.04 ± 0.60 d |
| Site 1B | 0.98 ± 0.56 a | 2.32 ± 1.34 c | 0.32 ± 0.18 ab | 1.19 ± 0.69 d | |
| Site 1C | 1.44 ± 0.83 a | 2.75 ± 1.89 c | 0.25 ± 0.14 ab | 2.38 ± 1.37 c | |
| Site 2 | 0.82 ± 0.47 a | 2.35 ± 1.36 c | 0.17 ± 0.10 c | 1.27 ± 0.73 d | |
| Week 4 | Site 1A | 0.99 ± 0.57 a | 3.48 ± 2.01 d | 1.45 ± 0.84 a | 2.41 ± 1.39 c |
| Site 1B | 0.32 ± 0.18 cd | 3.26 ± 1.88 d | 0.43 ± 0.25 ab | 2.82 ± 1.63 a | |
| Site 1C | 0.10 ± 0.06 c | 3.66 ± 2.11 d | 0.89 ± 0.51 cd | 2.76 ± 1.59 a | |
| Site 2 | 0.08 ± 0.05 c | 3.48 ± 2.01 d | 0.22 ± 0.13 ab | 3.19 ± 1.84 ab | |
| Week 5 | Site 1A | 0.13 ± 0.07 c | 2.18 ± 1.26 c | 0.75 ± 0.43 b | 3.55 ± 2.05 ab |
| Site 1B | 0.18 ± 0.10 c | 3.14 ± 1.81 d | 0.35 ± 0.20 ab | 2.55 ± 1.47 a | |
| Site 1C | 0.05 ± 0.03 c | 3.37 ± 1.94 d | 0.43 ± 0.25 ab | 3.37 ± 1.95 ab | |
| Site 2 | 0.07 ± 0.04 c | 3.36 ± 1.91 d | 0.28 ± 0.16 ab | 3.85 ± 2.22 e | |
| Soil pH | Electrical Conductivity | Moisture Content | Organic Carbon | |
|---|---|---|---|---|
| Urease | 0.175 | 0.054 | −0.297 | 0.021 |
| Invertase | −0.070 | −0.155 | 0.403 ** | −0.329 * |
| Catalase | 0.257 | 0.089 | 0.443 ** | 0.177 |
| Phosphatase | 0.128 | 0.082 | 0. 146 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maphuhla, N.G.; Lewu, F.B.; Oyedeji, O.O. The Effects of Physicochemical Parameters on Analysed Soil Enzyme Activity from Alice Landfill Site. Int. J. Environ. Res. Public Health 2021, 18, 221. https://doi.org/10.3390/ijerph18010221
Maphuhla NG, Lewu FB, Oyedeji OO. The Effects of Physicochemical Parameters on Analysed Soil Enzyme Activity from Alice Landfill Site. International Journal of Environmental Research and Public Health. 2021; 18(1):221. https://doi.org/10.3390/ijerph18010221
Chicago/Turabian StyleMaphuhla, Nontobeko Gloria, Francis Bayo Lewu, and Opeoluwa Oyehan Oyedeji. 2021. "The Effects of Physicochemical Parameters on Analysed Soil Enzyme Activity from Alice Landfill Site" International Journal of Environmental Research and Public Health 18, no. 1: 221. https://doi.org/10.3390/ijerph18010221
APA StyleMaphuhla, N. G., Lewu, F. B., & Oyedeji, O. O. (2021). The Effects of Physicochemical Parameters on Analysed Soil Enzyme Activity from Alice Landfill Site. International Journal of Environmental Research and Public Health, 18(1), 221. https://doi.org/10.3390/ijerph18010221





