Synthesis of NiCo2O4 Nanostructures and Their Electrochemial Properties for Glucose Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Chemical Bath Synthesis of NiCo-Layered Double Hydroxide and NiCo2O4
2.3. Material Characterizations and Electrochemical Measurements
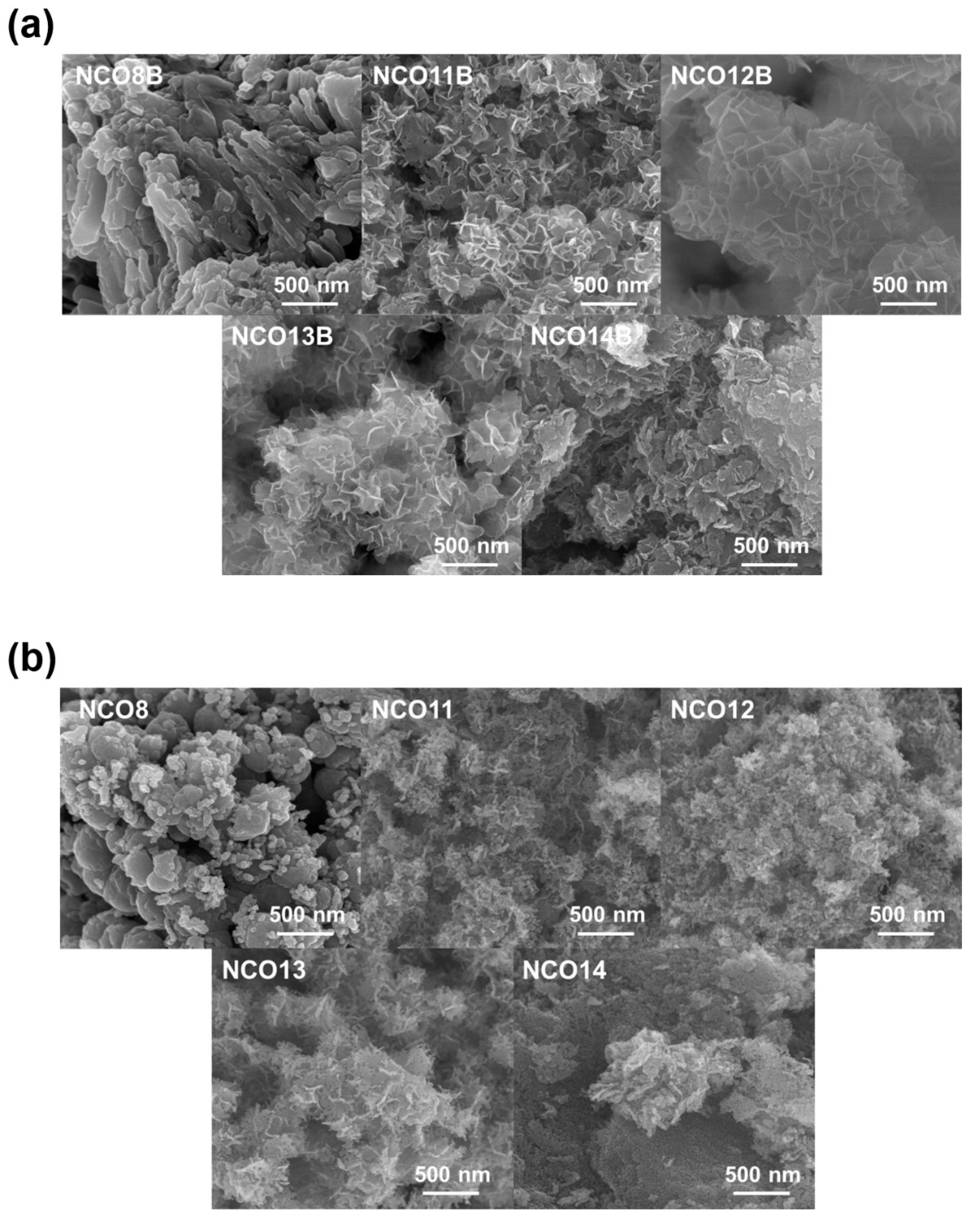
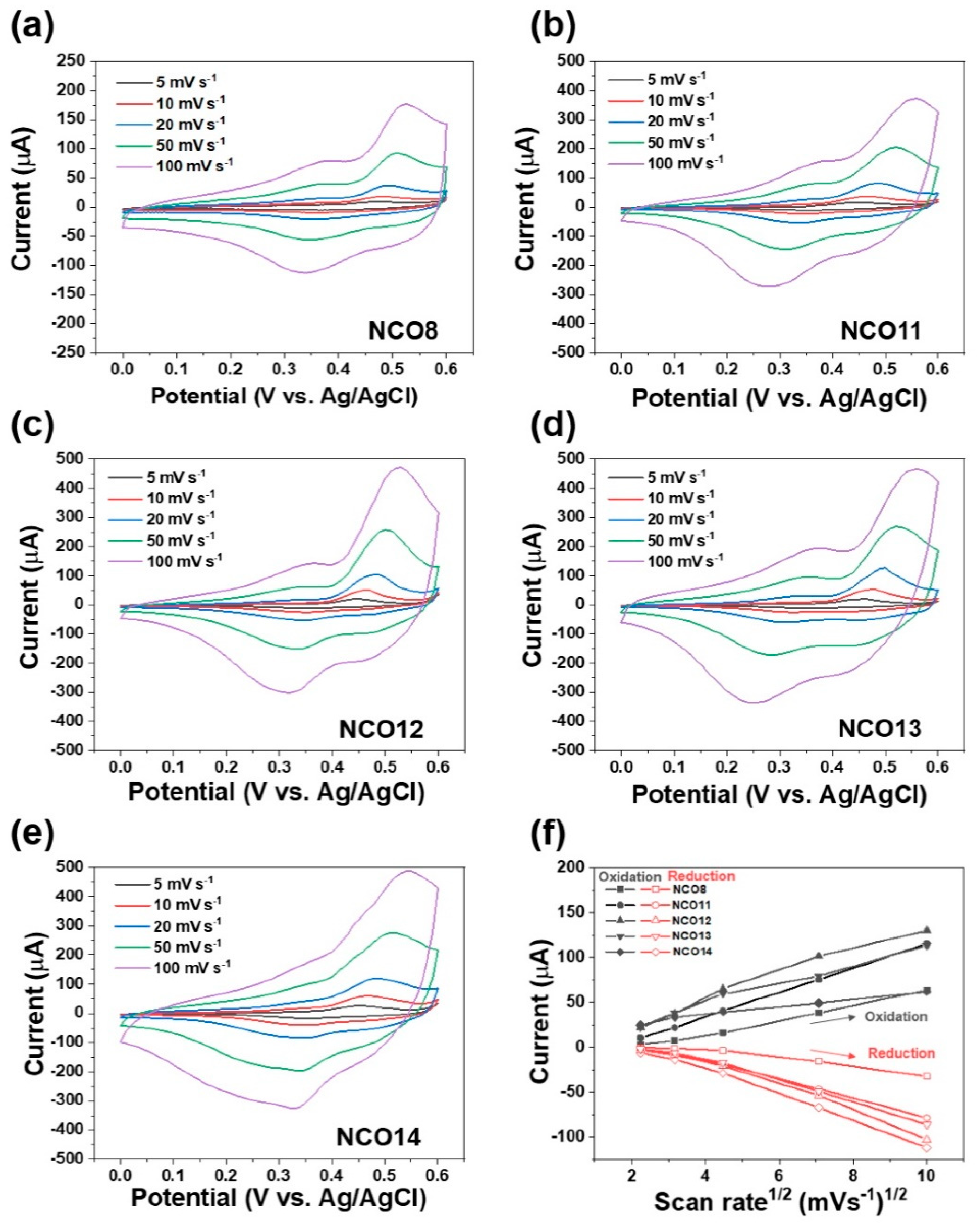
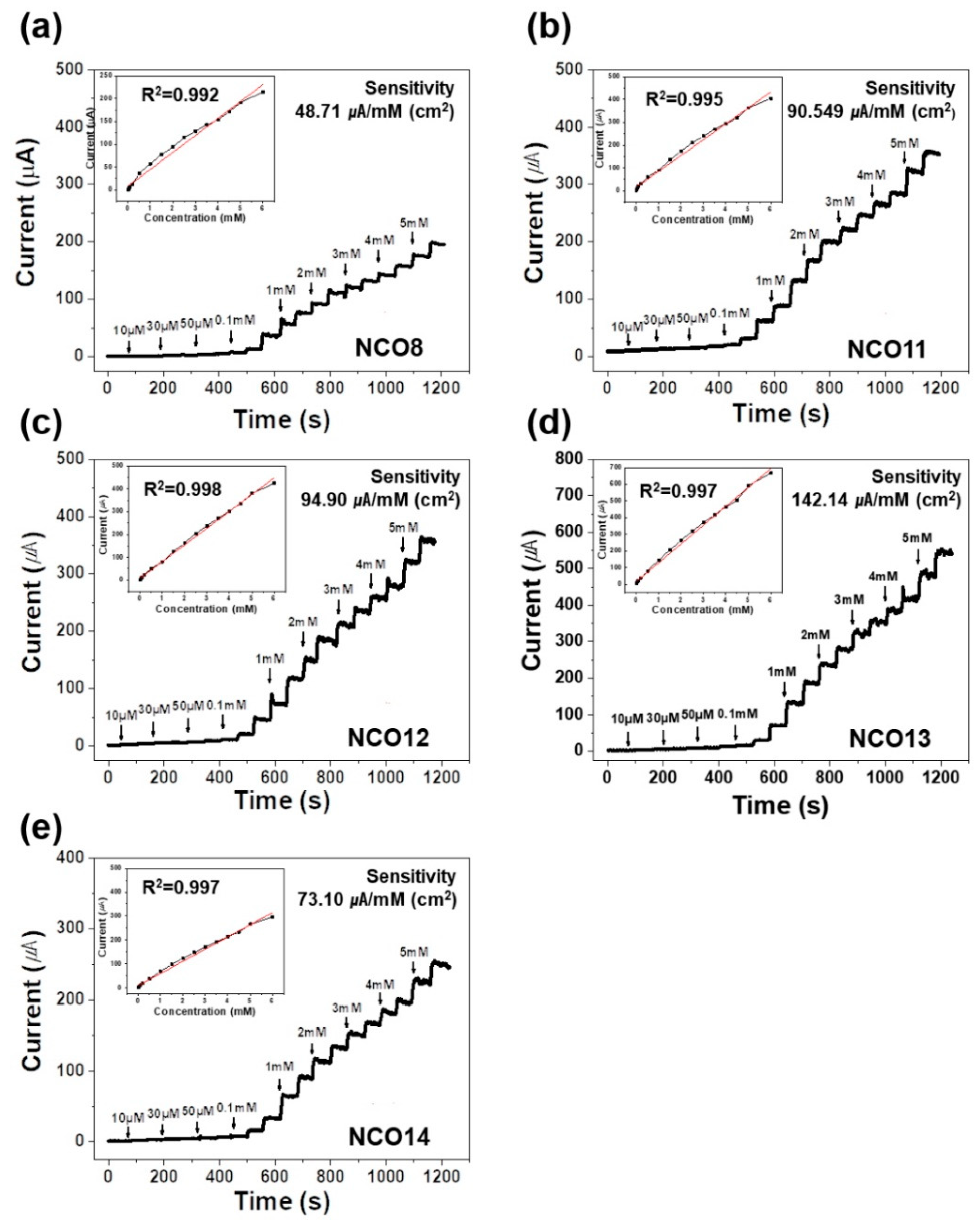
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cho, N.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Song, S.O.; Song, Y.D.; Nam, J.Y.; Park, K.H.; Yoon, J.H.; Son, K.M.; Lim, D.H. Epidemiology of type 1 diabetes mellitus in Korea through an investigation of the national registration project of type 1 diabetes for the reimbursement of glucometer strips with additional analyses using claims data. Diabetes. Metab. J. 2016, 40, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallon, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Ensafi, A.A.; Zandi-Atashbar, N.; Rezaei, B.; Ghiaci, M.; Taghizadeh, M. Silver nanoparticles decorated carboxylate functionalized SiO2, new nanocomposites for non-enzymatic detection of glucose and hydrogen peroxide. Electrochim. Acta. 2016, 214, 208–216. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Yao, H.; Liao, Y.; Lingley, A.R.; Afanasiev, A.; Lähdesmäki, I.; Otis, B.P.; Parviz, B.A. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J. Micromech. Microeng. 2012, 22, 075007. [Google Scholar] [CrossRef]
- Kim, D.M.; Moon, J.M.; Lee, W.C.; Yoon, J.H.; Choi, C.S.; Shim, Y.B. A potentiometric non-enzymatic glucose sensor using a molecularly imprinted layer bonded on a conducting polymer. Biosens. Bioelectron. 2017, 91, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S.; Hur, S.H. A highly sensitive enzyme-free glucose sensor based on Co3O4 nanoflowers and 3D graphene oxide hydrogel fabricated via hydrothermal synthesis. Sens. Actuators B Chem. 2016, 223, 76–82. [Google Scholar]
- Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis. 2013, 13, 983–988. [Google Scholar] [CrossRef]
- Jia, W.Z.; Wang, K.; Zhu, Z.J.; Song, H.T.; Xia, X.H. One-step immobilization of glucose oxidase in a silica matrix on a Pt electrode by an electrochemically induced sol−gel process. Langmuir 2007, 23, 11896–11900. [Google Scholar] [CrossRef] [PubMed]
- Bo, X.; Ndamanisha, J.C.; Bai, J.; Guo, L. Nonenzymatic amperometric sensor of hydrogen peroxide and glucose based on Pt nanoparticles/ordered mesoporous carbon nanocomposite. Talanta. 2010, 82, 85–91. [Google Scholar] [CrossRef]
- Yuan, J.H.; Wang, K.; Xia, X.H. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv Funct Mater. 2005, 15, 803–809. [Google Scholar] [CrossRef]
- Martins, A.; Ferreira, V.; Queirós, A.; Aroso, I.; Silva, F.; Feliu, J. Enantiomeric electro-oxidation of d-and l-glucose on chiral gold single crystal surfaces. Electrochem Commun. 2003, 5, 741–746. [Google Scholar] [CrossRef]
- Niu, X.; Lan, M.; Chen, C.; Zhao, H. Nonenzymatic electrochemical glucose sensor based on novel Pt–Pd nanoflakes. Talanta. 2012, 99, 1062–1067. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhu, Y.; Ji, X. NiCo2O4-based materials for electrochemical supercapacitors. J. Mater. Chem. A 2014, 2, 14759–14772. [Google Scholar] [CrossRef]
- Naik, K.K.; Kumar, S.; Rout, C.S. Electrodeposited spinel NiCo2O4 nanosheet arrays for glucose sensing application. RSC Adv. 2015, 5, 74585–74591. [Google Scholar] [CrossRef]
- Li, G.; Huo, H.; Xu, C. Ni0.31Co0.69S2 nanoparticles uniformly anchored on a porous reduced graphene oxide framework for a high-performance non-enzymatic glucose sensor. J. Mater. Chem. A 2015, 3, 4922–4930. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Gracia, M.; Gautier, J.L.; Rios, E.; Berry, F.J. Characterization of the nickel cobaltite, NiCo2O4, prepared by several methods: An XRD, XANES, EXAFS, and XPS study. J. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Cabo, M.; Pellicer, E.; Rossinyol, E.; Estrader, M.; López-Ortega, A.; Nogués, J.; Baró, M.D. Synthesis of compositionally graded nanocast NiO/NiCo2O4/Co3O4 mesoporous composites with tunable magnetic properties. J. Mater. Chem. 2010, 20, 7021–7028. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Sun, B.; Zhao, P.; Yao, M.; Hu, W.; Komarneni, S. Electrodeposition preparation of NiCo2O4 mesoporous film on ultrafine nickel wire for flexible asymmetric supercapacitors. Chem. Eng. J. 2018, 345, 31–38. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Li, X.; Xu, J. Fabrication of NiCo2O4 nanobelt by a chemical co-precipitation method for non-enzymatic glucose electrochemical sensor application. J. Alloys Compd. 2020, 154796. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Ye, J.; Wei, H.; Hao, J.; Mu, J.; Hussain, S. Facile synthesis of three-dimensional NiCo2O4 with different morphology for supercapacitors. RSC Adv. 2016, 6, 70077–70084. [Google Scholar] [CrossRef]
- Chi, B.; Li, J.; Han, Y.; Chen, Y. Effect of temperature on the preparation and electrocatalytic properties of a spinel NiCo2O4/Ni electrode. Int. J. Hydrog. Energy. 2004, 29, 605–610. [Google Scholar] [CrossRef]
- Guragain, D.; Zequine, C.; Poudel, T.; Neupane, D.; Gupta, R.K.; Mishra, S.R. Influence of Urea on the Synthesis of NiCo2O4 Nanostructure: Morphological and Electrochemical Studies. J. Nanosci. Nanotechnol. 2020, 20, 2526–2537. [Google Scholar] [CrossRef]
- Cui, B.; Lin, H.; Li, J.B.; Li, X.; Yang, J.; Tao, J. Core–ring structured NiCo2O4 nanoplatelets: Synthesis, characterization, and electrocatalytic applications. Adv. Funct. Mater. 2008, 18, 1440–1447. [Google Scholar] [CrossRef]
- Valdez, R.; Grotjahn, D.B.; Smith, D.K.; Quintana, J.M.; Olivas, A. Nanosheets of Co-(Ni and Fe) layered double hydroxides for electrocatalytic water oxidation reaction. Int. J. Electrochem. Sci. 2015, 10, 909–918. [Google Scholar]
- Hu, L.; Wu, L.; Liao, M.; Fang, X. High-Performance NiCo2O4 Nanofilm Photodetectors Fabricated by an Interfacial Self-Assembly Strategy. Adv. Mater. 2011, 23, 1988–1992. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Xiao, X.; Wang, Y. The electrochemical performances of NiCo2O4 nanoparticles synthesized by one-step solvothermal method. Ionics (Kiel) 2017, 23, 2457–2463. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Xu, J.; Jaber, F.; Musharavati, F.; Zalezhad, E.; Liu, J. Synthesis and Characterization of a NiCo2O4@NiCo2O4 Hierarchical Mesoporous Nanoflake Electrode for Supercapacitor Applications. Nanomaterials. 2020, 10, 1292. [Google Scholar] [CrossRef]
- Uke, S.J.; Chaudhari, G.N.; Bodade, A.B.; Mardikar, S.P. Morphology dependant electrochemical performance of hydrothermally synthesized NiCo2O4 nanomorphs. Mater. Sci. Technol. 2020, 3, 289–298. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.; Yuan, Y.; Jiang, Q.; Yan, C. Vertically Aligned NiCo2O4 Nanosheet-Encapsulated Carbon Fibers as a Self-Supported Electrode for Superior Li+ Storage Performance. Nanomaterials. 2019, 9, 1336. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Li, R.; Li, Z. Nickel–cobalt layered double hydroxide ultrathin nanoflakes decorated on graphene sheets with a 3D nanonetwork structure as supercapacitive materials. Mater. Res. Bull. 2014, 51, 97–104. [Google Scholar] [CrossRef]
- Song, Q.; Tang, Z.; Guo, H.; Chan, S.L.I. Structural characteristics of nickel hydroxide synthesized by a chemical precipitation route under different pH values. J. Power Sources. 2002, 112, 428–434. [Google Scholar] [CrossRef]
- Guo, Q.; Zeng, W.; Liu, S.; Li, Y. In situ formation of Co3O4 hollow nanocubes on carbon cloth-supported NiCo2O4 nanowires and their enhanced performance in non-enzymatic glucose sensing. Nanotechnology. 2020, 31, 265501. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, T.; Chen, X.; Lu, Y.; Yang, W. Hierarchical hollow urchin-like NiCo2O4 nanomaterial as electrocatalyst for oxygen evolution reaction in alkaline medium. Power Sources. 2014, 268, 341–348. [Google Scholar] [CrossRef]
- Yu, Z.; Li, H.; Zhang, X.; Liu, N.; Tan, W.; Zhang, X.; Zhang, L. Facile synthesis of NiCo2O4@Polyaniline core–shell nanocomposite for sensitive determination of glucose. Biosens. Bioelectron. 2016, 75, 161–165. [Google Scholar] [CrossRef]
- Hassanpoor, S.; Aghely, F. Hierarchically self-assembled NiCo2O4 nanopins as a high-performance supercapacitor cathodic material: A morphology controlled study. RSC Adv. 2020, 10, 35235–35244. [Google Scholar] [CrossRef]
- Zhan, J.; Cai, M.; Zhang, C.; Wang, C. Synthesis of mesoporous NiCo2O4 fibers and their electrocatalytic activity on direct oxidation of ethanol in alkaline media. Electrochim. Acta. 2015, 154, 70–76. [Google Scholar] [CrossRef]
- Yu, H.; Jin, J.; Jian, X.; Wang, Y.; Qi, G.C. Preparation of cobalt oxide nanoclusters/overoxidized polypyrrole composite film modified electrode and its application in nonenzymatic glucose sensing. Electroanalysis 2013, 25, 1665–1674. [Google Scholar] [CrossRef]
- Pasta, M.; La Mantia, F.; Cui, Y. Mechanism of glucose electrochemical oxidation on gold surface. Electrochim. Acta. 2010, 55, 5561–5568. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Multifunctional porous NiCo2O4 nanorods: Sensitive enzymeless glucose detection and supercapacitor properties with impedance spectroscopic investigations. New J. Chem. 2017, 41, 9299–9313. [Google Scholar] [CrossRef]
- Amin, B.G.; Masud, J.; Nath, M. A non-enzymatic glucose sensor based on a CoNi2Se4/rGO nanocomposite with ultrahigh sensitivity at low working potential. J. Mater. Chem. B. 2019, 7, 2338–2348. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Mao, Y.; Wen, C.; Zhao, P. A simple electrochemical route to access amorphous Co-Ni hydroxide for non-enzymatic glucose sensing. Nanoscale Res. Lett. 2019, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Zhang, J.; Ding, Y.; Gu, S.; Hu, P.; Hu, Z. Rectangular flake-like mesoporous NiCo2O4 as enzyme mimic for glucose biosensing and biofuel cell. Sci. China Mater. 2017, 60, 766–776. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Cao, Y.; Chen, Y.; Peng, J.; Lai, X.; Tu, J. Fast synthesis of porous NiCo2O4 hollow nanospheres for a high-sensitivity non-enzymatic glucose sensor. Appl. Surf. Sci. 2017, 396, 804–811. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.E.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Jiménez-Fiérrez, F.; González-Sánchez, M.I.; Jiménez-Pérez, R.; Iniesta, J.; Valero, E. Glucose Biosensor Based on Disposable Activated Carbon Electrodes Modified with Platinum Nanoparticles Electrodeposited on Poly (Azure A). Sensors. 2020, 20, 4489. [Google Scholar] [CrossRef]
- Gong, X.; Gu, Y.; Zhang, F.; Liu, Z.; Li, Y.; Chen, G.; Wang, B. High-performance non-enzymatic glucose sensors based on CoNiCu alloy nanotubes arrays prepared by electrodeposition. Front. Mater. 2019, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Madhuri, R.; Sharma, P.K. Probing the shape-specific electrochemical properties of cobalt oxide nanostructures for their application as selective and sensitive non-enzymatic glucose sensors. J. Mater. Chem. C. 2017, 5, 6497–6505. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Su, L.; Bellagamba, M.; Zhang, H.; Lei, Y. Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosens. Bioelectron. 2010, 26, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, X.; Ye, Y.; Sun, L.; Song, Y. Nickel-cobalt nanostructures coated reduced graphene oxide nanocomposite electrode for nonenzymatic glucose biosensing. Electrochim. Acta. 2016, 114, 484–493. [Google Scholar] [CrossRef]
- Ma, G.; Yang, M.; Li, C.; Tan, H.; Deng, L.; Xie, S.; Song, Y. Preparation of spinel nickel-cobalt oxide nanowrinkles/reduced graphene oxide hybrid for nonenzymatic glucose detection at physiological level. Electrochim. Acta. 2016, 220, 545–553. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Guo, Z.; Hu, Z.; Xue, Z.; Lu, X. Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively. Biosens. Bioelectron. 2017, 87, 101–107. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Du, X.; Chen, Y.; Dong, W.; Han, B.; Chen, Q. Facile fabrication of Pt-Ag bimetallic nanoparticles decorated reduced graphene oxide for highly sensitive non-enzymatic hydrogen peroxide sensing. Talanta 2016, 159, 280–286. [Google Scholar] [CrossRef]
- Kannan, P.K.; Hu, C.; Morgan, H.; Rout, C.S. One-Step Electrodeposition of NiCo2S4 Nanosheets on Patterned Platinum Electrodes for Non-Enzymatic Glucose Sensing. Chem. Asian. J. 2016, 11, 1837–1841. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Hui, K.S.; Hui, K.N. Flower-like copper cobaltite nanosheets on graphite paper as high-performance supercapacitor electrodes and enzymeless glucose sensors. ACS Appl. Mater. Interfaces. 2016, 8, 3258–3267. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Zhang, L.; Zhang, H.; Lei, Y. Preparation, characterization and application of novel conductive NiO–CdO nanofibers with dislocation feature. J. Mater. Chem. 2012, 22, 980–986. [Google Scholar] [CrossRef]


| Electrode | Method | Sensitivity (μA/mM (cm2)) | Linear Range (mM) | Correlation Coefficient (R2) | Detection Limit (μM) | Refs. |
|---|---|---|---|---|---|---|
| NCO8 | CA | 48.71 | 0.01–6 | 0.992 | 0.539 | This work |
| NCO11 | CA | 90.549 | 0.01–6 | 0.995 | 0.393 | This work |
| NCO12 | CA | 94.90 | 0.01–6 | 0.998 | 0.0503 | This work |
| NCO13 | CA | 142.14 | 0.01–6 | 0.997 | 0.0433 | This work |
| NCO14 | CA | 73.10 | 0.01–6 | 0.996 | 0.0475 | This work |
| NiCo2O4/CNT | CA | 66.15 | 0.02–12.12 | 0.99 | 5 | [52] |
| NiCo2O4/rGO | CA | 548.9 | 0.005–8.56 | 0.99 | 2 | [53] |
| CuCo2O4 | CA | 3.625 | Up to 0.32 | - | 5 | [56] |
| NiO | CA | 32.91 | Up to 1.94 | 1.28 | [57] | |
| Co3O4 | CA | 36.25 | Up to 2.04 | 0.97 | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, K.-b.; Park, K.R.; Kim, K.M.; Hyun, S.-k.; Jeon, J.-e.; Song, Y.S.; Park, S.-k.; Moon, K.-i.; Ahn, C.; Lim, S.-c.; et al. Synthesis of NiCo2O4 Nanostructures and Their Electrochemial Properties for Glucose Detection. Nanomaterials 2021, 11, 55. https://doi.org/10.3390/nano11010055
Jang K-b, Park KR, Kim KM, Hyun S-k, Jeon J-e, Song YS, Park S-k, Moon K-i, Ahn C, Lim S-c, et al. Synthesis of NiCo2O4 Nanostructures and Their Electrochemial Properties for Glucose Detection. Nanomaterials. 2021; 11(1):55. https://doi.org/10.3390/nano11010055
Chicago/Turabian StyleJang, Kyu-bong, Kyoung Ryeol Park, Kang Min Kim, Soong-keun Hyun, Jae-eun Jeon, Young Sik Song, Soo-keun Park, Kyoung-il Moon, Chisung Ahn, Sung-chul Lim, and et al. 2021. "Synthesis of NiCo2O4 Nanostructures and Their Electrochemial Properties for Glucose Detection" Nanomaterials 11, no. 1: 55. https://doi.org/10.3390/nano11010055
APA StyleJang, K.-b., Park, K. R., Kim, K. M., Hyun, S.-k., Jeon, J.-e., Song, Y. S., Park, S.-k., Moon, K.-i., Ahn, C., Lim, S.-c., Lee, J., Kim, J. C., Han, H., & Mhin, S. (2021). Synthesis of NiCo2O4 Nanostructures and Their Electrochemial Properties for Glucose Detection. Nanomaterials, 11(1), 55. https://doi.org/10.3390/nano11010055






