Abstract
The acute respiratory syndrome caused by the novel coronavirus (SARS-CoV-2) caused severe panic all over the world. The coronavirus (COVID-19) outbreak has already brought massive human suffering and major economic disruption and unfortunately, there is no specific treatment for COVID-19 so far. Herbal medicines and purified natural products can provide a rich resource for novel antiviral drugs. Therefore, in this review, we focused on the sterols and triterpenes as potential candidates derived from natural sources with well-reported in vitro efficacy against numerous types of viruses. Moreover, we compiled from these reviewed compounds a library of 162 sterols and triterpenes that was subjected to a computer-aided virtual screening against the active sites of the recently reported SARS-CoV-2 protein targets. Interestingly, the results suggested some compounds as potential drug candidates for the development of anti-SARS-CoV-2 therapeutics.
1. Introduction
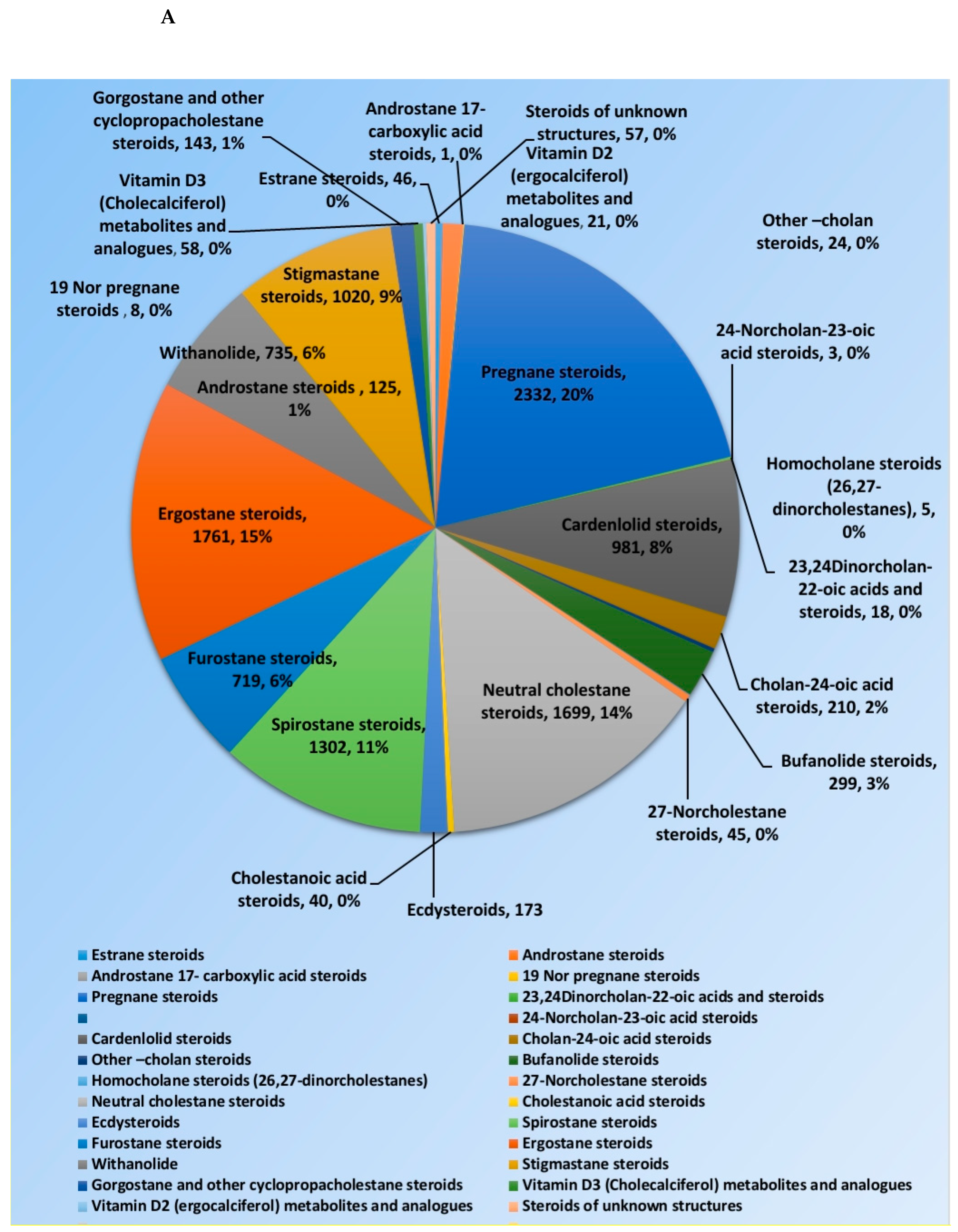
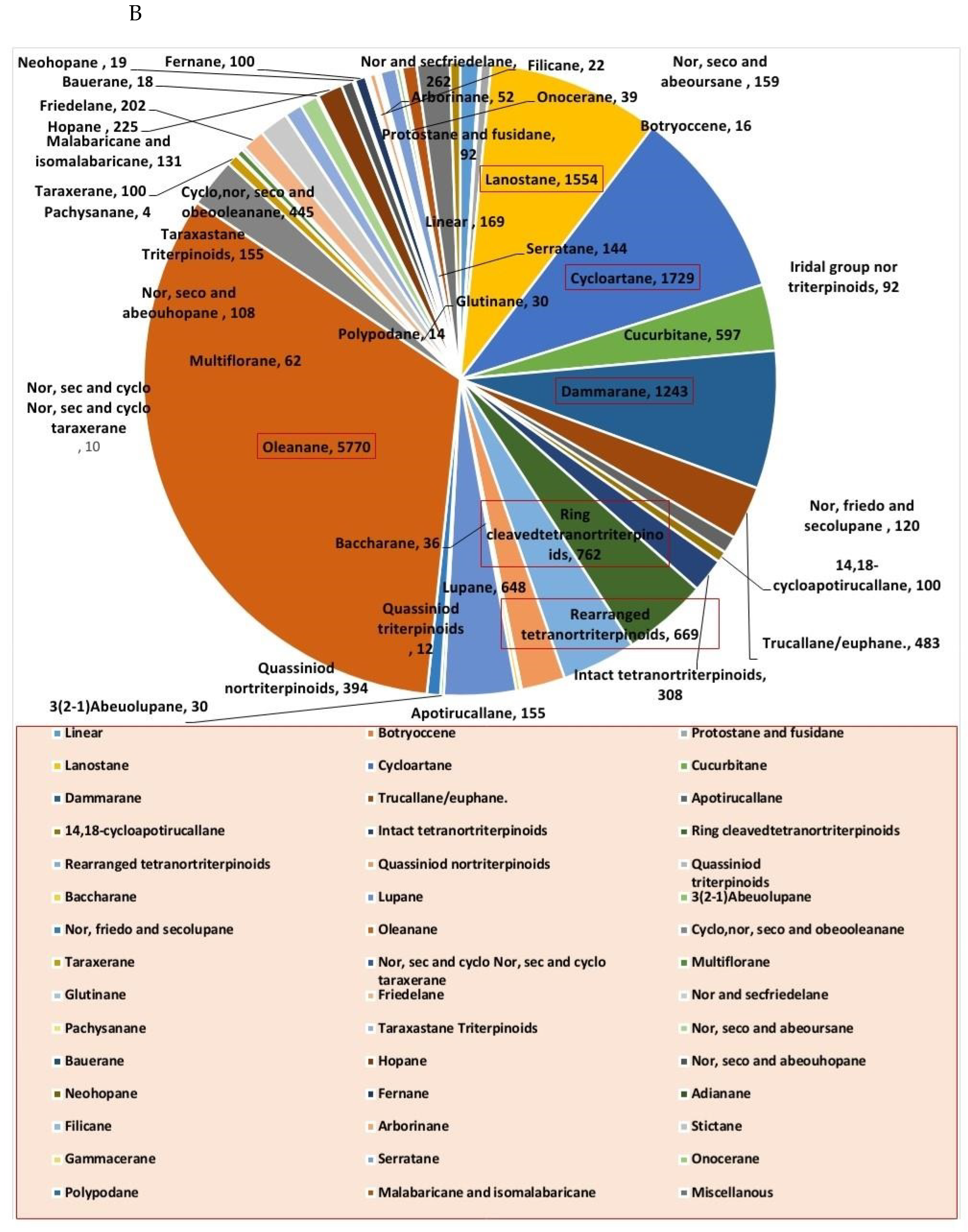
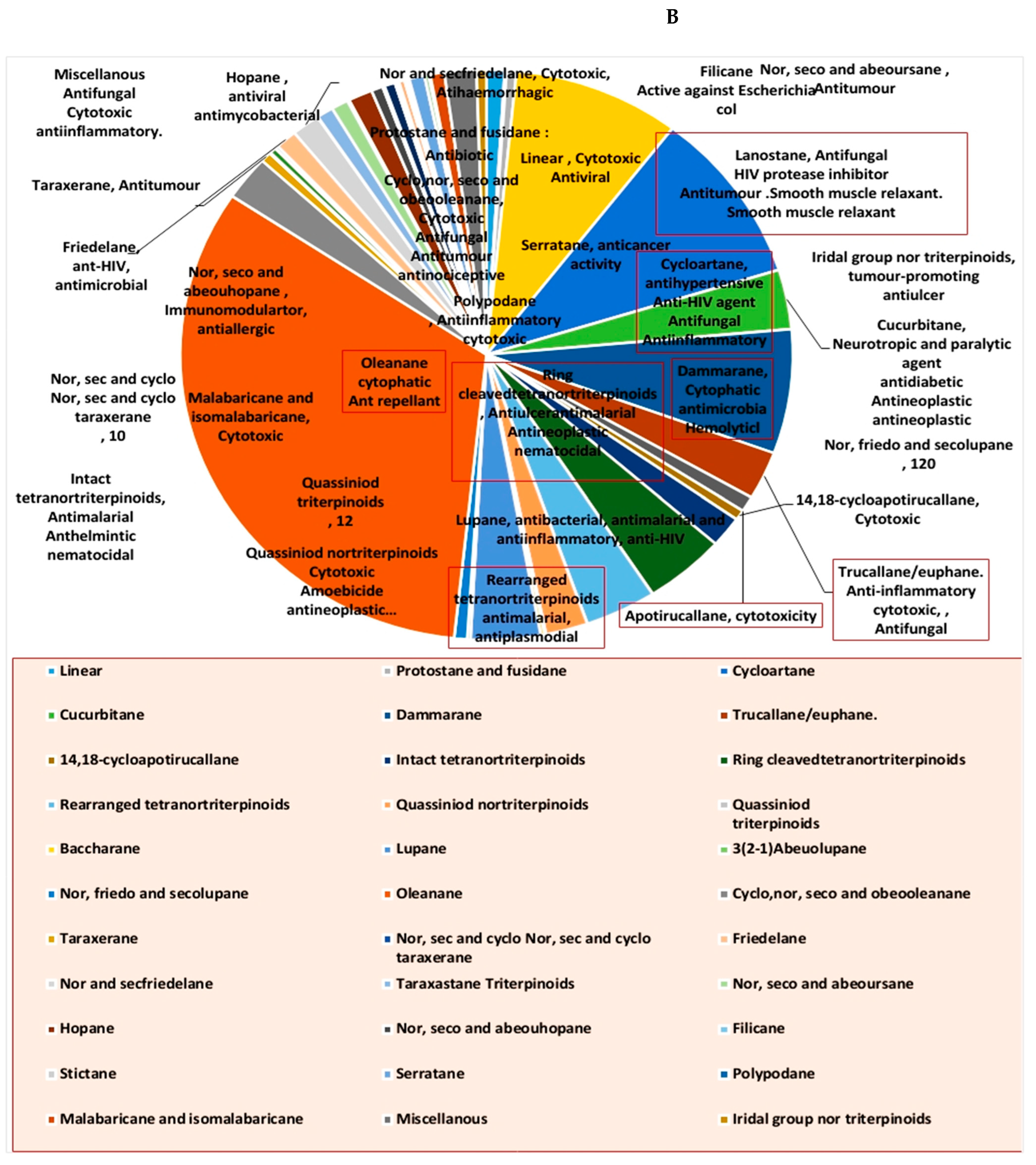
Steroids and triterpenes are considered common natural product classes that are widespread in different marine and terrestrial natural sources (e.g., plants, animals, and microorganisms) [1,2,3]. Additionally, they comprise many sub-classes with enormous chemical diversity. These two classes of compounds have been provided several successful drugs for various ailments since the discovery of digoxin in 1785, like cortisol, fusidic acid, carbenoxolone, and β-Aescin [4]. These compounds possess a myriad of biological activities, digoxin used in the treatment of heart failure and atrial fibrillation [5], fusidic acid used as topical antibiotics [6]. Carbenoxolone enhances peripheral insulin sensitivity [7] and treatment of gastric ulcer [8] and β-Aescin used in the treatment of human hepatocellular carcinoma SMMC-7721 cells [9]. Steroids and triterpenes possess various potential antiviral properties such as anti-Herpes simplex virus activity [10], anti-hepatitis B activity [11], anti-HIV1 and 2, AIDS, and hepatitis C virus activities [12]. Steroids class consists of 25 chemical subclasses with about 11,825 compounds that have been previously reported [13] (Figure 1A) While, triterpenoids class of compounds consists of 47 chemical subclasses with about 18,864 chemical compounds that were previously, isolated, and identified from different natural sources as shown in (Figure 1B).
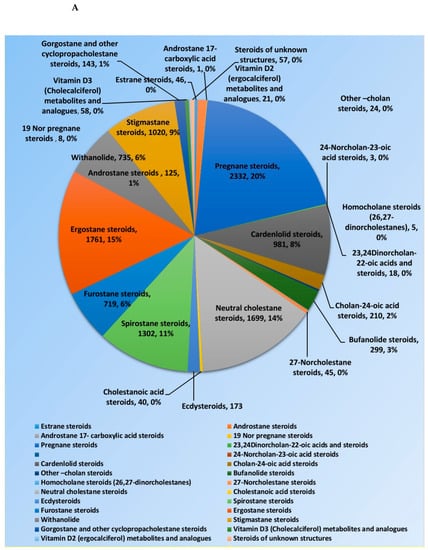
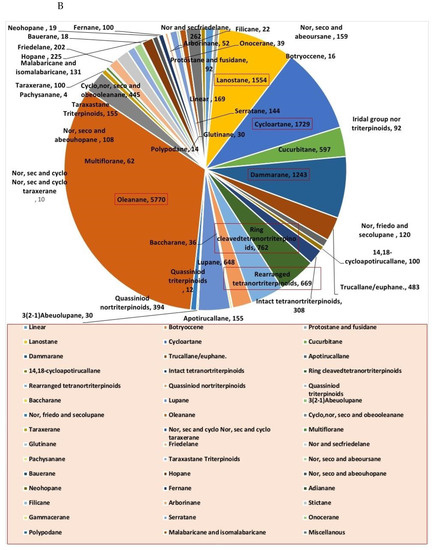
Figure 1.
Pie charts indicating the distribution of chemical subclasses of steroids (A) and triterpenes (B).
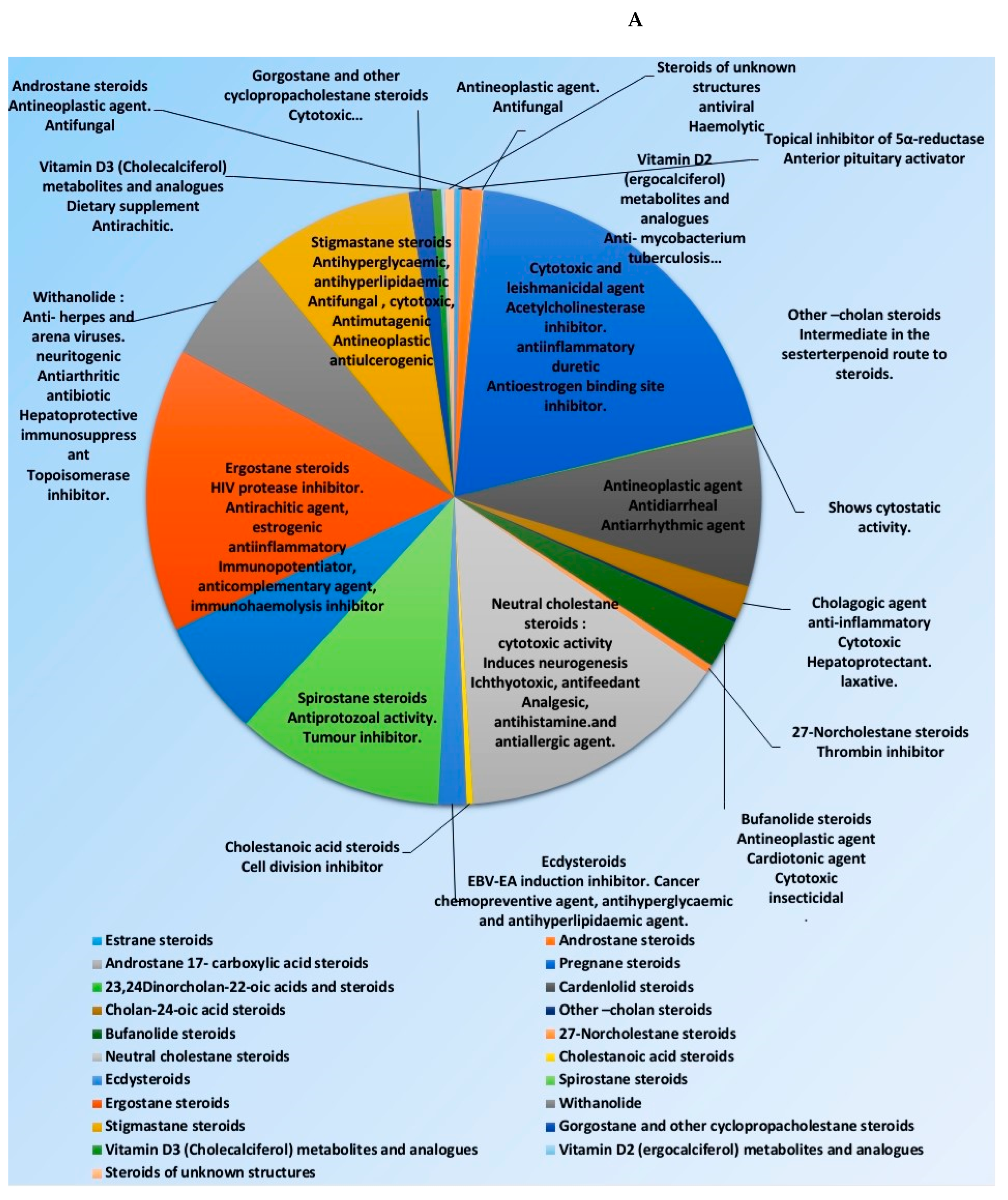
Many representatives from both classes (i.e., steroids and triterpenoids) have been reported for a wide spectrum of pharmacological activity (e.g., antimicrobial, anticancer, and anti-inflammatory activities) (Figure 2). Our current review aims to shed light on the antiviral potential of these interesting secondary metabolites classes (i.e., steroids and triterpenes) illustrating their mode of actions either directly (i.e., direct viral inhibition) or indirectly (i.e., boosting the host immune response, reducing the inflammatory and antagonize some host-based molecular targets used by viruses for cell entry and multiplication). We also investigated the probability of these reviewed triterpenes and sterols to provide promising candidates for the development of effective therapeutics towards the current SARS-CoV-2 pandemic by molecular docking experiments against the currently available protein targets (either viral or human-based targets).
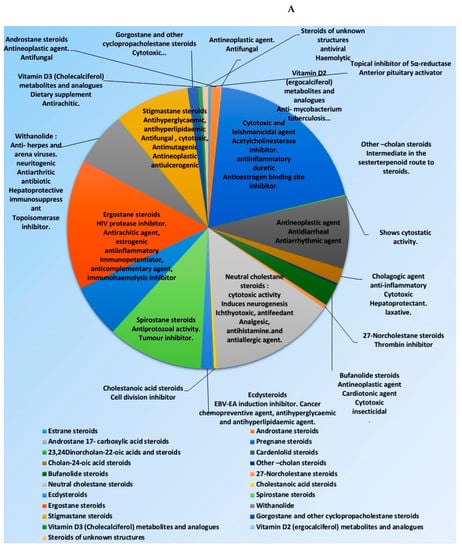
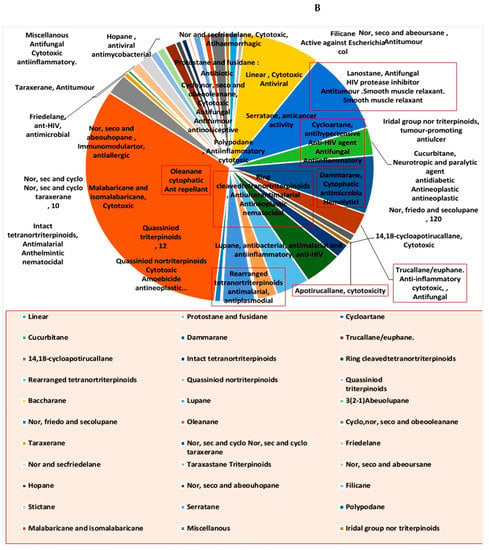
Figure 2.
Pie charts indicating the biological activities of steroids (A) and triterpenes (B).
2. Methodology
2.1. Databases
The review article search was conducted in the following databases: Research gate (https://www.researchgate.net/search), Science Direct (https://www.sciencedirect.com), PubMed (https://pubmed.ncbi.nlm.nih.gov), Scopus (https://www.scopus.com), Web of Science (https://clarivate.libguides.com/webofscienceplatform/alldb), Google Scholar (https://scholar.google.com), and DNP (Dictionary of Natural Products) database. The keywords “antiviral steroids and triterpenes” were paired with “natural products”, “marine drugs”, “medicinal plants”, “herbal drugs”, “crude extracts”, “fungi”, or “synthetic derivatives of natural products” to obtain published records till 2020.
2.2. Molecular Docking
Docking study was carried out against all available crystal structure (Figure 2) of SARS CoV-2 proteins (3 proteins) in Protein Data Bank (PDB) (along with other four human proteins (4 proteins) involved in the entry and possessing of the virus (Table 1) using Autodock Vina docking machine [14]. The viral crystal structures are SARS-CoV-2 main protease (Mpro) (PDB ID: 6LU7), papain-like protease (PLpro) (PDB ID: 6WXR)) which are key viral cysteine proteases essential for the viral replication inside the host cell, and viral ADP ribose phosphatase (ARP) (PDB ID: 6W02) which is essential for the viral protection against the ADP-ribosylation activated by the innate immune system of the host cells. The four human targets are adaptor protein 2 associated kinase.

Table 1.
Binding free energies in kcal/mol of the top-scoring triterpenes against 7 viral and non-viral targets (3 viral-based and 4 human-based).
(AAK1) (PDB ID: 4WSQ) and cyclin G-associated kinase (GAK) (PDB ID: 4Y8D) are two important proteins for the viral entry into the host cell [15]. Moreover, the two host-based proteases furin (PDB ID: 6EQX) and cathepsin L (PDB ID: 2YJC) that are involved in viral spike protein (S-protein) activation).
The applied docking protocol deals with the protein as a rigid structure and the tested compound as a flexible structure during its computations. The co-crystallized ligands were used to determine the binding sites. The ligand-to-binding-site shape matching root means square (RMSD) threshold was set to 2.0 Å. The interaction energies were determined using the Charmm Force Field (v.1.02) with 10.0 Å as a non-bonded cutoff distance and distance-dependent dielectric. Then, 5.0 Å was set as an energy grid extending from the binding site. The tested compounds were energy-minimized inside the selected binding pocket. The editing and visualization of the generated binding poses were performed using Pymol software [16].
3. Findings and Discussion
3.1. Anti-HIV Agents
Sterol and triterpenes reported as anti-HIV candidates have been reported extensively in the literature, where they exhibited multiple modes of action as antiviral.
3.1.1. Reverse Transcriptase Inhibitors
Reverse transcriptase is a key enzyme in retroviruses like HIV (i.e., essential for the viral replication process), and hence it has been extensively studied for the development of anti-HIV therapeutics. Several sterols and triterpenes have been reported as potent HIV reverse transcriptase inhibitors during the last 20 years.
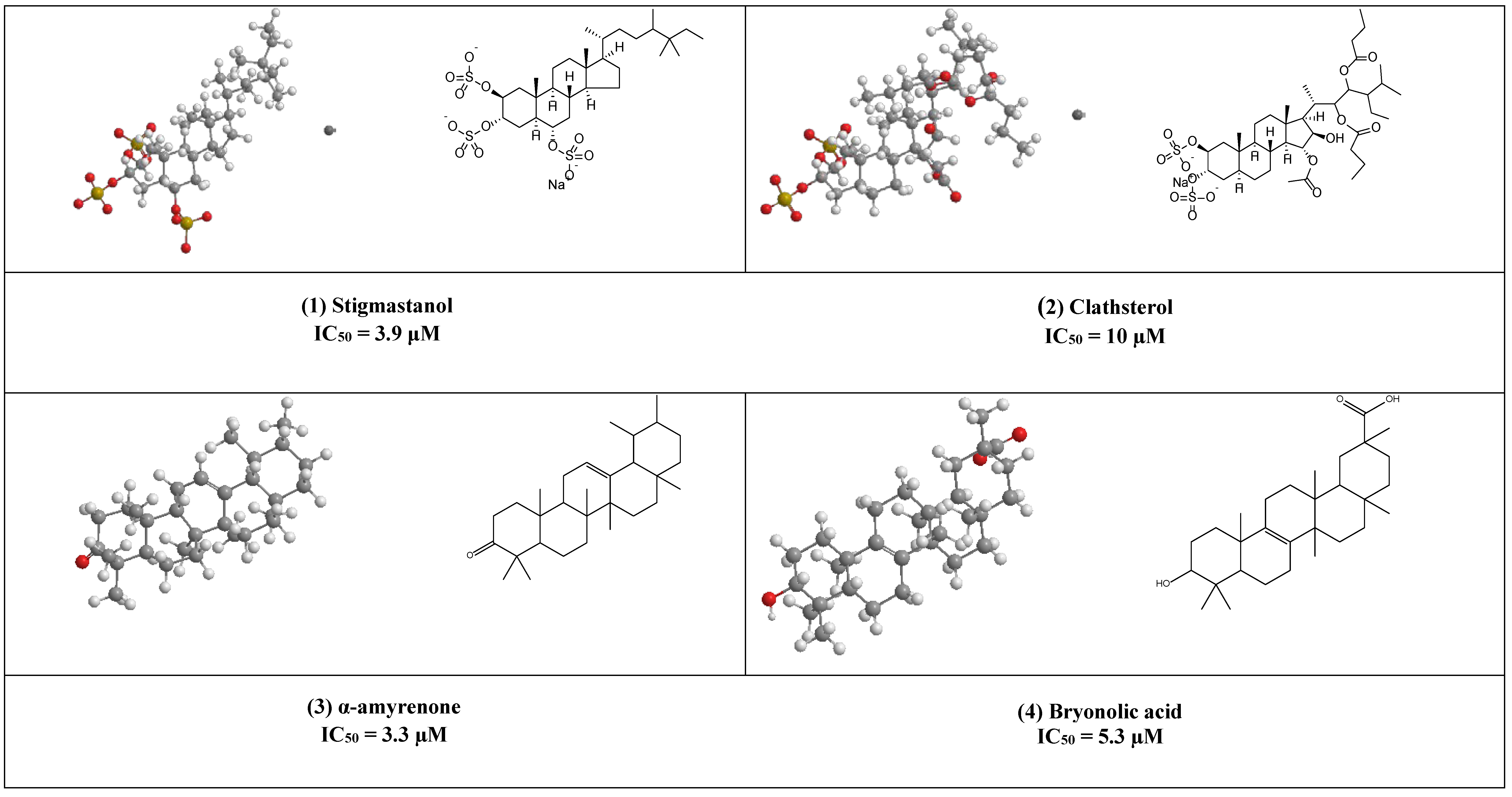
Stigmastanol (1) is a common sterol in many plants and present in large quantities in rice bran, where it can be easily isolated and purified. This sulfated sterol has been reported to inhibit HIV-’s reverse transcriptase competitively with IC50 = 3.9 μM. [17]. Clathsterol (2) the major sterol in Clathria species has been found to inhibit HIV-1 reverse transcriptase activity at a concentration of 10µM [18]. Furthermore, concise synthesis of the clathsterol steroidal core has also been explained [19].
Both α, β- amyrenone (3) are triterpene derivatives of the ursane and oleanane series, as shown in (Figure 3). They were commonly isolated from Burseraceae family species such as Protium heptaphyllum, Protium. opacum var. opacum, Protium. giganteum; Trattinnickia glaziovii, and Trattinnickia. peruviana.
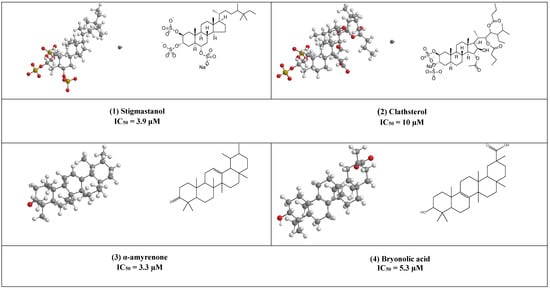
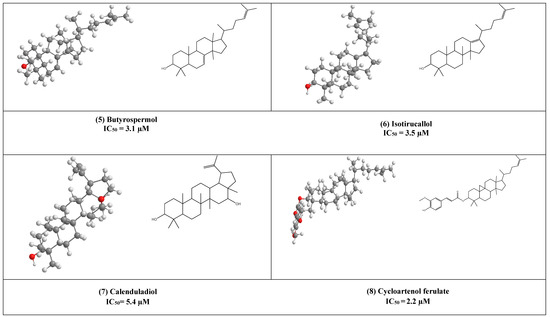
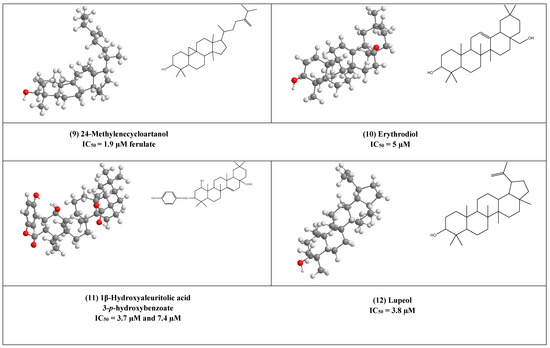
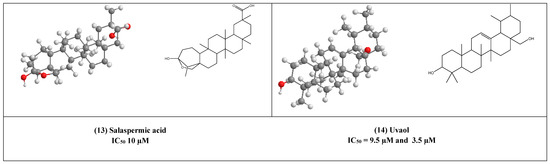
Figure 3.
The 2D and 3D chemical structures of virus reverse transcriptase inhibitors.
Both compounds have been reported to possess several pharmacological effects (e.g., anti-inflammatory, antinociceptive activities together with lipase, α-glucosidase, and α-amylase, inhibitory activities) [20]. In addition, α-amyrenone has also shown strong virucidal activity against HIV-1 reverse transcriptase in a low IC50 value equal to 3.3 µM.
Bryonolic acid (4) is a pentacyclic triterpenoid found in the family Cucurbitaceae. It has many remarkable potentials for the treatment of different diseases (e.g., allergic and inflammatory diseases) [21,22]. Additionally, it has shown anti-HIV activity by inhibiting the reverse transcriptase enzyme (IC50 5.3 µM) [23].
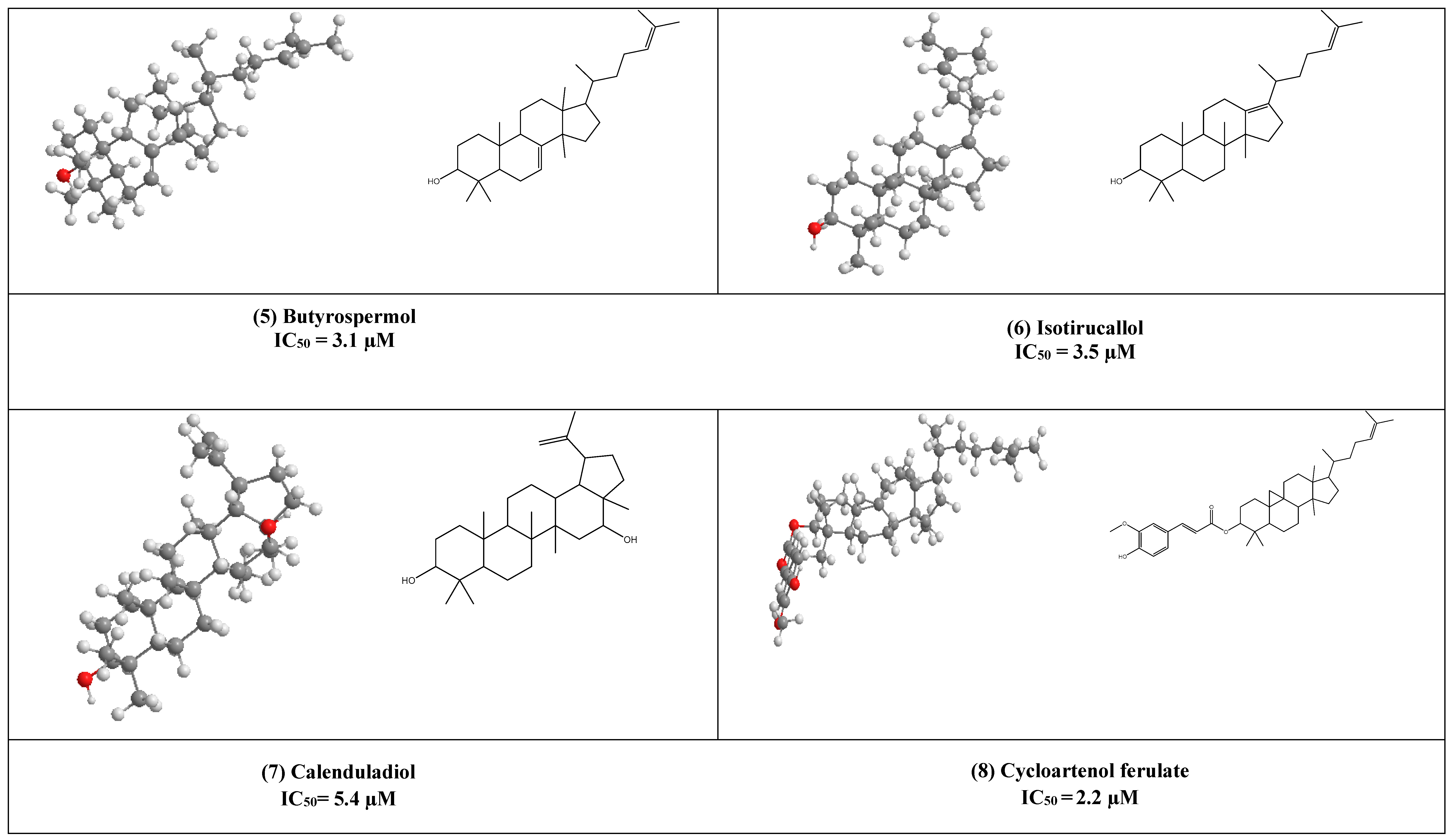
Butyrospermol (5) and Isotirucallol (6) are euphane and dammarane types triterpenes, respectively (Figure 3). Both compounds have been reported to be major metabolites in Camellia japonica and Camellia. sasanqua. Moreover, they were able to inhibit HIV-1 reverse transcriptase with IC50 values of 3.1 µM and 3.5 µM, respectively [17].
Another potent inhibitor of the HIV-1 reverse transcriptase enzyme is calenduladiol (7), which is a lupine type pentacyclic triterpene that found in Calendula officinalis. Calenduladiol could potentially inhibit the viral HIV-1 reverse transcriptase with IC50 equal to 5.4 µM.
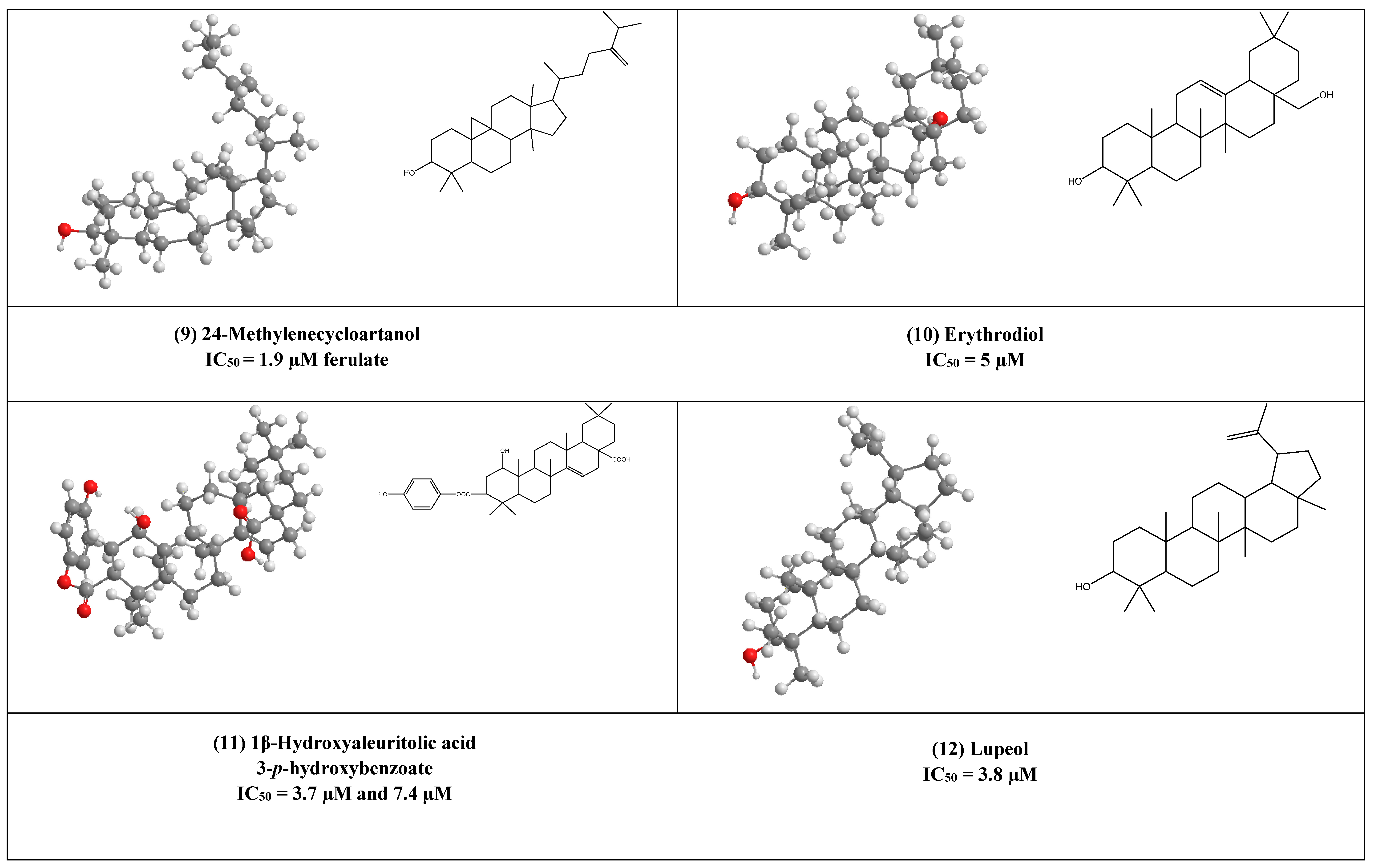
Two active cycloartane type pentacyclic triterpenes compounds of rice bran, cycloartenol ferulate (8), and 24-Methylenecycloartanol ferulate (9) displayed potent anti-HIV through competitive inhibition of reverse transcriptase with (IC50 2.2 µM and 1.9 µM, respectively) [17].
Erythrodiol (10), is the precursor of pentacyclic triterpenic acids that were isolated from Olea europaea. This compound has displayed antiviral activity against HIV-1 reverse transcriptase with an IC50 value of 5 µM.
1β-Hydroxyaleuritolic acid 3-p-hydroxybenzoate (11), a pentacyclic triterpene as shown in (Figure 1), was isolated from Maprounnea Africana. The compound is considered a potent inhibitor of HIV-1 reverse transcriptase with IC50 3.7 µM [24].
Lupeol (12) is a pentacyclic triterpene that is widely distributed in edible vegetables and fruits. Research over about three decades has displayed numerous remarkable pharmacological activities of lupeol. Several in vitro and preclinical animal studies have suggested potential anti-inflammatory, immunomodulatory, antiproliferative, and cholesterol-lowering agents [25,26,27,28,29,30,31]. The anti-inflammatory and immunomodulatory activity of this compound has been linked to its regulation of several cytokines expression (e.g., like IL-2, IL4, IL5, Ilβ), [30], and hence it can help as an adjuvant therapeutic agent in the treatment of COVID-19 to control the cytokine storm associated with this pandemic disease [32].
Furthermore, Akihisa et al. reported that lupeol strongly can inhibit HIV-1 reverse transcriptase in IC50 value of 3.8 µM [16].
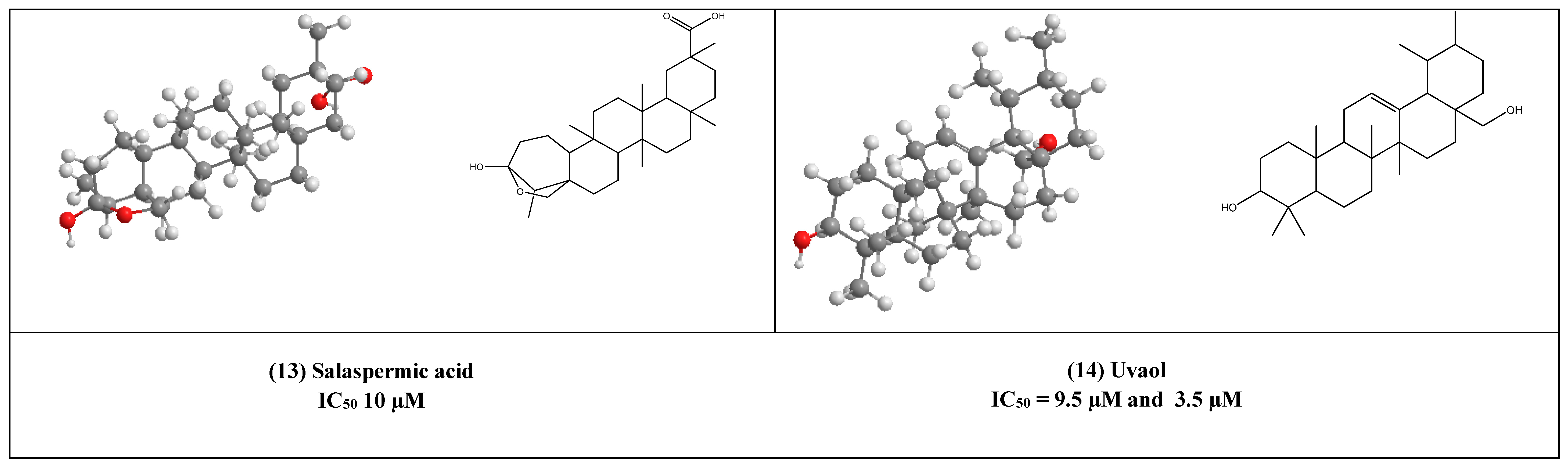
Salaspermic acid (13), and oleanane type triterpene derivative that has been found to act as an inhibitor of HIV reverse transcriptase in H9 lymphocyte cells (IC50 10 µM). This triterpene has been reported as the major metabolite of the roots of Triterygium wilfordii [33,34].
Uvaol (14), an olive-derived lupine type pentacyclic triterpene, was also found to inhibit HIV maturation through inhibiting HIV-1 reverse transcriptase in IC50 value of 9.5 µM [35].
3.1.2. Protease Inhibitors
Proteases are common viral hydrolytic enzymes. They are usually involved in viral entry and initiation of the viral replication process. Additionally, other human-based proteases are utilized by the majority of infectious viruses in their host cell attachment and entry [36]. Thus, pan-protease inhibitors (e.g., camostat) have shown broad-spectrum antiviral activity including the currently spread SARS-CoV-2 [36].
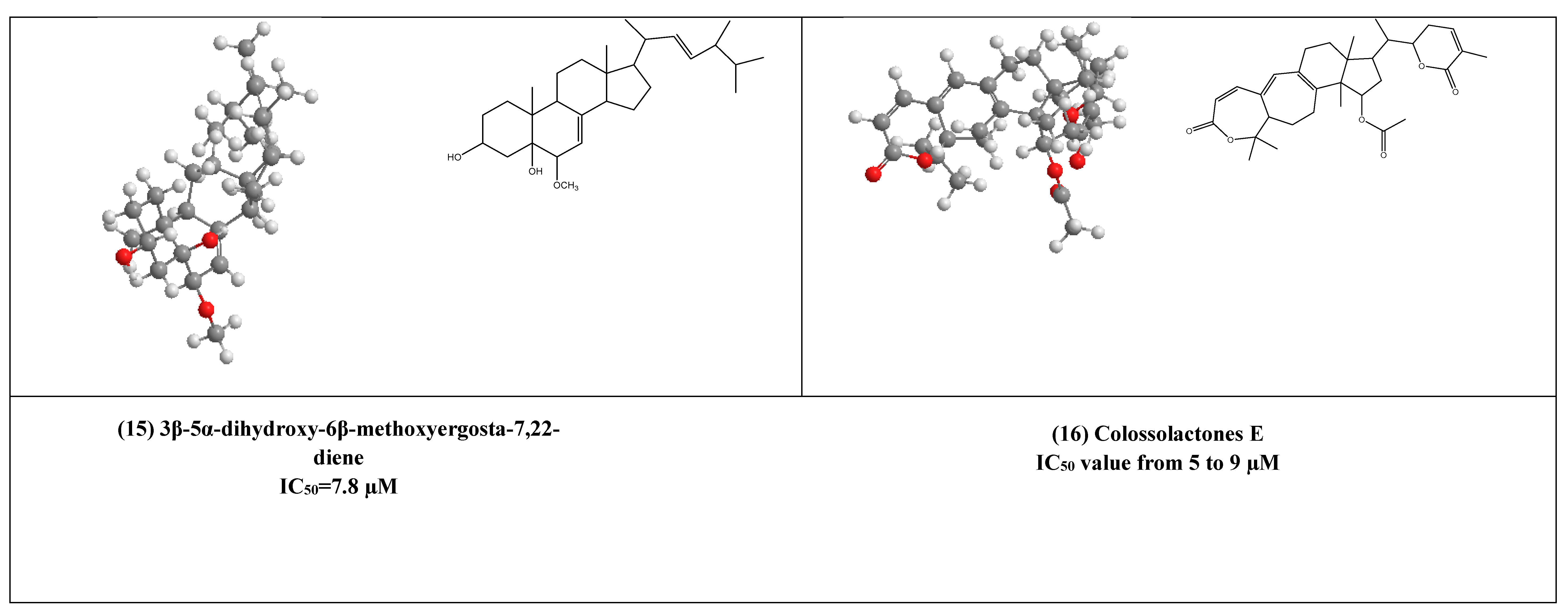
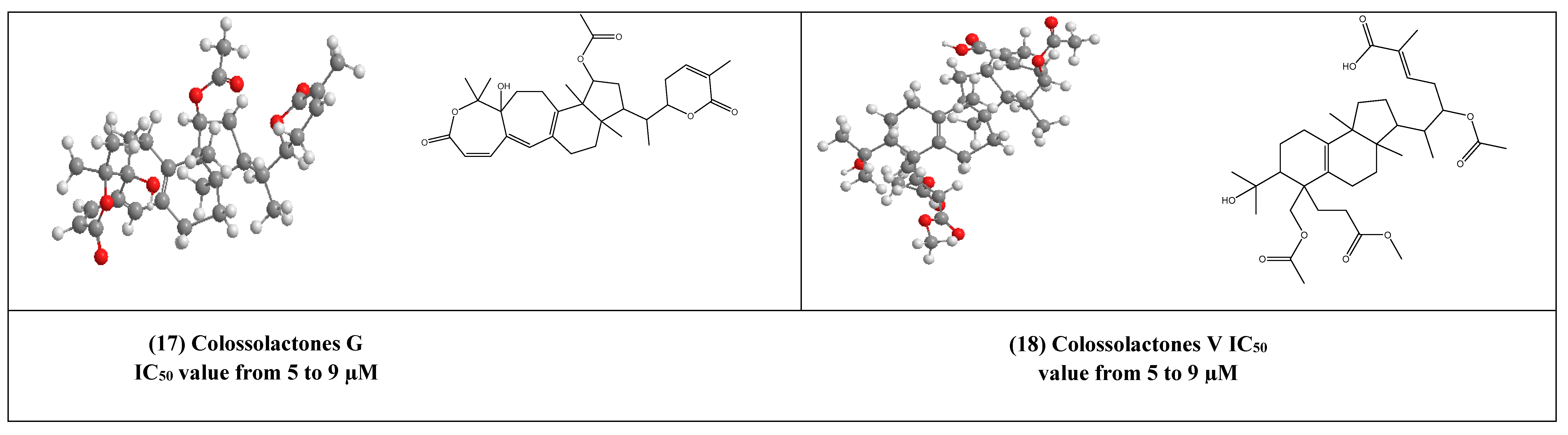
Some lanostane-type triterpenes isolated from the medicinal mushrooms Ganoderma species have been shown in vitro inhibitory effects against HIV-1 protease. For example, 3β-5α-dihydroxy-6β-methoxyergosta-7,22-diene (15) (Figure 4) [37] was isolated from an edible mushroom species called G. lucidum and was reported as a potent non-competitive HIV-1 protease inhibitor (IC50 = 7.8 µM). Additionally, three G.colosum-derived lanostane type triterpenes namely, colossolactones E, G, and V (16–18) (Figure 4) [38] as having been displayed anti-HIV-1 protease with IC50 values ranged from 5 to 9 µM, respectively.
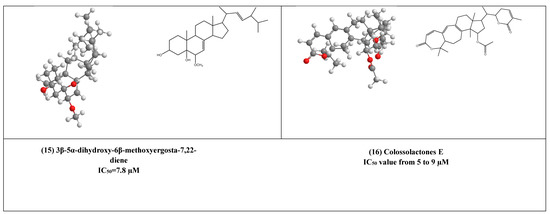
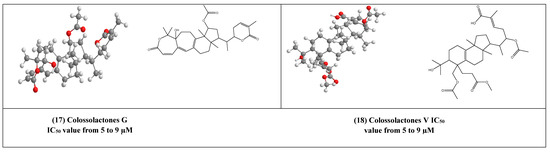
Figure 4.
The 2D and 3D chemical structures of virus protease inhibitors.
In addition to being a potent HIV reverse transcriptase [34], uvaol (14) was also found to inhibit HIV-1 protease with an IC50 value of 3.5 µM [35].
3.1.3. DNA Polymerase Inhibitors
The Maprounnea africana-derived pentacyclic triterpene,1β-hydroxyaleuritolic acid 3-p-hydroxybenzoate (11) has been also revealed potent inhibitory activity toward DNA polymerase with IC50 equal to 7.4 µM in addition to its anti-HIV-1 reverse transcriptase activity [24].
3.2. Replication Inhibitors:
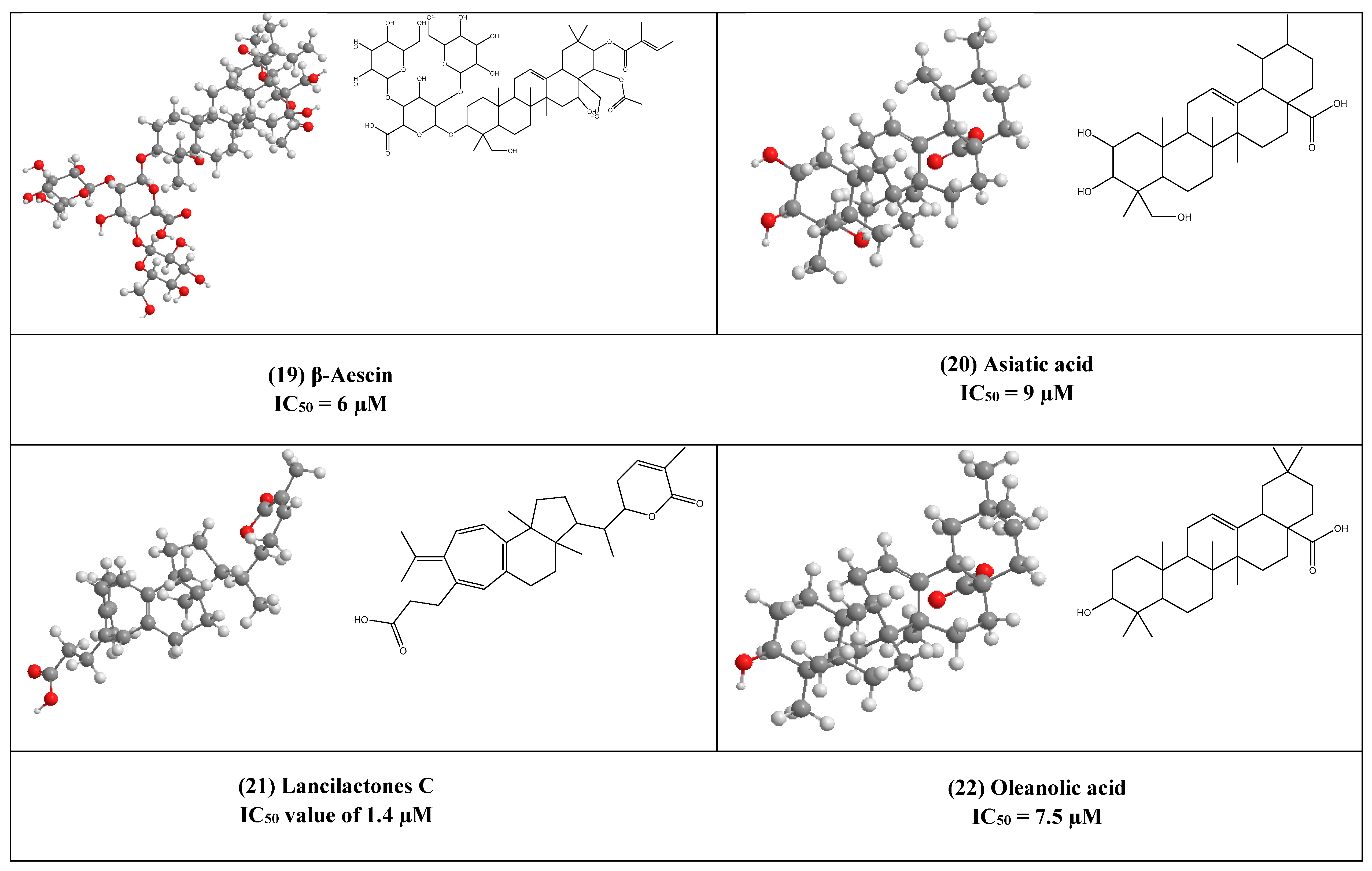
β-Aescin (19) is a pentacyclic triterpene that was isolated from Aesculus hippocastranum (Hippocastanaceae) seed extract. β-Aescin was reported to exhibit anti-oedematous, anti-inflammatory, and antioxidative effects [39,40]. It has been reported that β-Aescin inhibits NF- ƙB activation and cytokines production in LPS-treated mice and numerous tumor cells, macrophages, and endothelial cells [41,42]. Additionally, this inhibition was also found to play an important role in inhibiting Herpes Simplex virus-1 (HSV-1) replication with an IC50 value of 1.5 and 2.4 µM was calculated in Human corneal-limbal epithelial (HCLE) and normal human conjunctival epithelial cell line (NHC) cells. The virucidal activity of β-aescin could be due to its ability to interfere with cell membranes and cholesterol homeostasis.
Moreover, this glycosylated triterpene (i.e., β-aescin) has been also reported to inhibit the replication of SARS-CoV-1 in vitro (IC50 6 µM).
Asiatic acid (20) is a naturally occurring aglycone of ursane-type pentacyclic triterpenoids. It has been previously reported from a wide range of plants e.g., Actinida argute roots, Centella Asiatica leaves, and Combretum laxum Stems [43]. It has poly-pharmacological properties like antioxidant, anti-inflammatory. Moreover, it affects and regulates apoptosis that contributes to its therapeutic effects in various diseases. Numerous in vivo and in vitro studies reported that asiatic acid (20) influences many enzymes, receptors, growth, and transcription factors, in addition to several cell signaling cascades. Moreover, it inhibits both HIV-1 and enterovirus 71 (EV71) replications in acutely infected host cells with IC50 equal to 9 and 20 µM, respectively [43,44].
The triterpene lactone lancilactones C (21), which was isolated from the roots of Kadsura lancilimba (Schizandraceae), has been inhibited HIV replication with an IC50 value of 1.4 µM [45].
Oleanolic acid (OA) (22) is a pentacyclic triterpenoid that possesses a variety of interesting biological activities (e.g., anticancer, antimicrobial, and anti-inflammatory activities) [46]. This common triterpene has been previously reported from more than 1600 different plant species [47,48]. OA has been found to inhibit HIV-1 replication in acutely infected H9 lymphocyte cells (IC50 7.5µM) [44,49]. synthesized derivatives of OA by the modification of C12-C13 double bond yielding a series of new compounds that was 3-fold more active than OA [49].
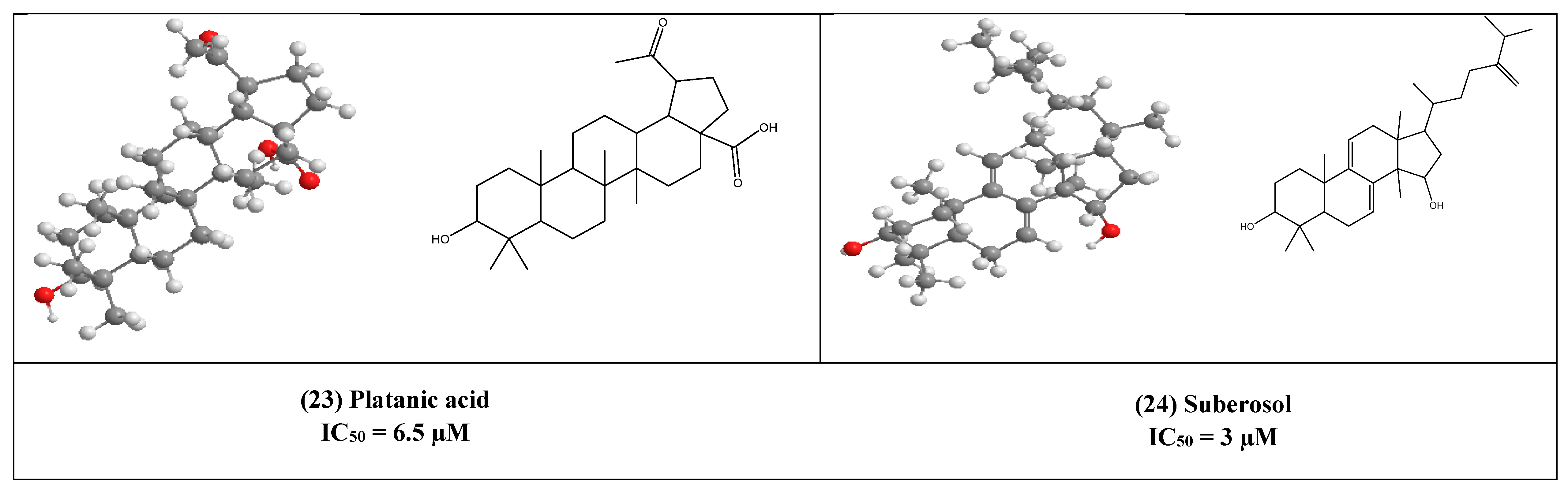
Platanic acid (23) is a pentacyclic triterpenoid as shown in (Figure 5), that was isolated from the leaves of Syzigium claviflorum (Myrtaceae) [33]. It showed an inhibitory effect on HIV replication with an IC50 of 6.5 µM, while its IC50 for inhibition of uninfected H9 cell growth was 90 µM.
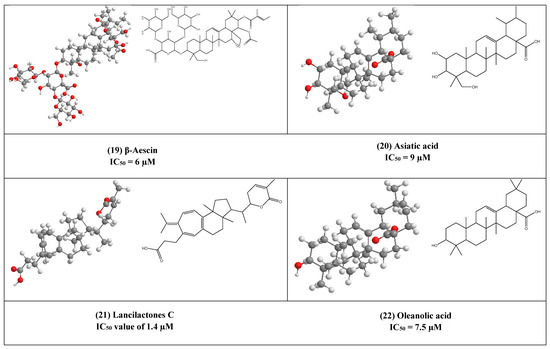
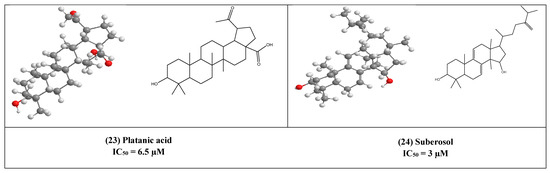
Figure 5.
The 2D and 3D chemical structures of virus replication inhibitors.
Salaspermic acid (13) is an oleanane-type triterpene derivative, which was isolated from the roots of Triterygium wilfordii [33,34]. It was previously mentioned to have inhibitory activity against HIV reverse transcriptase. Moreover, it has an anti-HIV replication in H9 lymphocyte cells with an IC50 value of 10 µM.
Another lanostane-type triterpene namely, suberosol (24) has shown inhibitory activity against HIV replication in H9 lymphocyte cells with IC50 equal to 3 µM. This compound has been previously isolated from Polyalthia suberosa (Annonaceae) [50].
5. In Silico Investigation
To suggest possible anti-SARS-CoV-2 candidates, the 162 compounds reviewed in this investigation were subjected to a docking study against both virus-based and human-based molecular targets (three and four targets, respectively) that their structures are currently available at protein data bank website (PDB, https://www.rcsb.org/). Six triterpenes were retrieved as top hits for one or more targets depending on the binding energies they scored (i.e., got binding energy lower than −6 kcal/mol) and their interactions with the reported key amino acid residues (Table 1 and Figure 7).
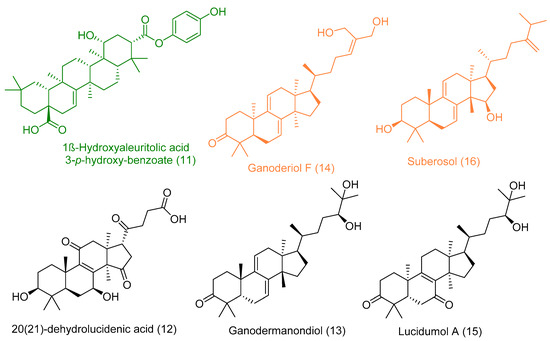
Figure 7.
Top-scoring triterpenes retrieved from docking against both viral and human-based targets. Hits for 5 targets (Green color), hits for 2 targets (Orange color), and hits for only one target (Black color).
Both SARS-CoV-2 main protease (Mpro) and papain-like protease (PLpro) are key viral cysteine proteases essential for the viral replication inside the host cell, where they activate the replication complex that in turn initiate the viral RNA replication [15].
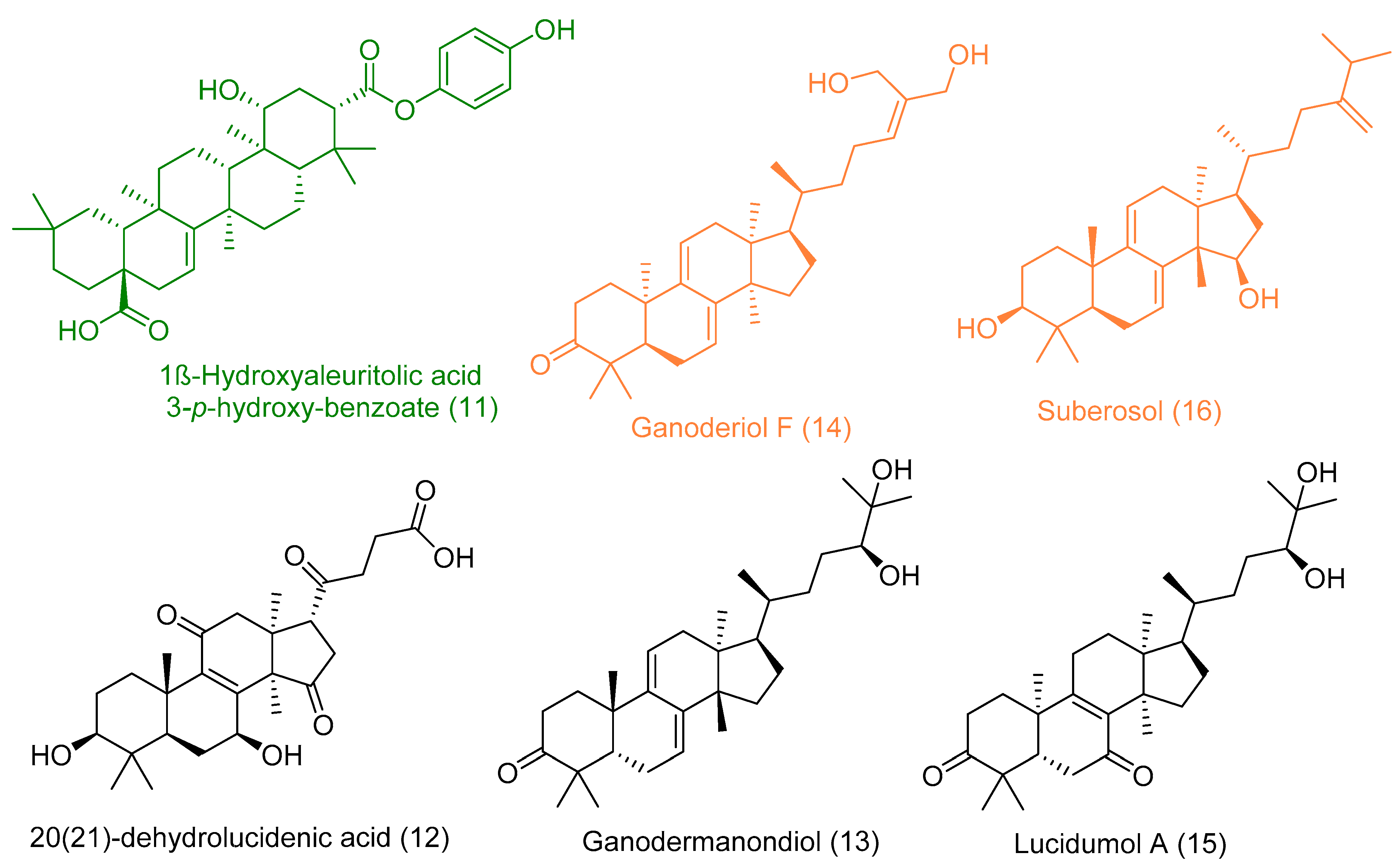
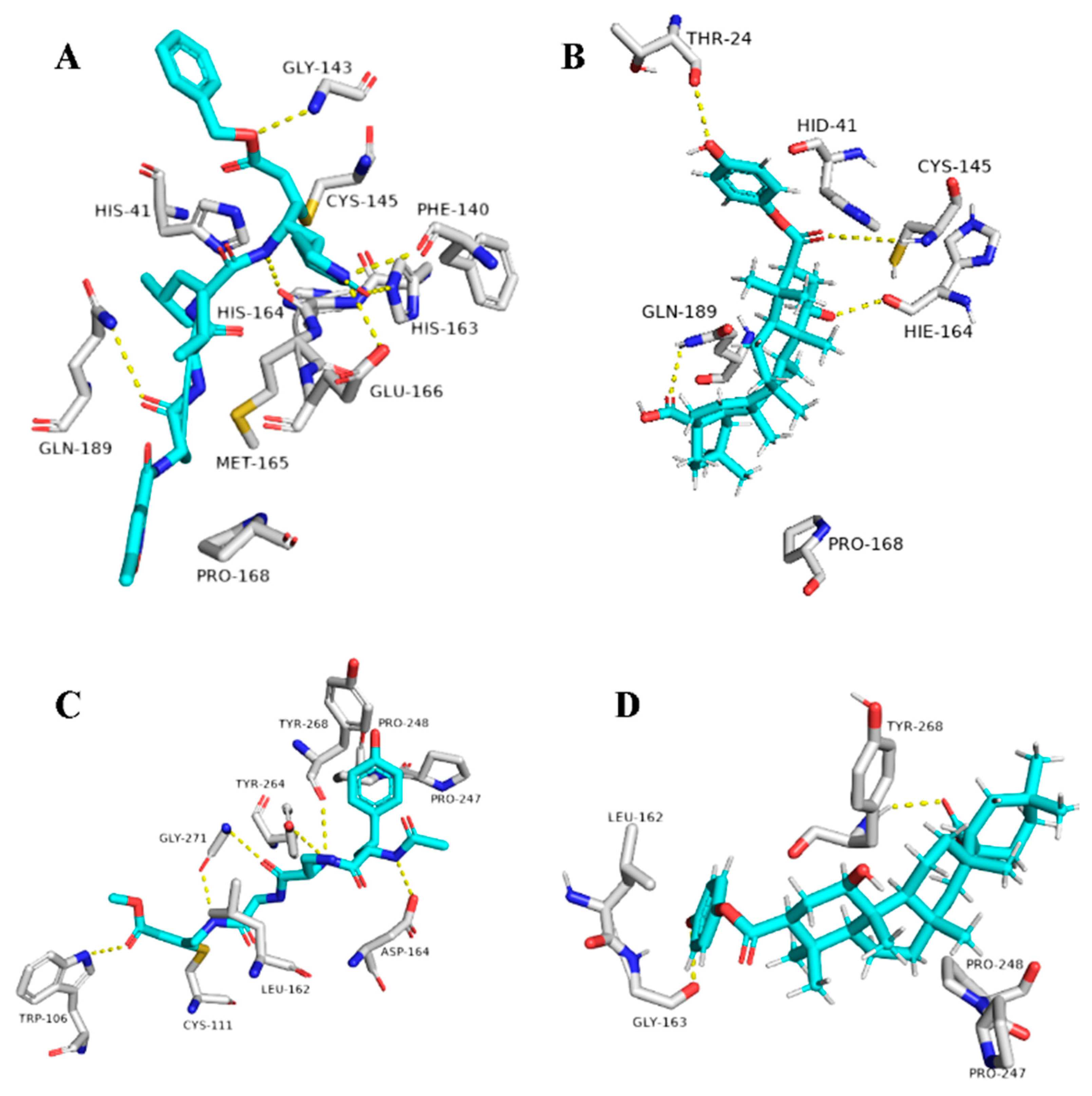
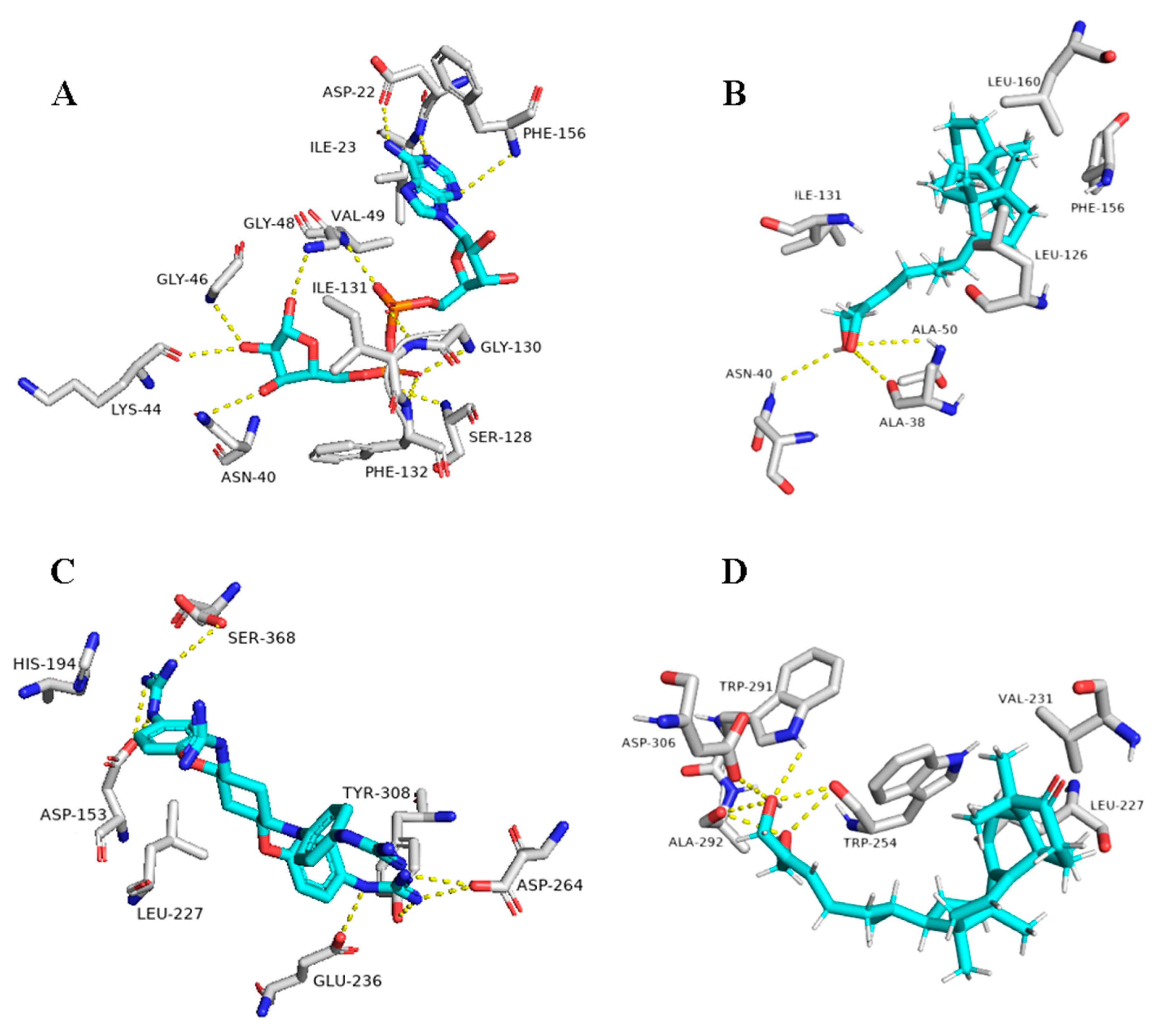
The triterpene 1β-hydroxyaleuritolic acid 3-p-hydroxybenzoate (11), which was previously isolated from the roots of Maprounea Africana [89], was the only compound that got interesting binding scores against the viral main protease (Mpro) and papain-like protease(PLpro) (binding scores of −8.5 and -8.0 kcal/mol, respectively, Table X). This compound was able to form four strong hydrogen bonds (<2.5 Å) and with four different amino acid residues inside the Mpro’s active site (Table 1 and Figure 8), three of them have been reported previously as essential ones for the enzyme’s inhibition [36]. Additionally, it was able to form two hydrophobic interactions with HID-41 and PRO-168 that have been also reported to be involved in the interactions with the co-crystallized inhibitors. Regarding PLpro, this compound achieved very good fitting inside the enzyme’s active site with 2 H-bond and 4 hydrophobic interactions with reported key amino acid residues (Table 1 and Figure 8) [90].
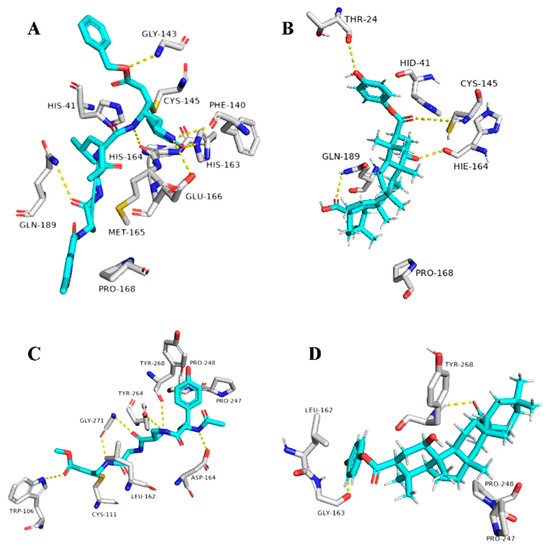
Figure 8.
Binding modes of 1ß-Hydroxyaleuritolic acid 3-p-hydroxybenzoate (11) inside SARS-CoV-2 targets. (B,D) Interactions inside both Mpro and PLpro, respectively. (A,C) co-crystallized ligands of both Mpro and PLpro, respectively.
In addition to the viral target proteins, 1ß-Hydroxyaleuritolic acid 3-p-hydroxybenzoate was able to give interesting docking scores with many non-viral targets like adaptor protein 2 associated kinase (AAK1), furin, and cathepsin L (Binding energy = −9.1, −8.7, and −8.1 kcal/mol, respectively).
AAK1 together with cyclin G-associated kinase (GAK) are considered two key proteins involved in the viral endocytosis process, and hence inhibition of these kinases can prevent the viral entry inside the host cells [91]. On the other hand, furin and cathepsin L are two host-based proteases that are widely distributed in the human lungs. Both enzymes have been reported to be involved in the activation of the viral spike protein (S-protein), which is considered a key step in the viral-host cell attachment and subsequently, viral entry [92]. Thus, targeting these host-based proteins can provide potential anti-COVID-19 therapeutics.
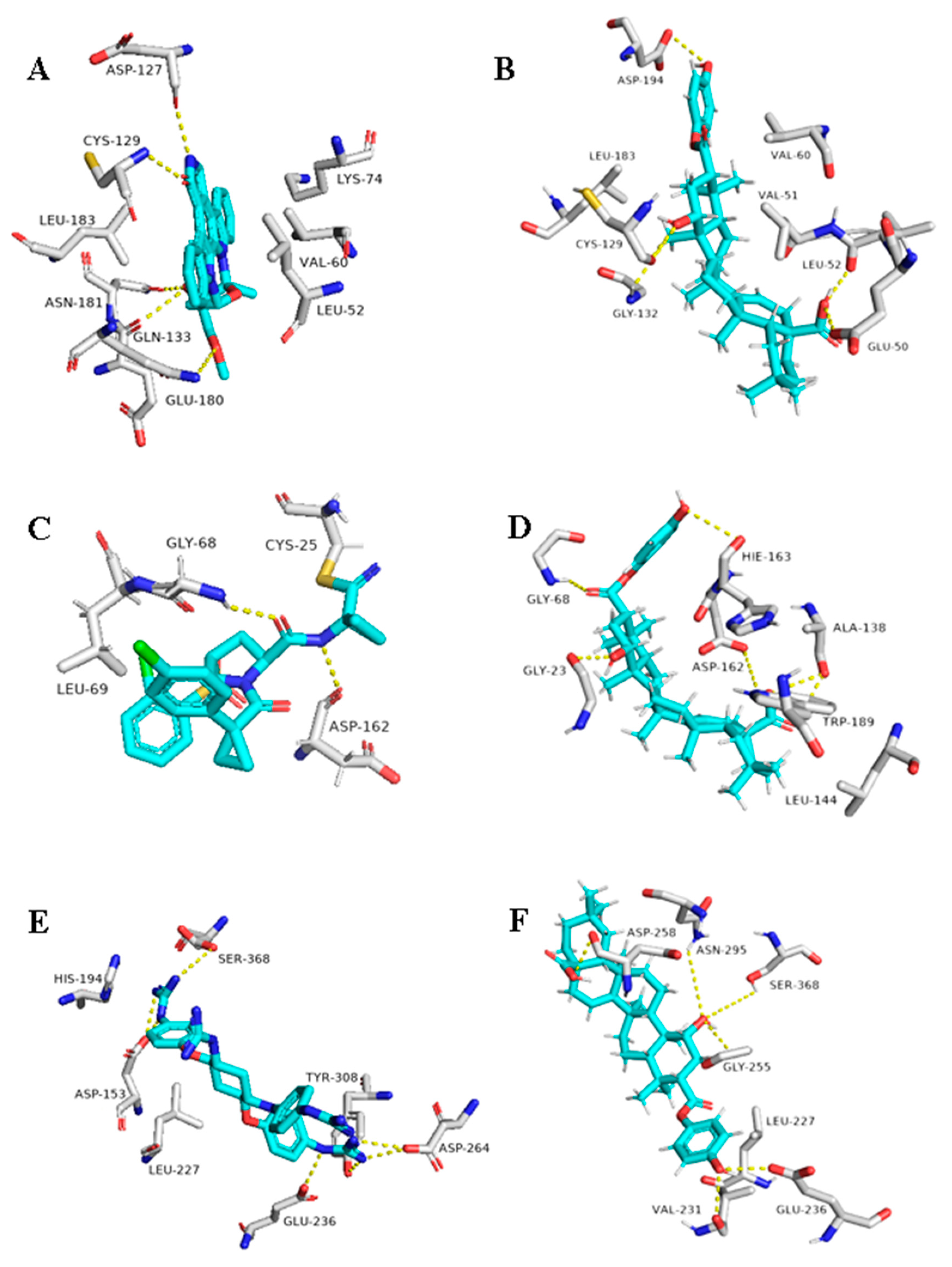
As illustrated in Table 1 and (Figure 9), 1ß-Hydroxyaleuritolic acid 3-p-hydroxybenzoate (11) was able to fit itself inside the AAK1’s active site through 5 H-bonds with 5 different amino acid residues together with hydrophobic interactions with another three. Moreover, it accommodated itself inside the active sites of both furin and cathepsin L via the formation of 6 H-bonds. Interestingly, 1ß-Hydroxyaleuritolic acid 3-p-hydroxybenzoate (11) has been also found to be a potent inhibitor of HIV-1 reverse transcriptase and DNA polymerase with IC50 values of 3.7 and 7.4 µM, respectively [24] (Figure 9).
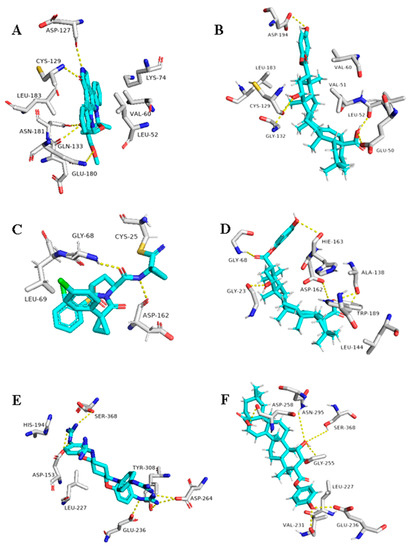
Figure 9.
Binding modes of 1ß-Hydroxyaleuritolic acid 3-p-hydroxybenzoate (11) inside the human-based targets. (B,D,F) Interactions inside both AAK1, cathepsin L, and furin, respectively. (A,C,E) co-crystallized ligands of AAK1, cathepsin L, and furin, respectively.
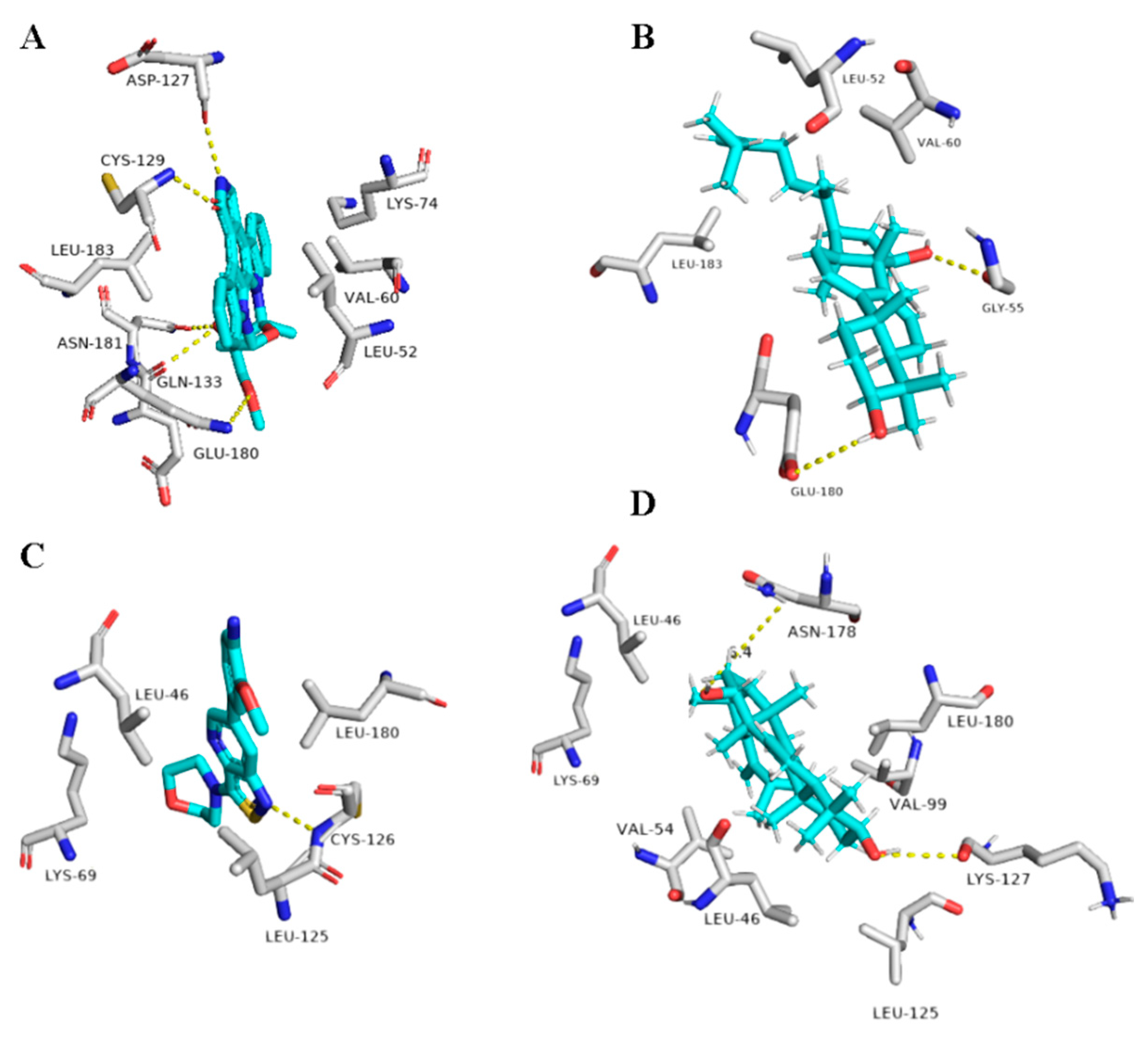
The next top scoring compound was ganoderiol F (14). It is an antitumor triterpene produced by Ganoderma lucidum, and its best binding scores were achieved against the viral ADP ribose phosphatase (ARP) and furin (−8.0 and −8.3 kcal/mol, respectively, Table 1). ARP was suggested to be involved in the viral protection against the ADP-ribosylation activated by the innate immune system of the host cells, and hence, this protein can be considered a relevant drug target [93,94].
Ganoderiol F (14) was able to form only three H-bonds inside ARP’s active site, however, it interacted with other four amino acid residues through hydrophobic interactions (Table 1 and Figure 10).
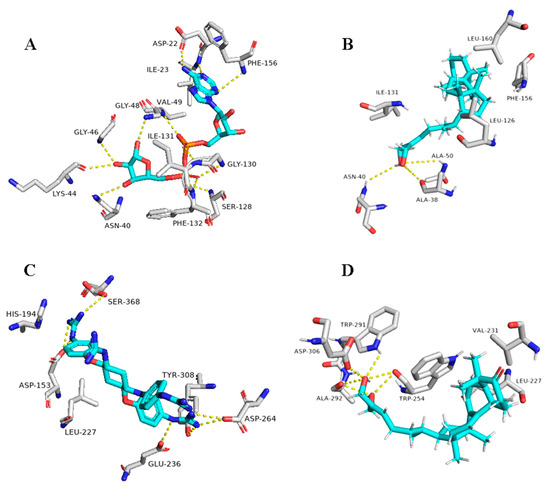
Figure 10.
Binding modes of Ganoderiol F (14) inside the viral and human-based targets. (B,D) Interactions inside both ARP and furin, respectively. (A,C) co-crystallized ligands of ARP and furin, respectively.
Unlike 1ß-Hydroxyaleuritolic acid 3-p-hydroxybenzoate (11), ganoderiol F (14) interacted with only four adjacent amino acid residues inside furin’s active site (Table 1 and Figure 10), however, its hydrophenanthren body was imbedded inside a hydrophobic pocket (LEU-227, VAL-231, and TRP-254).
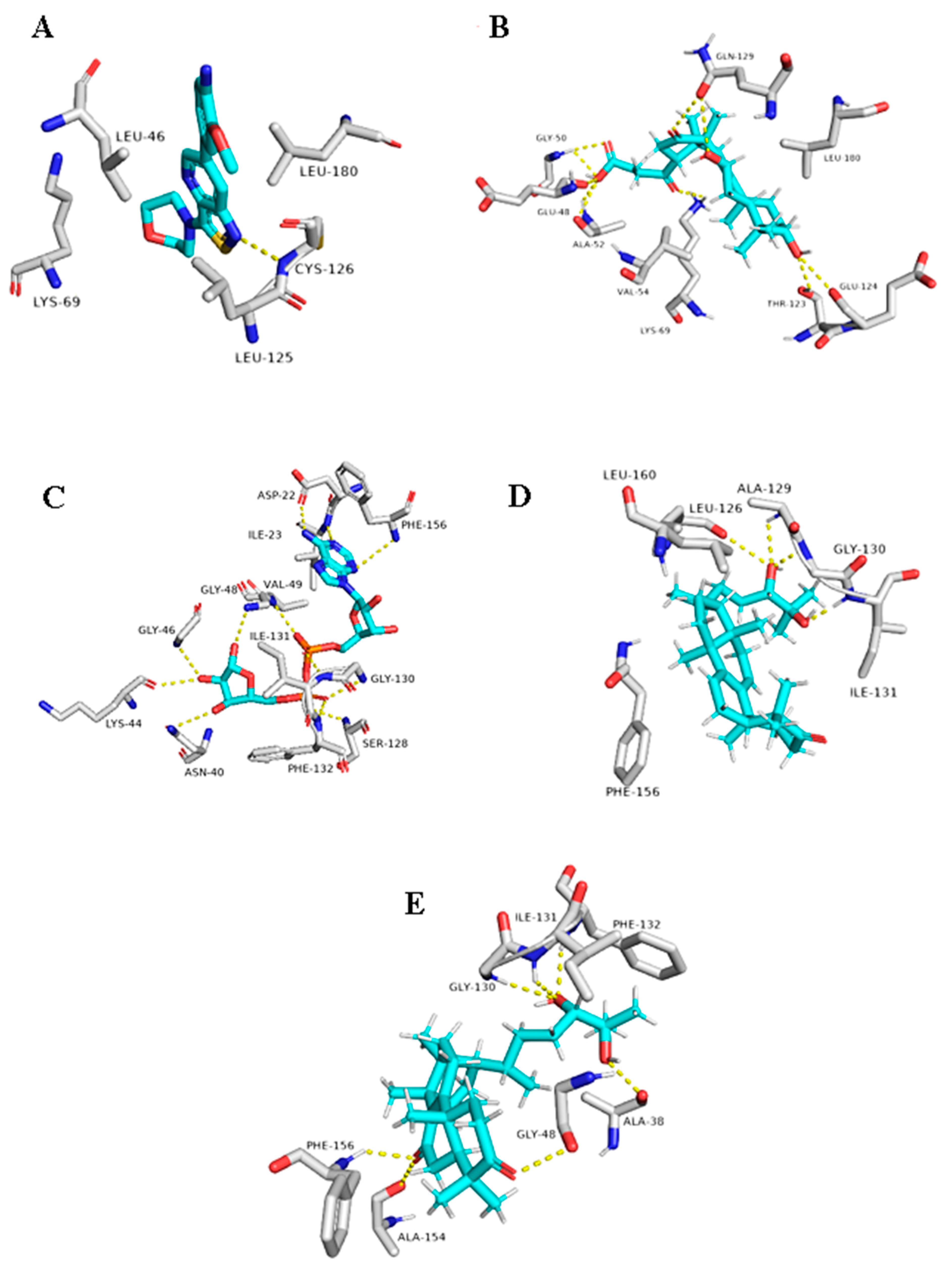
Regarding the triterpene suberosol (16), which has been previously isolated from Polyalthia suberosa [50], and shown anti-HIV activity, was also able to get high docking scores against 2 targets, AAK1 and GAK (−9.0 and −8.2 kcal/mol, respectively). Both of these targets are human-based kinases involved in the viral endocytosis regulation [91]. Interestingly, this compound has been shown in vitro antiviral activity against HIV [50]. This compound was able to interact inside both AAK1 and GAKs active site via H-bond (2 interactions) and hydrophobic interactions (three and five interactions, respectively) (Table 1 and Figure 11).
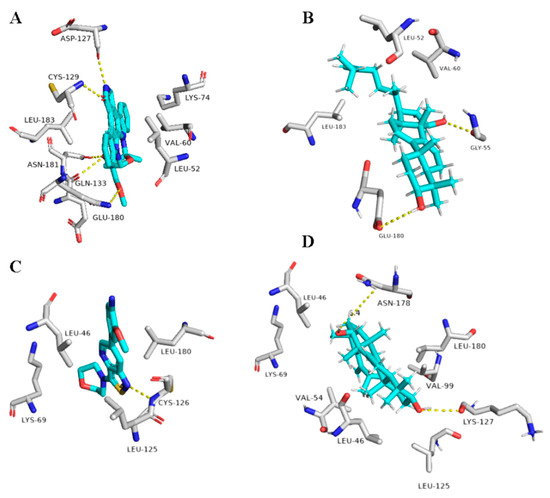
Figure 11.
Binding modes of Suberosol (16) inside the human-based targets. (B,D) Interactions inside both AAK1 and GAK, respectively. (A,C) co-crystallized ligands of AAK1 and GAK, respectively.
The G. lucidum-derived triterpene 20(21)-dehydrolucidenic acid (12) has been shown previously in vitro antiviral activity against both HIV and HCV [95]. In addition, it gave an interesting docking score with the human-based target GAK (Binding energy = −8.1 kcal/mol). Being a polyhydroxylated triterpene, 20(21)-dehydrolucidenic acid (12) was able to interact with six amino acid residues inside the enzyme’s active site through 9 H-bonds together with two hydrophobic interactions with VAL-54 and LEU-180 (Figure 11).
The last two triterpenes ganodermanondiol (13) and lucidumol A (15) have been also derived from G. lucidum, where the former compound has shown inhibitory activity against HIV (IC50 90 µm) [96]. Both triterpenes sowed interesting binding energies against the viral target ARP (Binding energy = −8.3 and −9.6, respectively). Additionally, they interacted with most of the key amino acid residues [94], particularly, lucidumol A (15) (Table 1 and Figure 12).
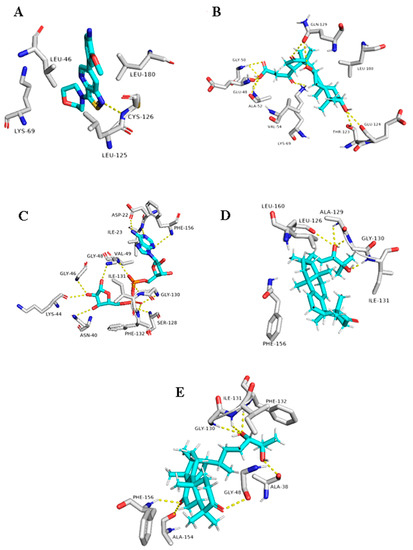
Figure 12.
Binding modes of 20(21)-dehydrolucidenic acid (B) (12), Ganodermanondiol (D) (13), and Lucidumol A (E) (15) inside the viral and human-based targets (GAK and ARP, respectively). (A,C) co-crystallized ligands of GAK and ARP, respectively.
Judging from the previous discussion, it can be concluded that the triterpene class of compounds, particularly, the polyhydroxylated ones, can be utilized in the development of multi-target inhibitors against COVID-19. Additionally, four top-scoring compounds out of six have been reported previously from G. lucidum indicating that this mushroom could be a valuable source and good starting point for exploring anti-SARS-CoV-2 therapeutics.
6. Conclusions
Sterols and triterpenes comprise a huge class of compounds with different biological activities, where mainly they act as anti-cancer, anti-inflammatory, immunomodulatory, and anti-viral. In this review, we were focusing on the antiviral hits, in natural form or derivatized ones that showed potent activities as anti-HIV and showing up their mechanisms of activities that may link these compounds to be a potent hit for the treatment of COVID-19.
We can be concluded that some compounds can be a good hit for SARS-2, for example, 1β-Hydroxyaleuritolic acid 3-p-hydroxybenzoate (11) that acting as reverse transcriptase inhibitors, and at the same time it showed a protease inhibitor too; moreover, when the virtual docking of the previously collected compounds against different molecular targets, it was the only compound that got interesting binding scores against the viral main protease (Mpro) and papain-like protease(PLpro) (binding scores of -8.5 and -8.0 kcal/mol, respectively). This compound was able to form four strong hydrogen bonds (< 2.5 Å) and with four different amino acid residues inside the Mpro’s active site, three of them have been reported previously as essential ones for the enzyme’s inhibition which conformed to its powerful antiviral activity and can be considered a role model for inhibition of COVID-19. In the same manner, Lupeol (12) and Uvaol (14) showed powerful reverse transcriptase inhibitors. Salaspermic acid (13) showed inhibitory activity against HIV via reverse transcriptase and it has replication inhibition activity.
On the other hand, if we are talking about the replication inhibitors, we can conclude that β-Aescin (19) is the one of choice, as it was reported that it inhibits NF- ƙB activation and cytokines production as well as, the inhibition of the replication of SARS-CoV-1 in vitro (IC50 6 µM), suggest it may be able to inhibit SARS-CoV-2 too.
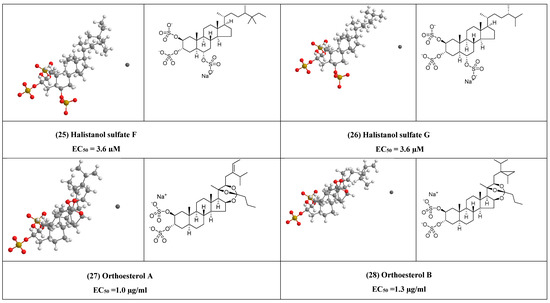
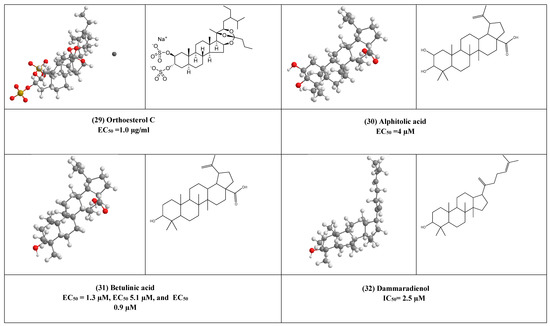
Some of the sulfated sterols e.g halistanol sulfate F (25) and halistanol sulfate G (26) exhibited antiviral activity against HIV-1, this may suggest the importance of the sulfate group in the inhibition of the virus. Alphitolic acid (30) showed an effect quite similar to that of dexamethasone in inhibition of the nuclear factor kappa-B (NF-κB), or suppressing the secretion of pro-inflammatory cytokines such as cytokines suppressor effect of alphitolic acid (30) can help in reducing the severe inflammatory syndrome associated with COVID-19.
The previous discussion showed that not only the triterpenes are active but also their semi-synthetic derivatives e.g., betulinic acid (31) as the antiviral activity of betulinic acid and its derivatives also have been studied against influenza A, herpes simplex type 1 (HSV-1), and ECHO-6 enterovirus. It has been found that the addition of ureides or the C-28 amide group to betulinic acid increases its anti-HSV-1 activity Moreover, the 3-oxime derivatives of betulonic acid were more active, and this increase the number of active hits and widen their activities.
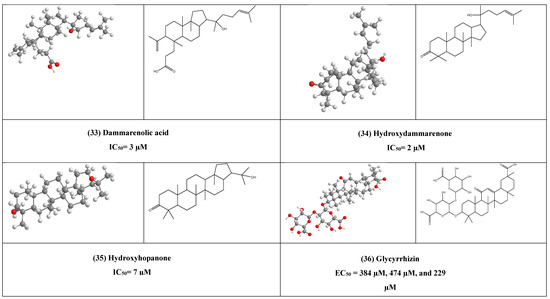
One of the most common triterpenes is glycyrrhizin (36) and its metabolites, such as glycyrrhetic acid (GA), reported having the ability to inhibit the cytopathic effect of numerous DNA and RNA viruses such as vaccinia, HSV-1, Newcastle disease, and vesicular stomatitis virus (VSV) in vitro. In vitro studies also showed that glycyrrhizin stopped the secretion of HBV surface antigen (HBsAg) from hepatocytes infected with HBV and also prohibited the viral cytopathic effect and the virus-specific antigen expression in HIV-infected MT-4 cells and inhibited virus replication in cultures of leukocytes from HIV-infected patients and it worth mentioning that, glycyrrhizin can inhibit SARS CoV entry to Vero cells.
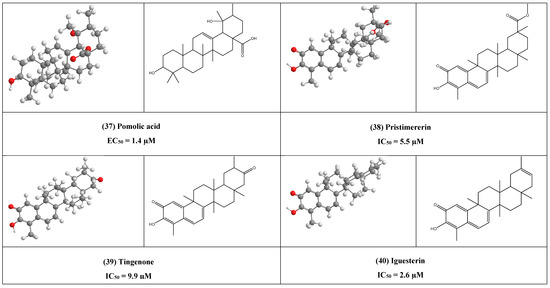
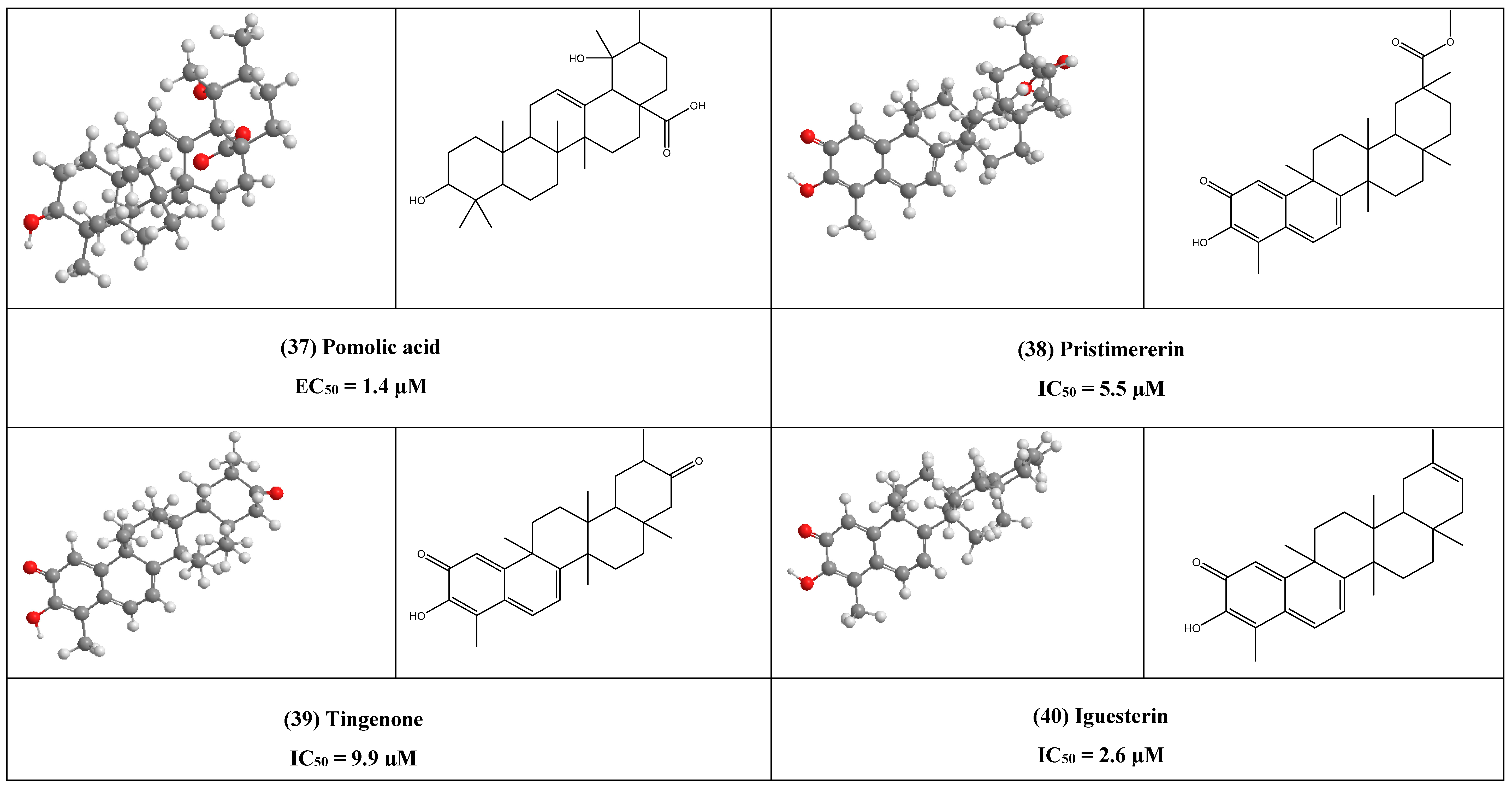
Concerning Mpro one of the most widespread targets for COVID-19, it was found the quinone-methide triterpenes, pristimererin, tingenone, and iguesterin (38–40) have shown potent inhibitory effect against SARS-CoV 3CLpro, this may contribute to the quinone group as through docking study ganoderiol F, that having similar quinone group in the same position showed the next scoring potency to the top 1β-Hydroxyaleuritolic acid 3-p-hydroxybenzoate (11).
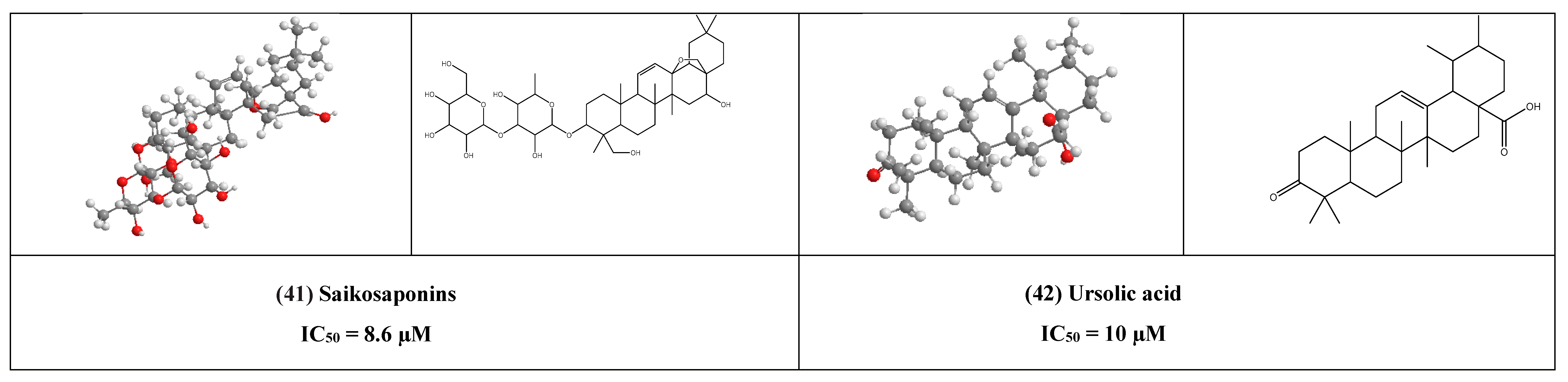
Finally, we would like to highlight the activity of ursolic acid (UA) (42) that was found to have antiviral activity in-vitro against rotavirus infections and it inhibited rotavirus replication, this may suggest its ability to inhibit some of coronaviruses effects when attacking the GIT in similar mechanisms.
Author Contributions
Conceptualization, N.H.S., K.A.Y. and A.M.S.; methodology, N.H.S., K.A.Y. and A.M.S.; software, N.H.S., K.A.Y. and A.M.S.; validation, L.B., T.O., H.M.H. and U.R.A.; formal analysis, N.H.S., K.A.Y., A.M.S., L.B., T.O., H.M.H. and U.R.A.; investigation, N.H.S., K.A.Y., A.M.S., L.B., T.O., H.M.H. and U.R.A.; resources, N.H.S., K.A.Y., A.M.S., L.B., T.O., H.M.H. and U.R.A.; data curation, L.B., T.O., H.M.H. and U.R.A.; writing—original draft preparation, N.H.S., K.A.Y. and A.M.S.; writing—review and editing, N.H.S., K.A.Y. and A.M.S.; visualization, L.B., T.O., H.M.H. and U.R.A.; supervision, L.B., T.O., H.M.H. and U.R.A.; project administration, L.B., T.O., H.M.H. and U.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank Deraya University for supporting the work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Salendra, L.; Lin, X.; Chen, W.; Pang, X.; Luo, X.; Long, J.; Liao, S.; Wang, J.; Zhou, X.; Liu, Y. Cytotoxicity of polyketides and steroids isolated from the sponge-associated fungus Penicillium citrinum SCSIO 41017. Nat. Prod. Res. 2019, 1478–6419, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Terent’ev, A.O.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Pounina, T.A.; Dembitsky, V.M. Hydroperoxy steroids and triterpenoids derived from plant and fungi: Origin, structures, and biological activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Withering, W. An Account of the Foxglove, and Some of Its Medical Uses; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Krim, S.R.; Vivo, R.P.; Perez, J.; Inklab, M.; Tenner Jr, T.; Hodgson, J. Digoxin: Current use and approach to toxicity. Am. J. Med Sci. 2008, 336, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Russell, A. Mupirocin, fusidic acid and bacitracin: Activity, action and clinical uses of three topical antibiotics. Vet. Dermatol. 1999, 10, 225–240. [Google Scholar] [CrossRef]
- Mori, R.C.; da Silva, T.P.; Campello, R.S.; Machado, U.F. Carbenoxolone enhances peripheral insulin sensitivity and GLUT4 expression in skeletal muscle of obese rats: Potential participation of UBC9 protein. Life Sci. 2019, 229, 157–165. [Google Scholar] [CrossRef]
- Doll, R.; Hill, I.; Hutton, C. Treatment of gastric ulcer with carbenoxolone sodium and oestrogens. Gut 1965, 6, 19. [Google Scholar] [CrossRef]
- Ming, Z.; Hu, Y.; Qiu, Y.; Cao, L.; Zhang, X. Synergistic effects of β-aescin and 5-fluorouracil in human hepatocellular carcinoma SMMC-7721 cells. Phytomedicine 2010, 17, 575–580. [Google Scholar] [CrossRef]
- Shamsabadipour, S.; Ghanadian, M.; Saeedi, H.; Rahimnejad, M.R.; Mohammadi-Kamalabadi, M.; Ayatollahi, S.M.; Salimzadeh, L. Triterpenes and steroids from Euphorbia denticulata Lam. with anti-Herpes symplex virus activity. Iran. J. Pharm. Res. 2013, 12, 759. [Google Scholar]
- Zhou, W.B.; Tao, J.Y.; Xu, H.M.; Chen, K.L.; Zeng, G.Z.; Ji, C.J.; Zhang, Y.M.; Tan, N.H. Three new antiviral triterpenes from Aster tataricus. Z. Nat. B 2010, 65, 1393–1396. [Google Scholar] [CrossRef]
- Rezanka, T.; Siristova, L.; Sigler, K. Sterols and triterpenoids with antiviral activity. Anti Infect. Agents Med. Chem. 2009, 8, 193–210. [Google Scholar] [CrossRef]
- Buckingham, J. (Ed.) Dictionary of Natural Products; Chapman and Hall: London, UK, 1994. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.M.; Alhadrami, H.A.; El-Gendy, A.O.; Shamikh, Y.I.; Belbahri, L.; Hassan, H.M.; Abdelmohsen, U.R.; Rateb, M.E. Microbial natural products as potential inhibitors of SARS-CoV-2 main protease (Mpro). Microorganisms 2020, 8, 970. [Google Scholar] [CrossRef] [PubMed]
- Lill, M.A.; Danielson, M.L. Computer-aided drug design platform using PyMOL. J. Comput. Aided Mol. Des. 2011, 25, 13–19. [Google Scholar] [CrossRef]
- Akihisa, T.; Ogihara, J.; Kato, J.; Yasukawa, K.; Ukiya, M.; Yamanouchi, S.; Oishi, K. Inhibitory effects of triterpenoids and sterols on human immunodeficiency virus-1 reverse transcriptase. Lipids 2001, 36, 507–512. [Google Scholar] [CrossRef]
- Kim, S.-K.; Van Ta, Q. Chapter 17—Bioactive Sterols from Marine Resources and Their Potential Benefits for Human Health. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 65, pp. 261–268. [Google Scholar]
- Cong, R.; Zhang, Y.; Tian, W. A concise synthesis of the steroidal core of clathsterol. Tetrahedron Lett. 2010, 51, 3890–3892. [Google Scholar] [CrossRef]
- Quintão, N.L.M.; Rocha, L.W.; Silva, G.F.; Reichert, S.; Claudino, V.D.; Lucinda-Silva, R.M.; Malheiros, A.; Souza, M.M.D.; Bellé Bresolin, T.M.; Machado, M.D.S. Contribution of α, β-Amyrenone to the Anti-Inflammatory and Antihypersensitivity Effects of Aleurites moluccana (L.) Willd. Biomed Res. Int. 2014, 2014, 636839. [Google Scholar]
- Tanaka, S.; Uno, C.; Akimoto, M.; Tabata, M.; Honda, C.; Kamisako, W. Anti-allergic effect of bryonolic acid from Luffa cylindrica cell suspension cultures. Planta Med. 1991, 57, 527–530. [Google Scholar] [CrossRef]
- Lertphadungkit, P.; Suksiriworapong, J.; Satitpatipan, V.; Sirikantaramas, S.; Wongrakpanich, A.; Bunsupa, S. Enhanced Production of Bryonolic Acid in Trichosanthes cucumerina L. (Thai Cultivar) Cell Cultures by Elicitors and Their Biological Activities. Plants 2020, 9, 709. [Google Scholar] [CrossRef]
- Akihisa, T.; Yamamoto, K.; Tamura, T.; Kimura, Y.; Iida, T.; Nambara, T.; Chang, F.C. Triterpenoid Ketones from Lingnania chungii MoClure: Arborinone, friedelin and glutinone. Chem. Pharm. Bull. 1992, 40, 789–791. [Google Scholar] [CrossRef]
- Pengsuparp, T.; Cai, L.; Constant, H.; Fong, H.H.; Lin, L.-Z.; Kinghorn, A.D.; Pezzuto, J.M.; Cordell, G.A.; Ingolfsdöttir, K.; Wagner, H. Mechanistic evaluation of new plant-derived compounds that inhibit HIV-1 reverse transcriptase. J. Nat. Prod. 1995, 58, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Al-Rehaily, A.J.; El-Tahir, K.E.; Mossa, J.S.; Rafatullah, S. Pharmacological studies of various extracts and the major constituent, lupeol, obtained from hexane extract of Teclea nobilis in rodents. Nat. Prod. Sci. 2001, 7, 76–82. [Google Scholar]
- Fernández, A.; Alvarez, A.; García, M.D.; Sáenz, M.T. Anti-inflammatory effect of Pimenta racemosa var. ozua and isolation of the triterpene lupeol. Il Farm. 2001, 56, 335–338. [Google Scholar] [CrossRef]
- Fernández, M.A.; de las Heras, B.; Garcia, M.D.; Sáenz, M.T.; Villar, A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J. Pharm. Pharmacol. 2001, 53, 1533–1539. [Google Scholar] [CrossRef]
- Chaturvedi, P.K.; Bhui, K.; Shukla, Y. Lupeol: Connotations for chemoprevention. Cancer Lett. 2008, 263, 1–13. [Google Scholar] [CrossRef]
- Sudhahar, V.; Kumar, S.A.; Varalakshmi, P.; Sujatha, V. Protective effect of lupeol and lupeol linoleate in hypercholesterolemia associated renal damage. Mol. Cell. Biochem. 2008, 317, 11. [Google Scholar] [CrossRef]
- Sudhahar, V.; Veena, C.K.; Varalakshmi, P. Antiurolithic effect of lupeol and lupeol linoleate in experimental hyperoxaluria. J. Nat. Prod. 2008, 71, 1509–1512. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; Collaboration, H.A.S. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033. [Google Scholar] [CrossRef]
- Perez, R. Antiviral activity of compounds isolated from plants. Pharm. Biol. 2003, 41, 107–157. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral activities of oleanolic acid and its analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.M.; Khattab, A.R.; AboulMagd, A.M.; Hassan, H.M.; Rateb, M.E.; Zaid, H.; Abdelmohsen, U.R. Nature as a treasure trove of potential anti-SARS-CoV drug leads: A structural/mechanistic rationale. RSC Adv. 2020, 10, 19790–19802. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Sujarit, K.; Pattananandecha, T.; Saenjum, C.; Lumyong, S. Natural Bioactive Compounds from Fungi as Potential Candidates for Protease Inhibitors and Immunomodulators to Apply for Coronaviruses. Molecules 2020, 25, 1800. [Google Scholar] [CrossRef] [PubMed]
- El Dine, R.S.; El Halawany, A.M.; Ma, C.-M.; Hattori, M. Anti-HIV-1 protease activity of lanostane triterpenes from the vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2008, 71, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Ernst, E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst. Rev. 2012, 1465–1858. [Google Scholar] [CrossRef]
- Domanski, D.; Zegrocka-Stendel, O.; Perzanowska, A.; Dutkiewicz, M.; Kowalewska, M.; Grabowska, I.; Maciejko, D.; Fogtman, A.; Dadlez, M.; Koziak, K. Molecular mechanism for cellular response to β-escin and its therapeutic implications. PLoS ONE 2016, 11, e0164365. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.; Sun, F.; Jiang, N.; Fan, H.; Wang, T.; Li, Z.; He, J.; Fu, F. Escin exerts synergistic anti-inflammatory effects with low doses of glucocorticoids in vivo and in vitro. Phytomedicine 2011, 18, 272–277. [Google Scholar] [CrossRef]
- Subramanya, S.B.; Venkataraman, B.; Meeran, M.F.N.; Goyal, S.N.; Patil, C.R.; Ojha, S. Therapeutic potential of plants and plant derived phytochemicals against acetaminophen-induced liver injury. Int. J. Mol. Sci. 2018, 19, 3776. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Wang, H.-K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-F.; Zhang, S.-X.; Wang, H.-K.; Zhang, S.-Y.; Sun, Q.-Z.; Cosentino, L.M.; Lee, K.-H. Novel anti-HIV lancilactone C and related triterpenes from Kadsura lancilimba. J. Nat. Prod. 1999, 62, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Oprean, C.; Mioc, M.; Csányi, E.; Ambrus, R.; Bojin, F.; Tatu, C.; Cristea, M.; Ivan, A.; Danciu, C.; Dehelean, C. Improvement of ursolic and oleanolic acids’ antitumor activity by complexation with hydrophilic cyclodextrins. Biomed. Pharmacother. 2016, 83, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zeng, H.; Wang, Y.; Fan, X.; Xu, C.; Deng, R.; Zhou, X.; Bi, H.; Huang, M. Low dose of oleanolic acid protects against lithocholic acid–induced cholestasis in mice: Potential involvement of nuclear factor-E2-related factor 2-mediated upregulation of multidrug resistance-associated proteins. Drug Metab. Dispos. 2014, 42, 844–852. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Shen, J.-K.; Wang, H.-K.; Cosentino, L.M.; Lee, K.-H. Synthesis and anti-HIV activity of oleanolic acid derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 3115–3118. [Google Scholar] [CrossRef]
- Li, H.-Y.; Sun, N.-J.; Kashiwada, Y.; Sun, L.; Snider, J.V.; Cosentino, L.M.; Lee, K.-H. Anti-AIDS agents, 9. Suberosol, a new C31 lanostane-type triterpene and anti-HIV principle from Polyalthia suberosa. J. Nat. Prod. 1993, 56, 1130–1133. [Google Scholar] [CrossRef]
- Bifulco, G.; Bruno, I.; Minale, L.; Riccio, R. Novel HIV-inhibitory halistanol sulfates F-H from a marine sponge, Pseudoaxinissa digitata. J. Nat. Prod. 1994, 57, 164–167. [Google Scholar] [CrossRef]
- Koehn, F.E.; Gunasekera, M.; Cross, S.S. New antiviral sterol disulfate ortho esters from the marine sponge Petrosia weinbergi. J. Org. Chem. 1991, 56, 1322–1325. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Gao, W.; Wang, H. Study on chemical composition, anti-inflammatory and anti-microbial activities of extracts from Chinese pear fruit (Pyrus bretschneideri Rehd.). Food Chem. Toxicol. 2012, 50, 3673–3679. [Google Scholar] [CrossRef]
- Moghaddam, M.G.; Faujan Bin, H.; Ahmad, F.H.; Kermani, A.S. Biological Activity of Betulinic Acid: A Review. Pharmacol. Pharm. 2012, 3, 119–123. [Google Scholar] [CrossRef]
- Yu, D.; Wild, C.T.; Martin, D.E.; Morris-Natschke, S.L.; Chen, C.-H.; Allaway, G.P.; Lee, K.-H. The discovery of a class of novel HIV-1 maturation inhibitors and their potential in the therapy of HIV. Expert Opin. Investig. Drugs 2005, 14, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Kandefer-Szerszen, M. Antitumor and antiviral activity of pentacyclic triterpenes. Mini Rev. Org. Chem. 2014, 11, 262–268. [Google Scholar] [CrossRef]
- Soler, F.; Poujade, C.; Evers, M.; Carry, J.-C.; Hénin, Y.; Bousseau, A.; Huet, T.; Pauwels, R.; De Clercq, E.; Mayaux, J.-F. Betulinic acid derivatives: A new class of specific inhibitors of human immunodeficiency virus type 1 entry. J. Med. Chem. 1996, 39, 1069–1083. [Google Scholar] [CrossRef]
- Qian, K.; Morris-Natschke, S.L.; Lee, K.H. HIV entry inhibitors and their potential in HIV therapy. Med. Res. Rev. 2009, 29, 369–393. [Google Scholar] [CrossRef]
- Pohjala, L.; Alakurtti, S.; Ahola, T.; Yli-Kauhaluoma, J.; Tammela, P. Betulin-derived compounds as inhibitors of alphavirus replication. J. Nat. Prod. 2009, 72, 1917–1926. [Google Scholar] [CrossRef]
- Pavlova, N.; Savinova, O.; Nikolaeva, S.; Boreko, E.; Flekhter, O. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia 2003, 74, 489–492. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Karachurina, L.T.; Nigmatullina, L.R.; Sapozhnikova, T.A.; Baltina, L.A.; Zarudii, F.S.; Galin, F.Z.; Spirikhin, L.V.; Tolstikov, G.A.; Plyasunova, O.A. Synthesis and pharmacological activity of betulin dinicotinate. Russ. J. Bioorg. Chem. 2002, 28, 494–500. [Google Scholar] [CrossRef]
- Baltina, L.A.; Flekhter, O.B.; Nigmatullina, L.R.; Boreko, E.I.; Pavlova, N.I.; Nikolaeve, S.N.; Savinova, O.V.; Tolstikov, G.A. Lupane triterpenes and derivatives with antiviral activity. Bioorg. Med. Chem. Lett. 2003, 13, 3549–3552. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef]
- Pompei, R.; Flore, O.; Marccialis, M.A.; Pani, A.; Loddo, B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 1979, 281, 689–690. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Shigeta, S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antivir. Res. 1987, 7, 99–107. [Google Scholar] [CrossRef]
- Crance, J.M.; Scaramozzino, N.; Jouan, A.; Garin, D. Interferon, ribavirin, 6-azauridine and glycyrrhizin: Antiviral compounds active against pathogenic flaviviruses. Antivir. Res. 2003, 58, 73–79. [Google Scholar] [CrossRef]
- Lin, J.-C.; Cherng, J.-M.; Hung, M.-S.; Baltina, L.A.; Baltina, L.; Kondratenko, R. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: Structure–activity relationships. Antivir. Res. 2008, 79, 6–11. [Google Scholar] [CrossRef]
- Takahara, T.; Watanabe, A.; Shiraki, K. Effects of glycyrrhizin on hepatitis B surface antigen: A biochemical and morphological study. J. Hepatol. 1994, 21, 601–609. [Google Scholar] [CrossRef]
- Sasaki, H.; Takei, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology 2002, 70, 229–236. [Google Scholar] [CrossRef]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef]
- Wolkerstorfer, A.; Kurz, H.; Bachhofner, N.; Szolar, O.H. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antivir. Res. 2009, 83, 171–178. [Google Scholar] [CrossRef]
- Sui, X.; Yin, J.; Ren, X. Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antivir. Res. 2010, 85, 346–353. [Google Scholar] [CrossRef]
- Hardy, M.E.; Hendricks, J.M.; Paulson, J.M.; Faunce, N.R. 18 β-glycyrrhetinic acid inhibits rotavirus replication in culture. Virol. J. 2012, 9, 1–7. [Google Scholar] [CrossRef]
- Kang, H.; Lieberman, P.M. Mechanism of glycyrrhizic acid inhibition of Kaposi’s sarcoma-associated herpesvirus: Disruption of CTCF-cohesin-mediated RNA polymerase II pausing and sister chromatid cohesion. J. Virol. 2011, 85, 11159–11169. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, U.A.; Masoud, M.S.; Nawaz, Z.; Riazuddin, S. Glycyrrhizin as antiviral agent against Hepatitis C Virus. J. Transl. Med. 2011, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chama, M.A.; Dziwornu, G.A.; Waibel, R.; Osei-Safo, D.; Addae-Mensah, I.; Otchere, J.; Wilson, M. Isolation, characterization, and anthelminthic activities of a novel dichapetalin and other constituents of Dichapetalum filicaule. Pharm. Biol. 2016, 54, 1179–1188. [Google Scholar] [PubMed]
- Rojano, B.; Saez, J.; Schinella, G.; Quijano, J.; Vélez, E.; Gil, A.; Notario, R. Experimental and theoretical determination of the antioxidant properties of isoespintanol (2-Isopropyl-3, 6-dimethoxy-5-methylphenol). J. Mol. Struct. 2008, 877, 1–6. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Park, S.-J.; Kim, Y.M.; Lee, J.-Y.; Seo, W.D.; Chang, J.S.; Park, K.H.; Rho, M.-C.; Lee, W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorg. Med. Chem. Lett. 2010, 20, 1873–1876. [Google Scholar] [CrossRef]
- Nakanishi, K.; Takahashi, Y.; Budzikiewicz, H. Pristimerin. Spectroscopic Properties of the Dienone—Phenol-Type Rearrangement Products and Other Derivatives1. J. Org. Chem. 1965, 30, 1729–1734. [Google Scholar] [CrossRef]
- Monache, F.; Bettólo, G.; de Lima, O.; D’Albuquerque, I.; de Barros, C.J. The structure of tingenone, a quinonoid triterpene related to pristimerin. J. Chem. Soc. Perkin Trans. 1973, 22, 2725. [Google Scholar] [CrossRef]
- Itokawa, H.; Shirota, O.; Ikuta, H.; Morita, H.; Takeya, K.; Iitaka, Y. Triterpenes fromMaytenus ilicifolia. Phytochemistry 1991, 30, 3713–3716. [Google Scholar] [CrossRef]
- Pomponi, M.; Delle Monache, F. “Rearrangements of Tingenone.” IV. Researches on Quinonoid Triterpenes; Real Sociedad Española de Química: Madrid, Spain, 1974. [Google Scholar]
- Sotanaphun, U.; Lipipun, V.; Suttisri, R.; Bavovada, R. Antimicrobial activity and stability of tingenone derivatives. Planta Med. 1999, 65, 450–452. [Google Scholar] [CrossRef]
- Sotanaphun, U.; Lipipun, V.; Yaipakdee, P.; Bavovada, R. New acidic-rearranged compounds from tingenone derivatives and their biological activity. Pharm. Biol. 2005, 43, 39–46. [Google Scholar] [CrossRef][Green Version]
- Ito, M.; Nakashima, H.; Baba, M.; Pauwels, R.; De Clercq, E.; Shigeta, S.; Yamamoto, N. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)]. Antivir. Res. 1987, 7, 127–137. [Google Scholar] [CrossRef]
- Ushio, Y.; Abe, H. Inactivation of measles virus and herpes simplex virus by saikosaponin d. Planta Med. 1992, 58, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.S.; Sinsheimer, J.E.; Cochran, K.W. Antiviral activity of triterpenoid saponins containing acylated β-amyrin aglycones. J. Pharm. Sci. 1974, 63, 471–473. [Google Scholar] [PubMed]
- Amoros, M.; Fauconnier, B.; Girre, R. In vitro antiviral activity of a saponin from Anagallis arvensis, Primulaceae, against herpes simplex virus and poliovirus. Antivir. Res. 1987, 8, 13–25. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Fullas, F.; Brown, D.M.; Wani, M.C.; Wall, M.E.; Cai, L.; Mar, W.; Lee, S.K.; Luo, Y.; Zaw, K. Isolation and structural elucidation of pentacyclic triterpenoids from Maprounea africana. J. Nat. Prod. 1995, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rut, W.; Lv, Z.; Zmudzinski, M.; Patchett, S.; Nayak, D.; Snipas, S.J.; El Oualid, F.; Bekes, M.; Huang, T.T.; Drag, M. Activity profiling and structures of inhibitor-bound SARS-CoV-2-PLpro protease provides a framework for anti-COVID-19 drug design. bioRxiv 2020, 6, eabd4596. [Google Scholar]
- Bekerman, E.; Neveu, G.; Shulla, A.; Brannan, J.; Pu, S.-Y.; Wang, S.; Xiao, F.; Barouch-Bentov, R.; Bakken, R.R.; Mateo, R. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J. Clin. Investig. 2017, 127, 1338–1352. [Google Scholar] [CrossRef]
- Gil, C.; Ginex, T.; Maestro, I.; Nozal, V.; Barrado-Gil, L.; Cuesta-Geijo, M.A.; Urquiza, J.; Ramírez, D.; Alonso, C.; Campillo, N.E. COVID-19: Drug targets and potential treatments. J. Med. Chem. 2020, 63, 12359–12386. [Google Scholar] [CrossRef]
- Cantini, F.; Banci, L.; Altincekic, N.; Bains, J.; Dhamotharan, K.; Fuks, C.; Fürtig, B.; Gande, S.; Hargittay, B.; Hengesbach, M. 1 H, 13 C, and 15 N backbone chemical shift assignments of the apo and the ADP-ribose bound forms of the macrodomain of SARS-CoV-2 non-structural protein 3b. Biomol. NMR Assign. 2020, 14, 339–346. [Google Scholar] [CrossRef]
- Michalska, K.; Kim, Y.; Jedrzejczak, R.; Maltseva, N.I.; Stols, L.; Endres, M.; Joachimiak, A. Crystal structures of SARS-CoV-2 ADP-ribose phosphatase (ADRP): From the apo form to ligand complexes. bioRxiv 2020, 7, 814–824. [Google Scholar]
- Hattori, M.; Ma, C.-M.; Wei, Y.; Dine, R.; Sato, N. Survey of anti-HIV and anti-HCV compounds from Natural sources. Can Chem. Trans. 2013, 1, 116–140. [Google Scholar]
- Min, B.-S.; Nakamura, N.; Miyashiro, H.; BAE, K.-W.; Hattori, M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998, 46, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).