Perfluorooctanesulfonic Acid Detection Using Molecularly Imprinted Polyaniline on a Paper Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
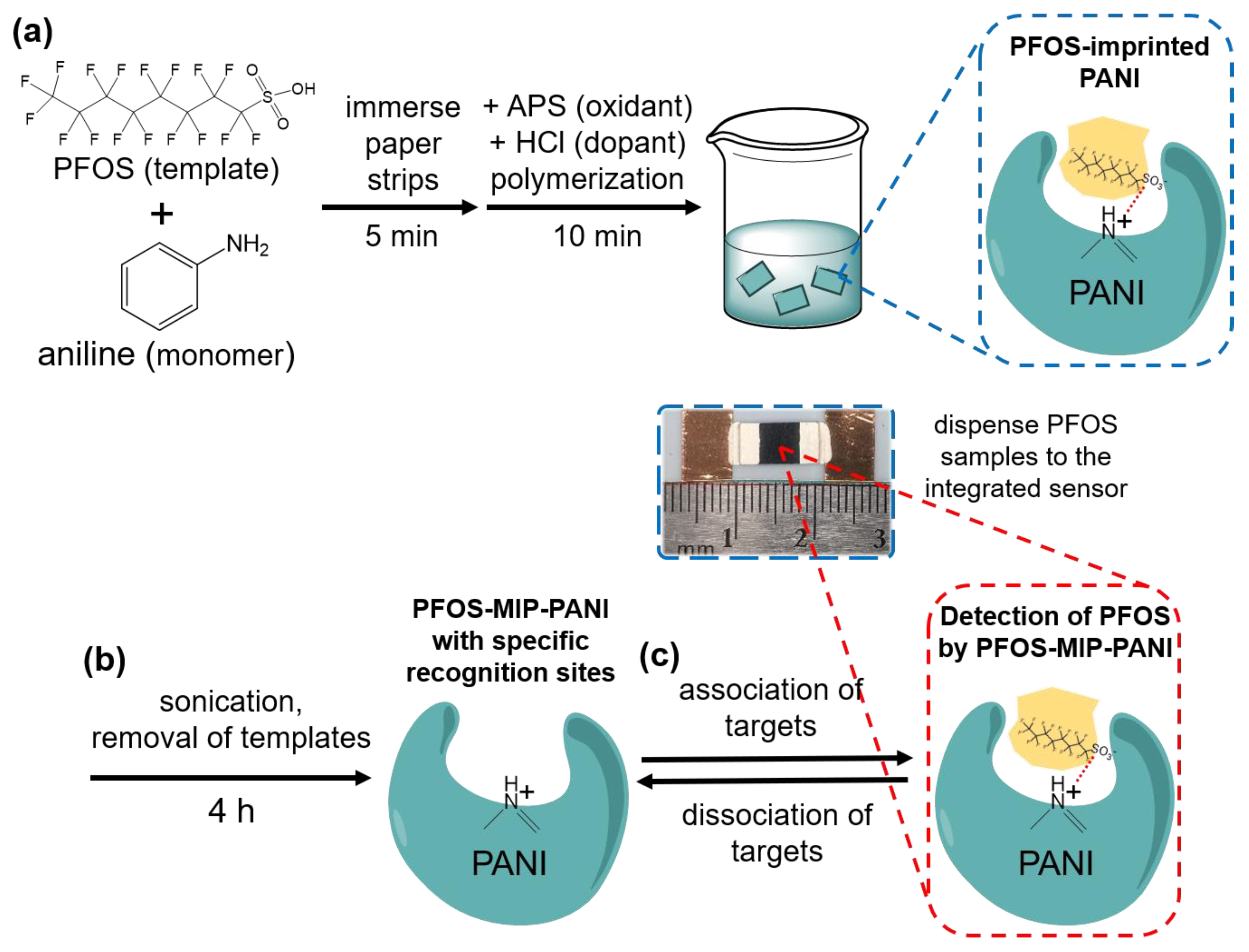
2.2. Synthesis of Molecularly Imprinted Polyaniline on Paper
2.3. PFOS Paper Sensor Fabrication
2.4. PFOS Exposure and Resistivity Measurement
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.6. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.7. Scanning Electron Microscopy (SEM) Analysis
3. Results
3.1. ATR-FTIR Spectra
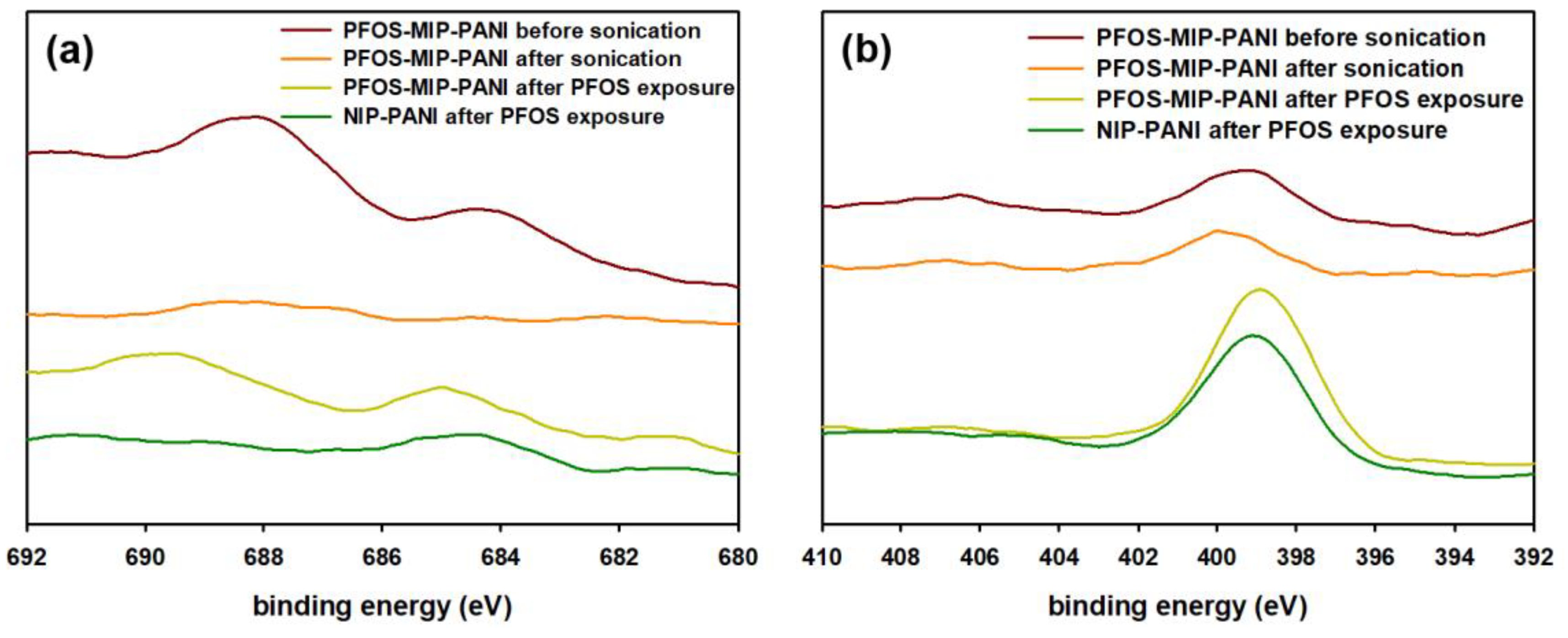
3.2. XPS Spectra
3.3. Scanning Electron Microscopy Images
3.4. PFOS Detection on PFOS-MIP-PANI Electrodes
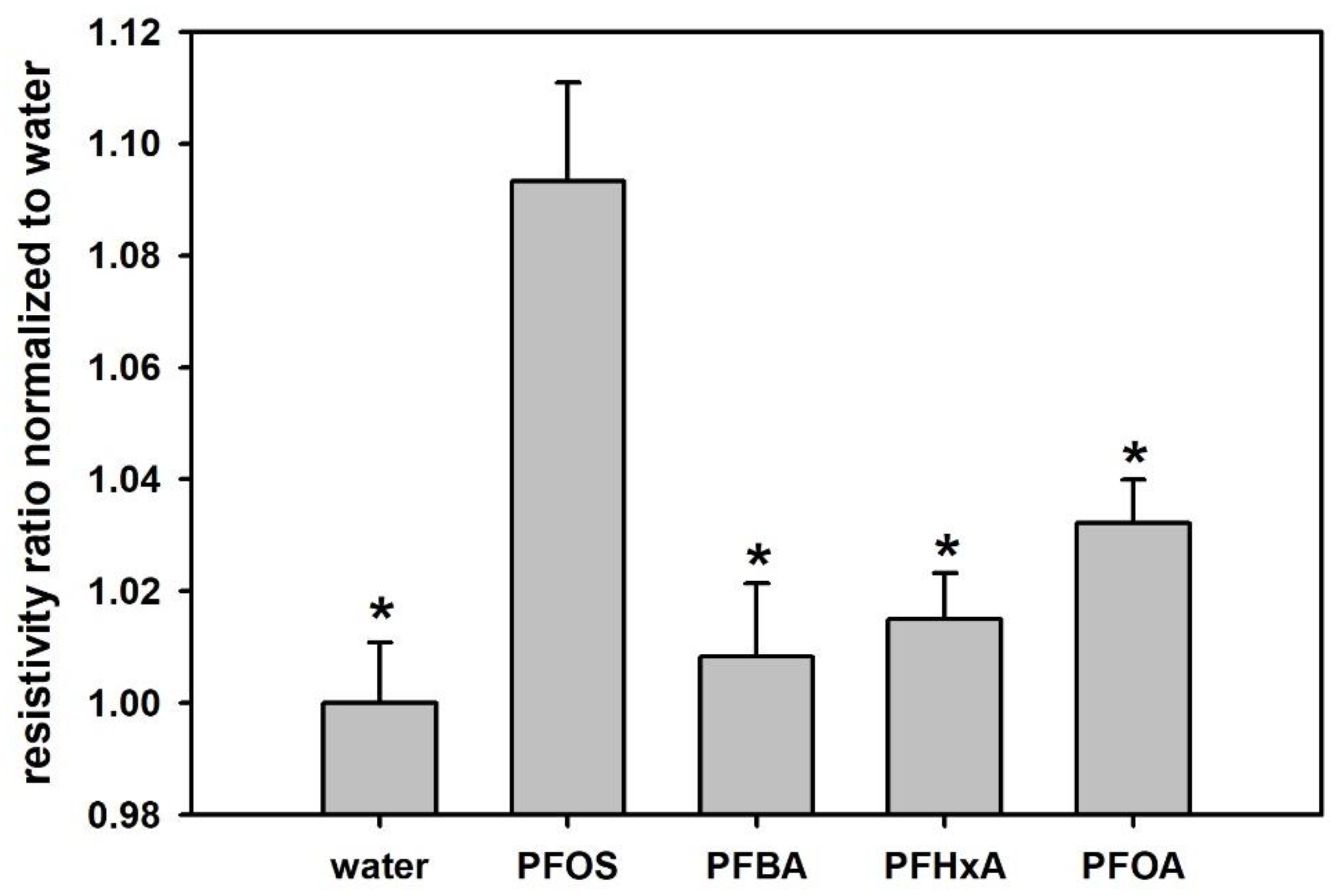
3.5. Selectivity among Relevant PFCs
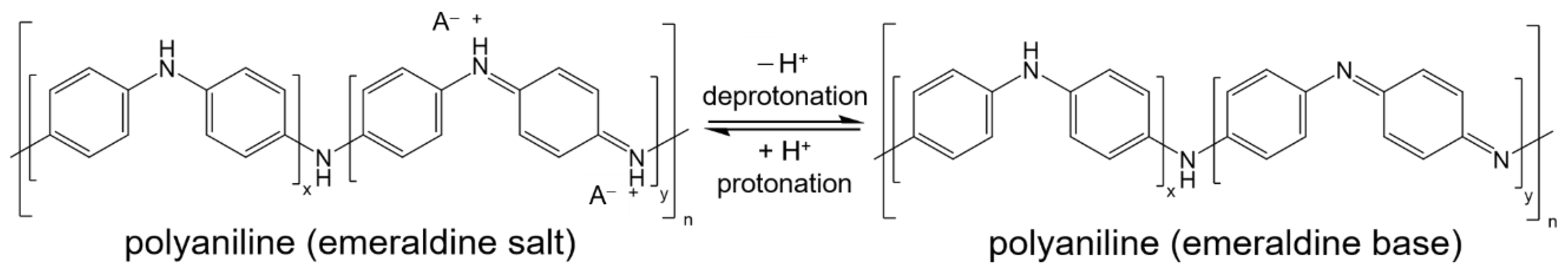
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, H.; Peng, H.; Guan, J.; Liu, Y.; Dai, J.; Su, R.; Guo, Z.; Chen, Y.; Hu, Q.; Yuan, B.; et al. Photodegradation of Gaseous Toluene by Vacuum Ultraviolet Light: Performance and Mechanism. Eng. Sci. 2020, 9, 68–76. [Google Scholar] [CrossRef]
- Nidamanuri, N.; Li, Y.; Li, Q.; Dong, M.; Boni, B.O.O.; Lamboni, L.; Bakadia, B.M.; Hussein, S.A.; Yang, G. Graphene and Graphene Oxide-Based Membranes for Gas Separation. Eng. Sci. 2020, 9, 3–16. [Google Scholar] [CrossRef]
- Xiang, X.; Pan, F.; Li, Y. Flower-like Bismuth Metal-Organic Frameworks Grown on Carbon Paper as a Free-Standing Electrode for Efficient Electrochemical Sensing of Cd2+ and Pb2+ in Water. Eng. Sci. 2018, 3, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Jain, B.; Singh, A.K.; Hashmi, A.; Susan, A.B.H.; Lellouche, J.-P. Surfactant-assisted cerium oxide and its catalytic activity towards Fenton process for non-degradable dye. Adv. Compos. Hybrid Mater. 2020, 3, 430–441. [Google Scholar] [CrossRef]
- Singh, N.; Jana, S.; Singh, G.P.; Dey, R.K. Graphene-supported TiO2: Study of promotion of charge carrier in photocatalytic water splitting and methylene blue dye degradation. Adv. Compos. Hybrid Mater. 2020, 3, 127–140. [Google Scholar] [CrossRef]
- Görgün, N.; Özer, Ç.; Polat, K. A new catalyst material from electrospun PVDF-HFP nanofibers by using magnetron-sputter coating for the treatment of dye-polluted waters. Adv. Compos. Hybrid Mater. 2019, 2, 423–430. [Google Scholar] [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Xu, C.; Chen, H.; Jiang, F. Adsorption of perflourooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) on polyaniline nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2015, 479, 60–67. [Google Scholar] [CrossRef]
- Zushi, Y.; Takeda, T.; Masunaga, S. Existence of nonpoint source of perfluorinated compounds and their loads in the Tsurumi River basin, Japan. Chemosphere 2008, 71, 1566–1573. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Peer Reviewed: Perfluorochemical Surfactants in the Environment. Environ. Sci. Technol. 2002, 36, 146A–152A. [Google Scholar] [CrossRef] [Green Version]
- Butt, C.M.; Berger, U.; Bossi, R.; Tomy, G.T. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci. Total. Environ. 2010, 408, 2936–2965. [Google Scholar] [CrossRef]
- Biegel, L.B.; Hurtt, M.E.; Frame, S.R.; O’Connor, J.C.; Cook, J.C. Mechanisms of Extrahepatic Tumor Induction by Peroxisome Proliferators in Male CD Rats. Toxicol. Sci. 2001, 60, 44–55. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [Green Version]
- Thomford, P. 104-Week Dietary Chronic Toxicity and Carcinogenicity Study with Perfluorooctane Sulfonic Acid Potassium Salt (PFOS; T-6295) in Rats; Final Report, 3M T-6295 Covance Study No. 6329-183; 3M: St. Paul, MN, USA, 2002; Volume I–IX. [Google Scholar]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef]
- DeWitt, J.; Peden-Adams, M.M.; Keller, J.M.; Germolec, D.R. Immunotoxicity of Perfluorinated Compounds: Recent Developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar] [CrossRef]
- Wójcik, A.; Perczyk, P.; Wydro, P.; Broniatowski, M. Effects of water soluble perfluorinated pollutants on phospholipids in model soil decomposer membranes. Biochim. Biophys. Acta BBA Biomembr. 2018, 1860, 2576–2587. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Technical Fact Sheet—Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA); US Environmental Protection Agency: Washington, DC, USA, 2017; pp. 1–8.
- Interstate Technology Regulatory Council. Regulations, Guidance and Advisories for Per-and Polyfluoroalkyl Substances (PFAS); ITRC: Washington, DC, USA, 2017. [Google Scholar]
- UNEP. Stockholm Convention on Persistent Organic Pollutants (POPs); UNEP: Geneva, Switzerland, 2009. [Google Scholar]
- Wu, M.; Sun, R.; Wang, M.; Liang, H.; Ma, S.; Sihan, M.; Xia, X.; Ma, J.; Tang, L.; Sun, Y.; et al. Analysis of perfluorinated compounds in human serum from the general population in Shanghai by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Chemosphere 2017, 168, 100–105. [Google Scholar] [CrossRef]
- Poboży, E.; Król, E.; Wójcik, L.; Wachowicz, M.; Trojanowicz, M. HPLC determination of perfluorinated carboxylic acids with fluorescence detection. Microchim. Acta 2010, 172, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Mitroshkov, A.V.; Zhong, L.; Thomas, L.M. Analysis of Perfluorinated, Pharmaceutical, Personal Care Compounds and Heavy Metals in Waste Water Sludge Using GC-MS/MS and Multicollector ICP-MS; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2019.
- Scott, B.F.; Moody, C.A.; Spencer, C.; Small, J.M.; Muir, D.C.G.; Mabury, S.A. Analysis for Perfluorocarboxylic Acids/Anions in Surface Waters and Precipitation Using GC−MS and Analysis of PFOA from Large-Volume Samples. Environ. Sci. Technol. 2006, 40, 6405–6410. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, P.; Xiong, J.; Gao, L.; Tana, K. A sensitive and selective triple-channel optical assay based on red-emissive carbon dots for the determination of PFOS. Microchem. J. 2019, 145, 388–396. [Google Scholar] [CrossRef]
- Cheng, Z.; Dong, H.; Liang, J.; Zhang, F.; Chen, X.; Du, L.; Tana, K. Highly selective fluorescent visual detection of perfluorooctane sulfonate via blue fluorescent carbon dots and berberine chloride hydrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 262–269. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, X.; Dong, Z.; Wang, L.; Megharaj, M.; Naidu, R. Smartphone app-based/portable sensor for the detection of fluoro-surfactant PFOA. Chemosphere 2018, 191, 381–388. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, B.; Jiang, X.; Li, J. Current Progress of Nanomaterials in Molecularly Imprinted Electrochemical Sensing. Crit. Rev. Anal. Chem. 2017, 48, 15–32. [Google Scholar] [CrossRef]
- Pauling, L. A Theory of the Structure and Process of Formation of Antibodies *. J. Am. Chem. Soc. 2005, 62, 2643–2657. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Saianand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Tsai, T.-C.; Han, H.-Z.; Cheng, C.-C.; Chen, L.-C.; Chang, H.-C.; Chen, J.-J.J. Modification of platinum microelectrode with molecularly imprinted over-oxidized polypyrrole for dopamine measurement in rat striatum. Sens. Actuators B Chem. 2012, 171, 93–101. [Google Scholar] [CrossRef]
- Lattach, Y.; Fourati, N.; Zerrouki, C.; Fougnion, J.-M.; Garnier, F.; Pernelle, C.; Remita, S. Molecularly imprinted surface acoustic wave sensors: The synergy of electrochemical and gravimetric transductions in chemical recognition processes. Electrochim. Acta 2012, 73, 36–44. [Google Scholar] [CrossRef]
- Paik, P.; Gedanken, A.; Mastai, Y. Chiral-mesoporous-polypyrrole nanoparticles: Its chiral recognition abilities and use in enantioselective separation. J. Mater. Chem. 2010, 20, 4085–4093. [Google Scholar] [CrossRef]
- Ho, K.-C.; Yeh, W.-M.; Tung, T.-S.; Liao, J.-Y. Amperometric detection of morphine based on poly(3,4-ethylenedioxythiophene) immobilized molecularly imprinted polymer particles prepared by precipitation polymerization. Anal. Chim. Acta 2005, 542, 90–96. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, D.; Yu, J. A molecule-imprinted polyaniline membrane modified on carbon fiber for detection of glycine. Bio Med Mater. Eng. 2014, 24, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Huang, J.; Wu, Y.; Sun, J.; Wei, W.; Liu, X. Synthesis of hydrophilic and conductive molecularly imprinted polyaniline particles for the sensitive and selective protein detection. Biosens. Bioelectron. 2017, 94, 39–46. [Google Scholar] [CrossRef]
- Shin, Y.J.; Kameoka, J. Amperometric cholesterol biosensor using layer-by-layer adsorption technique onto electrospun polyaniline nanofibers. J. Ind. Eng. Chem. 2012, 18, 193–197. [Google Scholar] [CrossRef]
- Li, S.; Yang, C.; Sarwar, S.; Nautiyal, A.; Zhang, P.; Du, H.; Liu, N.; Yin, J.; Deng, K.; Zhang, X. Facile synthesis of nanostructured polyaniline in ionic liquids for high solubility and enhanced electrochemical properties. Adv. Compos. Hybrid Mater. 2019, 2, 279–288. [Google Scholar] [CrossRef]
- Ingle, R.V.; Shaikh, S.F.; Bhujbal, P.K.; Pathan, H.M.; Tabhane, V.A. Polyaniline Doped with Protonic Acids: Optical and Morphological Studies. ES Mater. Manuf. 2020, 8, 54–59. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, X.; Cao, D. Biomass-derived nitrogen-doped porous carbons (NPC) and NPC/ polyaniline composites as high performance supercapacitor materials. Eng. Sci. 2018, 1, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, W.; Yin, R.; Huang, X.; Qian, L. A Highly Porous Polyaniline-Graphene Composite Used for Electrochemical Supercapacitors. Eng. Sci. 2018, 3, 89–95. [Google Scholar] [CrossRef]
- Li, S.; Jasim, A.; Zhao, W.; Fu, L.; Ullah, M.W.; Shi, Z.; Yang, G. Fabrication of pH-electroactive Bacterial Cellulose/Polyaniline Hydrogel for the Development of a Controlled Drug Release System. ES Mater. Manuf. 2018, 1, 41–49. [Google Scholar] [CrossRef]
- Majumdar, S.; Saikia, U.; Mahanta, D. Polyaniline-Coated Filter Papers: Cost Effective Hybrid Materials for Adsorption of Dyes. J. Chem. Eng. Data 2015, 60, 3382–3391. [Google Scholar] [CrossRef]
- Luo, J.; Sun, J.; Huang, J.; Liu, X. Preparation of water-compatible molecular imprinted conductive polyaniline nanoparticles using polymeric micelle as nanoreactor for enhanced paracetamol detection. Chem. Eng. J. 2016, 283, 1118–1126. [Google Scholar] [CrossRef]
- Shi, L.; Hou, A.; Chen, L.; Wang, Z. Electrochemical sensor prepared from molecularly imprinted polymer for recognition of TNT. Polym. Compos. 2014, 36, 1280–1285. [Google Scholar] [CrossRef]
- Chen, Z.; Wright, C.; Dincel, O.; Chi, T.-Y.; Kameoka, J. A Low-Cost Paper Glucose Sensor with Molecularly Imprinted Polyaniline Electrode. Sensors 2020, 20, 1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Chi, T.-Y.; Dincel, O.; Tong, L.; Kameoka, J. A Low-cost and Enzyme-free Glucose Paper Sensor. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Institute of Electrical and Electronics Engineers (IEEE): Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 4097–4100. [Google Scholar]
- Pidenko, P.S.; Pidenko, S.A.; Skibina, Y.S.; Zacharevich, A.M.; Drozd, D.D.; Goryacheva, I.Y.; Burmistrova, N.A. Molecularly imprinted polyaniline for detection of horseradish peroxidase. Anal. Bioanal. Chem. 2020, 412, 6509–6517. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, G.; Ge, L.; Ge, S.; Yu, J.; Yan, M. Photoelectrochemical lab-on-paper device based on molecularly imprinted polymer and porous Au-paper electrode. Analyst 2013, 138, 4802–4811. [Google Scholar] [CrossRef]
- Tsuji, M.; Mori, Y.; Kanda, H.; Ito, T.; Hidaka, T.; Kakamu, T.; Kumagai, T.; Hayakawa, T.; Osaki, Y.; Fukushima, T. Development of simple HPLC/UV with a column-switching method for the determination of nicotine and cotinine in hair samples. Health 2013, 5, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Vogeser, M.; Seger, C. A decade of HPLC–MS/MS in the routine clinical laboratory—Goals for further developments. Clin. Biochem. 2008, 41, 649–662. [Google Scholar] [CrossRef]
- Liu, X.; Mwangi, M.; Li, X.; O’Brien, M.; Whitesides, G.M. Paper-based piezoresistive MEMS sensors. Lab A Chip 2011, 11, 2189–2196. [Google Scholar] [CrossRef] [Green Version]
- Armah, S.M.; Ferruzzi, M.G.; Gletsu-Miller, N. Feasibility of Mass-Spectrometry to Lower Cost and Blood Volume Requirements for Assessment of B Vitamins in Patients Undergoing Bariatric Surgery. Metabolites 2020, 10, 240. [Google Scholar] [CrossRef]
- Määttänen, A.; Vanamo, U.; Ihalainen, P.; Pulkkinen, P.; Tenhu, H.; Bobacka, J.; Peltonen, J. A low-cost paper-based inkjet-printed platform for electrochemical analyses. Sens. Actuators B Chem. 2013, 177, 153–162. [Google Scholar] [CrossRef]
- Rida, A.; Yang, L.; Vyas, R.; Tentzeris, M.M. Conductive Inkjet-Printed Antennas on Flexible Low-Cost Paper-Based Substrates for RFID and WSN Applications. IEEE Antennas Propag. Mag. 2009, 51, 13–23. [Google Scholar] [CrossRef]
- Gerbers, R.; Foellscher, W.; Chen, H.; Anagnostopoulos, C.; Faghri, M. A new paper-based platform technology for point-of-care diagnostics. Lab A Chip 2014, 14, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Nijhuis, C.A.; Gong, J.; Chen, X.; Kumachev, A.; Martinez, A.W.; Narovlyansky, M.; Whitesides, G.M. Electrochemical sensing in paper-based microfluidic devices. Lab A Chip 2009, 10, 477–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozuelo, M.; Blondeau, P.; Novell, M.; Andrade, F.J.; Rius, F.; Riu, J. Paper-based chemiresistor for detection of ultralow concentrations of protein. Biosens. Bioelectron. 2013, 49, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, S.; Fan, W.; Miao, Y.-E.; Liu, T. Filter paper-derived carbon fiber/polyaniline composite paper for high energy storage applications. Compos. Sci. Technol. 2014, 101, 152–158. [Google Scholar] [CrossRef]
- Wang, H.; Liu, D.; Du, P.; Liu, P. Flexible and robust amino-functionalized glass fiber filter paper/polyaniline composite films as free-standing tensile-tolerant electrodes for high performance supercapacitors. Electrochim. Acta 2017, 228, 371–379. [Google Scholar] [CrossRef]
- Borysiak, M.D.; Thompson, M.J.; Posner, J.D. Translating diagnostic assays from the laboratory to the clinic: Analytical and clinical metrics for device development and evaluation. Lab A Chip 2016, 16, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Vogel, W.; Chu, P.P. Polyaniline coated active carbon as binary catalysts support for direct methanol fuel cell. ECS Trans. 2009, 19, 127. [Google Scholar] [CrossRef]
- Lal, S.; Tripathi, S.; Sood, N.; Khosla, S. Impact of the concentration of multiwall carbon nanotubes on polyaniline. J. Inf. Disp. 2014, 15, 111–117. [Google Scholar] [CrossRef]
- Gao, X.; Chorover, J. Adsorption of perfluorooctanoic acid and perfluorooctanesulfonic acid to iron oxide surfaces as studied by flow-through ATR-FTIR spectroscopy. Environ. Chem. 2012, 9, 148–157. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Tesolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1993, 70, A25. [Google Scholar]
- Mohtasebi, A.; Chowdhury, T.; Hsu, L.H.H.; Biesinger, M.C.; Kruse, P. Interfacial Charge Transfer between Phenyl-Capped Aniline Tetramer Films and Iron Oxide Surfaces. J. Phys. Chem. C 2016, 120, 29248–29263. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, T.-Y.; Chen, Z.; Kameoka, J. Perfluorooctanesulfonic Acid Detection Using Molecularly Imprinted Polyaniline on a Paper Substrate. Sensors 2020, 20, 7301. https://doi.org/10.3390/s20247301
Chi T-Y, Chen Z, Kameoka J. Perfluorooctanesulfonic Acid Detection Using Molecularly Imprinted Polyaniline on a Paper Substrate. Sensors. 2020; 20(24):7301. https://doi.org/10.3390/s20247301
Chicago/Turabian StyleChi, Ting-Yen, Zheyuan Chen, and Jun Kameoka. 2020. "Perfluorooctanesulfonic Acid Detection Using Molecularly Imprinted Polyaniline on a Paper Substrate" Sensors 20, no. 24: 7301. https://doi.org/10.3390/s20247301





