Abstract
Emerging evidence indicates that the dysregulation of cellular redox homeostasis and chronic inflammatory processes are implicated in the pathogenesis of kidney and brain disorders. In this light, endogenous dipeptide carnosine (β-alanyl-L-histidine) and hydrogen sulfide (H2S) exert cytoprotective actions through the modulation of redox-dependent resilience pathways during oxidative stress and inflammation. Several recent studies have elucidated a functional crosstalk occurring between kidney and the brain. The pathophysiological link of this crosstalk is represented by oxidative stress and inflammatory processes which contribute to the high prevalence of neuropsychiatric disorders, cognitive impairment, and dementia during the natural history of chronic kidney disease. Herein, we provide an overview of the main pathophysiological mechanisms related to high levels of pro-inflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and neurotoxins, which play a critical role in the kidney–brain crosstalk. The present paper also explores the respective role of H2S and carnosine in the modulation of oxidative stress and inflammation in the kidney–brain axis. It suggests that these activities are likely mediated, at least in part, via hormetic processes, involving Nrf2 (Nuclear factor-like 2), Hsp 70 (heat shock protein 70), SIRT-1 (Sirtuin-1), Trx (Thioredoxin), and the glutathione system. Metabolic interactions at the kidney and brain axis level operate in controlling and reducing oxidant-induced inflammatory damage and therefore, can be a promising potential therapeutic target to reduce the severity of renal and brain injuries in humans.
1. Introduction
The dysregulation of cellular redox homeostasis and chronic inflammatory processes represent interdependent factors implicated in the pathogenesis of multiple diseases, including atherosclerosis and cardiovascular diseases, neurodegenerative diseases, chronic kidney disease, diabetes, cancer, and aging [1,2]. Both inflammation and oxidative stress are orchestrated to accentuate each other and to induce progressive damage. Thus, their complex interrelations should be considered when evaluating novel therapies [2]. In this regard, many small molecules able to modulate endogenous cellular defense mechanisms and biochemical pathways of cytoprotection have demonstrated a major role for cellular protection in neurodegenerative disorders such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Multiple Sclerosis (MS), and chronic inflammatory diseases such as Type 2 Diabetes mellitus (T2DM) [1]. The biological relevance of carnosine and its derivates and their metabolism is only partially understood but there is evidence that a group of histidine-containing dipeptides (HDPs) have a protective function in disease progression. Carnosine inhibits glycation [3], functions as a carbonyl scavenger [4,5,6], acts as an ion-chelating agent, especially for copper(II) and zinc(II) [7], and as an ACE inhibitor [8,9]. Several studies demonstrated a multifunctional antioxidant activity of carnosine [10,11,12,13], anserine, and homocarnosine [14,15]. Moreover, it is suggested that these activities are likely mediated, at least in part, via hormetic processes, involving Nrf2, Hsp70, Sirt-1, Trx, and the glutathione system [16]. The evidence to support this general perspective is strongly suggestive but limited in specificity. For example, Nrf2 activation can follow a hormetic biphasic dose response in various cell types [17,18]. There is also a substantial number of papers demonstrating the occurrence of H2S-induced hormetic dose responses in a broad variety of cell types such as cardiac [19], bone marrow stem cells [20], mammary epithelia cells [21], endothelial cells [22], lymphocytes [23], hepatocytes [24] as well as the brain [25] and neuro-stem cells [26]. However, research is needed to further explore whether and to what extent kidney–brain crosstalk occurs, its underlying mechanisms, whether effective treatment could be formulated, and how these factors may be related to hormetic processes. Despite these limitations, there is substantial documentation in the experimental literature that low doses of X-rays prevent the occurrence of diabetes-induced renal toxicity via the activation of the Nrf2 pathway. On the other hand, when blocking this activation, the protection disappears [27]. In a parallel manner, low dose X-ray treatment induces the development of a generalized anti-inflammatory phenotype that reduces arthritic inflammation in humans for prolonged periods [28,29]. Calabrese et al. (2019) [30] report that low dose X-ray treatments reduced inflammatory processes in numerous organs including the brain. Since Nrf2 activation is commonly induced via X-rays to affect these protective processes, it is likely that physical and/or chemical activators of Nrf2, such as H2S, may have clinical application within the context of the kidney–brain axis, as therapeutic modulation of these protective pathways can be mutually beneficial. The brain and the kidney interact strongly, and patients with neuropsychiatric disorders have a higher frequency of renal diseases. The crosstalk between the two organs may be caused by inflammatory processes via cytokine/chemokine release, oxidative stress via production of ROS, and factors related to the renin–angiotensin system [31].
Inflammation and oxidative stress are tightly linked and are emerging as key factors in several chronic diseases. Understanding the interrelation between oxidative stress and inflammation and the antioxidative and anti-inflammatory protective effects of carnosine and H2S in kidney and brain pathophysiology may lead to new therapeutic interventions and are now discussed.
2. Carnosine Signaling in Kidney and Brain
Carnosine (ß-alanyl-L-histidine), anserine (ß-alanyl-Nπ-methyl-histidine), homocarnosine (γ-aminobutyryl acid-L-histidine), and ophidine/balenine (ß-alanyl-Nτ-methyl-histidine) belong to the HDPs. These dipeptides are found in humans, mammals, fish, and amphibia and their ratio and concentrations vary widely. In mammals and humans, carnosine seems to be the most important dipeptide, with the highest concentrations in the muscle (up to 20 mM), mostly together with either anserine or ophidine in different ratios [32]. Their abundant presence in muscles has been linked to their ability to counteract the massive production of lactic acid occurring during intense muscle activity, thus preventing a decrease in cytosolic pH [33]. Carnosine is also present in the kidney of mice [34,35], retina [36], liver [37], and spleen [38]. The highest carnosine concentrations in the brain were found in the olfactory system and carnosine levels are comparable to those usually found in skeletal muscle. However, homocarnosine is the most prevalent dipeptide in the mammalian brain [39] and this has been attributed to the bioavailability of GABA (γ-aminobutyric acid), the non-proteinogenic precursor of homocarnosine, exclusively present in the brain areas [40].
2.1. Carnosine in Cell Lines
Evidence from studies on cerebral cell cultures showed that carnosine is produced by glial cells [39]. The extensive distribution of glial cells in the brain and spinal cord suggest a broad spectrum of function and a diffuse presence of carnosine and its related dipeptides in the central nervous systems [40]. Carnosine is metabolized by two carnosinases, members of the M20 family of metalloproteases. The two isoforms carnosinase 1 (CN1) and carnosinase 2 (CN2) are structurally similar, but have enough varying properties. CN1 has a narrow substrate spectrum for histidine-containing dipeptides, such as carnosine, anserine, and homocarnosine. Conversely, the cytosolic isoform CN2 degrades a great number of dipeptides, but not homocarnosine [41]. The human kidney possesses an intrinsic carnosine metabolism. Distribution of carnosinase varies within the nephron regulating renal physiology. In the distal tubules, where a continuous low pH is required, the high levels of CN1 assure the removal of carnosine, which has high pH buffering capacity. Furthermore, the high levels of CN1 mRNA, proteins, and enzyme activities measured in podocytes, and the consistently low levels found in endothelial cells, suggest a cell-specific role of carnosine metabolism [42]. Carnosine prevented protein oxidation induced by glucose oxidase. In addition, carnosine proved to reduce both poly(ADP-ribose) polymerase-1 (PARP-1) and poly(ADP-ribose) polymerase-2 (PARP-2) activation induced by oxidative stress [43]. In contrast, carnosine is only transported slowly into renal cells [34], it can penetrate neurons [44], and several neuroprotective effects have been reported for carnosine [45]. Carnosine has the ability to protect neuronal cells against ischemic injury and oxidative stress [46]. In astrocytes, pre-treatment with carnosine reduced the overexpression of inducible isoform nitric oxide synthases (iNOS) caused by nitrosative stress. The direct link with nitric oxide is one of the possible mechanisms by which carnosine is able to neutralize the pathological effects of nitrosative stress [47]. Preston et al. [48] first reported a protective effect of carnosine on the cellular damage induced by amyloid-beta (Aβ) toxicity in the rat brain.
2.2. Carnosine in Animal Models
In rodents, the effects of carnosine supplementation have been extensively studied, especially in diabetes [49] and in neurological functions [40]. In diabetic mice and rats, renal carnosine metabolism is altered and exogenous carnosine intake exerts a range of nephroprotective effects such as a reduction in proteinuria, renal vasculopathy, and podocyte loss [34,35]. Carnosine can mitigate nitrite-induced metabolic alterations and oxidative damage in Wistar rats by increasing plasma GSH levels and major antioxidant defense enzymes, such as superoxide dismutase (SOD), glutathione reductase, or glutathione peroxidase [50] and decreasing inflammatory molecules, such as TNF-α and CRP (C-reactive protein) levels [51]. Recently, a carnosinase-resistant carnosine derivate was shown to prevent the onset of diabetic nephropathy in diabetic (db/db) mice by promoting renal inflammation and injury [52]. In stimulated murine macrophages, carnosine decreased apparent NO formation and enabled the modulation of macrophage-mediated inflammation processes [53]. In mice with chronic methylglyoxal administration, the generated hepatic and plasma oxidative stress was suppressed by carnosine treatment [54]. In Zucker obese rats, the beneficial effects of carnosine seem to be mediated by disruption of the advanced lipoxidation/glycation end products–receptor for advanced glycation end products (ALEs/AGEs-RAGE)–pro-inflammatory axis [55]. Intraperitoneal injection of carnosine protected against white matter damage caused by chronic cerebral ischemia in mice, likely by reducing oligodendroglial cell loss [56]. Carnosine inhibited microglia activation and cortical neuron apoptosis in a rat model of experimental subarachnoid hemorrhage [57]. In salsolinol-induced neurotoxicity in rats, cytotoxicity was reverted by treatment with carnosine. The latter has normalized the levels of malonaldehyde, glutathione, superoxide dismutase, and catalase [58]. In STZ-induced diabetic rats, carnosine treatment ameliorated learning and memory disturbances through modulation of the NF-κB/Nrf2/HO-1 (nuclear factor kappa-light-chain-enhancer of activated B cells/Nuclear factor-like 2/heme oxygenase-1) signaling cascade, with suppression of oxidative stress, neuroinflammation, astrogliosis, and enhancement of cholinergic function [59].
2.3. Carnosine in Clinical Settings
Increased concentrations of carnosine or homocarnosine due to carnosinemia caused by serum carnosinase deficiency seem to be clinically irrelevant under physiological conditions, but it has been reported that increased levels may be of therapeutic relevance, particularly in conditions exacerbated by oxidative stress. In humans, the half-life of carnosine in the human circulation is minutes only, even in subjects with low CN1 activity and protein content [60], but surprisingly, carnosine supplementation in humans showed beneficial effects. Besides treatment options in cancer, cataracts, and cachexia, several studies showed the beneficial effect of carnosine on glucose metabolism and neurological functions. Carnosine normalized glucose intolerance and reduced 2-h insulin levels after an oral glucose tolerance test (OGTT) in a subgroup of individuals with impaired glucose tolerance [61]. Recently, it was shown that carnosine lowered fasting glucose, serum levels of triglycerides, AGEs, and TNF-α without changing sRAGE, IL-6, and IL-1β levels in type 2 diabetes patients [62]. In pediatric patients with diabetic nephropathy, oral supplementation with L-Carnosine reduced oxidative stress, increased antioxidant levels and low malondialdehyde levels, and improved glycemic control (decreased HbA1c, insulin resistance, increased insulin secretion, and β-cell mass). It also improved renal function [63]. CN1 inhibitors or medications increasing internal synthesis are currently being developed [64]. In human plasma, all HDPs are present at very low concentrations, with the highest levels of homocarnosine, while the mean concentration of carnosine and ophidine/balenine was below the detection limit [65].
HDP can also improve neurological diseases. Topiramate has been described to increase brain GABA and homocarnosine levels that could contribute to enhance its potent antiepileptic action in patients with complex partial seizures. A randomized double-blind placebo-controlled study on 75 patients with schizophrenia has demonstrated that carnosine (2 g/day) adjunctive to basic therapy improves cognitive functions [66]. Carnosine plays a protective role in neurodegenerative disorders through several mechanisms. Many other studies confirmed these effects, suggesting that carnosine reverses the neurotoxicity induced by Aβ (1–42) by inhibiting glutamatergic activity [12]. In spite of its function as a molecular chaperone, carnosine has recently been proposed to inhibit Aβ aggregation by interfering with the propensity of the peptide to form backbone hydrogen bonds near residues with key roles in fibrillogenesis [67]. A pilot study with 52 patients diagnosed with moderate Alzheimer’s disease and treated with the acetylcholinesterase inhibitor donepezil reported that the group that received carnosine (along with an antioxidant cocktail) showed improvements in their Mini-Mental Status Exam II scores, while the group that continued to receive only donepezil treatment maintained similar scores [68]. Moreover, a recent double-blind, randomized clinical trial of 43 autistic patients has demonstrated that 500 mg of carnosine improved sleep duration and parasomnia subscales [69]. In addition, another recent randomized, double-blind, placebo-controlled trial has suggested that 250 mg of oral carnosine supplementation exerts protective effects against cognitive decline in APOE4 (+) mild cognitive impairment (MCI) patients [70]. In addition, a double-blind randomized controlled trial of 60 AD patients preserve verbal episodic memory, probably owing to inflammatory chemokine CCL24 suppression in the blood [71]. Carnosine has been also proposed as a potential drug for the treatment of Parkinson’s disease. It has been described to enhance the efficacy of the levodopa (L-DOPA)-based treatment of Parkinson’s disease and to inhibit the a-synuclein oligomerization induced by the Cu, Zn-SOD, and hydrogen peroxide system [72]. The oxidative and nitrosative stresses represent other characteristic aspects of neurodegeneration which could be regulated by carnosine. Interestingly, carnosine treatment induced a prominent reduction in intraneuronal Aβ in the hippocampus of transgenic mice, but failed to decrease phospho-tau immunoreactive levels and to restore long-term memory deficits. Thus, the reduction in Aβ by carnosine appears to be not sufficient to produce an appreciable cognitive improvement. Furthermore, lower plasma levels of carnosine have also been revealed in Alzheimer disease patients with respect to age-matched controls [73].
3. Hydrogen Sulfide Signaling
Hydrogen sulfide (H2S) is a small gaseous molecule with profound biological effects within living organisms. It exerts key roles in cytoprotection, inflammation, vascular function, neurological systems, mitochondrial function, energy metabolism, and ageing [74,75]. However, H2S was originally known for its deleterious effects on health and the environment. First, in 1700, Italian physician Bernardino Ramazzini [76] described a severe ocular irritation and inflammation in sewer workers, caused by an unspecified volatile acid. Later, the chemical composition of H2S was elucidated and its association with ocular adverse effects and intoxication in sewer workers was recognized. For over a century, studies focused on its major toxic effects—e.g., inhibition of cytochrome c oxidase, carbonic anhydrase, monoamine oxidase, and sodium/potassium-ATPase (NaC/KC ATPase) [77]. The image of H2S was revolutionized when Kimura, in 1996, revealed its role as an endogenous neuromodulator [78]. Recently, H2S was classified as the third gasotransmitter along with NO and carbon monoxide (CO). H2S is enzymatically released in our body and it has a highly regulated metabolism. It is freely permeable to membranes and exerts specific physiological functions in several systems that can be mimicked by H2S donors applied exogenously [79]. In mammals, hydrogen sulfide (H2S) is primarily produced by two cytosolic pyridoxal-5′-phosphate-dependent enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), which use the substrates homocysteine and L-cysteine (Figure 1).
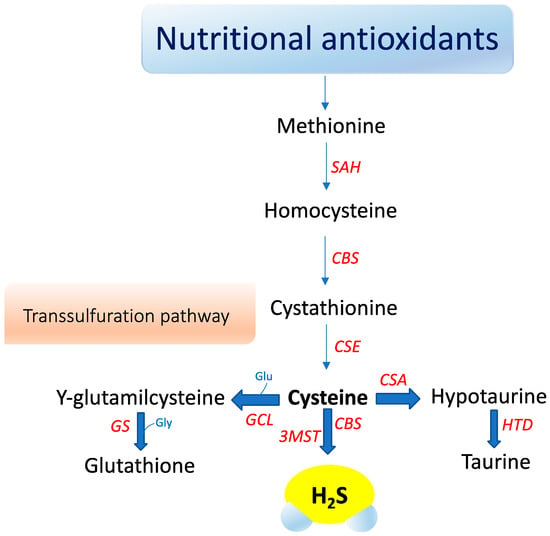
Figure 1.
Nutraceutical support of intracellular H2S synthesis via cysteine metabolism. S-adenosyl homocysteine hydrolase (SAH), Cystathionine-β-synthase (CBS), 3-mercaptopyruvate sulfurtransferase (3-MST), Cystathionine gamma lyase (CSE), Cysteine sulfinate (CSA), Hypotaurine dehydrogenase (HTD), Glutamate cysteine ligase (GCL), Glutathione synthetase (GS), Glycine (Gly).
A third enzyme, 3-mercaptopyruvate sulfurtransferase (3-MST), catalyzes H2S production mainly in mitochondria by the conversion of 3-mercaptopyruvate to pyruvate and H2S [80]. Recently, an additional biosynthetic pathway has been described for the production of hydrogen sulfide from D-cysteine involving 3-mercaptopyruvate sulfurtransferase and D-amino acid oxidase that operates predominantly in the cerebellum and the kidney, i.e., within the KB axis [81]. D-cysteine is mainly adsorbed with food and derives from L-cysteine racemization during food processing. This novel pathway is of particular interest since supplementation with D-cysteine showed to protect renal cortex cells and cerebellum cells (i.e., KB axis) more efficiently than L-cysteine [81]. Furthermore, it was reported that gut microbiota would be another source of H2S that might influence health and function [81]. The contribution of each of these enzymes to net H2S production is dictated by its presence and relative tissue concentration, which varies in a cell-specific manner [81,82,83]. CBS is the major H2S-producing enzyme in the brain, while CSE is significantly expressed in the mammalian cardiovascular system and respiratory system and it seems to be the main H2S-forming enzyme in the liver, kidney, and pancreas [84].
Oxidative stress seems to influence CSE and CBS in a different manner. Reactive oxygen species appear to induce CSE expression, whereas they clearly suppress the transcription of the human CBS gene [85]. Eventually, H2S is metabolized to sulfite in the mitochondria by thiosulfate reductase, and then, is oxidized to thiosulfate and sulfate by sulfite oxidase. The sulfates are excreted in the urine [80]. Interestingly, urinary sulfates have been used as markers of H2S plasma levels and increased urinary sulfate concentrations have been correlated with a decreased risk of renal events in type 2 diabetic patients with nephropathy [86]. However, urinary sulfate and thiosulfate are not specific markers for endogenous H2S formation, and can also be the products of exogenous H2S production by sulfate-reducing bacteria in the gut [86]. H2S acts independently of any specific transporters by mechanisms not fully understood. S-sulfhydration, a novel posttranslational modification, is emerging as a mechanism responsible for many biological effects mediated by H2S [75,87]. H2S sulfhydrates protein thiol groups by transferring its sulfhydryl group to the cysteine residue of targeted proteins. Furthermore, H2S is oxidized in biological systems to polysulfides, which are now increasingly recognized as effectors of the H2S signaling mechanism [88]. H2S S-sulfhydration of enzymes, transcription factors, and ion channels has been described accounting for several protective effects of H2S, ranging from response to inflammation to cytoprotection [82,89]. For instance, H2S attenuates inflammation through the S-sulfhydration of Nuclear Factor-kappa B (NF-κB) [90]. H2S increases the antioxidative properties of cells by sulfhydration of Kelch-like ECH-associated protein 1 (Keap1), leading to its dissociation from Nrf2, which translocates in the nucleus and binds to the antioxidant response element (ARE) promoting antioxidant gene transcription, such as GCLM, GCLC, and glutathione reductase [91]. S-sulfhydration may be involved in the augment of the life span of Caenorhabditis elegans induced by H2S through sirtuins [92]. H2S may also inhibit mitochondria ROS production through sulfhydration of p66Shc [91]. In this regard, gut microbiota has attracted considerable interest for its role in microbial-mediated ROS generation, which might influence many signaling and homeostatic processes. Notably, lactobacilli ROS-mediated signaling has been described to induce Nrf2, opening the prospect that probiotic bacteria may elicit beneficial effects on disease states that involve Nrf2, including diabetes and neurodegenerative diseases [93]. In particular, in the (nod-like receptor) NLR family, the nod-like receptor pyrin domain-containing 3 (NLRP3) inflammasome has been reported to play a pathogenic role in the initiation and progression of metabolic and neurodegenerative diseases [94,95]. Recently, in vitro and in vivo studies showed that H2S mitigated lipopolysaccharide (LPS)-induced sepsis against oxidative stress and inflammation damage mediated by the NADPH oxidase 4 (Nox4) pathway [96], inhibiting the vicious cycle of NLRP3 inflammasome and oxidative stress in human retinal pigment epithelial cells [97] and in hypertensive rats [98]. In addition, H2S mediated effects on neuroinflammation and Aβ1-42 production by suppressing the activation of STAT3 and cathepsin S [99]. H2S suppresses oxidative stress-induced mtROS production and NLRP3 inflammasome activation via S-sulfhydrating c-Jun at cysteine 269 in macrophages [100].
4. H2S as a Signaling Mediator in the Kidney–Brain Axis
Over the past few years, a crosstalk between kidney and brain emerged, as elucidated through the modulation of H2S for neuroprotection, particularly to unveil the pathophysiological mechanisms of oxidative and inflammatory stress. Consistent with this notion H2S, as a biological signaling mediator, is involved in various functions, including antioxidant, neuromodulatory, regulation of vascular tone, cytoprotective, anti-inflammatory, modulator of immune response, oxygen sensing, angiogenesis, mitochondrial bioenergetics, and blood–brain barrier permeability [101]. The kidney and brain are vital organs exposed to high-volume blood flow and thus, are highly susceptible to vascular damage [102]. Normal renal function regulates whole-body homeostasis, including neuronal homeostasis [103]. Therefore, it was well recognized that oxidative stress and inflammation are common outcomes strongly associated with both kidney and brain dysfunctions, leading to the development and progression of several diseases, including acute kidney disease (AKD), chronic kidney disease (CKD), neuropsychiatric disorders, and cognitive dementia [104,105]. Recent data have demonstrated that patients with CKD, especially at advanced stages, are highly susceptible to developing neuropsychiatric disorders (i.e., depression and anxiety) and cognitive impairment (i.e., Alzheimer and Parkinson diseases) [31]. In the kidney, H2S induces important diuretic, natriuretic, and kaliuretic effects by raising glomerular filtration rate and inhibiting tubular sodium re-absorption [106,107]. Notably, under hypoxic conditions, H2S functions as an oxygen sensor in the renal medulla that restores oxygen balance by enhancing medullary blood flow, decreasing energy requirements for tubular transport and directly suppressing mitochondrial respiration [107]. Since medullary hypoxia is a common feature of CKD, H2S deficiency can lead to progression of CKD by limiting this significant adaptive mechanism [108].
4.1. Anti-Inflammatory Role of H2S
Inflammation is a common feature in brain and kidney injuries, and it is quite reasonable to assume that inflammatory mediators may amplify the kidney–brain crosstalk. Particularly, the nucleotide-binding oligomerization domain NLRP3 inflammasome, a multiprotein complex, is involved in the pathogenesis of inflammation in chronic kidney and brain diseases [109,110]. NLRP3 induces the production of many proinflammatory cytokines and chemokines such as IL-1β, IL-6, TNF-α, transforming growth factor-β (TGF-β), and NF-κB, which are frequently associated with the pathogenesis of CKD [111]. These cytokines then initiate or amplify different downstream signaling pathways and drive proinflammatory factors [112], leading to cellular damage, such as autophagy dysfunction and ROS production [113]. In the kidney, generation and activation of the NLRP3 inflammasome have been reported to occur not only in immune cells like dendritic cells [114] and infiltrating macrophages [115], but also in other renal cells such as tubular epithelial cells [116] and podocytes [117]. Several human studies reported that the inflammatory response triggered by CKD may contribute toward increasing proinflammatory cytokines that can cross the blood–brain barrier (BBB) to reach the CNS and lead to neuroinflammation such as reported with neuropsychiatric and neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, major depressive disorder, and schizophrenia. This reinforces the concept of kidney–brain inflammatory crosstalk [118,119,120]. Accordingly, these pro-inflammatory molecules operate in the brain to induce common symptoms of sickness, including loss of appetite, sleepiness, withdrawal from normal social activities, fever, aching joints, fatigue, embezzlement of cognition, malaise, inattention, and depression [121]. In this context, H2S possess powerful anti-inflammatory, antioxidant, and anti-apoptotic effects [122]. On the other hand, H2S depletion may contribute to the progression of CKD and cognitive disorders [123,124]. Moreover, accumulating evidence reported that H2S appears to play a crucial role in the modulation of the immune response by regulating posttranslational modification of the NF-kB pathway in vitro and in vivo [125,126]. In the brain, H2S protects neurons from apoptosis and degeneration by inducing anti-inflammatory effects and upregulating antioxidant enzymes [127]. Additionally, treatment of H2S in the APP/PS1 (human amyloid precursor protein and presenilin 1) mouse model of AD reduced cognitive impairment and mitigated oxidative stress [128]. Moreover, H2S mediated the process of altered blood pressure in response to changes in serum homocysteine levels by blocking the activation of extracellular signal-regulated kinase 1/2 (ERK1/2)-STAT3 signaling pathway [129], exerted antidepressant effects by the induction of the mTORC1-TrkB-AMPA receptor pathway [130,131] as well as prevented cisplatin-induced nephrotoxicity [132]. Furthermore, NaHS, an exogenous H2S donor, also inhibits macrophage pro-inflammatory cytokine production, as well as cyclooxygenase-2 and nitric oxide production, and decreases macrophage motility. In addition, recent in vivo studies reported that NaHS ameliorates CKD-mediated brain dysfunctions through interaction with NO signaling in the hippocampus [133], as well as improves apoptosis, inflammation, and autophagy, exerting nephroprotective effects against CKD in rats [134,135].
4.2. Antioxidant Role of H2S
During pathological conditions, there is a mutual upregulation between factors promoting inflammation and oxidative stress, which sustains CDK progression and neurological disorders [136]. Under physiological conditions, ROS provides beneficial effects, regulating cellular stress responses by redox-sensitive signaling pathways. Indeed, ROS control cellular growth, differentiation, and migration; regulate vascular tone and cellular adhesion, leading to the production of iNOS at the transcriptional and posttranscriptional level by redox-dependent NF-κB or mitogen-activated protein kinases (MAPKs); modulate immune response; and control angiogenesis and apoptosis [137]. When ROS generation is prolonged or excessive, harmful consequences are observed with peculiar changes in cellular proteins, lipids, and ribonucleic acids, leading to cell dysfunction or death. Numerous enzymes with antioxidant activity are involved in neutralizing ROS, including superoxide dismutase (SOD), γ-glutamyltransferase (GGT), glutathione (GSH), glutathione reductase (GSSG-Rd), glutathione peroxidase (GSH-Px), glutathione S-transferase (GST), catalase (CAT), and nuclear factor erythroid 2-related factor 2 (Nrf2) [138]. All these antioxidant pathways are differently expressed in various cells and organs including kidney and brain tissues [139,140]. Intriguingly, recent evidence highlighted the complex role of H2S as a direct antioxidant scavenger to inhibit ROS generation and the inflammatory process in the pathogenesis of kidney and brain injuries [141,142]. Additionally, the formation of free radicals (i.e., ROS and RNS) as signaling molecules to promote inflammation induces the activation of the transcription of NF-κB by upregulating the production of pro-inflammatory cytokines and chemokines, leading to the recruitment and activation of leukocytes and resident cells [143]. The “oxidative” linkage between CKD and its complications is achieved through several pathophysiological mechanisms, including mitochondrial dysfunction [144], uremic neurotoxin-induced endothelial nitric oxide synthase (eNOS) uncoupling [145], and increased nicotinamide adenine dinucleotide phosphate-oxidases (NADPH oxidases (NOX)) activity [146], myeloperoxidase (MPO) [147], but also antioxidant enzymes depletion due to dietary restriction, and/or decreased intestinal absorption [148]. In particular, preclinical and clinical studies confirmed beneficial effects by upregulation of the Nrf2-HO1 antioxidant pathway during acute and chronic kidney diseases [149,150]. In addition, activation of the glutathione peroxidase pathway reduced not only the oxidative stress but also the inflammatory process induced by the uremic neurotoxins on the endothelium during CKD [151]. On the other hand, glutathione peroxidase deficiency in kidney diseases contributed to the development of cardiac diseases risk due to an increase in ROS generation and inflammatory pathways [152]. Furthermore, CKD patients also display hypovitaminosis D [153] as well as hypoalbuminemia [154] and zinc deficiency [155].
Fascinatingly evidence demonstrated that NaHS suppresses the expression of the ROS-generating enzyme, NADPH oxidase (NOX) and its essential subunit, Rac-1, in cultured vascular smooth muscle cells [156]. Likewise, NaHS has been shown to lower NOX-4 expression and potentiates the antioxidant effects of apocyanin, N-acetyl-L-cysteine, catalase, superoxide dismutase, and GSH, in the brain endothelial cells [157]. In addition, H2S can scavenge and/or degrade lipid peroxides [158] and increases the production of GSH [148]. Moreover, intraperitoneal application of NaHS to pregnant rats protects fetal brains from ischemia-reperfusion injury by restoring the glutathione levels reduced by ischemia-reperfusion [156]. However, experimental evidence demonstrated the scavenging effect of H2S in the presence of intracellular glutathione concentration between 1 and 10 mM, and 1 and 100 μM of cysteine in neurons [159]. Thus, the suppression of oxidative stress by increased levels of glutathione should be more effective than ROS scavenging by H2S itself.
GSH is a ubiquitous thiol tripeptide composed of cysteine, glycine, and glutamate, existing often as a reduced form, and it is synthesized from cysteine. Moreover, H2S also facilitates the transport of cysteine into cells. Glutathione is produced by two enzymes, glutamate cysteine ligase (GCL) (γ-glutamylcysteine synthase (γ-GCS)), which is a rate-limiting enzyme in the production of γ-glutamyl cysteine from glutamate and cysteine, and glutathione synthetase (GS), which produces glutathione by adding glycine to γ-glutamyl cysteine. GSH reduces disulfide bonds formed within cytoplasmic proteins to cysteines by serving as an electron donor. In this process, GSH is converted to its oxidized form, glutathione disulfide (GSSG). Intracellular cysteine plays an important role in cellular homeostasis as a precursor for protein synthesis, and for the production of GSH, hydrogen sulfide (H2S), and taurine. Cysteine exists as two unstable redox forms in the body: the oxidized form cystine and the reduced form cysteine. The extracellular cystine form is carried into cells through the cystine/glutamate antiporter system, after which cysteine is reduced and ready for GSH synthesis. The release of H2S into the extracellular space promotes the reduction of cystine to cysteine, increasing the amount of cysteine available as a substrate for GSH synthesis, and improving cystine transport [156,160,161]. Another significant mechanism for the effect of H2S on GSH may be by enhancing glutamate uptake [162]. Interestingly, kidney and brain tissues contain significant amounts of γ-glutamyl transpeptidase and γ-glutamyl cyclotransferase and nevertheless, maintain an appreciable concentration of glutathione. This suggested to us that these organs might also possess the enzymatic equipment needed for the synthesis of glutathione. Many in vivo studies have demonstrated that the kidney–brain axis contains a high concentration of both γ-glutamylcysteine synthetase and glutathione synthetase [163,164]. The presence of these enzymes in kidney and brain enables a series of catalytic events involving the synthesis and degradation of glutathione, and the coupled uptake and release of free amino acids from γ-glutamyl linkage. These reactions are steps in a cyclical process referred to as the “γ-glutamylcycle” (Figure 2).
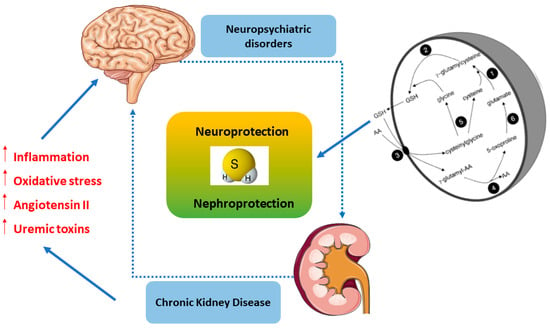
Figure 2.
The kidney–brain crosstalk by the H2S signaling pathway. γ-glutamyl transpeptidase (γGGT) (3), γ-glutamyl cyclotransferase (4), dipeptidase (5), oxoprolinase (6), γ-glutamyl-cysteine synthase (1), and glutathione synthetase (2) operate in the Meister cycle to generate glutathione (GSH) and internalize amino acids (AA).
A great number of accumulated data indicate that oxidative stress and inflammation are major contributing factors of renin–angiotensin system (RAS) imbalance both in peripheral and brain tissues [165,166]. The potential homeostatic role between kidney–brain crosstalk by RAS to regulate sodium/water balance and maintain normal blood pressure has been purported [167]. The first end-product of this system related to a biological activity is the octapeptide angiotensin (ANG) II, synthesized by the angiotensin converting enzyme (ACE). This pathway may also be involved in neurocognitive performance decline [168]. The role of RAS peptides in the interaction between the kidney and brain is supported by studies showing that treatment with ACE inhibitors and type I receptor (AT1) blockers, besides exerting renoprotection, also have beneficial actions in neurodegenerative disorders [169]. Dysfunctional RAS activation induces angiotensin II release, increasing systemic vascular resistance [170]. It has been established that treatment with captopril reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinson’s disease [171]. Analogue results were obtained with the administration of AT1 receptor antagonists in patients and in experimental models of Alzheimer’s disease, Parkinson’s disease, stroke, traumatic brain injury, and spinal cord injury [172]. Surprisingly, exogeneous H2S administration regulates renin release by downregulating the intracellular cAMP level in several cell types [173] and decreases protein expression of AT1R [174], resulting in renoprotection from kidney diseases. Moreover, H2S treatment inhibits the upregulation of renin level in renovascular hypertensive rats and reduces the intracellular cAMP level in primary cultures of renin-rich kidney cells [175]. In addition, H2S blocks forskolin-induced renin degranulation in mast cells by lowering the intracellular cAMP level, thus protecting against isoproterenol (ISO)-induced heart failure [176]. Likewise, H2S therapy protects multiple organs including the heart, kidney, and blood vessels and improves exercise capacity, coupled with inactivation of RAS in a murine model of transverse aortic constriction-induced heart failure [177]. Recent in vivo studies suggested that treatment with NaHS, increases renal H2S concentrations, restores NO bioavailability, and blocks RAS in the kidney, thus promoting vasodilatation to prevent the development of hypertension in rats [178]. In addition, H2S mitigates the development of diabetic nephropathy through suppressing RAS activity in diabetic rats [179].
As a rationale for neuropsychiatric disorders secondary to kidney damage, known as the “vascular theory”, kidney and brain hemodynamic analogies occur, both being low resistance end organs exposed to high-volume blood flow and, consequently, more vulnerable to vascular damage [180]. Accordingly, magnetic resonance imaging (MRI) studies have shown high occurrence of silent brain infarction in patients with CKD, supporting the vascular theory [181]. Consistent with this, approximately 50% of CKD patients present ischemic white matter lesions in the MRI compared with 10% of the general population [182]. The loss of a direct correlation between known vascular risk factors, like diabetes and hypertension with CKD-related cognitive decline [183], the onset of neuropsychiatric comorbidities in pediatric patients with CKD preceding the vascular damage [184], as well as inconsistent findings regarding antihypertensive drugs’ beneficial effects in cognition [185], may indicate that other mechanisms underlie CKD-associated brain dysfunctions. Cerebrovascular injuries in CKD have also been associated with the retention of uremic toxins along with electrolyte imbalance, which ultimately leads to neuropsychiatric diseases, especially cognitive impairment and dementia [186]. Importantly, high neurotoxin levels (up to 10-fold higher in CKD patients than in controls) of guanidino compounds were present in brain regions that play a determinant role in cognition, such as the thalamus, the mammillary bodies, and the cerebral cortex [187]. A recent study reported several uremic toxins that potentially mediate the interactions between kidney and brain, which in turn, may influence brain homeostasis. Convincingly, uric acid, indoxyl sulfate, p-cresyl sulfate, IL-1β, IL-6, TNF, and parathyroid hormone have a strong impact on cognition in uremic conditions [188]. Interestingly, uremic neurotoxic effects seem to be also mediated by guanidino compounds, including creatinine, guanidine, guanidinosuccinic acid, and methylguanidine. Indeed, some studies reported that guanidine compounds affect the CNS by inhibition of GABA(A) receptors and concomitant activation of N-methyl-d-aspartate (NMDA) receptors [189,190]. Moreover, other studies showed that uremic mice decrease central dopamine turnover in the striatum, mesencephalon, and hypothalamus, which was correlated with the impairment of motor activity [191].
In this scenario, H2S plays a beneficial role by inhibiting uremic toxins release in vitro and in vivo. During uremia, CSE activity and also H2S are significantly decreased in blood mononuclear cells from uremic patients on hemodialysis [192]. On the contrary, its metabolic-related compounds such as cystathionine, homolanthionine, and lanthionine are significantly raised [193]. CSE inhibition could be due to a uremic toxin, lanthionine, which is able to decrease H2S production in hepatoma cells. Recent in vitro studies suggested that slow-releasing H2S donors, such as diallyl disulfide (DADS) and diallyl trisulfide (DATS) enhance levels of alkaline phosphatase, osteopontin, osteocalcin, and collagen type I that are downregulated in human mesenchymal stem cells derived from serum of uremic patients in hemodialysis [194]. Although the potential role of uremic toxins in mediating the kidney–brain crosstalk has become clearer over the past years, the role exerted by H2S on these neurotoxic compounds and how it may directly or indirectly influence these compounds and restore the CNS function remain still elusive.
Moreover, hyperhomocysteinemia is a clinical hallmark in patients with CKD or AKI, in the latter setting often caused by ischemia-reperfusion. It causes arteriolar constriction, arterial stiffness, and endothelial damage [195]. H2S treatment in rodents attenuates renovascular damage by reducing hyperhomocysteinemia [196]. The pathophysiology of chronic kidney disease and neuropsychiatric disorders has been commonly associated with mechanisms related to the decreased availability of brain-derived neurotropic factor (BDNF) for renal and neuronal failure [197]. BDNF exerts traditional antidepressant actions, and its deletion in the hippocampus weakens antidepressant behavioral responses [198]. Interestingly, epidemiological and experimental studies point to a potential role of the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA) and BDNF in neuropsychiatric disorders, in particularly in depression [199]. BDNF regulates the growth and maintenance of the neuronal system as well as neuronal plasticity, like long-term potentiation of learning [200], which has been shown to be reduced in major depressive disorders [201]. Several preclinical and clinical data reported that an increase in ADMA infusion alone causes a marked reduction in serum BDNF levels leading to behavioral changes and depression of CKD in 11 hemodialyzed patients as well as in nephrectomized rats. Thus, ADMA is considered a uremic toxin that acts as an endogenous inhibitor of NO [197]. Recently, it has been reported that BDNF and tropomyosin receptor kinase B (TrkB) are expressed in podocytes and in the zebrafish model, being essential for actin polymerization and cell survival [202]. Moreover, a recent study demonstrated that BDNF is required for glomerular development, morphology, and function, and the expression of BDNF and KIM-1 is highly correlated in urine cells of CKD patients. Therefore, BDNF mRNA in urine cells could serve as a potential prognostic biomarker for CKD [203]. Some studies demonstrated that H2S reversed the decrease in TrKB receptors against chronic unpredictable mild stress (CUMS)-induced hippocampal oxidative stress, demonstrating the critical role of neurotrophic signaling in the antidepressant effects mediated by H2S [204]. These outcomes are consistent with premises that H2S exerted neuroprotective effects against formaldehyde-induced toxicity in PC12 cells [205] as well as against homocysteine-induced ER stress and neuronal apoptosis in the hippocampus of rat via the BDNF–TrKB pathway [206]. Taken together, the data indicate that the interactions between kidney and brain are complex and multifaceted, thus justifying the significant neuropsychiatric comorbidity observed in patients with CKD. Despite much research efforts, to date, kidney–brain crosstalk is still an area with excitingly few publications, especially as regards the molecular mechanisms underlying CDK and brain damage. Consistent with this, H2S signaling could represent a novel direct link between kidney and brain diseases, conferring protection, and limiting neuropsychiatric disorder occurrence in CKD patients.
5. H2S and Diabetes
H2S is abundant in kidney and is generated mainly by CBS and CSE. In the glomeruli, CSE is the main H2S-producing enzyme expressed by endothelial cells, mesangial cells, and podocytes [207], while both CBS and CSE have been reported to be expressed on renal proximal tubules [78]. H2S is important in kidney for its role in homocysteine metabolism, regulation of GFR and of urinary sodium and potassium excretion [76]. In a murine model of renovascular hypertension, NaHS, a donor of H2S, suppressed the upregulation of renin mRNA by downregulating cAMP levels [208]. Emerging evidence indicates an active role of H2S in diabetes and in diabetic nephropathy (DN). CSE expression is upregulated in liver and pancreas in streptozotocin-induced diabetic rats, a model for type-1 diabetes. Insulin treatment reduced CSE expression [81]. CSE is also overexpressed in pancreatic β-cells in Zucker diabetic fatty rats, a model for type-2 diabetes [209]. On the other hand, renal expression of CBS and CSE enzymes which synthetize H2S, has been reported downregulated both in patients and in animal models of diabetes [76]. Hyperglycemia impairs cell redox homeostasis increasing ROS generation, reducing the activities of endogenous antioxidants such as GSH and SOD and downregulating Nrf-2, which control the expression of protective enzymes [207]. In addition, hyperglycemia-induced oxidative stress reduced CBS and CSE expression by upregulating MMP-9. These effects have been reported in cultured mesangial cells [210] and in diabetic rats [211] treated with high glucose and were reversed by treatment with NaHS [78]. H2S have been also reported to attenuate renovascular remodeling in DN through its regulatory action on MPP-9 and NADPH oxidase 4 (NOX4) [212,213]. In renal epithelial cells, NaHS reversed the inactivation of AMPK, responsible for the cascade that leads to hyperactivation of mTOR and subsequent matrix deposition [214]. Later, it was demonstrated that H2S inhibits NOX4 expression and matrix deposition by activation of iNOS and NO production [94]. Interestingly NO in turn is able to induce CSE and H2S production, suggesting a crosstalk interaction between the two gasotransmitters [215]. H2S have demonstrated an antifibrotic effect in renal tubular epithelial cells by attenuating the TGF-beta1-induced epithelial-to-mesenchymal transition (EMT) through both ERK-dependent and β-catenin-dependent pathways [216]. In addition, H2S accelerates wound healing in diabetic rats [217]. H2S levels and H2S-producing enzymes were found decreased in plasma, urine, and kidney of aging mice. Chronic H2S donor (NaHS) treatment could attenuate oxidative stress levels and renal tubular interstitial collagen deposition and restores H2S production in aging kidney. These protective effects may refer to Nrf2 activation and transcription of antioxidant proteins, including HO-1, SIRT1, SOD1, and SOD2, which are upregulated in the ageing kidney after NaHS treatment [218].
6. H2S and Brain
In the brain, CBS is the main H2S-producing enzyme, present in both neurons and astrocytes [219], and it was expressed mainly in the hippocampus and cerebellum when compared with the cerebral cortex and brain stem [220]. Furthermore, H2S production in astrocytes is approximately 10-fold higher than in cultured microglial and neuronal cells, suggesting that astrocytes are the main brain cells producing H2S [1]. H2S and sulfhydration play significant roles in the optimal functioning of the nervous system. For instance, H2S takes part in the regulation of intracellular Ca2+ in neurons and microglia, maintains pH homeostasis [76], and induces hippocampal long-term potentiation in active synapsis by activating N-methyl-D-aspartate (NMDA) receptors [221]. By regulating the NR2B subunit of NMDA receptors, NaHS treatment improved the cognitive dysfunction associated with hepatic ischemia/reperfusion in hippocampus of rats [222]. Various studies demonstrated the protective effects of H2S on neurons against oxidative stress [223]. H2S is unlikely to scavenge oxidants by itself due to its low endogenous concentration [83], whereas it seems to exert potent antioxidant effects indirectly regulating GSH production through the upregulation of the Keap1/Nrf2/ARE pathway. In addition, in vitro and in vivo models demonstrated that activation of Nrf2 in astrocytes provides protection also in neurons, prompting the hypothesis that this effect is the primary factor leading to neuroprotection of both cortical and motor neurons [1]. Notably, both D-Cys and L-Cys, substrates for H2S synthesis showed to protect the neurons of the cerebellum from hydrogen peroxide (H2O2)-induced oxidative stress [81]. In human cultured neuron cells, H2S inhibits peroxynitrite, acting as an endogenous peroxynitrite scavenger [1]. Dysregulation of H2S metabolism has been described in different neurological diseases. Hu et al. showed reduced levels of H2S in striatum and substantia nigra in two different murine models of Parkinson’s disease [224]. NaHS treatment attenuated neuronal loss, lipid oxidation, accumulation of inflammatory markers, and improved movement dysfunction [224]. In addition, H2S sulfhydration plays a relevant role in Parkinson’s disease by activating the neuroprotective ubiquitin E3 ligase, parkin, responsible for clearance of toxic, misfolded proteins [82]. Parkin sulfhydration was found markedly depleted in the brains of patients with Parkinson’s disease, suggesting that this loss may be pathologic and hydrogen sulfide donors may be therapeutic [225]. Likewise, H2S levels were found reduced in the plasma of patients with Alzheimer disease (AD) compared to controls, and H2S levels correlated negatively with the severity of the disease [226]. Depletion of H2S and concomitant increase in homocysteine levels in AD have been attributed to a lack of S-adenosylmethionine (SAM), an allosteric activator of CBS. Moreover, NaHS treatments in murine models of AD reduced oxidative stress through Nrf-2 and provided protection against homocysteine-induced cognitive dysfunction [227]. In addition, recent studies have shown that inhalation of H2S protected against PD-induced movement dysfunction and prevented neuronal apoptosis and microglia activation in the nigrostriatal region. CBS overexpression has also been reported to protect against the 6-hydroxydopamine-induced model of PD [228]. On the other hand, H2S production by CBS has been found elevated in amyotrophic lateral sclerosis (ALS) both in mice tissues and cerebrospinal fluid of ALS patients. Similarly, high H2S levels have been observed in trisomy of chromosome 21 trisomy (Down syndrome), on which the CBS gene is located [82]. It is well documented in the literature that H2S exerts either antioxidant and anti-inflammatory effects or prooxidant and pro-inflammatory effects depending on its local concentration. Following a bell-shaped dose–response curve, H2S produces protective effects at a lower concentration and a variety of deleterious/cytotoxic effects at higher concentrations [83,91]. The varying effects of H2S reported in several lines of evidence could be due to the dual effects of H2S [82]. This has been widely suggested in investigations concerning various types of inflammatory processes, vasorelaxation/tension in aortic tissue, cell proliferation/apoptosis, as well as in the case of tumor promotion and inhibition. Since the concentration of H2S can vary considerably both within and between tissues and under differing conditions, it is likely that H2S could affect a complex array of biological responses that may challenge the capacity to make definitive biomedical and/or clinical interpretations or predictions. Li and Moore [229] closed their recent insightful review of the role of H2S in health and disease with a statement probing their articulated biological conundrum of how does one molecule display such widely differing effects and could it be due to different effects at differing concentrations. The answer to this seminal question is found within the framework of a hormetic dose–response interaction that is independent of biological model, tissue, and endpoint [1].
7. H2S Redox Signaling and Resilience
Emerging evidence has highlighted the crucial role of H2S in maintaining redox homeostasis, which occurs by modulating levels of cellular antioxidant enzymes and increasing the expression of the transcription factor nuclear erythroid-related factor 2 (Nrf2) during oxidative stress. The latter is an intracellular excess of reactive oxygen species (ROS) relative to depletion of antioxidant capacity of the cell [230]. Interestingly, Nrf2 is a master regulator of redox cellular stress response in various pathological states [1,231]. Under physiological conditions, Nrf2 is localized in the cytosol and regulated by its inhibitor Kelch-like ECH-associated protein 1 (Keap1). Recently, much evidence has demonstrated that H2S induces cytoprotection against oxidative stress by the stimulation of Nrf2, which accumulates and translocates into the nucleus where it binds to the antioxidant response element (ARE) inducing the transcription of multiple target genes, including phase II detoxification enzymes such as NAD(P)H: quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HO-1), thioredoxin, γ-glutamylcysteine synthetase, and glutathione S-transferase (GST) [232]. In line with this observation, Nrf2 encodes the vitagene antioxidant pathway which exists to counteract different forms of stress (e.g., oxidative, environmental, and mitochondrial stress). Vitagenes include heat shock protein 70 (Hsp70), heme oxygenase 1 (HO-1), γ-glutamylcysteine synthetase (γ-GCs), thioredoxin (Trx), and sirtuins (SIRTs) (Figure 3) [1,233,234,235] as biomarkers for stress adaptation, cross-tolerance, and resilience underlying hormesis or preconditioning [236] (Figure 3).
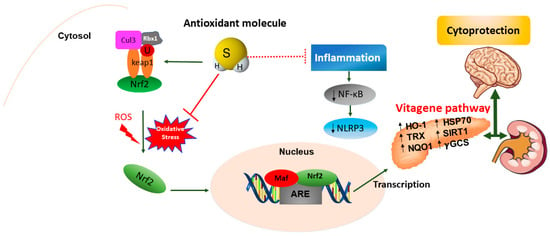
Figure 3.
The modulation of the Nrf2-vitagene pathway by H2S. In physiological conditions, Nrf2 is bound to its inhibitor Keap1 and is restricted to the cytosol where it undergoes ubiquitination and proteasomal degradation via association with the Cul3-Rbx1-based E3/ubiquitin ligase complex. Under stress conditions, Nrf2 is released from Keap1 and is translocated into the nucleus where it binds to the phase 2 of ARE in heterodimeric combination with the Maf transcription factor in the DNA promoter region. The H2S antioxidant molecule blocks oxidative stress and NLRP3 inflammasome cascade by activating Nrf2 nuclear translocation and the transcription of cytoprotective (phase 2) vitagenes. The upregulation of the vitagene pathway such as HO-1, Hsp70, Trx, sirtuin Sirt1, NQO1, and γ-GCS improves brain health in neurological disorders. Nuclear factor-erythroid 2 p45-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), antioxidant response element (ARE), heme-oxygenase 1 (HO-1), heat shock protein 70 (Hsp70), thioredoxin (Trx), sirtuin 1 (Sirt1), NAD(P)H: quinone oxidoreductase 1 (NQO1), γ-glutamylcysteine synthetase (γ-GCS).
For instance, the benefits of hormetic stimuli are well documented, such as preconditioning, dietary restriction as well as intermittent fasting in regulating endogenous H2S production by the conserved trans-sulfuration pathway (TSP) in a dose–response manner during ischemia reperfusion (I/R) injury [237,238,239]. Moreover, H2S can also act as a mimetic of dietary restriction and extend lifespan in yeast and worms [240]. Intriguingly, a growing number of studies have demonstrated that H2S decreases the ROS level in cardiomyocytes under ischemia/reperfusion injury in the setting of diabetes by relieving oxidative stress, and the ability of H2S to upregulate cellular antioxidants in the heart in a Nrf2-dependent manner [241,242]. Especially H2S-mediated cardioprotection increases in antioxidant (i.e., HO-1, Trx, Hsp70, and Hsp90) and anti-apoptotic (i.e., Bcl-2, Bcl-xL, and COX-2) signaling in a mouse model of pharmacological preconditioning [241]. These findings are consistent with the integrated view that cellular resilience is mediated by many distinct pathways that converge upon mitochondria to supply the extra energy for building resilience. Accordingly, several research groups have characterized the effect of H2S on mitochondrial activity and cellular bioenergetics [242,243,244]. These studies mentioned above demonstrated that H2S induces a U-shaped biological dose–response concentration typical of hormetic compounds in in vitro and in vivo models, with activation at lower concentrations and inhibition at higher concentrations [245]. Thus, the toxic and therapeutic effects of H2S depend on its endogenous concentration. Specifically, H2S is synthesized endogenously and mainly metabolized by a mitochondrial sulfide-oxidizing pathway including sulfide quinone oxidoreductase (SQR) and utilizes GSH as a thiophilic acceptor to produce GSSH [246]. Therefore, H2S produced in the mitochondria scavenges ROS [156]. In principle, H2S is a potent inhibitor of oxidative phosphorylation (OXPHOS) by its well-known ability to inhibit cytochrome c oxidase (COX) [247]. On the other hand, H2S therapy has been observed to preserve mitochondrial function in the heart muscle of rodents after I/R injury [238,248]. It has been well established that a low level of H2S administration increases the phosphorylation of protein serine/threonine kinase B (Akt) and enhances the nuclear localization of two transcription factors, nuclear respiratory factors-1 and -2, which are involved in increasing the levels of endogenous antioxidants, attenuating apoptosis, increasing mitochondrial biogenesis, and confer resilience against cellular stress [249]. Recently, the modulation of vitagenes elicited through H2S antioxidant molecule has taken on a considerable importance in preserving redox homeostasis, mitochondrial stress, protein quality control, and enhancing resilience in a hormetic dose–response manner during metabolic diseases such as diabetes and its complications. In this context, H2S provides antioxidant function by enhancing γ-glutamyl synthetase activity and upregulation of cysteine transport, restoring the levels of GSH in glutamate-mediated oxidative stress [148]. Some experimental studies suggested that administration of H2S attenuates high glucose-induced elevation in ROS production in renal mesangial cells and diabetic rat kidneys [250]. In addition, H2S treatment exerts protective effects in the kidney of type 1 diabetic rats, related to the suppression of oxidative stress through upregulation of superoxide dismutase (SOD) activity [251]. Interestingly, H2S mitigates diabetic renal damage by blocking mitochondrial Ca2+ permeability through the N-methyl-d-aspartate receptor-R1 (NMDA-R1) pathway [252]. Based on the concept of hormesis [253], in which low levels of H2S confer cytoprotective actions against oxidative stress, it can also cause toxicity when present in high concentrations [1,254]. Under oxidative stress, reactive sulfur species (RSS) are generated that can act as aggressive oxidizing agents. This phenomenon is mainly due to the high concentration of the H2S signaling molecule [255]. Thus, efforts have been made to identify suitable exogenous H2S donors. Indeed, some in vitro studies indicate that a high concentration of 0.1 and 1 mM NaHS induces pro-oxidant effects such as lipid peroxidation and protein carbonylation in human plasma [256]. In addition, in vivo studies demonstrate that NaHS improves diabetic state-induced muscle atrophy by increasing Akt/mTOR signaling and decreasing the expression of myostatin and the FoxO1/MuRF1/atrogin-dependent pathway [257]. Recent studies revealed a novel H2S-induced posttranslational modification, termed protein S-sulfhydration (also known as S-persulfidation) [87,258]. During this process, the –SH group of cysteine residues becomes covalently converted to a –SSH group, which can result in changes in the activity of the protein. This process importantly contributes to physiological and pathophysiological H2S-signaling. In this context, the S-sulfhydration reaction with H2S or hydrogen polysulfide (H2Sn) participation plays a crucial regulatory role in the biogenesis of RSS from endogenous and exogenous precursors and on regulatory properties of this reaction. On the other hand, these reversibly oxidized –SH groups are under the control of intracellular antioxidant pathways such as glutathione (GSH), cysteine (Cys), and thioredoxin (Trx) that actively participate to restore redox H2S homeostasis [259]. It is noteworthy that excessive levels of H2S can be countered by activation of the sulfide oxidation pathway, which involves the enzyme sulfide quinone oxidoreductase that oxidizes H2S and reduces coenzyme Q [260]. Several studies have reported that S-sulfhydration is one mechanism where H2S interacts directly with the Nrf2 pathway. In this respect, H2S has been shown to S-sulfhydrate Keap1 at the cysteine-151 residue, leading to Nrf2 dissociation and increased nuclear translocation and expression of antioxidant genes through binding to promoters’ ARE sites [232]. Furthermore, H2S can S-sulfhydrate Keap1 at the cysteine-226 and cysteine-613 residues, leading to Keap1 inactivation, Nrf2 release, and activation of Nrf2-dependent gene expression [261]. Additionally, NaHS upregulated Nrf2 nuclear translocation, and the transcription of the two key downstream antioxidant genes peroxiredoxin-1 and NAD(P)H dehydrogenase quinone 1 [262]. Most studies indicate that insufficient endogenous H2S concentration is implicated to increase oxidative stress leading to type 2 diabetes in mice and in humans [263,264]. Conversely, exogenous H2S promoted antioxidant effects by increasing Keap-1 and suppressing its ubiquitination, facilitating ubiquitin aggregate clearance via autophagy in the hearts of db/db mice [265]. In addition, recent studies demonstrated that H2S, in the form of a NaHS donor, may reduce high glucose-induced oxidative stress, inflammation, and apoptosis by activating the Nrf2/ARE pathway and may exert anti-apoptotic effects in diabetic myocardium by inhibiting c-Jun N-terminal kinase (JNK) and p38 MAPK pathways and activating PI3K/Akt signaling in vitro and in vivo [266]. Nevertheless, other recent studies showed that slow-releasing H2S donor, GYY4137, induces cardioprotection against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis via activation of the PHLPP-1/Akt/Nrf2 pathway in mice [267], inhibition of the STAT3/HIF-1α signaling pathway [268], and activation of the AMPK (5′AMP-activated protein kinase)/mTOR signal pathway in high glucose-induced H9c2 cardiomyocyte damage [269]. Moreover, the GYY4137 donor exerts anti-inflammatory effects by suppression of the NF-kB and MAPK signaling pathway in Coxsackie virus B3-infected rat cardiomyocytes [270] as well as attenuating the development of diabetic cardiomyopathy via FoxO1 signaling pathway in vitro and in vivo [271]. Recent findings reported that the H2S–Nrf2–antioxidant proteins axis protects renal tubular epithelial cells and rescues cells from the native hibernator and from lipid peroxidation-mediated cell death under reoxygenation conditions [272]. Recently, Kimura et al. provided novel insights into generated potential redox regulators cysteine- and glutathione-persulfide species (Cys-SSH, GSSH) as well as signaling molecules such as H2S2 and H2S3 produced by 3MST in the presence of physiological concentrations of cysteine and glutathione, to maintain neuronal transmission, vascular tone, cytoprotection, inflammation, and oxygen-sensing [273]. In this scenario, it has been shown that H2S2 along with H2S3 shields neuronal cells from oxidative as well as carbonyl stress through exerting reduced synthesis of glutathione [101]. Additional studies suggested that H2Sn rather that H2S regulates the activity of the tumor suppressor phosphatase and tensin homolog (PTEN) and reduces blood pressure by dilating vascular smooth muscle [274] through the modulation of the Nrf2 pathway [275]. Interestingly, H2Sn activates transient receptor potential ankyrin 1 (TRPA1) channels by sulfhydrating two cysteine residues at the amino terminus of the channels [276] and the species also facilitates the translocation of Nrf2 to the nucleus to upregulate antioxidant genes by sulfhydrating its binding partner Keap1 to release Nrf2 [275]. It is well known that decreased endogenous H2S levels, redox imbalance, and oxidative damage are closely correlated with disease severity and progression in cardiac, neurological, pulmonary, gastric, nephrological, hepatic diseases, as well as in aging. Notably, Xie and coworkers detect significantly lower levels of plasma H2S in diabetic mice that is corrected by administration of exogenous H2S donor GYY4137 [277]. Intriguingly, GYY4137 exerts anti-atherogenic [277] and inflammatory effects [278] via the Nrf2/HO-1 pathway in mice. In addition, H2S induced NRF2 activity, the upregulation of antioxidant genes HO-1, Trx-1, and GSH, and the reduction in inflammation response by suppressing the NF-κB pathway [279] as well as apoptosis in rat models [280]. In these conditions of inflammation, activation of the cellular stress response represents an essential system that requires upregulation of the antioxidant vitagene pathway to preserve resilience and redox homeostasis of the cell in various pathological conditions [1,281,282,283]. Within this context, silent mating type information regulator 2 homolog 1 (SIRT1) is a NAD+-dependent deacetylase of lysine residue of the target protein. Mammals have seven different sirtuins, SIRT1–SIRT7 [284]. SIRT1 extends lifespan [285] and improves cell tolerance to inhibit environmental stress [286]. Recently, it has been shown that H2S is a novel SIRT1 activator by direct sulfhydration. Numerous experimental studies in vitro and in vivo indicate protective effects of H2S signaling through activation of Sirtuin’s family in different pathologies and, in particular, in type 2 diabetes and related complications. In this regard, NaHS increased SIRT1 and reversed biochemical, apoptotic, oxidant, and pathologic parameters characteristic of diabetic nephropathy, at a dose of 100 µmol/kg/day [287]. In vivo studies reported that hydrogen sulfide ameliorated reperfusion-induced oxidative stress and mitochondrial dysfunction via activation of Sirtuin3 signaling, thereby decreasing lung ischemia-reperfusion damage in rats with a model of type II diabetes [288]. Endogenous CSE/H2S directly sulfhydrated SIRT1, enhanced SIRT1 binding to zinc ion, then promoted its deacetylation activity, and increased SIRT1 stability, thus reducing atherosclerotic plaque formation [289]. In vitro studies suggested that H2S attenuates CSE-induced cellular senescence and apoptosis by improving mitochondrial function and reducing oxidative stress in alveolar epithelial cells [290] as well as protects against hyperglycemia-induced neuronal senescence and neurotoxicity in the mouse hippocampal cell line [291] via upregulation of the SIRT1 pathway. Compelling evidence reported that H2S plays a peculiar role in protecting the ageing kidney from antifibrosis and anti-apoptosis through the regulation of redox homeostasis. Consistent with this, SIRT1, which emerges as a major lifespan regulator, has been widely investigated in the cardiovascular system and nervous system, but it is rare in the urinary system. Recent studies have demonstrated that a low concentration of NaHS (25 μmol/L) could directly induce SIRT1 activation in a cell-free system, whereas chronic treatment of NaHS (50 μmol/kg/day) selectively improves the expression but not the activity of SIRT1 in the ageing kidney. SIRT1 also regulates lipid metabolism by modulating a great variety of signaling pathways such as PPAR-α, LXR, FXR, and SREBP signals [292]. It is noteworthy that SIRT3 is a crucial regulator of mitochondrial function. SIRT3 catalyzes the deacetylation of mitochondrial proteins, which in turn affects mitochondrial energy metabolism. SIRT3 is regulated by nutritional status and metabolic stress. In this context, a recent in vivo study has demonstrated that exogenous H2S supplement attenuated isoproterenol-(ISO-)-induced myocardial hypertrophy through SIRT3 protein in mice [293]. It is well observed that chronic NaHS treatment modulates Nrf2 downstream genes such as HO-1, SOD1, and SOD2, consequently enhancing resistance to oxidative stress in the ageing kidney. Chronic exogenous H2S treatment could protect the ageing kidney by reducing oxidative stress, decreasing collagen deposition, and enhancing Nrf2 nuclear translocation, as well as increasing endogenous H2S production. Reactive oxygen species are constantly generated during metabolic diseases such diabetes. Among various signals activated by ROS, the thioredoxin pathway is among the extensively investigated signaling cascades that mediate oxidative cell proliferation, inflammation, senescence, and survival [294,295]. Trx represent the primary defense mechanism against oxidative stress. It contains two redox-active cysteine residues that protect protein against unwarranted oxidant-mediated inter- or intra-molecular disulfide bond formation. Some studies have demonstrated that H2S exerts antioxidative effects on the cells through the regulation of the redox state of Trx and interference with the ASK1/P38 signaling pathway [296]. Importantly, H2S modulates cellular redox signaling via direct S-sulfhydration of the Nrf2-Trx pathway. Consistent with this notion, Trx is the major regulator enzyme of intracellular persulfidation that cleaves disulfides in proteins and acts as an S-denitrosylase [297]. Especially, cysteine-32 within Trx is responsible for the direct interaction of Trx and S-sulfhydrated proteins following the breakage of the hydropersulfide group [298]. Likewise, Trx can desulfhydrate the protein tyrosine phosphatases 1B (PTP1B), restoring endoplasmic reticulum (ER) stress response in an in vitro setting [299]. Several studies revealed that H2S preconditioning could protect mice against cerebral I/R injury by the induction of HSP70 and the PI3K/Akt/Nrf2 pathway [300]. The heme oxygenase 1 (HO-1), also referred to as Hsp32, is induced by various oxidative stimuli, including ROS, RNS, and RSS. Accordingly, HO-1 has been recognized also as dynamic sensors of cellular oxidative stress and modulators of redox homeostasis throughout the phylogenetic spectrum. In this light, H2S exerts a protective role by upregulating the antioxidant enzyme HO-1 expression in human kidney cells [301] (Figure 4).
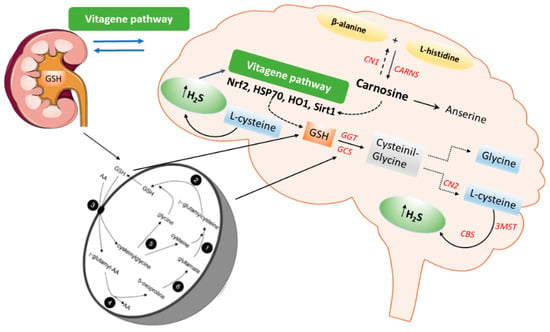
Figure 4.
Schematic representation of the carnosine, glutathione, and hydrogen sulfide (H2S) pathways in the kidney-brain axis. γ-glutamyl transpeptidase (GGT) (3), γ-glutamyl cyclotransferase (4), dipeptidase (5), oxoprolinase (6), γ-glutamyl-cysteine synthase (1), and glutathione synthetase (2) operate in the Meister cycle to generate glutathione (GSH) and internalize amino acids (AA). GSH interacts with Cystathionine-β-synthase (CBS) and 3-mercaptopyruvate sulfurtransferase (3MST) to produce H2S.
In addition, recent in vivo studies reveal the nephroprotective effects of H2S on renal tissue through upregulation of antioxidant proteins and anti-inflammatory cytokines, as well as the expression of eNOS and iNOS via induction of the Nrf2/HO-1 pathway in renal injury [302] and in the spinal cord of rats [303]. Moreover, H2S could attenuate high glucose-induced myocardial injury in rat cardiomyocytes by suppressing the Wnt/β-catenin pathway and upregulating the expression of HO-1 and NQO1 [304]. In addition, H2S protected renal tissue against ischemia-reperfusion injury-induced lipid peroxidation, inflammation, and apoptosis, which may be attributed to the upregulation of HSP 70, HO-1, and HSP 27 [305]. H2S reduces myocardial fibrosis in diabetic rats, which is related to the inhibition of protein kinase Cα (PKCα), upregulation of HSP70 expression [306], and downregulation of the JAK/STAT signaling pathway [307]. Finally, H2S protects cells from oxidative stress [308] and delays programmed cell death by increasing the levels of antioxidant glutathione and HO1 expression [309]. Taken together, the data above convincingly indicate the crucial role of H2S as a potent antioxidant molecule that, at low concentrations, induces protective actions exploited through the redox modulation of the Nrf2 vitagene signaling pathway which may provide a novel potential therapeutic approach to confer resilience against oxidative stress, inflammation as well as apoptosis during pathological conditions such as diabetes and related complications.
8. Analytical Approaches to Quantify H2S in Biological Matrices
Hydrogen sulfide, present in mammalian tissues, plays a vital role in physiological and pathophysiological processes. The long identified toxic gas, which has also been confirmed as the third gaseous signaling molecule following NO and CO, plays important roles in various physiological and pathological processes. However, striking differences with orders of magnitude were observed for the detected hydrogen sulfide concentrations in biological matrices among different measurements in the literature, which lead to the uncertainty for examination of the biological relevance of hydrogen sulfide. The monitoring of the quality control of H2S donor drugs and the study on the pathophysiology and pharmacology mechanism of H2S on experimental animals and even on humans require for detection methods with high sensitivity, selectivity, precision, and accuracy. Various techniques for the analysis of low concentrations of H2S have been developed over the past 50 years. Some of the older methods are still used by health departments, but due to their very low sensitivity, they cannot be used in the quantification of very low concentrations of this gas in mammalian tissues useful for scientific research. Also, due to the variety of chemical species with multiple properties, make it difficult accurate and reliable measurements of hydrogen sulfide in biological matrix [310]. Among the various methods that have been established for the measurement of endogenous hydrogen sulfide, the most widely used are: colorimetry [311], gas chromatography [312], electrochemical measurements by electrodes selective for sulfide (ion-selective electrodes, ISEs) [313], polarographic sensors [314], estimation by fluorescent probes [315], and high performance liquid chromatography (HPLC coupled with ultraviolet, fluorescence or electrochemical detection) [316,317]. Currently, fluorescence derivatization is the most used method. Hydrogen sulfide has two possibilities to perform nucleophilic substitution on fluorescence probes to form a fluorescent derivative that can be detected using a fluorimeter coupled with high performance liquid chromatography (HPLC) [318,319]. For example, monobromobimane (MBB) is a common fluorescent reagent that reacts rapidly and completely with thiol groups [320,321]. However, these methods have obvious limitations, such as complex preparation processes, low specificity and sensitivity, and time-consuming procedures [310]. Another analytical technique considered most effective today for the analysis of substances at such low concentrations is MS/MS, the analysis with triple quadrupole mass spectrometry in particular. Due to its low molecular weight and simple chemical structure, hydrogen sulfide is not suitable for direct quantification by triple quadruple mass spectrometry and thus, chemical derivatization was used before detection by LC-MS/MS [322,323,324]. Compared to all other methods, HPLC-MS/MS methods can determine concentrations in the order of 10−9–10−10 molar, the derivatization reactions allow high selectivity, and with the use of deuterated standards, it can calculate the recovery very simply. Therefore, in particular as regards those studies aimed to define the signaling role of H2S, HPLC-MS/MS methods, based on derivatization probes, are the most promising methodology to address the need to detect very small amounts of H2S in mammalian tissues and in other biological matrices.
9. Conclusions
This evaluation has demonstrated that both H2S and carnosine can modulate oxidative stress and inflammation in the brain and the kidney, suppressing damage interactive synergies between these two organs. Substantial evidence has emerged that these two agents independently activate the Nrf2 transcription factor, which then leads to the formation of acquired resilience following the quantitative features of the hormetic dose response. These processes also may involve the occurrence of transcription factor crosstalk that is both dose- and biological context-sensitive. The process is a general one, and is proposed to account for observations that hormetic effects are independent of biological model, cell type, level of biological organization, inducing agent, and endpoint measured. The emerging findings of H2S and carnosine are consistent with similar mechanistic and dose response findings for a wide range of chemopreventive agents such as sulforaphane and EGCG that mediate their protective effects via Nrf2 activation within the context of the hormetic dose response.
Author Contributions
Conceptualization, V.C., A.T.S., V.P., C.P.S., S.S. and E.J.C.; resources M.S., M.L.O., V.C. and S.M.; writing—original draft preparation, V.C., A.T.S., M.S, G.D., V.P., C.P.S. and E.J.C.; writing—review and editing, V.C., A.T.S., V.P., C.P.S., V.G., S.S. and E.J.C.; visualization, V.C., A.T.S., V.P., C.P.S. and E.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from “Piano di incentivi per la Ricerca, Linea Intervento 2 and Linea Intervento 3 PIACERI, 2020–2022”, University of Catania, Italy (VC, ATS); and from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project number 236360313—SFB 1118 (V.P., CPS). CPS is a member of the European Rare Kidney Disease Reference Network, ERKNet.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular Stress Responses, The Hormesis Paradigm, and Vitagenes: Novel Targets for Therapeutic Intervention in Neurodegenerative Disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longov. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Alhamdani, M.; Al-Azzawie, H.F.; Abbas, F.K. Decreased formation of advanced glycation end-products in peritoneal fluid by carnosine and related peptides. Perit. Dial. Int. 2007, 27, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; De Buhr, S.; Beijer, B.; Lindner, T.; Wischnjow, A.; Kender, Z.; Peters, V.; Kopf, S.; Haberkorn, U.; et al. A scavenger peptide prevents methylglyoxal induced pain in mice. Biochim. Biophys. Acta 2017, 1863, 654–662. [Google Scholar] [CrossRef]
- Colzani, M.; De Maddis, D.; Casali, G.; Carini, M.; Vistoli, G.; Aldini, G. Reactivity, Selectivity, and Reaction Mechanisms of Aminoguanidine, Hydralazine, Pyridoxamine, and Carnosine as Sequestering Agents of Reactive Carbonyl Species: A Comparative Study. ChemMedChem 2016, 11, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Weigand, T.; Singler, B.; Fleming, T.; Nawroth, P.; Klika, K.D.; Thiel, C.; Baelde, H.; Garbade, S.F.; Wagner, A.H.; Hecker, M.; et al. Carnosine Catalyzes the Formation of the Oligo/Polymeric Products of Methylglyoxal. Cell. Physiol. Biochem. 2018, 46, 713–726. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Chapter carnosine and its possible roles in nutrition and health. Adv. Food Nutr. Res. 2009, 57, 87–154. [Google Scholar]
- Hou, W.; Chen, H.J.; Lin, Y.H. Antioxidant peptides with Angiotensin converting enzyme inhibitory activities and applications for Angiotensin converting enzyme purification. J. Agric. Food Chem. 2003, 51, 1706–17093. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ueno, A.; Nishikawa, Y. Interactions between carnosine and captopril on free radical scavenging activity and angiotensin-converting enzyme activity in vitro. Yakugaku Zasshi 2006, 126, 37–42. [Google Scholar] [CrossRef]
- Decker, E.A.; Livisay, S.A.; Zhou, S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry 2000, 65, 766–770. [Google Scholar]
- Mozdzan, M.; Szemraj, J.; Rysz, J.; Nowak, D. Antioxidant properties of carnosine re-evaluated with oxidizing systems involving iron and copper ions. Basic Clin. Pharmacol. Toxicol. 2005, 96, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Velez, S.; Nair, N.G.; Reddy, V.P. Transition metal ion binding studies of carnosine and histidine: Biologically relevant antioxidants. Colloids Surf. B Biointerfaces 2008, 66, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Mancuso, C.; Pennisi, G.; Calafato, S.; Bellia, F.; Bates, T.E.; Giuffrida Stella, A.M.; Schapira, T.; Dinkova Kostova, A.T.; et al. Cellular stress response: A novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neuroch Res. 2008, 33, 2444–2471. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Facino, R.M.; Beretta, G.; Carini, M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: From structural studies to therapeutic perspectives. Biofactors 2005, 24, 77–87. [Google Scholar] [CrossRef]
- Grasso, G.I.; Arena, G.; Bellia, F.; Rizzarelli, E.; Vecchio, G. Copper(II)-chelating homocarnosine glycoconjugate as a new multifunctional compound. J. Inorg. Biochem. 2014, 131, 56–63. [Google Scholar] [CrossRef]
- Liu, W.; Wang, D.; Liu, K.; Sun, X. Nrf as a converging node for cellular signaling pathways of gasotransmitters. Med. Hypotheses 2012, 79, 308–310. [Google Scholar] [CrossRef]
- Bogen, K.T. Low dose rese response for in vitro Nrf2 ARE activation in human HepG2 cells. Dose Response 2017, 15, 1559325817699696. [Google Scholar] [CrossRef]
- Jing, X.; Wei, X.; Ren, R.; Wang, L.; Ahang, X.; Lou, H. Neuroprotective effects of tans chinone I against 6OHDA-induced oxidative stress in cellular and mouse model of Parkinson’s Disease through upregulating Nrf2. Neurochem. Res. 2016, 41, 779–786. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, X.; Kang, B.; Xu, J.; Wu, L.; Hong, J.; Zhang, Y.; Ni, X.; Wang, Z. Hydrogen sulfide attenuates myocardial hypoxia-reoxygenation injury by inhibiting autophage via mTOR activation. Cell Physiol. Biochem. 2015, 37, 2444–2453. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Li, T.; Yuan, L.; Liu, H.; Wang, X.; Wang, F.; Wang, S.; Hao, A.; Liu, D.; et al. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget 2016, 7, 58089–58104. [Google Scholar] [CrossRef]
- Zhang, J.; Yr, J.; Yuan, C.; Fu, Q.; Zhang, F.; Zhu, X.; Wang, L.; Go, P.; Shu, G.; Jiang, Q.; et al. Exogenous H2S exerts biphasic effects on porcine mammary epithelial cells proliferation through P13K/Akt-mTOR signaling pathway. J. Cell Physiol. 2018, 233, 7071–7081. [Google Scholar] [CrossRef]
- Cai, W.J.; Wang, M.J.; Moore, P.K.; Jin, H.M.; Yao, T.; Zu, Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2017, 76, 29–40. [Google Scholar] [CrossRef]
- Han, Y.; Zeng, F.; Tan, G.; Yang, C.; Tang, H.; Luo, Y.; Feng, J.; Xiong, H.; Guo, Q. Hydrogen sulfide inhibits abnormal proliferation of lymphocytes via AKT/GSK3B signal pathway in systemic lupus erythematosus patients. Cell. Physiol. Biochem. 2013, 31, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, Z.; Wu, L. Oxidative stress effects of soluble sulfide on human hepatocytes cell line L02. International. J. Environ. Res. Public Health 2019, 16, 1662. [Google Scholar] [CrossRef]
- Yang, B.; Bai, Y.; Yin, C.; Xing, G.; Wang, S.; Li, F.; Bian, J.; Aschner, M.; Lu, R. Activation of autophagic flux and the Nrf2/ARE signaling pathway by hydrogen sulfide protects against acrylonitrile-induced neurotoxicity in primary rat astrocytes. Arch. Toxicol. 2018, 92, 2093–2108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, D.X.; Wang, F.W.; Zhang, Q.; Du, Z.X.; Zhan, J.M.; Yuan, Q.H.; Ling, E.A.; Hao, A.J. L-cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H2S pathway. Neuroscience 2013, 237, 106–117. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, C.; Chen, X.; Wang, Z.; Wan, Z.; Jia, L.; Liu, Q.; Wang, Y.; Li, W.; Cui, J.; et al. Repetitive exposure to low-dose X-irradiation attenuates testicular apoptosis in type diabetic rats, likely via Akt-mediated Nrf2 activation. Mol. Cell. Endocrinol. 2016, 422, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Calabrese, V. Low dose radiation therapy (LDRT) is effective in the treatment of arthritis: Animal model findings. Int. J. Radiat. Biol. 2013, 89, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Calabrese, V. Reduction of arthritic symptoms by low dose radiation therapy (LDRT) is associated with an anti-inflammatory phenotype. Int. J. Radiat. Biol. 2013, 89, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Kozumbo, W.J. Radiotherapy treatment of human inflammatory disease and conditions: Optimal dose. Human Exp. Toxicol. 2019, 38, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.S.; Cordeiro, T.M.; Dos Santos Lacerda Soares, T.M.; Ferreira, R.N.; Simões ESilva, A.C. Kidney-brain axis inflammatory cross-talk: From bench to bedside. Clin. Sci. 2017, 131, 1093–1105. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Abe, H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry 2000, 65, 757–765. [Google Scholar] [PubMed]
- Peters, V.; Schmitt, C.P.; Zschocke, J.; Gross, M.L.; Brismar, K.; Forsberg, E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids 2012, 42, 2411–2416. [Google Scholar] [CrossRef]
- Peters, V.; Yard, B.; Schmitt, C.P. Carnosine and Diabetic Nephropathy. Curr. Med. Chem. 2020, 27, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Pfister, F.; Riedl, E.; Wang, Q.; Vom Hagen, F.; Deinzer, M.; Harmsen, M.C.; Molema, G.; Yard, B.; Feng, Y.; Hammes, H.P. Oral carnosine supplementation prevents vascular damage in experimental diabetic retinopathy. Cell. Physiol. Biochem. 2011, 28, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Mong, M.C.; Chao, C.Y.; Yin, M.C. Histidine and carnosine alleviated hepatic steatosis in mice consumed high saturated fat diet. Eur. J. Pharmacol. 2011, 653, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Jiang, H.; Hu, Y.; Keep, R.F.; Smith, D.E. Influence of genetic knockout of Pept2 on the in vivo disposition of endogenous and exogenous carnosine in wild-type and Pept2 null mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R986–R991. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K. Carnosine and homocarnosine, the forgotten, enigmatic peptides of the brain. Neuroch Res. 2005, 30, 1339–1345. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Cuzzocrea, S.; Calabrese, V.; Rizzarelli, E. Neuroprotective features of carnosine in oxidative driven diseases. Mol. Aspects Med. 2011, 32, 258–266. [Google Scholar] [CrossRef]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6251–6531. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Klessens, C.Q.; Baelde, H.J.; Singler, B.; Veraar, K.A.M.; Zutinic, A.; Drozak, J.; Zschocke, J.; Schmitt, C.P.; De Heer, E. Intrinsic carnosine metabolism in the human kidney. Amino Acids 2015, 47, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Spina-Purrello, V.; Giliberto, S.; Barresi, V.; Nicoletti, V.G.; Giuffrida Stella, A.M.; Rizzarelli, E. Modulation of PARP-and PARP-expression by L-carnosine and trehalose after LPS and INFgamma-induced oxidative stress. Neurochem. Res. 2010, 35, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Lopachev, A.V.; Lopacheva, O.M.; Abaimov, D.A.; Koroleva, O.V.; Vladychenskaya, E.A.; Erukhimovich, A.A.; Fedorova, T.N. Neuroprotective Effect of Carnosine on Primary Culture of Rat Cerebellar Cells under Oxidative Stress. Biochemistry 2016, 81, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Tanaka, K.I.; Kato-Negishi, M. Zinc, Carnosine, and Neurodegenerative Diseases. Nutrients 2018, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Sato, M.; Matsumoto, T.; Kadooka, K.; Hasegawa, T.; Fujimura, T.; Katakura, Y. Mechanisms of carnosine-induced activation of neuronal cells. Biosci. Biotechnol. Biochem. 2018, 82, 683–688. [Google Scholar] [CrossRef]
- Calabrese, V.; Colombrita, C.; Guagliano, E.; Sapienza, M.; Ravagna, A.; Cardile, V.; Scapagnini, G.; Santoro, A.M.; Mangiameli, A.; Butterfield, D.A.; et al. Protective effect of carnosine during nitrosative stress in astroglial cell cultures. Neurochem. Res. 2005, 30, 797–807. [Google Scholar] [CrossRef]
- Preston, J.E.; Hipkiss, A.R.; Himsworth, D.T.; Romero, I.A.; Abbott, J.N. Toxic effects of beta-amyloid (25–35) on immortalised rat brain endothelial cell: Protection by carnosine, homocarnosine and beta-alanine. Neurosci. Lett. 1998, 242, 105–108. [Google Scholar] [CrossRef]
- Peters, V.; Zschocke, J.; Schmitt, C.P. Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J. Inherit. Metab. Dis. 2018, 41, 39–47. [Google Scholar] [CrossRef]
- Ansari, F.A.; Mahmood, R. Carnosine and N-acetyl cysteine protect against sodium nitrite-induced oxidative stress in rat blood. Cell Boil. Int. 2018, 42, 281–293. [Google Scholar] [CrossRef]
- Fadda, L.M.; Attia, H.A.; Al-Rasheed, N.M.; Ali, H.M.; Aldossari, M. Attenuation of DNA damage and mRNA gene expression in hypoxic rats using natural antioxidants. J. Biochem. Mol. Toxicol. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Blasetti Fantauzzi, C.; Pesce, C.M.; Giaccari, A.; Salomone, E.; Lapolla, A.; Orioli, M.; Aldini, G.; Pugliese, G. FL-926-16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice. Br. J. Pharmacol. 2018, 175, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; De Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine RAW 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Z.; Kalaz, E.B.; Aydin, A.F.; Soluk-Tekkesin, M.; Dogru-Abbasoglu, S.; Uysal, M.; Kocak-Toker, N. The effect of carnosine on methylglyoxal-induced oxidative stress in rats. Arch. Physiol. Biochem. 2017, 123, 192–198. [Google Scholar] [CrossRef]
- Aldini, G.; Vistoli, G.; Stefek, M.; Chondrogianni, N.; Grune, T.; Sereikaite, J.; Sadowska-Bartosz, I.; Bartosz, G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013, 47, 93–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bo, S.H.; Lu, X.T.; Xu, A.J.; Zhang, J. Protective effects of carnosine on white matter damage induced by chronic cerebral hypoperfusion. Neural Regen Res. 2016, 11, 1438–1444. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Sun, B.L.; Yang, M.F.; Li, D.W.; Fang, J.; Zhang, S. Carnosine attenuates early brain injury through its antioxidative and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Cell. Mol. Neurobiol. 2015, 35, 147–157. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, L.; Zhang, L.R. Neuroprotective effect of carnosine against salsolinol-induced Parkinson’s disease. Exp. Ther. Med. 2017, 14, 664–670. [Google Scholar] [CrossRef]
- Ahshin-Majd, S.; Zamani, S.; Kiamari, T.; Kiasalari, Z.; Baluchnejadmojarad, T.; Roghani, M. Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: Possible involved mechanisms. Peptides 2016, 86, 102–111. [Google Scholar] [CrossRef]
- Baguet, A.; Everaert, I.; Yard, B.; Peters, V.; Zschocke, J.; Zutinic, A.; De Heer, E.; Podgorski, T.; Domaszewska, K.; Derave, W. Does low serum carnosinase activity favor high-intensity exercise capacity? J. Appl. Physiol. 2014, 116, 553–559. [Google Scholar] [CrossRef]
- De Courten, B.; Jakubova, M.; De Courten, M.P.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovic, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S.; et al. Ukropcova B: Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity 2016, 24, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Houjeghani, S.; Kheirouri, S.; Faraji, E.; Jafarabadi, M.A. L-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor-alpha levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018, 49, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Elbarbary, N.S.; Ismail, E.A.R.; El-Naggar, A.R.; Hamouda, M.H.; El-Hamamsy, M. The effect of weeks carnosine supplementation on renal functional integrity and oxidative stress in pediatric patients with diabetic nephropathy: A randomized placebo-controlled trial. Pediatric Diabetes 2018, 19, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hauske, S.J.; Zhang, S.; Rodriguez-Niño, A.; Albrecht, T.; Pastene, D.O.; Van den Born, J.; Van Goor, H.; Ruf, S.; Kohlmann, M.; et al. Identification and characterisation of carnostatine (SAN9812), a potent and selective carnosinase (CN1) inhibitor with in vivo activity. Amino Acids 2019, 51, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Jansen, E.E.; Jakobs, C.; Riedl, E.; Janssen, B.; Yard, B.A.; Wedel, J.; Hoffmann, G.F.; Zschocke, J.; Gotthardt, D.; et al. Anserine inhibits carnosine degradation but in human serum carnosinase (CN1) is not correlated with histidine dipeptide concentration. Clin. Chim. Acta 2011, 412, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Chengappa, K.N.; Turkin, S.R.; DeSanti, S.; Bowie, C.R.; Brar, J.S.; Schlicht, P.J.; Murphy, S.L.; Hetrick, M.L.; Bilder, R.; Fleet, D. A preliminary, randomized, double-blind, placebo-controlled trial of L-carnosine to improve cognition in schizophrenia. Schizoph Res. 2012, 142, 145–152. [Google Scholar] [CrossRef]
- Attanasio, F.; Convertino, M.; Magno, A.; Caflisch, A.; Corazza, A.; Haridas, H.; Esposito, G.; Cataldo, S.; Pignataro, B.; Milardi, D.; et al. Carnosine Inhibits Aβ (42) Aggregation by Perturbing the H-bond Network in and Around the Central Hydrophobic Cluster. Chembiochem 2013, 14, 583–592. [Google Scholar] [CrossRef]
- Cornelli, U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neuro-Degener Dis. 2010, 7, 193–202. [Google Scholar] [CrossRef]
- Mehrazad-Saber, Z.; Kheirouri, S.; Noorazar, S.G. Effects of l-Carnosine Supplementation on Sleep Disorders and Disease Severity in Autistic Children: A Randomized, Controlled Clinical Trial. Basic Clin. Pharmacol. Toxicol. 2018, 123, 72–77. [Google Scholar] [CrossRef]
- Masuoka, N.; Yoshimine, C.; Hori, M.; Tanaka, M.; Asada, T.; Abe, K.; Hisatsune, T. Effects of Anserine/Carnosine Supplementation on Mild Cognitive Impairment with APOE4. Nutrients 2019, 11, 1626. [Google Scholar] [CrossRef]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/Carnosine Supplementation Suppresses the Expression of the Inflammatory Chemokine CCL24 in Peripheral Blood Mononuclear Cells from Elderly People. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.; Fedorova, T.; Stepanova, M.; Dobrotvorskaya, I.; Kozlova, E.; Boldanova, N.; Bagyeva, G.; Ivanova-Smolenskaya, I.; Illarioshkin, S. Carnosine increases efficiency of DOPA therapy of Parkinson’s disease: A pilot study. Rejuvenation Res. 2008, 11, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef]
- Kimura, H.; Shibuya, N.; Kimura, Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal. 2012, 17, 45–57. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Ramazzini, B. Diseases of Workers–De Morbis Artificum Diatriba–1713. Am. J. Public Health 2001, 91, 1380–1382. [Google Scholar] [CrossRef]
- Szabo, C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef]
- Yamamoto, J.; Sato, W.; Kosugi, T.; Yamamoto, T.; Kimura, T.; Taniguchi, S.; Kojima, H.; Maruyama, S.; Imai, E.; Matsuo, S.; et al. Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clin. Exp. Nephrol. 2013, 17, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem. Pharmacol. 2018, 149, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Longen, S.; Beck, K.F.; Pfeilschifter, J. H2S-induced thiol-based redox switches: Biochemistry and functional relevance for inflammatory diseases. Pharmacol. Res. 2016, 111, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, H.; Li, S.; Yang, G. Regulation of cystathionine gamma-lyase/H2S system and its pathological implication. Front. Biosci. 2014, 19, 1355–1369. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Van den Born, J.C.; Frenay, A.R.; Bakker, S.J.; Pasch, A.; Hillebrands, J.L.; Lambers Heerspink, H.J.; Van Goor, H. High urinary sulfate concentration is associated with reduced risk of renal disease progression in type diabetes. Nitric Oxide 2016, 55–56, 18–24. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef]
- Koike, S.; Ogasawara, Y.; Shibuya, N.; Kimura, H.; Ishii, K. Polysulfide exerts a protective effect against cytotoxicity caused by t-buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett. 2013, 587, 3548–3555. [Google Scholar] [CrossRef]
- Meng, G.; Zhao, S.; Xie, L.; Han, Y.; Ji, Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br. J. Pharmacol. 2018, 175, 1146–1156. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell. 2012, 45, 13–24. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Liu, Y.; Bian, J.S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid. Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Roth, M.B. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2007, 104, 20618–20622. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Neish, A.S. Redox Signaling Mediated by the Gut Microbiota. Free Radic. Res. 2013, 47, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Adijiang, A.; Vandanmagsar, B.; Burk, D.; Ravussin, A.; Dixit, V.D. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology 2011, 152, 4039–4045. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Tan, L. The NLRP inflammasome in Alzheimer’s disease. Mol. Neurobiol. 2013, 48, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Pan, L.L.; Long, F.; Wu, W.J.; Yan, D.; Xu, P.; Liu, S.Y.; Qin, M.; Jia, W.W.; Liu, X.H.; et al. Endogenous Hydrogen Sulfide Ameliorates NOX4 Induced Oxidative Stress in LPS-Stimulated Macrophages and Mice. Cell. Physiol. Biochem. 2018, 47, 458–474. [Google Scholar] [CrossRef]
- Wang, P.; Chen, F.; Wang, W.; Zhang, X.D. Hydrogen Sulfide Attenuates High Glucose-Induced Human Retinal Pigment Epithelial Cell Inflammation by Inhibiting ROS Formation and NLRP3 Inflammasome Activation. Mediat. Inflamm. 2019, 2019, 8908960. [Google Scholar] [CrossRef]
- Li, J.; Teng, X.; Jin, S.; Dong, J.; Guo, Q.; Tian, D.; Wu, Y. Hydrogen sulfide improves endothelial dysfunction by inhibiting the vicious cycle of NLRP3 inflammasome and oxidative stress in spontaneously hypertensive rats. J. Hypertens. 2019, 37, 1633–1643. [Google Scholar] [CrossRef]
- Cao, L.; Cao, X.; Zhou, Y.; Nagpure, B.V.; Wu, Z.Y.; Hu, L.F.; Yang, Y.; Sethi, G.; Moore, P.K.; Bian, J.S. Hydrogen sulfide inhibits ATP-induced neuroinflammation and Aβ 1-42 synthesis by suppressing the activation of STAT3 and cathepsin S. Brain Behav. Immun. 2018, 73, 603–614. [Google Scholar] [CrossRef]
- Lin, Z.; Altaf, N.; Li, C.; Chen, M.; Pan, L.; Wang, D.; Xie, L.; Zheng, Y.; Fu, H.; Han, Y.; et al. Hydrogen sulfide attenuates oxidative stress-induced NLRP3 inflammasome activation via S-sulfhydrating c-Jun at Cys269 in macrophages. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2890–2900. [Google Scholar] [CrossRef]
- Kimura, H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Horiuchi, M. Clinical interaction between brain and kidney in small vessel disease. Cardiol. Res. Pract. 2011, 2011, 306189. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Kiernan, M.C.; Murray, A.; Rosner, M.H.; Ronco, C. Kidney–brain crosstalk in the acute and chronic setting. Nat. Rev. Nephrol. 2015, 11, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.; Silva, A.C.; Miranda, A.S.; Rocha, N.P.; Teixeira, A.L. Neuropsychiatric Disorders in Chronic Kidney Disease. Front. Pharmacol. 2019, 16, 10–932. [Google Scholar]
- Etgen, T.; Chonchol, M.; Forstl, H.; Sander, D. Chronic kidney disease and cognitive impairment: A systematic review and meta-analysis. Am. J. Nephrol. 2012, 35, 474–482. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, D.Y.; Mariappan, M.M.; Feliers, D.; Ghosh-Choudhury, G.; Abboud, H.E.; Gorin, Y.; Kasinath, B.S. Hydrogen sulfide inhibits high glucose-induced NADPH oxidase expression and matrix increase by recruiting inducible nitric oxide synthase in kidney proximal tubular epithelial cells. J. Biol. Chem. 2017, 292, 5665–5675. [Google Scholar] [CrossRef]
- Xia, M.; Chen, L.; Muh, R.W.; Li, P.-L.; Li, N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J. Pharmacol. Exp. Ther. 2009, 329, 1056–1062. [Google Scholar] [CrossRef]
- Bełtowski, J. Hypoxia in the renal medulla: Implications for hydrogen sulfide signaling. J. Pharmacol. Exp. Ther. 2010, 334, 358–363. [Google Scholar] [CrossRef]
- Hutton, H.L.; Ooi, J.D.; Holdsworth, S.R.; Kitchingm, A.R. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology 2016, 21, 736–744. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Z.; Wei, X.; Han, H.; Meng, X.; Zhang, Y.; Shi, W.; Li, F.; Xin, T.; Pang, Q.; et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J. Cereb. Blood Flow Metab. 2014, 34, 660–667. [Google Scholar] [CrossRef]
- Granata, S.; Masola, V.; Zoratti, E.; Scupoli, M.T.; Baruzzi, A.; Messa, M.; Sallustio, F.; Gesualdo, L.; Lupo, A.; Zaza, G. NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS ONE 2015, 10, e0122272. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.; Dietrich, D.; Martin, P.; Palmer, G.; Gabay, C. The interleukin (IL)-cytokine family–balance between agonists and antagonists in inflammatory diseases. Cytokine 2015, 76, 25–37. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1betadependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, S.-H.; Kim, Y.-G.; Kim, S.-Y.; Seo, J.-W.; Choi, Y.-W.; Kim, D.-J.; Jeong, K.-H.; Lee, T.; Ihm, C.-G.; et al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2015, 308, F993–F1003. [Google Scholar] [CrossRef]
- Kasimsetty, S.G.; DeWolf, S.E.; Shigeoka, A.A.; McKay, D.B. Regulation of TLR2 and NLRP3 in primary murine renal tubular epithelial cells. Nephron Clin. Pract. 2014, 127, 119–123. [Google Scholar] [CrossRef]
- Abais, J.M.; Zhang, C.; Xia, M.; Liu, Q.; Gehr, T.W.; Boini, K.M.; Li, P. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid. Redox Signal. 2013, 18, 1537–1548. [Google Scholar] [CrossRef]
- Ghosh, S.; Wu, M.D.; Shaftel, S.S.; Kyrkanides, S.; LaFerla, F.M.; Olschowka, J.A.; O’Banion, M.K. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J. Neurosci. 2013, 33, 5053–5064. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef]
- Salama, M.; Farrag, S.M.; Abulasrar, S.A.; Amin, M.M.; Ali, A.A.; Sheashaa, H.; Sobh, M.; Arias-Carrión, O. Up-regulation of TLR-4 in the brain after ischemic kidney-induced encephalopathy in the rat. CNS Neurol. Disord Drug Targets 2013, 12, 583–586. [Google Scholar] [CrossRef]
- Dantzer, R.; Kelley, K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007, 21, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Tu, C.; Zhao, J.; Ou, D.; Chen, G.; Liu, Y.; Xiao, X. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain Res. 2013, 1491, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, M.A.; Vaziri, N.D. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Vandini, E.; Ottani, A.; Zaffe, D.; Calevro, A.; Canalini, F.; Cavallini, G.M.; Rossi, R.; Guarini, S.; Giuliani, D. Mechanisms of Hydrogen Sulfide against the Progression of Severe Alzheimer’s Disease in Transgenic Mice at Different Ages. Pharmacology 2019, 103, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Feng, W.; Peh, M.T.; Peh, K.; Dymock, B.W.; Moore, P.K. A novel slow-releasing hydrogen sulfide donor, FW1256, exerts anti-inflammatory effects in mouse macrophages and in vivo. Pharmacol. Res. 2016, 113, 533–546. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, L.; Li, L.; Zhu, Y.; Wang, Z.; Shen, H.; Song, K. Hydrogen Sulfide Alleviates Peritoneal Fibrosis via Attenuating Inflammation and TGF-β1 Synthesis. Nephron 2015, 131, 210–219. [Google Scholar] [CrossRef]
- Olas, B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta 2015, 439, 212–218. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Liu, H.; Yin, C.; Li, X.; Gong, Q. Hydrogen sulfide ameliorates learning memory impairment in APP/PS1 transgenic mice: A novel mechanism mediated by the activation of Nrf2 pathway. Pharmacol. Biochem. Behav. 2016, 150–151, 207–216. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.Y.; Huang, Z.G.; Ma, Z.Y.; Xi, Y.; Wang, L.Y.; Sun, N.L. Endogenous hydrogen sulfide and ERK1/2-STAT3 signaling pathway may participate in the association between homocysteine and hypertension. J. Geriatr. Cardiol. 2019, 16, 822–834. [Google Scholar]
- Hou, X.Y.; Hu, Z.L.; Zhang, D.Z.; Lu, W.; Zhou, J.; Wu, P.F.; Guan, X.L.; Han, Q.Q.; Deng, S.L.; Zhang, H.; et al. Rapid Antidepressant Effect of Hydrogen Sulfide: Evidence for Activation of mTORC1-TrkB-AMPA Receptor Pathways. Antioxid. Redox Signal. 2017, 27, 472–488. [Google Scholar] [CrossRef]
- Shefa, U.; Kim, D.; Kim, M.S.; Jeong, N.Y.; Jung, J. Roles of Gasotransmitters in Synaptic Plasticity and Neuropsychiatric Conditions. Neural. Plast. 2018, 1824713. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xiong, S.; Zhou, Y.; Wu, Z.; Ding, L.; Zhu, Y.; Wood, M.E.; Whiteman, M.; Moore, P.K.; Bian, J.S. Renal Protective Effect of Hydrogen Sulfide in Cisplatin-Induced Nephrotoxicity. Antioxid. Redox Signal. 2018, 29, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Abazari, M.F.; Ghoraeian, P.; Torabinejad, S.; Nouri Aleagha, M.; Mirfallah Nassiri, R.; Tahmasebi, F.; Abedi, N.; Rajani, S.F.; Salarian, A.; et al. Ameliorative effects of hydrogen sulfide (NaHS) on chronic kidney disease-induced brain dysfunction in rats: Implication on role of nitric oxide (NO) signaling. Metab. Brain Dis. 2018, 33, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.K.; Azarnezhad, A.; Abazari, M.F.; Poorebrahim, M.; Ghoraeian, P.; Sanadgol, N.; Bokharaie, H.; Heydari, S.; Abbasi, A.; Kabiri, S.; et al. The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: Involvement of oxidative stress, autophagy and apoptosis. J. Cell. Physiol. 2019, 234, 11411–11423. [Google Scholar] [CrossRef] [PubMed]
- Ozatik, F.Y.; Teksen, Y.; Kadioglu, E.; Ozatik, O.; Bayat, Z. Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int. Urol. Nephrol. 2019, 51, 745–754. [Google Scholar] [CrossRef]
- Jabbari, B.; Vaziri, N.D. The nature, consequences, and management of neurological disorders in chronic kidney disease. Hemodial. Int. 2018, 22, 150–160. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Smeyne, M.; Smeyne, R.J. Glutathione metabolism and Parkinson’s disease. Free Radic. Biol. Med. 2013, 62, 13–25. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression. Am. J. Nephrol. 2017, 45, 473–483. [Google Scholar] [CrossRef]
- Tabassum, R.; Jeong, N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019, 16, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J. The smell of renal protection against chronic kidney disease: Hydrogen sulfide offers a potential stinky remedy. Pharmacol. Rep. 2018, 70, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018, 52, 507–543. [Google Scholar] [CrossRef] [PubMed]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef]
- Yu, M.; Kim, Y.J.; Kang, D.H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol. 2011, 6, 30–39. [Google Scholar] [CrossRef]
- Tbahriti, H.F.; Kaddous, A.; Bouchenak, M.; Mekki, K. Effect of different stages of chronic kidney disease and renal replacement therapies on oxidant-antioxidant balance in uremic patients. Biochem. Res. Int. 2013, 358985. [Google Scholar] [CrossRef]
- Kisic, B.; Miric, D.; Dragojevic, I.; Rasic, J.; Popovic, L. Role of Myeloperoxidase in Patients with Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2016, 2016, 1069743. [Google Scholar] [CrossRef]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef]
- Kim, H.J.; Vaziri, N.D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Physiol. 2010, 298, F662–F671. [Google Scholar] [CrossRef]
- Leaf, D.E.; Body, S.C.; Muehlschlegel, J.D.; McMahon, G.M.; Lichtner, P.; Collard, C.D.; Shernan, S.K.; Fox, A.A.; Waikar, S.S. Length polymorphisms in heme oxygenase-1 and AKI after cardiac surgery. J. Am. Soc. Nephrol. 2016, 27, 3291–3297. [Google Scholar] [CrossRef]
- Vera, M.; Torramade-Moix, S.; Martin-Rodriguez, S.; Cases, A.; Cruzado, J.M.; Rivera, J.; Escolar, G.; Palomo, M.; Diaz-Ricart, M. Antioxidant and Anti-Inflammatory Strategies Based on the Potentiation of Glutathione Peroxidase Activity Prevent Endothelial Dysfunction in Chronic Kidney Disease. Cell Physiol. Biochem. 2018, 51, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.; Abbott, M.; Abdi, M.; Fucci, Q.A.; Chauhan, N.; Mistri, M.; Proctor, B.; Chin, M.; Wang, B.; Yin, W.; et al. Pre-clinical model of severe glutathione peroxidase-deficiency and chronic kidney disease results in coronary artery thrombosis and depressed left ventricular function. Nephrol. Dial. Transplant. 2018, 33, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, C.; Ruzhytska, O.; Vettoretti, S.; Caldiroli, L.; Cozzolino, M.; Messa, P. Native Hypovitaminosis D in CKD Patients: From Experimental Evidence to Clinical Practice. Nutrients 2019, 11, 1918. [Google Scholar] [CrossRef]
- Shao, M.; Wang, S.; Parameswaran, P.K. Hypoalbuminemia: A risk factor for acute kidney injury development and progression to chronic kidney disease in critically ill patients. Int. Urol. Nephrol. 2017, 49, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Ayala-Macedo, G.; Sakihara, G.; Peralta, S.; Almaraz-Gómez, A.; Barrado, E.; Marugán-Miguelsanz, J.M. Effects of Zinc Supplementation on Nutritional Status in Children with Chronic Kidney Disease: A Randomized Trial. Nutrients 2019, 11, 2671. [Google Scholar] [CrossRef]
- Kimura, Y.; Goto, Y.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Tyagi, N.; Moshal, K.S.; Sen, U.; Vacek, T.P.; Kumar, M.; Hughes, W.M.; Kundu, S.; Tyagi, S.C. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid. Redox Signal. 2009, 11, 25–33. [Google Scholar] [CrossRef]
- Schreier, S.M.; Muellner, M.K.; Steinkellner, H.; Hermann, M.; Esterbauer, H.; Exner, M.; Gmeiner, B.M.; Kapiotis, S.; Laggner, H. Hydrogen sulfide scavenges the cytotoxic lipid oxidation product 4-HNE. Neurotox Res. 2010, 17, 249–256. [Google Scholar] [CrossRef]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef]
- Lee, Z.W.; Low, Y.L.; Huang, S.; Wang, T.; Deng, L.W. The cystathionine γ-lyase/hydrogen sulfide system maintains cellular glutathione status. Biochem. J. 2014, 460, 425–435. [Google Scholar] [CrossRef]
- Kimura, H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013, 63, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Hu, L.F.; Hu, G.; Bian, J.S. Hydrogen sulfide protects astrocytes against H2O2-induced neural injury via enhancing glutamate uptake. Free Radic. Biol. Med. 2008, 45, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, M.; Meister, A. The gamma-glutamyl cycle: A possible transport system for amino acids. Proc. Natl. Acad. Sci. USA 1970, 67, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Chauhan, V.; Chauhan, A. Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 89–95. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Chen, C.; Wang, J.; Zou, X.; Li, C.; Xu, Z.; Yang, X.; Shi, W.; Zeng, C. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J. Am. Heart Assoc. 2015, 4, e001559. [Google Scholar] [CrossRef]
- Tsuruya, K.; Yoshida, H. Brain Atrophy and Cognitive Impairment in Chronic Kidney Disease. Contrib. Nephrol. 2018, 196, 27–36. [Google Scholar]
- Lin, Y.C.; Chang, Y.H.; Yang, S.Y.; Wu, K.D.; Chu, T.S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Kaur, P.; Muthuraman, A.; Kaur, M. The implications of angiotensin-converting enzymes and their modulators in neurodegenerative disorders: Current and future perspectives. ACS Chem. Neurosci. 2015, 6, 508–521. [Google Scholar] [CrossRef]
- Kim, S.; Iwao, H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 2000, 52, 11–34. [Google Scholar]
- Lopez-Real, A.; Rey, P.; Soto-Otero, R.; Mendez-Alvarez, E.; Labandeira-Garcia, J.L. Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism. J. Neurosci. Res. 2005, 81, 865–873. [Google Scholar] [CrossRef]
- Villapol, S.; Saavedra, J.M. Neuroprotective effects of angiotensin receptor blockers. Am. J. Hypertens. 2015, 28, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Liu, Y.H.; Khin, E.S.; Bian, J.S. Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2008, 295, C1261–C1270. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Feng, X.; Xue, H.; Teng, X.; Jin, S.; Duan, X.; Xiao, L.; Wu, Y. Maternal renovascular hypertensive rats treatment with hydrogen sulfide increased the methylation of AT1b gene in offspring. Am. J. Hypertens. 2017, 30, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liu, Y.H.; Goh, H.S.; Wang, J.J.; Yong, Q.C.; Wang, R.; Bian, J.S. Hydrogen sulfide inhibits plasma renin activity. J. Am. Soc. Nephrol. 2010, 21, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Lu, M.; Xie, Z.Z.; Hua, F.; Xie, L.; Gao, J.H.; Koh, Y.H.; Bian, J.S. Hydrogen sulfide prevents heart failure development via inhibition of renin release from mast cells in isoproterenol-treated rats. Antioxid. Redox Signal. 2014, 20, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Organ, C.L.; Kang, J.; Polhemus, D.J.; Trivedi, R.K.; Sharp, T.E.; Jenkins, J.S.; Tao, Y.X.; Xian, M.; Lefer, D.J. Hydrogen sulfide attenuates renin angiotensin and aldosterone pathological signaling to preserve kidney function and improve exercise tolerance in heart failure. JACC Basic Transl. Sci. 2018, 3, 796–809. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Lu, P.C. Early short-term treatment with exogenous hydrogen sulfide postpones the transition from prehypertension to hypertension in spontaneously hypertensive rat. Clin. Exp. Hypertens. 2018, 40, 58–64. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, Y.; Zhan, Z.; Chen, J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J. Biol. Chem. 2014, 289, 28827–28834. [Google Scholar] [CrossRef]
- Karasavvidou, D.; Boutouyrie, P.; Kalaitzidis, R.; Kettab, H.; Pappas, K.; Stagikas, D.; Antonakis, N.; Tsalikakis, D.; Elisaf, M.; Laurent, S. Arterial damage and cognitive decline in chronic kidney disease patients. J. Clin. Hypertens. (Greenwich) 2018, 20, 1276–1284. [Google Scholar] [CrossRef]
- Martinez-Vea, A.; Salvadó, E.; Bardaji, A.; Gutierrez, C.; Ramos, A.; García, C.; Compte, T.; Peralta, C.; Broch, M.; Pastor, R.; et al. Silent cerebral white matter lesions and their relationship with vascular risk factors in middle-aged predialysis patients with CKD. Am. J. Kidney Dis. 2006, 47, 241–250. [Google Scholar] [CrossRef]
- Nakatani, T.; Naganuma, T.; Uchida, J.; Masuda, C.; Wada, S.; Sugimura, T.; Sugimura, K. Silent cerebral infarction in hemodialysis patients. Am. J. Nephrol. 2003, 23, 86–90. [Google Scholar] [CrossRef]
- Seliger, S.L.; Siscovick, D.S.; Stehman-Breen, C.O.; Gillen, D.L.; Fitzpatrick, A.; Bleyer, A.; Kuller, L.H. Moderate renal impairment and risk of dementia among older adults: The Cardiovascular Health Cognition Study. J. Am. Soc. Nephrol. 2004, 15, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.M.; Bouissou Morais Soares, C.M.; Teixeira, A.L.; Simoes-e-Silva, A.C.; Kummer, A.M. Anxiety, depression, resilience and quality of life in children and adolescents with pre-dialysis chronic kidney disease. Pediatr. Nephrol. 2015, 30, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Duron, E.; Hanon, O. Antihypertensive treatments, cognitive decline, and dementia. J. Alzheimers Dis. 2010, 20, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Kiernan, M.C. Neurological complications of chronic kidney disease. Nat. Rev. Neurol. 2009, 5, 542–551. [Google Scholar] [CrossRef]
- De Deyn, P.P.; Vanholder, R.; Eloot, S.; Glorieux, G. Guanidino compounds as uremic (neuro) toxins. Semin. Dial. 2009, 22, 340–345. [Google Scholar] [CrossRef]
- Watanabe, K.; Watanabe, T.; Nakayama, M. Cerebro-renal interactions: Impact of uremic toxins on cognitive function. Neurotoxicology 2014, 44, 184–193. [Google Scholar] [CrossRef]
- De Deyn, P.P.; D’Hooge, R.; Van Bogaert, P.P.; Marescau, B. Endogenous guanidino compounds as uremic neurotoxins. Kidney Int. Suppl. 2001, 78, S77–S83. [Google Scholar] [CrossRef]
- Hénaut, L.; Grissi, M.; Brazier, F.; Assem, M.; Poirot-Leclercq, S.; Lenglet, G.; Boudot, C.; Avondo, C.; Boullier, A.; Choukroun, G.; et al. Cellular and molecular mechanisms associated with ischemic stroke severity in female mice with chronic kidney disease. Sci. Rep. 2019, 9, 6432. [Google Scholar] [CrossRef]
- Adachi, N.; Lei, B.; Deshpande, G.; Seyfried, F.J.; Shimizu, I.; Nagaro, T.; Arai, T. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med. 2001, 27, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Luciano, M.G.; Ingrosso, D.; Pulzella, P.; Sepe, I.; Lanza, D.; Violetti, E.; Capasso, R.; Lombardi, C.; De Santo, N.G. Hydrogen sulphide-generating pathways in haemodialysis patients: A study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrol. Dial. Transplant. 2009, 24, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Di Nunzio, A.; Amoresano, A.; Pane, F.; Fontanarosa, C.; Pucci, P.; Vigorito, C.; Cirillo, G.; Zacchia, M.; Trepiccione, F.; et al. Divergent behavior of hydrogen sulfide pools and of the sulfur metabolite lanthionine, a novel uremic toxin, in dialysis patients. Biochimie 2016, 126, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lanza, D.; Perna, A.F.; Oliva, A.; Vanholder, R.; Pletinck, A.; Guastafierro, S.; Di Nunzio, A.; Vigorito, C.; Capasso, G.; Jankowski, V.; et al. Impact of the uremic milieu on the osteogenic potential of mesenchymal stem cells. PLoS ONE 2015, 10, e0116468. [Google Scholar] [CrossRef] [PubMed]
- Karmin, O.; Siow, Y.L. Metabolic Imbalance of Homocysteine and Hydrogen Sulfide in Kidney Disease. Curr. Med. Chem. 2018, 25, 367–377. [Google Scholar] [PubMed]
- Pushpakumar, S.; Kundu, S.; Sen, U. Hydrogen Sulfide Protects Hyperhomocysteinemia-Induced Renal Damage by Modulation of Caveolin and eNOS Interaction. Sci. Rep. 2019, 9, 2223. [Google Scholar] [CrossRef]
- Kielstein, H.; Suntharalingam, M.; Perthel, R.; Song, R.; Schneider, S.M.; Martens-Lobenhoffer, J.; Jäger, K.; Bode-Böger, S.M.; Kielstein, J.T. Role of the endogenous nitric oxide inhibitor asymmetric dimethylarginine (ADMA) and brain-derived neurotrophic factor (BDNF) in depression and behavioural changes: Clinical and preclinical data in chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 1699–1705. [Google Scholar] [CrossRef]
- Monteggia, L.M.; Barrot, M.; Powell, C.M.; Berton, O.; Galanis, V.; Gemelli, T.; Meuth, S.; Nagy, A.; Greene, R.W.; Nestler, E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA 2004, 101, 10827–10832. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analyses on associations (N = 9484). Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef]
- Gartner, A.; Staiger, V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc. Natl. Acad. Sci. USA 2002, 99, 6386–6391. [Google Scholar] [CrossRef]
- Karege, F.; Bondolfi, G.; Gervasoni, N.; Schwald, M.; Aubry, J.-M.; Bertschy, G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 2005, 57, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Changli, W.; Armelloni, S.; Zennaro, C.; Wei, C.; Corbelli, A.; Ikehata, M.; Berra, S.; Giardino, L.; Mattinzoli, D.; Watanabe, S.; et al. BDNF repairs podocyte damage by microRNA-mediated increase of actin polymerization. J. Pathol. 2015, 235, 731–744. [Google Scholar]
- Endlich, N.; Lange, T.; Kuhn, J.; Klemm, P.; Kotb, A.M.; Siegerist, F.; Kindt, F.; Lindenmeyer, M.T.; Cohen, C.D.; Kuss, A.W.; et al. BDNF: mRNA expression in urine cells of patients with chronic kidney disease and its role in kidney function. J. Cell. Mol. Med. 2018, 22, 5265–5277. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zou, W.; Wang, C.Y.; Chen, X.; Tan, H.Y.; Zeng, H.Y.; Zhang, P.; Gu, H.F.; Tang, X.Q. Hydrogen Sulfide Protects against Chronic Unpredictable Mild Stress-Induced Oxidative Stress in Hippocampus by Upregulation of BDNF-TrkB Pathway. Oxid. Med. Cell. Longev. 2016, 2153745. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.M.; Zhou, C.F.; Gao, S.L.; Tian, Y.; Wang, C.Y.; Wang, L.; Tang, X.Q. BDNF-TrkB pathway mediates neuroprotection of hydrogen sulfide against formaldehyde-induced toxicity to PC12 cells. PLoS ONE 2015, 10, e0119478. [Google Scholar] [CrossRef]
- Wei, H.J.; Xu, J.H.; Li, M.H.; Tang, J.P.; Zou, W.; Zhang, P.; Wang, L.; Wang, C.; Tang, X.Q. Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway. Acta Pharmacol. Sin. 2014, 35, 707–715. [Google Scholar] [CrossRef]
- Dugbartey, G.J. Diabetic nephropathy: A potential savior with ‘rotten-egg’ smell. Pharmacol. Rep. 2017, 69, 331–339. [Google Scholar] [CrossRef]
- Itani, H.; Liu, X.; Sarsour, E.H.; Goswami, P.C.; Born, E.; Keen, H.L.; Sigmund, C.D. Regulation of renin gene expression by oxidative stress. Hypertension 2009, 53, 1070–1076. [Google Scholar] [CrossRef]
- Wu, L.; Yang, W.; Jia, X.; Yang, G.; Duridanova, D.; Cao, K.; Wang, R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab. Investig. 2009, 89, 59–67. [Google Scholar] [CrossRef]
- Yuan, P.; Xue, H.; Zhou, L.; Qu, L.; Li, C.; Wang, Z.; Ni, J.; Yu, C.; Yao, T.; Huang, Y.; et al. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol. Dial. Transplant. 2011, 26, 2119–2126. [Google Scholar] [CrossRef]
- Safar, M.M.; Abdelsalam, R.M. H2S donors attenuate diabetic nephropathy in rats: Modulation of oxidant status and polyol pathway. Pharmacol. Rep. 2015, 67, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Gorin, Y.; Cavaglieri, R.C.; Khazim, K.; Lee, D.Y.; Bruno, F.; Thakur, S.; Fanti, P.; Szyndralewiez, C.; Barnes, J.L.; Block, K.; et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type diabetes. Am. J. Physiol. Renal. Physiol. 2015, 308, F1276–F1287. [Google Scholar] [CrossRef]
- Kundu, S.; Pushpakumar, S.B.; Tyagi, A.; Coley, D.; Sen, U. Hydrogen sulfide deficiency and diabetic renal remodeling: Role of matrix metalloproteinase-9. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1365–E1378. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Mariappan, M.M.; Feliers, D.; Cavaglieri, R.C.; Sataranatarajan, K.; Abboud, H.E.; Choudhury, G.G.; Kasinath, B.S. Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. J. Biol. Chem. 2012, 287, 4451–4461. [Google Scholar] [CrossRef]
- Feliers, D.; Lee, H.J.; Kasinath, B.S. Hydrogen Sulfide in Renal Physiology and Disease. Antioxid. Redox Signal. 2016, 25, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Peng, W.; Tao, J.; Lan, Z.; Hei, H.; Tian, L.; Pan, W.; Wang, L.; Zhang, X. Hydrogen Sulfide Inhibits Transforming Growth Factor-β1-Induced EMT via Wnt/Catenin Pathway. PLoS ONE 2016, 11, e0147018. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, W.; Chen, Q.; Jiang, Y.; Lu, X.; Zhao, X. Hydrogen sulfide accelerates wound healing in diabetic rats. Int. J. Clin. Exp. Pathol. 2015, 8, 5097–5104. [Google Scholar]
- Hou, C.L.; Wang, M.J.; Sun, C.; Huang, Y.; Jin, S.; Mu, X.P.; Chen, Y.; Zhu, Y.C. Protective Effects of Hydrogen Sulfide in the Ageing Kidney. Oxid. Med. Cell. Longev. 2016, 2016, 7570489. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Thomas, M.; Ghorpade, A.; Gendelman, H.E.; Banerjee, R.A. functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 2006, 281, 35785–35793. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.P.; Li, J.X.; Li, Q.; Wang, J. Effects of hydrogen sulfide on cognitive dysfunction and NR2B in rats. J. Surg. Res. 2016, 205, 426–431. [Google Scholar] [CrossRef]
- Tang, Z.J.; Zou, W.; Yuan, J.; Zhang, P.; Tian, Y.; Xiao, Z.F.; Li, M.H.; Wei, H.J.; Tang, X.Q. Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in streptozotocin-induced diabetic rats through inhibition of hippocampal oxidative stress. Behav. Pharmacol. 2015, 26, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Lu, M.; Tiong, C.X.; Dawe, G.S.; Hu, G.; Bian, J.S. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 2010, 9, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Vandiver, M.S.; Paul, B.D.; Xu, R.; Karuppagounder, S.; Rao, F.; Snowman, A.M.; Ko, H.S.; Lee, Y.I.; Dawson, V.L.; Dawson, T.M.; et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013, 4, 1626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Liu, X.Q.; Jiang, P.; Huang, H.; Yan, Y. Plasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer’s disease and vascular dementia and the significance thereof. Zhonghua Yi Xue Za Zhi 2008, 88, 2246–2249. [Google Scholar]
- Kumar, M.; Ray, R.S.; Sandhir, R. Hydrogen sulfide attenuates homocysteine-induced neurotoxicity by preventing mitochondrial dysfunctions and oxidative damage: In vitro and in vivo studies. Neurochem. Int. 2018, 120, 87–98. [Google Scholar] [CrossRef]
- Yin, W.L.; Yin, W.G.; Huang, B.S.; Wu, L.X. Neuroprotective effects of lentivirus-mediated cystathionine-betasynthase overexpression against 6-OHDA-induced Parkinson’s disease rats. Neurosci. Lett. 2017, 657, 45–52. [Google Scholar] [CrossRef]
- Li, L.; Moore, P.K. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol. Sci. 2008, 29, 84–90. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Amara, I.; Salah, A.; Timoumi, R.; Annabi, E.; Scuto, M.; Trovato, A.; Neffati, F.; Calabrese, V.; Abid-Essefi, S. Effect of di(2-ethylhexyl) phthalate on Nrf2-regulated glutathione homeostasis in mouse kidney. Cell Stress Chaperones. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Song, T.; Gu, Y.; Zhang, Y.; Cao, S.; Miao, Q.; Zhang, X.; Chen, H.; Gao, Y.; Zhang, L.; et al. Hydrogen sulfide alleviates liver injury via S-sulfhydrated-Keap1/Nrf2/LRP1 pathway. Hepatology 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Trovato Salinaro, A.; Maiolino, L.; Calabrese, V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2019, 21, 284. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Di Paola, R.; Cuzzocrea, S.; Calabrese, V. Anti-inflammatory and Anti-oxidant Activity of Hidrox® in Rotenone-Induced Parkinson’s Disease in Mice. Antioxidants 2020, 9, 824. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.T.; Maiolino, L.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium Erinaceus in rat brain: Relevance to Alzheimer’s disease pathogenesis. Immun. Ageing 2016, 9, 13–23. [Google Scholar] [CrossRef]
- Leak, R.K.; Calabrese, E.J.; Kozumbo, W.J.; Gidday, J.M.; Johnson, T.E.; Mitchell, J.R.; Ozaki, C.K.; Wetzker, R.; Bast, A.; Belz, R.G.; et al. Enhancing and Extending Biological Performance and Resilience. Dose Response 2018, 16, 1559325818784501. [Google Scholar] [CrossRef]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Treviño-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2015, 172, 1587–1606. [Google Scholar] [CrossRef]
- Ansari, M.; Kurian, G.A. Mechanism of Hydrogen Sulfide Preconditioning-Associated Protection Against Ischemia-Reperfusion Injury Differs in Diabetic Heart That Develops Myopathy. Cardiovasc. Toxicol. 2020, 20, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Hine, C.; Zhu, Y.; Hollenberg, A.N.; Mitchell, J.R. Dietary and Endocrine Regulation of Endogenous Hydrogen Sulfide Production: Implications for Longevity. Antioxid. Redox Signal. 2018, 28, 1483–1502. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Peake, B.F.; Nicholson, C.K.; Lambert, J.P.; Hood, R.L.; Amin, H.; Amin, S.; Calvert, J.W. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am. J. Physiol. Heart. Circ. Physiol. 2013, 304, H1215–H1224. [Google Scholar] [CrossRef] [PubMed]
- Módis, K.; Ju, Y.; Ahmad, A.; Untereiner, A.A.; Altaany, Z.; Wu, L.; Szabo, C.; Wang, R. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016, 113, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Ransy, C.; Módis, K.; Andriamihaja, M.; Murghes, B.; Coletta, C.; Olah, G.; Yanagi, K.; Bouillaud, F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part, I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014, 171, 2099–2122. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. Hormesis provides a generalized quantitative estimate of biological plasticity. J. Cell Commun. Signal. 2011, 5, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Calvert, J.W.; Coetzee, W.A.; Lefer, D. Novel Insights into Hydrogen Sulfide Mediated Cytoprotection. Antioxid. Redox Signal. 2010, 12, 1203–1217. [Google Scholar] [CrossRef]
- Xue, H.; Yuan, P.; Ni, J.; Li, C.; Shao, D.; Liu, J.; Shen, Y.; Wang, Z.; Zhou, L.; Zhang, W.; et al. H2S inhibits hyperglycemia-induced intrarenal renin-angiotensin system activation via attenuation of reactive oxygen species generation. PLoS ONE 2013, 8, e74366. [Google Scholar] [CrossRef]
- Yang, R.; Liu, X.F.; Ma, S.F.; Gao, Q.; Li, Z.H.; Jia, Q. Protective effect of hydrogen sulfide on kidneys of type 1 diabetic rats. J. Appl. Physiol. 2016, 32, 181–184. [Google Scholar]
- Papu John, A.S.; Kundu, S.; Pushpakumar, S.; Amin, M.; Tyagi, S.C.; Sen, U. Hydrogen sulfide inhibits Ca2+-induced mitochondrial permeability transition pore opening in type 1-diabetes. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E269–E283. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Santoro, A.; Trovato Salinaro, A.; Modafferi, S.; Scuto, M.; Albouchi, F.; Monti, D.; Giordano, J.; Zappia, M.; Franceschi, C.; et al. Hormetic approaches to the treatment of Parkinson’s disease: Perspectives and possibilities. J. Neurosci. Res. 2018, 96, 1641–1662. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.H.; Eghbal, M.A.; Hindmarsh, W.; Roth O’Brien, P.J. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev. 2006, 38, 733–744. [Google Scholar] [CrossRef]
- Giles, G.I.; Tasker, K.M.; Jacob, C. Hypothesis: The role of reactive sulfur species in oxidative stress. Free Radic. Biol. Med. 2001, 31, 1279–1283. [Google Scholar] [CrossRef]
- Olas, B.; Brodek, P.; Kontek, B. The Effect of Hydrogen Sulfide on Different Parameters of Human Plasma in the Presence or Absence of Exogenous Reactive Oxygen Species. Antioxidants 2018, 8, 610. [Google Scholar] [CrossRef]
- Bitar, M.S.; Nader, J.; Al-Ali, W.; Al Madhoun, A.; Arefanian, H.; Al-Mulla, F. Hydrogen Sulfide Donor NaHS Improves Metabolism and Reduces Muscle Atrophy in Type 2 Diabetes: Implication for Understanding Sarcopenic Pathophysiology. Oxid. Med. Cell. Longev. 2018, 2018, 6825452. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyderm, S.H. H2S: A Novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Yu, M.; Du, H.; Wang, B.; Chen, J.; Lu, F.; Peng, S.; Sun, Y.; Liu, N.; Sun, X.; Shiyun, D.; et al. Exogenous H2S Induces Hrd1 S-sulfhydration and Prevents CD36 Translocation via VAMP3 Ubiquitylation in Diabetic Hearts. Aging Dis. 2020, 11, 286–300. [Google Scholar] [CrossRef]
- Mimoun, S.; Andriamihaja, M.; Chaumontet, C.; Atanasiu, C.; Benamouzig, R.; Blouin, J.M.; Tomé, D.; Bouillaud, F.; Blachier, F. Detoxification of H2S by differentiated colonic epithelial cells: Implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxid. Redox Signal. 2012, 17, 1–10. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid. Redox Signal. 2013, 19, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Gambari, L.; Lisignoli, G.; Cattini, L.; Manferdini, C.; Facchini, A.; Grassi, F. Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via NRF2-dependent mechanism. Pharmacol. Res. 2014, 87, 99–112. [Google Scholar] [CrossRef]
- Cheng, Z.; Shen, X.; Jiang, X.; Shan, H.; Cimini, M.; Fang, P.; Ji, Y.; Park, J.Y.; Drosatos, K.; Yang, X.; et al. Hyperhomocysteinemia potentiates diabetes-impaired EDHF-induced vascular relaxation: Role of insufficient hydrogen sulfide. Redox Biol. 2018, 16, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J.; Wójcicka, G.; Jamroz-Wiśniewska, A. Hydrogen sulfide in the regulation of insulin secretion and insulin sensitivity: Implications for the pathogenesis and treatment of diabetes mellitus. Biochem. Pharmacol. 2018, 149, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tian, Z.; Sun, Y.; Lu, C.; Liu, N.; Gao, Z.; Zhang, L.; Dong, S.; Yang, F.; Zhong, X.; et al. Exogenous H2S facilitating ubiquitin aggregates clearance via autophagy attenuates type 2 diabetes-induced cardiomyopathy. Cell Death Dis. 2017, 8, e2992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; An, G.; Lu, X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin. Sci. 2015, 128, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wu, Y.; Meng, M.; Luo, M.; Zhao, H.; Sun, H.; Gao, S. GYY4137 protects against myocardial ischemia/reperfusion injury via activation of the PHLPP-1/Akt/Nrf2 signaling pathway in diabetic mice. J. Surg. Res. 2018, 225, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, Y.Q.; Zhang, L.; Zhang, H.; Zhang, S.W.; Zhang, Y.; Xuan, X.X.; Wang, M.J.; Zhang, J.Y. Exogenous hydrogen sulfide protects against high glucose-induced apoptosis and oxidative stress by inhibiting the STAT3/HIF-1α pathway in H9c2 cardiomyocytes. Exp. Ther. Med. 2019, 18, 3948–3958. [Google Scholar] [CrossRef]
- Wei, W.B.; Hu, X.; Zhuang, X.D.; Liao, L.Z.; Li, W.D. GYY4137, a novel hydrogen sulfide-releasing molecule, likely protects against high glucose-induced cytotoxicity by activation of the AMPK/mTOR signal pathway in H9c2 cells. Mol. Cell Biochem. 2014, 389, 249–256. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, H.; Du, Q.; Lin, W.; Liu, Y. GYY4137, a hydrogen sulfide-releasing molecule, inhibits the inflammatory response by suppressing the activation of nuclear factor-kappa B and mitogen-activated protein kinases in Coxsackie virus B3-infected rat cardiomyocytes. Mol. Med. Rep. 2015, 11, 1837–1844. [Google Scholar] [CrossRef]
- Ye, P.; Gu, Y.; Zhu, Y.R.; Chao, Y.L.; Kong, X.Q.; Luo, J.; Ren, X.M.; Zuo, G.F.; Zhang, D.M.; Chen, S.L. Exogenous hydrogen sulfide attenuates the development of diabetic cardiomyopathy via the FoxO1 pathway. J. Cell Physiol. 2018, 233, 9786–9798. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Nikolaou, E.; Liakopoulos, V.; Stefanidis, I. The H2S-Nrf2-Antioxidant Proteins Axis Protects Renal Tubular Epithelial Cells of the Native Hibernator Syrian Hamster from Reoxygenation-Induced Cell Death. Biology 2019, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Koike, S.; Shibuya, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci. Rep. 2017, 7, 10459. [Google Scholar] [CrossRef] [PubMed]
- Stubbert, D.; Prysyazhna, O.; Rudyk, O.; Scotcher, J.; Burgoyne, J.R.; Eaton, P. Protein kinase G Ialpha oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension 2014, 64, 1344–1351. [Google Scholar] [CrossRef]
- Shinkai, Y.; Abiko, Y.; Ida, T.; Miura, T.; Kakehashi, H.; Ishii, I.; Nishida, M.; Sawa, T.; Akaike, T.; Kumagai, Y. Reactive Sulfur Species-Mediated Activation of the Keap1-Nrf2 Pathway by 1,2-Naphthoquinone through Sulfenic Acids Formation under Oxidative Stress. Chem. Res. Toxicol. 2015, 28, 838–847. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Takahashi, K.; Tominaga, M.; Kimura, H.; Ohta, T. Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. Mol. Pain. 2015, 11, 24. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, Q.; Yang, S.; Huang, S.; Han, X.; Duan, J.; Pan, S.; Zhao, M.; Guo, S. GYY4137 attenuates LPS-induced acute lung injury via heme oxygenase-1modulation. Pulm. Pharmacol. Ther. 2019, 54, 77–86. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, T.; Yuan, Y.; Hu, N.; Tang, X. Hydrogen sulfide (H2S) attenuates uranium-induced acute nephrotoxicity through oxidative stress and inflammatory response via Nrf2-NF-kappaB pathways. Chem. Biol. Interact. 2015, 242, 353–362. [Google Scholar] [CrossRef]
- Jha, S.; Calvert, J.W.; Duranski, M.R.; Ramachandran, A.; Lefer, D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H801–H806. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Fronte, V.; Koverech, G.; Luca, M.; Serra, A.; Toscano, M.A.; Petralia, A.; et al. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: Relevance to Alzheimer’s disease pathogenesis. Neurotoxicology 2016, 53, 350–358. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Cornelius, C.; Koverech, G.; Koverech, A.; Scuto, M.; Lodato, F.; Fronte, V.; Muccilli, V.; Reibaldi, M.; Longo, A.; et al. Cellular stress response, redox status, and vitagenes in glaucoma: A systemic oxidant disorder linked to Alzheimer’s disease. Front. Pharmacol. 2014, 6, 5–129. [Google Scholar] [CrossRef]
- Calabrese, V.; Scapagnini, G.; Davinelli, S.; Koverech, G.; Koverech, A.; De Pasquale, C.; Salinaro, A.T.; Scuto, M.; Calabrese, E.J.; Genazzani, A.R. Sex hormonal regulation and hormesis in aging and longevity: Role of vitagenes. J. Cell Commun. Signal. 2014, 8, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Asakawa, A.; Morinaga, A.; Amitani, M.S.; Amitani, H.; Katsuura, G.; Sawada, Y.; Sudo, Y.; Uezono, Y.; Mochiki, E.; et al. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol. Psychiatry 2016, 21, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.H.; Taha, F.M.; Omar, H.S.; Elwi, H.M.; Abdelnasser, M. Hydrogen sulfide modulates SIRT1 and suppresses oxidative stress in diabetic nephropathy. Mol. Cell. Biochem. 2019, 457, 1–9. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, Y.; Meng, Q.; Lv, X.; Yue, Z.; Ding, W.; Liu, T.; Cui, X. Hydrogen sulfide attenuates lung ischemia-reperfusion injury through SIRT3-dependent regulation of mitochondrial function in type 2 diabetic rats. Surgery 2019, 165, 1014–1026. [Google Scholar] [CrossRef]
- Du, C.; Lin, X.; Xu, W.; Zheng, F.; Cai, J.; Yang, J.; Cui, Q.; Tang, C.; Cai, J.; Xu, G.; et al. Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetics Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxid. Redox Signal. 2019, 30, 84–197. [Google Scholar] [CrossRef]
- Guan, R.; Cai, Z.; Wang, J.; Ding, M.; Li, Z.; Xu, J.; Li, Y.; Li, J.; Yao, H.; Liu, W.; et al. Hydrogen sulfide attenuates mitochondrial dysfunction-induced cellular senescence and apoptosis in alveolar epithelial cells by upregulating sirtuin 1. Aging 2019, 11, 11844–11864. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Wang, C.Y.; Tang, Y.Y.; Huang, H.L.; Kang, X.; Li, X.; Xie, Y.R.; Tang, X.Q. Hydrogen Sulfide Inhibits High Glucose-Induced Neuronal Senescence by Improving Autophagic Flux via Up-regulation of SIRT1. Front. Mol. Neurosci. 2019, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, J.; Chen, Y.; Liu, L.; Xu, M.; Sun, L.; Luo, H.; Wang, Y.; Meng, G. Exogenous Hydrogen Sulfide Supplement Attenuates Isoproterenol-Induced Myocardial Hypertrophy in a Sirtuin 3-Dependent Manner. Oxid. Med. Cell. Longev. 2018, 2018, 9396089. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef]
- Amara, I.; Timoumi, R.; Annabi, E.; Di Rosa, G.; Scuto, M.; Najjar, M.F.; Calabrese, V.; Abid-Essefi, S. Di (2-ethylhexyl) phthalate targets the thioredoxin system and the oxidative branch of the pentose phosphate pathway in liver of Balb/c mice. Environ. Toxicol. 2020, 35, 78–86. [Google Scholar] [CrossRef]
- Mao, Z.; Huang, Y.; Zhang, Z.; Yang, X.; Zhang, X.; Huang, Y.; Sawada, N.; Mitsui, T.; Takeda, M.; Yao, J. Pharmacological levels of hydrogen sulfide inhibit oxidative cell injury through regulating the redox state of thioredoxin. Free Radic. Biol. Med. 2019, 134, 190–199. [Google Scholar] [CrossRef]
- Wedmann, R.; Onderka, C.; Wei, S.; Szijártó, I.A.; Miljkovic, J.L.; Mitrovic, A.; Lange, M.; Savitsky, S.; Yadav, P.K.; Torregrossa, R.; et al. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem. Sci. 2016, 7, 3414–3426. [Google Scholar] [CrossRef]
- Ju, Y.; Wu, L.; Yang, G. Thioredoxin regulation of protein S-desulfhydration. Biochem. Biophys. Rep. 2015, 5, 27–34. [Google Scholar] [CrossRef]
- Krishnan, N.; Fu, C.; Pappin, D.J.; Tonks, N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011, 4, ra86. [Google Scholar] [CrossRef]
- Ji, K.; Xue, L.; Cheng, J.; Bai, Y. Preconditioning of H2S inhalation protects against cerebral ischemia/reperfusion injury by induction of HSP70 through PI3K/Akt/Nrf2 pathway. Brain Res. Bull. 2016, 121, 68–74. [Google Scholar] [CrossRef]
- D’Araio, E.; Shaw, N.; Millward, A.; Demaine, A.; Whiteman, M.; Hodgkinson, A. Hydrogen sulfide induces heme oxygenase-in human kidney cells. Acta Diabetol. 2014, 51, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.M.; Elbassuoni, E.A.; Kamel, M.Y.; Ahmed, S.M. Hydrogen sulfide renal protective effects: Possible link between hydrogen sulfide and endogenous carbon monoxide in a rat model of renal injury. Cell Stress Chaperones. 2020, 25, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xie, K.; Chen, Y.; Wang, Y.; Wang, Y.; Lian, N.; Zhang, K.; Yu, Y. Nrf2/HO-1 signaling pathway participated in the protection of hydrogen sulfide on neuropathic pain in rats. Int. Immunopharmacol. 2019, 75, 105746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ye, M. Hydrogen Sulfide Attenuates High Glucose-induced Myocardial Injury in Rat Cardiomyocytes by Suppressing Wnt/beta-catenin Pathway. Curr. Med. Sci. 2019, 39, 938–946. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.H.; Zhu, H.C.; Wang, L.; Wang, Z.S.; Ning, J.Z.; Xiao, C.C. Hydrogen sulfide treatment protects against renal ischemia-reperfusion injury via induction of heat shock proteins in rats. Iran. J. Basic Med. Sci. 2019, 22, 99–105. [Google Scholar]
- Li, F.; Luo, J.; Wu, Z.; Xiao, T.; Zhang, J.; Yang, J. Effect of hydrogen sulfide on myocardial fibrosis and expression of PKCα and HSP70 in diabetic rats. ZhongNan Da Xue Xue Bao Yi Xue Ban 2015, 40, 1–5. [Google Scholar]
- Liu, M.; Li, Y.; Liang, B.; Li, Z.; Jiang, Z.; Chu, C.; Yang, J. Hydrogen sulphide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signalling pathway. Int. J. Mol. Med. 2018, 41, 1867–1876. [Google Scholar]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of Hydrogen Sulfide in NRF2-and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; Lai, D.; Sun, Y.; Samma, M.K.; Zhang, J.; Shen, W. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-expression. J. Plant Physiol. 2014, 171, 53–62. [Google Scholar] [CrossRef]
- Nagy, P.; Pálinkás, Z.; Nagy, A.; Budai, B.; Tóth, I.; Vasas, A. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim. Biophys. Acta 2014, 1840, 876–891. [Google Scholar] [CrossRef]
- Sugahara, S.; Suzuki, M.; Kamiya, H.; Yamamuro, M.; Semura, H.; Senga, Y.; Egawa, M.; Seike, Y. Colorimetric Determination of Sulfide in Microsamples. Anal. Sci. 2016, 32, 1129–1131. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Morris, G.F.; Hidiroglou, M. Rapid estimation of sulfide in rumen and blood with a sulfide-specific ion electrode. Microchem. J. 1980, 25, 388–395. [Google Scholar]
- Doeller, J.E.; Isbell, T.S.; Benavides, G.; Koenitzer, J.; Patel, H.; Patel, R.P.; Lancaster, J.R.; Darley-Usmar, V.; Kraus, D.W. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal. Biochem. 2005, 341, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Karpus, J.; Kabil, O.; Zhang, S.-Y.; Zhu, H.-L.; Banerjee, R.; Zhao, J.; He, C. Selective fluorescent probes for live-cell monitoring of sulphide. Nat. Commun. 2011, 2, 495. [Google Scholar] [CrossRef]
- Wintner, E.; Deckwerth, T.L.; Langston, W.; Bengtsson, A.; Leviten, D.; Hill, P.; Insko, A.M.; Dumpit, R.; VandenEkart, E.; Toombs, C.F.; et al. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br. J. Pharmacol. 2010, 160, 941–957. [Google Scholar] [CrossRef]
- Shen, X.; Pattillo, C.B.; Pardue, S.; Bir, S.C.; Wang, R.; Kevil, C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011, 50, 1021–1031. [Google Scholar] [CrossRef]
- Shen, X.; Peter, E.A.; Bir, S.; Wang, R.; Kevil, C.G. Analytical measurement of discrete hydrogen sulfide pools in biological specimens. Free Radic. Biol. Med. 2012, 52, 2276–2283. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, D.; Ma, F.; Yang, S.; Tan, B.; Xin, H.; Gu, X.; Chen, X.; Chen, S.; Mao, Y.; et al. Novel Angiogenic Activity and Molecular Mechanisms of ZYZ-803, a Slow-Releasing Hydrogen Sulfide-Nitric Oxide Hybrid Molecule. Antioxid. Redox Signal. 2016, 25, 498–514. [Google Scholar] [CrossRef]
- Togawa, T.; Ogawa, M.; Nawata, M.; Ogasawara, Y.; Kawanabe, K.; Tanabe, S. High performance liquid chromatographic determination of bound sulfide and sulfite and thiosulfate at their low levels in human serum by pre-column fluorescence derivatization with monobromobimane. Chem. Pharm. Bull. 1992, 40, 3000–3004. [Google Scholar] [CrossRef]
- Fahey, R.C.; Newton, G.L.; Dorian, R.; Kosower, E.M. Analysis of biological thiols: Quantitative determination of thiols at the picomole level based upon derivatization with monobromobimanes and separation by cation-exchange chromatography. Anal. Biochem. 1981, 111, 357–365. [Google Scholar] [CrossRef]
- Tan, B.; Jin, S.; Sun, J.; Gu, Z.; Sun, X.; Zhu, Y.; Huo, K.; Cao, Z.; Yang, P.; Xin, X.; et al. New method for quantification of gasotransmitter hydrogen sulfide in biological matrices by LC-MS/MS. Sci. Rep. 2017, 7, 46278. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Wu, S.; Meng, X.-G.; Jiang, J.; Zheng, M.-Y.; Fan, G. A simple liquid chromatography tandem mass spectrometric method for fast detection of hydrogen sulfide based on thiolysis of 7-nitro-2,1,3-benzoxadiazole ether. J. Chromatogr. A 2020, 1625, 461243. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Baeza-Garza, C.D.; Logan, A.; Rosa, T.; Wedmann, R.; Prime, T.A.; Martin, J.L.; Saeb-Parsy, K.; Krieg, T.; Filipovic, M.R.; et al. Assessment of H2S in vivo using the newly developed mitochondria-targeted mass spectrometry probe MitoA. J. Biol. Chem. 2017, 292, 7761–7773. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).