Molecular Dynamics Study of Anti-Wear Erosion and Corrosion Protection of PTFE/Al2O3 (010) Coating Composite in Water Hydraulic Valves
Abstract
:1. Introduction
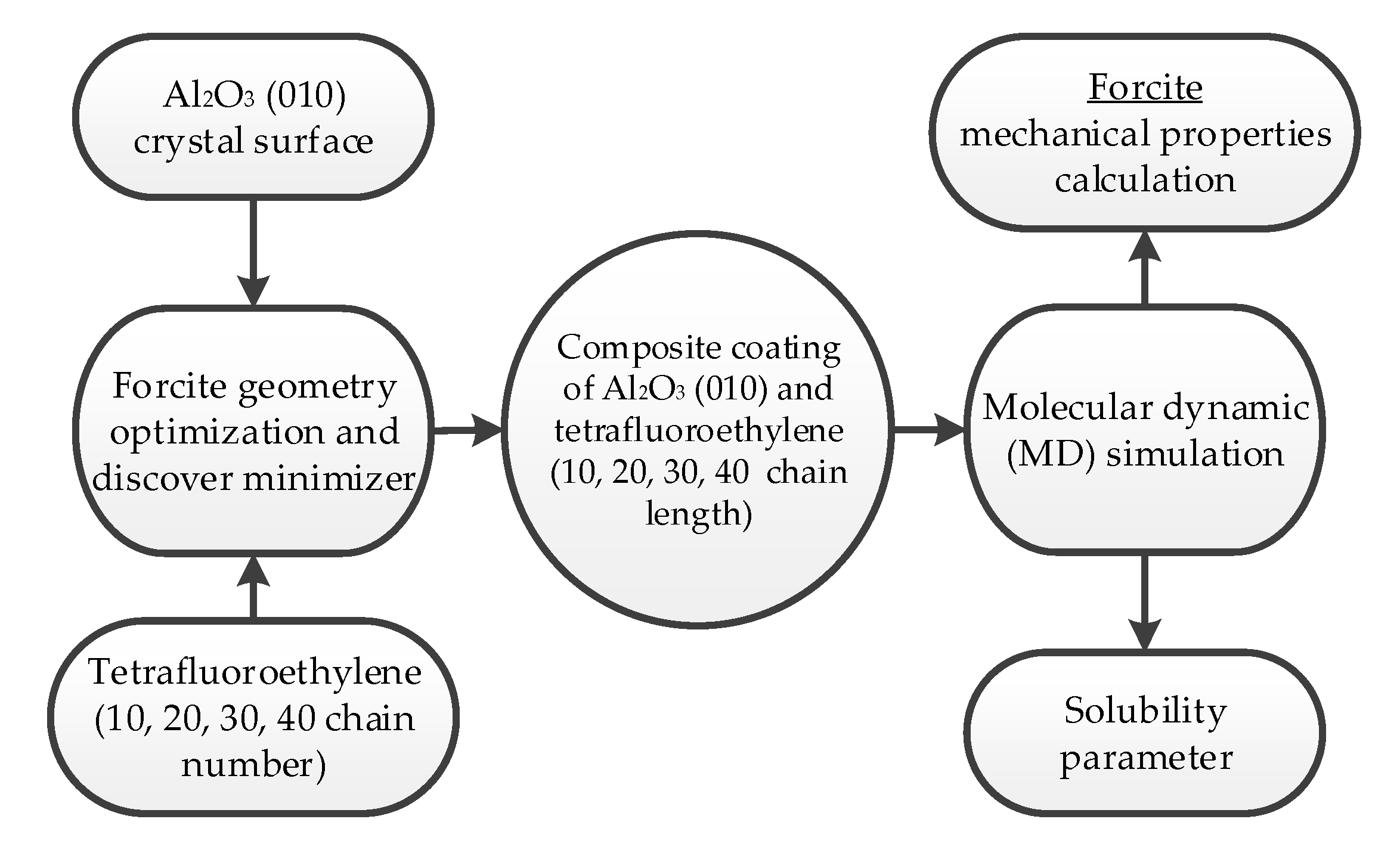
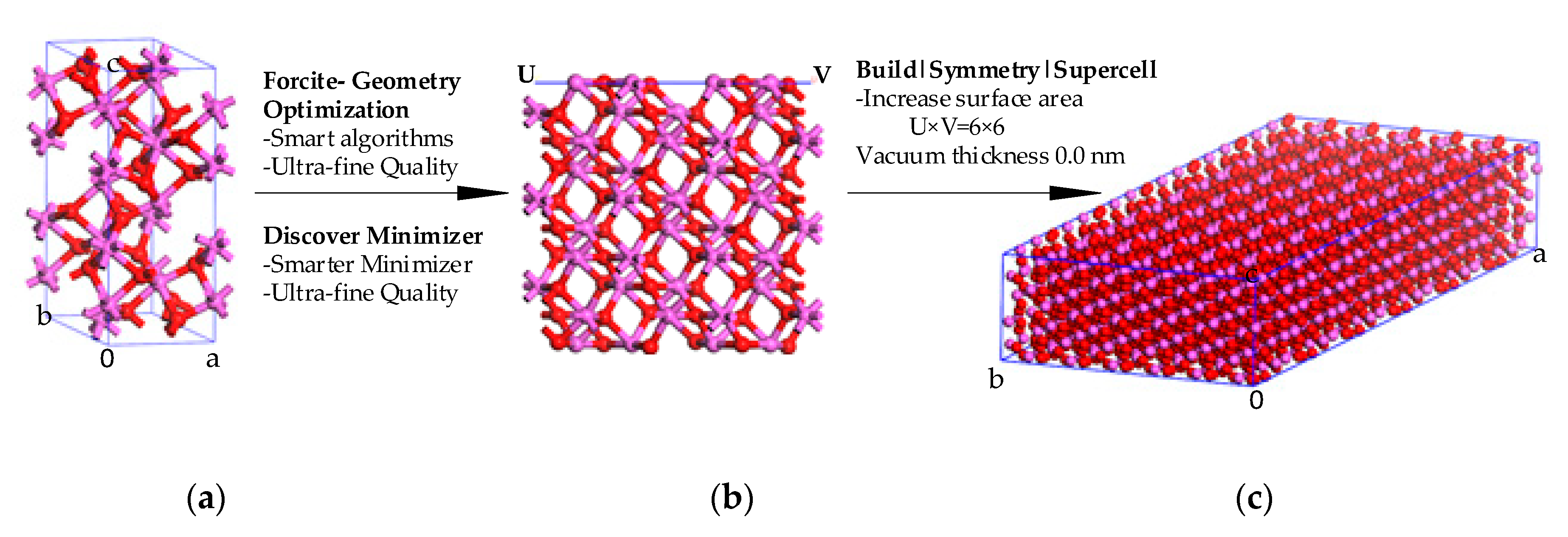
2. Method and Approach
Molecular Dynamics (MD) Simulation and Properties Calculation
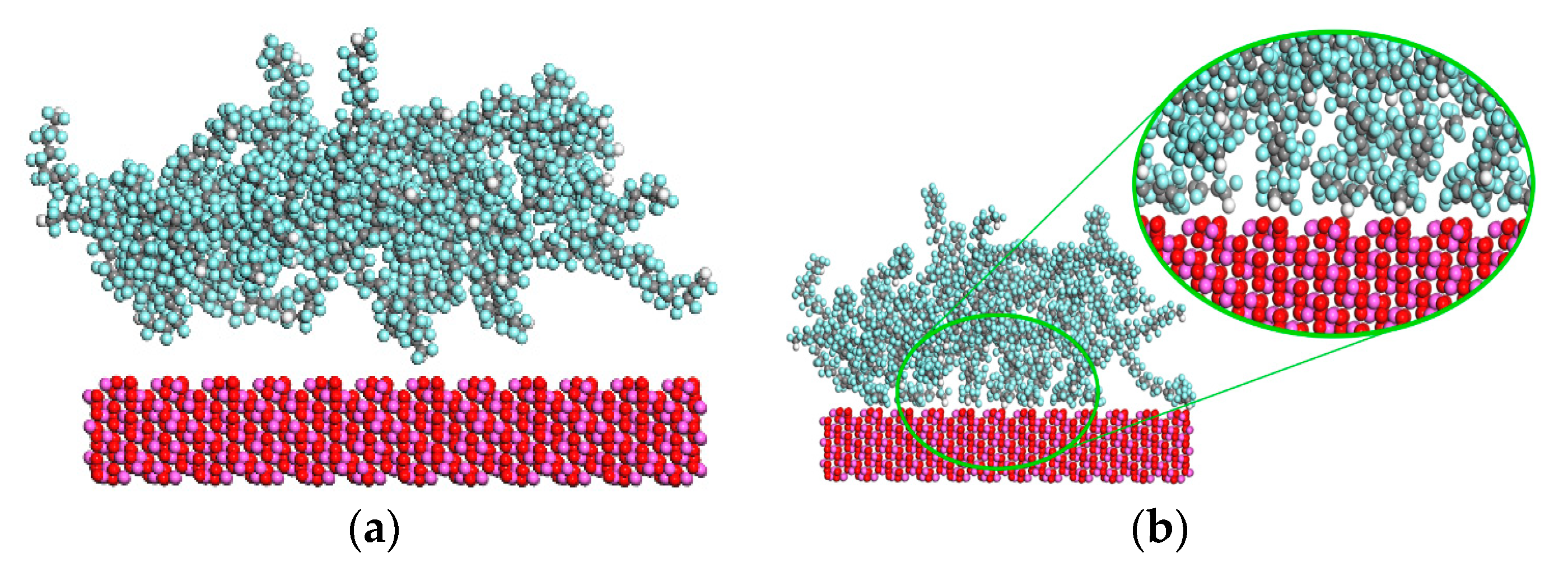
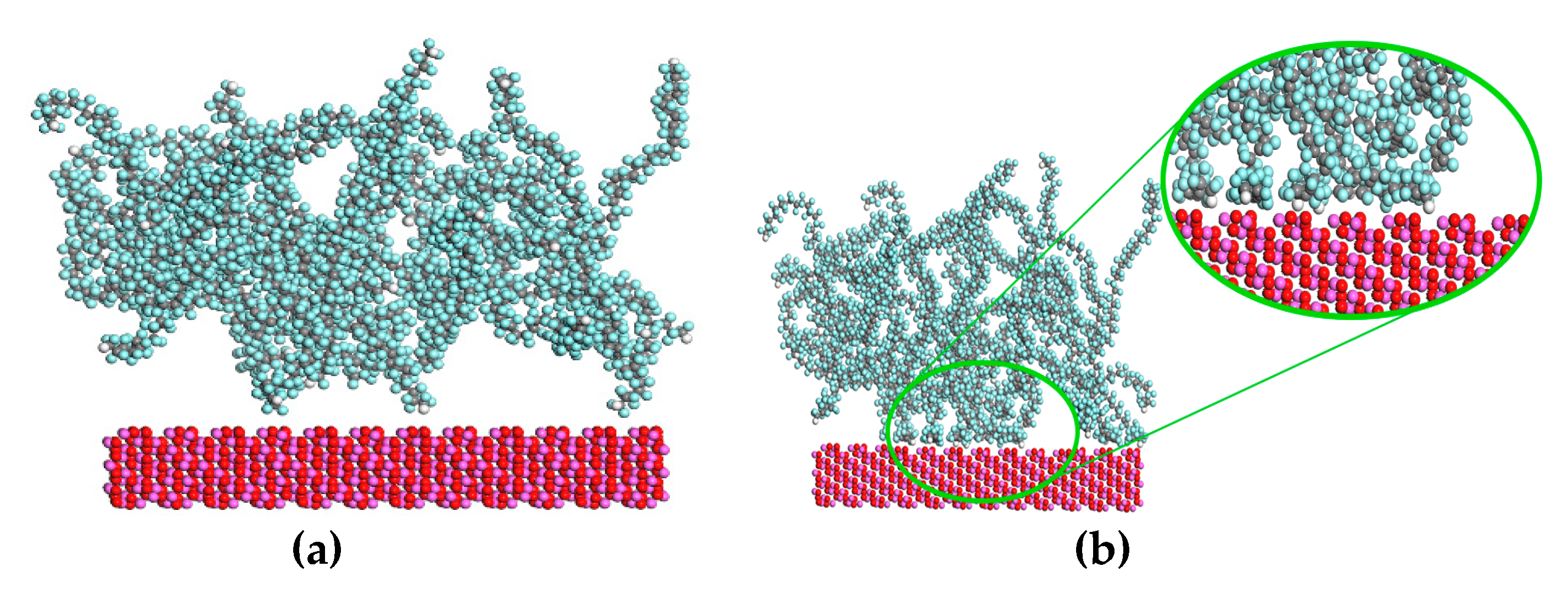
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Micu, L.M.; Lazar, I.; Circiumaru, A.; Bordeasu, I.; Pirvulescu, L.D.; Hluscu, M. New results regarding cavitation behavior of polymers modified with anorganic substances coated on bronze surfaces. Mater. Plast. 2018, 55, 460–463. [Google Scholar] [CrossRef]
- Zheng, H.-W.; Shu, X.-Y.; Li, Y.; Zhao, J.-P. Mechanical properties of Fe-based amorphous–crystalline composite: A molecular dynamics simulation and experimental study. Rare Met. 2018. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, H.; Yang, J.; Liu, R.; Zhou, Z.; Hao, T.; Nie, Y. Molecular simulations of microscopic mechanism of the effects of chain length on stereocomplex formation in polymer blends. Comput. Mater. Sci. 2020, 172, 109297. [Google Scholar] [CrossRef]
- Chen, B.; O’Mahony, J.A. Impact of glucose polymer chain length on heat and physical stability of milk protein-carbohydrate nutritional beverages. Food Chem. 2016, 211, 474–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Lu, L.; Rabczuk, T. The tensile and shear failure behavior dependence on chain length and temperature in amorphous polymers. Comput. Mater. Sci. 2015, 96, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Pacułt, J.; Rams-Baron, M.; Chrząszcz, B.; Jachowicz, R.; Paluch, M. Effect of polymer chain length on the physical stability of amorphous drug–polymer blends at ambient pressure. Mol. Pharm. 2018, 15, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Mlela, M.K.; Xu, H.; Sun, F.; Wang, H.; Madenge, G.D. Material analysis and molecular dynamics simulation for cavitation erosion and corrosion suppression in water hydraulic valves. Materials 2020, 13, 453. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Bahuleyan, B.K.; Johnson, R.P.; Shchipunov, Y.A.; Suh, H.; Ha, C.-S.; Kim, I. Morphology-tunable architectures constructed by supramolecular assemblies of α-diimine compound: Fabrication and application as multifunctional host systems. J. Mater. Chem. 2011, 21, 17938–17945. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar] [CrossRef]
- Wang, J.; Jin, S.; Chen, S.; Li, L.; Wang, D.; Lu, Z.; Wang, N.; Wang, J. Molecular dynamic simulations for FOX-7 and FOX-7 based PBXs. J. Mol. Model. 2018, 24, 145. [Google Scholar] [CrossRef]
- Chung, D.H.; Buessem, W.R. The Voigt-Reuss-Hill (VRH) Approximation and the Elastic Moduli of Polycrystalline ZnO, TiO2 (Rutile), and α-Al2O3. J. Appl. Phys. 1968, 39, 2777–2782. [Google Scholar] [CrossRef]
- Hua, X.; Wang, L.; Yang, S.J.P. Molecular dynamics simulation of improving the physical properties of polytetrafluoroethylene cable insulation materials by boron nitride nanoparticle under moisture-temperature-electric fields conditions. Polymers 2019, 11, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, I.D.; Awang, M.; Mamat, O.; Shaari, Z.B. First-principles calculations of the structural, mechanical and thermodynamics properties of cubic zirconia. World J. Nano Sci. Eng. 2014, 4, 97. [Google Scholar] [CrossRef] [Green Version]
- Greaves, G.N.; Greer, A.L.; Lakes, R.S.; Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 2011, 10, 823–837. [Google Scholar] [CrossRef]
- Rosato, D.; Rosato, D. DESIGN PARAMETER. In Plastics Engineered Product Design; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 161–197. [Google Scholar]
- Polymer Properties Database. Relationship between Moduli of Elasticity. 2020. Available online: http://polymerdatabase.com/polymer%20physics/Moduli.html (accessed on 2 July 2020).
- Gatt, R.; Grima, J. Negative compressibility. Phys. Status Solidi (RRL)-Rapid Res. Lett. 2008, 2, 236–238. [Google Scholar] [CrossRef]
- Lakes, R.; Wojciechowski, K.W. Negative compressibility, negative Poisson’s ratio, and stability. Phys. Status Solidi (B) 2008, 245, 545–551. [Google Scholar] [CrossRef]
- Cairns, A.B.; Goodwin, A.L. Negative linear compressibility. Phys. Chem. Chem. Phys. 2015, 17, 20449–20465. [Google Scholar] [CrossRef] [Green Version]
- Schuëller, G.I. Computational Methods in Stochastic Dynamics, 1st ed.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Corrosionpedia. Mass Density. 2019. Available online: https://www.corrosionpedia.com/definition/1562/mass-density (accessed on 24 May 2020).
- Zhou, X.; Hu, G. Analytic model of elastic metamaterials with local resonances. Phys. Rev. B 2009, 79, 195109. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Martin, J.L. Cavitation erosion of materials. Int. Met. Rev. 1986, 31, 1–26. [Google Scholar] [CrossRef]
- Wang, Y.; Lakes, R. Composites with inclusions of negative bulk modulus: Extreme damping and negative Poisson’s ratio. J. Compos. Mater. 2005, 39. [Google Scholar] [CrossRef]
- Moore, B.; Jaglinski, T.; Stone, D.S.; Lakes, R.S. Negative incremental bulk modulus in foams. Philos. Mag. Lett. 2006, 86, 651–659. [Google Scholar] [CrossRef]
- Su, Y.C.; Sun, C.T. Design of double negativity elastic metamaterial. Int. J. Smart Nano Mater. 2015, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Lai, Y.; Zhang, Z.-Q. Elastic metamaterials with simultaneously negative effective shear modulus and mass density. Phys. Rev. Lett. 2011, 107, 105506. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.B. The Hildebrand solubility parameters, cohesive energy densities and internal energies of 1-alkyl-3-methylimidazolium-based room temperature ionic liquids. Chem. Commun. 2005, 27, 3469–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]


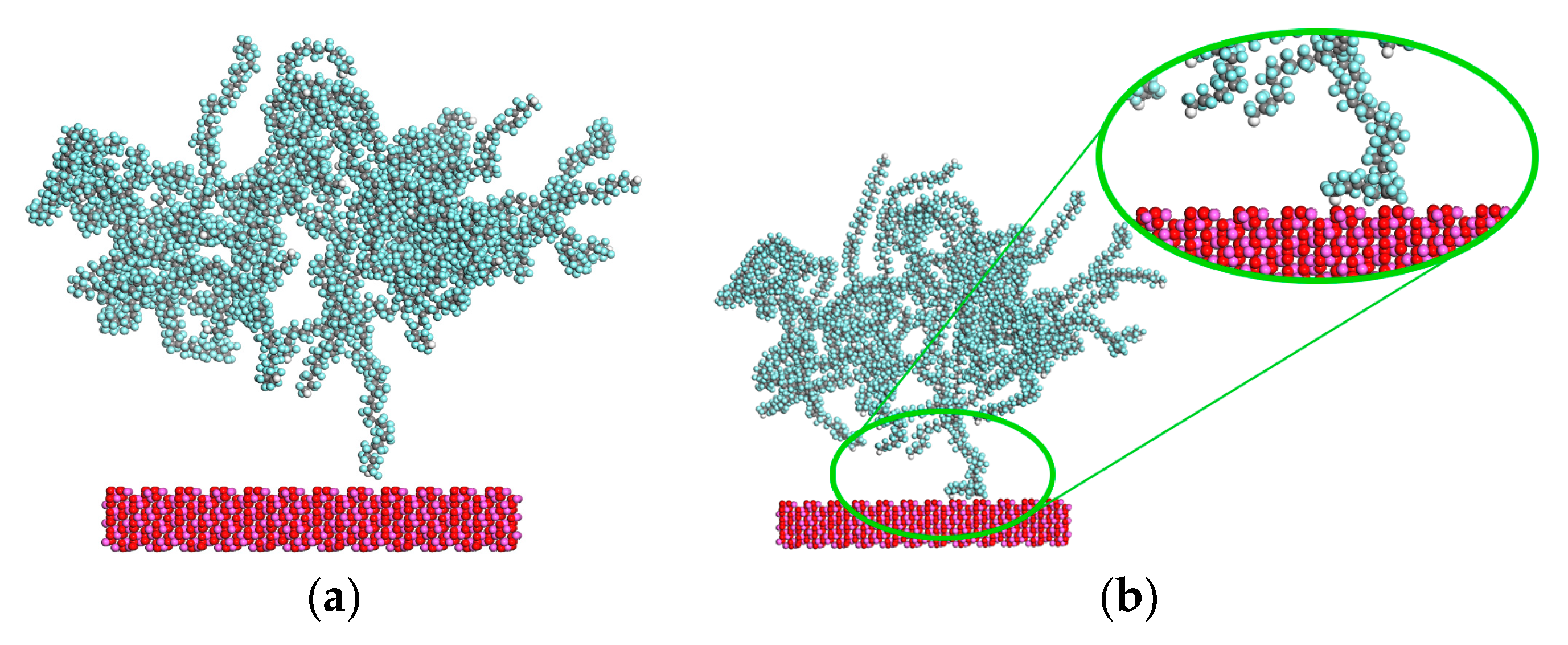
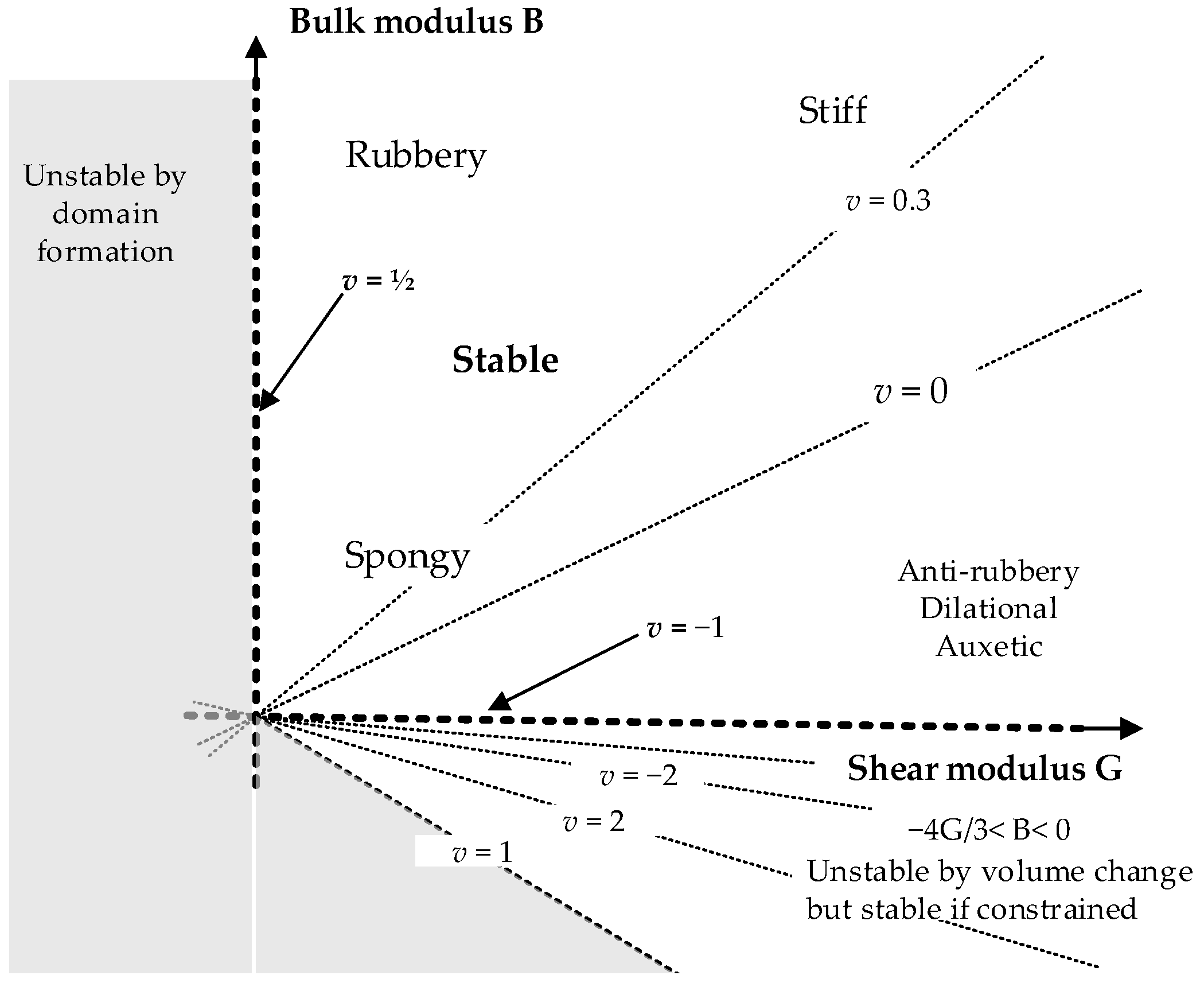

| Chain Length (K) | Number of Chains | Number of Configuration | Amorphous Cell Parameter (nm) |
|---|---|---|---|
| 10 | 10 | 2 | a = 7.795, b = 2.855, c = 0.747 |
| 20 | 10 | 2 | a = 7.795, b = 2.855, c = 1.493 |
| 30 | 10 | 2 | a = 7.795, b = 2.855, c = 2.239 |
| 40 | 10 | 2 | a = 7.795, b = 2.855, c = 2.985 |
| Coating of an Amorphous Cell of K10 Chain Length of PTFE on Al2O3 (010) | ||||||
| Cij (GPa) | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | −1276.94 | −2274.16 | −3758.69 | −627.14 | 333.72 | −253.96 |
| 2 | −2274.16 | −3386.35 | −4816.39 | −490.81 | 411.54 | −323.10 |
| 3 | −3758.69 | −4816.39 | −6567.59 | −520.05 | 478.95 | −328.83 |
| 4 | −627.14 | −490.81 | −520.05 | 72.10 | 14.45 | 75.11 |
| 5 | 333.72 | 411.54 | 478.95 | 14.4466 | −21.22 | 2.47 |
| 6 | −253.96 | −323.10 | −328.83 | 75.11 | 2.47 | −127.57 |
| Coating of an Amorphous Cell of K20 Chain Length of PTFE on Al2O3 (010) | ||||||
| Cij (GPa) | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | 407.85 | 1468.53 | −1695.44 | 2793.44 | −587.12 | 1229.29 |
| 2 | 1468.54 | 2051.47 | −81.20 | 2679.19 | −532.33 | 851.38 |
| 3 | −1695.44 | −81.20 | −4798.43 | 3515.44 | −712.54 | 1884.61 |
| 4 | 2793.44 | 2679.19 | 3515.45 | 158.28 | 35.72 | −171.47 |
| 5 | −587.12 | −532.33 | −712.53 | 35.72 | −29.93 | −23.67 |
| 6 | 1229.29 | 851.38 | 1884.61 | −171.47 | −23.67 | −422.33 |
| Coating of an Amorphous Cell of K30 Chain Length of PTFE on Al2O3 (010) | ||||||
| Cij (GPa) | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | 6455.40 | 2093.16 | 368.40 | −344.27 | 2804.84 | −7985.71 |
| 2 | 2093.16 | −2217.00 | −4349.66 | −15.84 | 2656.07 | −7700.60 |
| 3 | 368.34 | −4349.66 | −9322.88 | −122.49 | 3771.29 | −9737.98 |
| 4 | −344.27 | −15.83 | −122.49 | −15.789 | −34.00 | 414.38 |
| 5 | 2804.84 | 2656.07 | 3771.29 | −34.009 | −47.43 | −144.01 |
| 6 | −7985.71 | −7700.60 | −9737.98 | 414.38 | −144.01 | 531.12 |
| Coating of an Amorphous cell of K40 chain Length of PTFE on Al2O3 (010) | ||||||
| Cij (GPa) | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | 685.48 | 587.11 | −111.84 | −1777.92 | 892.86 | −448.15 |
| 2 | 587.11 | 453.74 | −440.53 | −1917.85 | 969.05 | −419.62 |
| 3 | −111.84 | −440.53 | −1064.19 | −1594.49 | 880.50 | −540.85 |
| 4 | −1777.92 | −1917.85 | −1594.49 | 131.19 | −7.49 | −61.23 |
| 5 | 892.86 | 969.05 | 880.50 | −7.49 | −90.60 | 81.79 |
| 6 | −448.15 | −419.62 | −540.84 | −61.23 | 81.79 | 102.39 |
| Property | K10 Chain Length | K20 Chain Length | K30 Chain Length | K40 Chain Length |
|---|---|---|---|---|
| Binding Energy, Eb (kJ/mol) | 6267.16 | 4271.05 | 3428.92 | 590.58 |
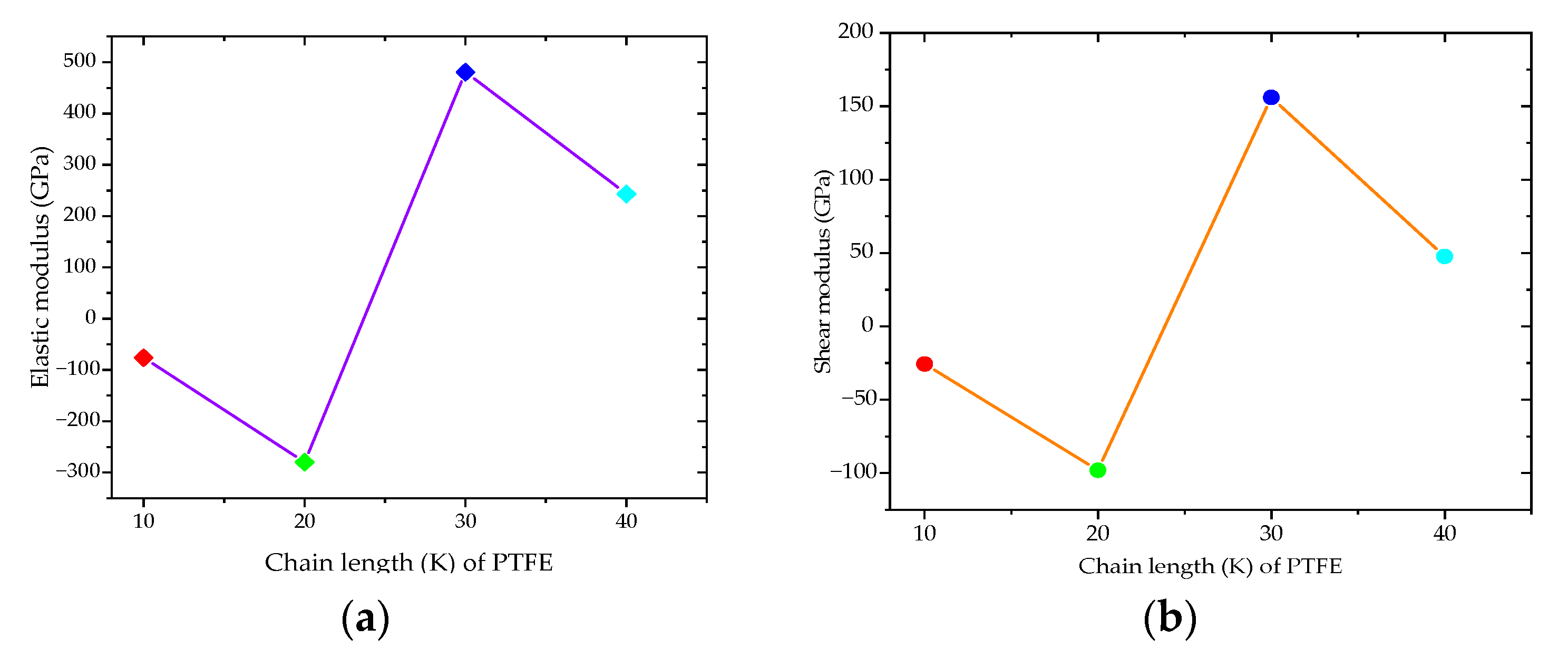
| Elastic Modulus, E (GPa) | −76.52 | −279.89 | 481.04 | 243.28 |
| Shear Modulus, G (GPa) | −25.56 | −97.99 | 155.96 | 47.66 |
| Bulk Modulus, B (GPa) | −3709.54 | −649.04 | −1902.78 | −38.54 |
| Poisson’s Ratio, v (GPa) | 0.49 | 0.42 | 0.54 | 1.55 |
| Chain Length (K) | CED (J/m3) × 109 | Solubility Parameter (J/cm3)0.5 |
|---|---|---|
| 10 | 6.885 ± 0.00076 | 82.974 ± 0.005 |
| 20 | 4.370 ± 0.00104 | 66.110 ± 0.008 |
| 30 | 2.691 ± 0.00077 | 51.876 ± 0.007 |
| 40 | 0.9 ± 0.00078 | 30.000 ± 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlela, M.K.; Xu, H.; Wang, H. Molecular Dynamics Study of Anti-Wear Erosion and Corrosion Protection of PTFE/Al2O3 (010) Coating Composite in Water Hydraulic Valves. Coatings 2020, 10, 1214. https://doi.org/10.3390/coatings10121214
Mlela MK, Xu H, Wang H. Molecular Dynamics Study of Anti-Wear Erosion and Corrosion Protection of PTFE/Al2O3 (010) Coating Composite in Water Hydraulic Valves. Coatings. 2020; 10(12):1214. https://doi.org/10.3390/coatings10121214
Chicago/Turabian StyleMlela, Masoud Kamoleka, He Xu, and Haihang Wang. 2020. "Molecular Dynamics Study of Anti-Wear Erosion and Corrosion Protection of PTFE/Al2O3 (010) Coating Composite in Water Hydraulic Valves" Coatings 10, no. 12: 1214. https://doi.org/10.3390/coatings10121214





