Social Behaviour of Horses in Response to Vocalisations of Predators
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Note
2.2. Animals
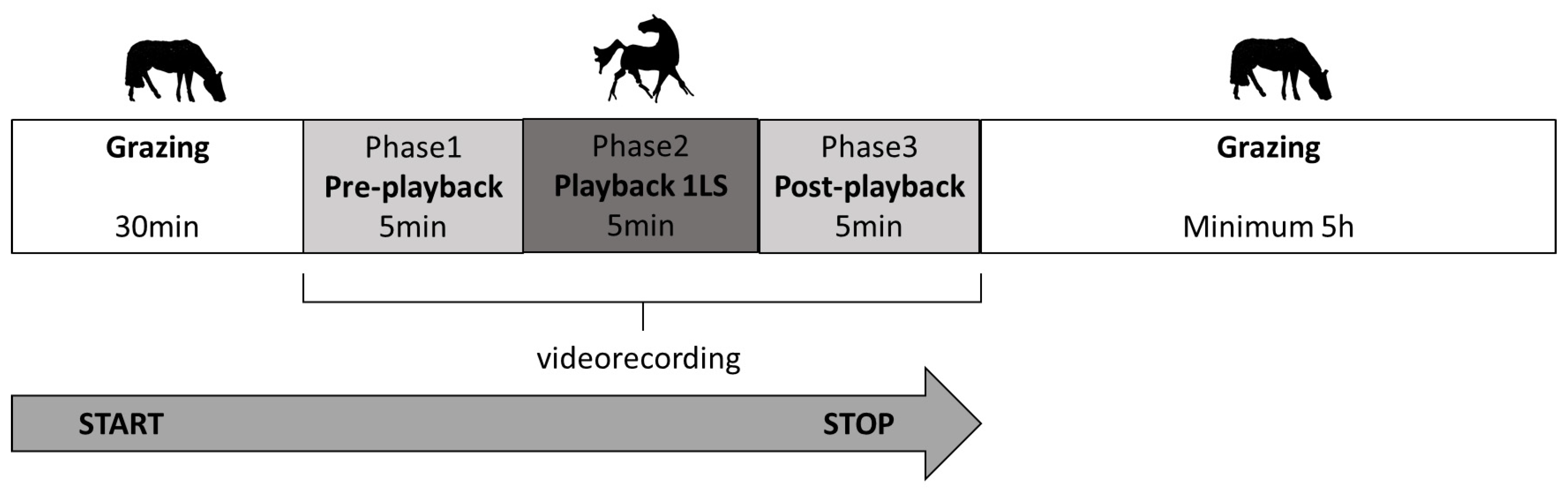
2.3. Experiments
2.4. Behavioural Variables
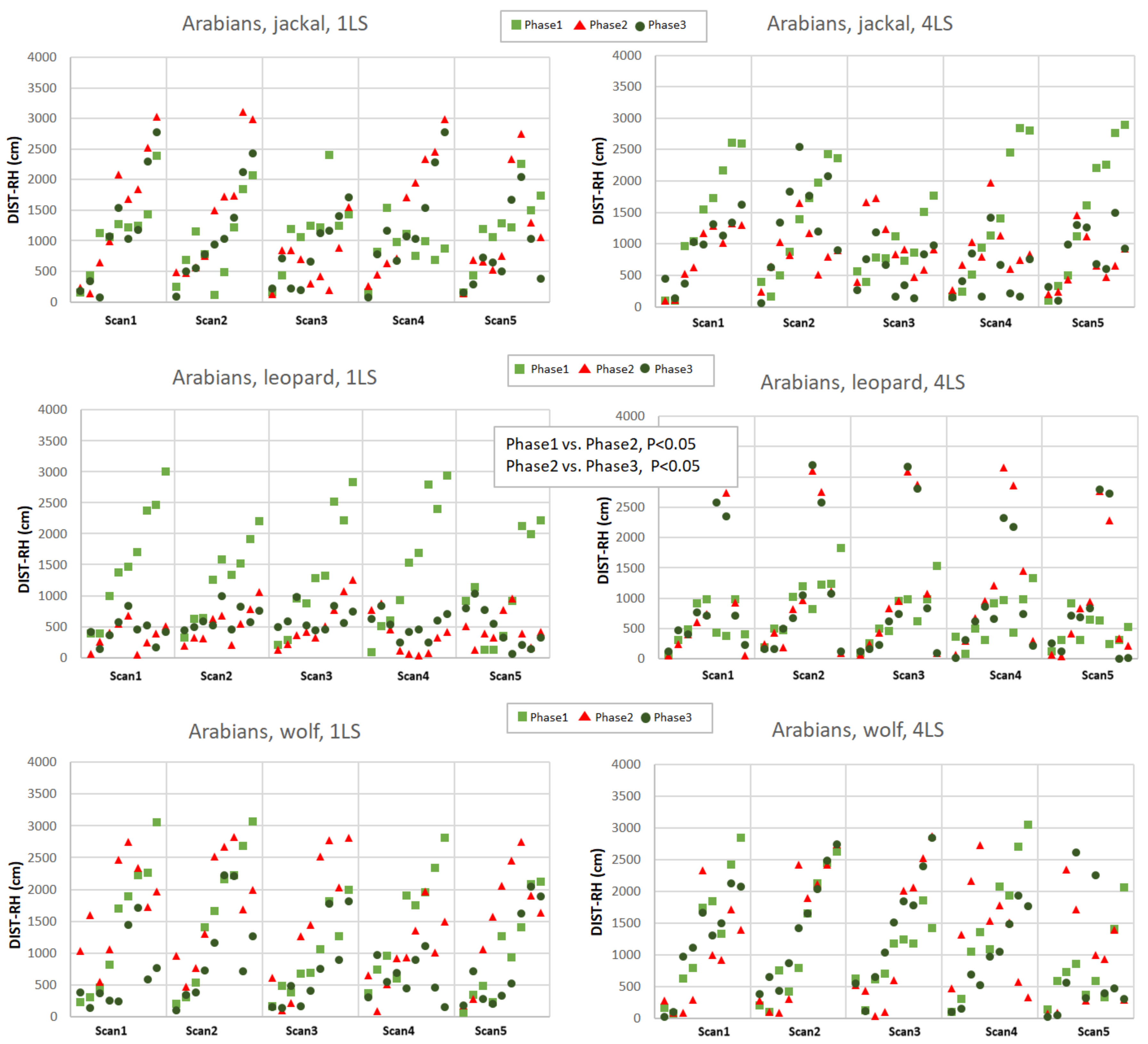
2.5. Distance from the Reference Horse and the LS
2.6. Statistical Analyses
3. Results
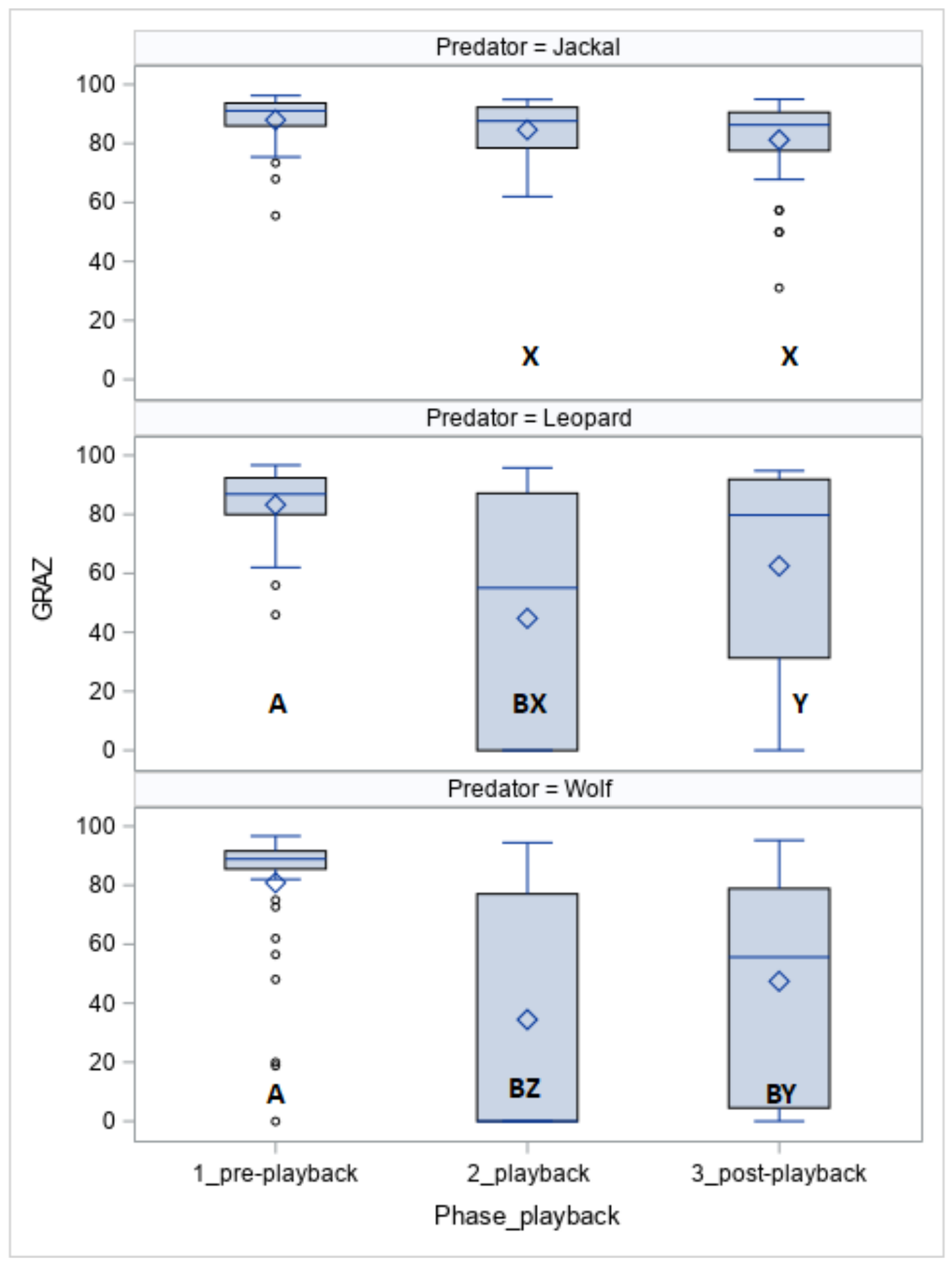
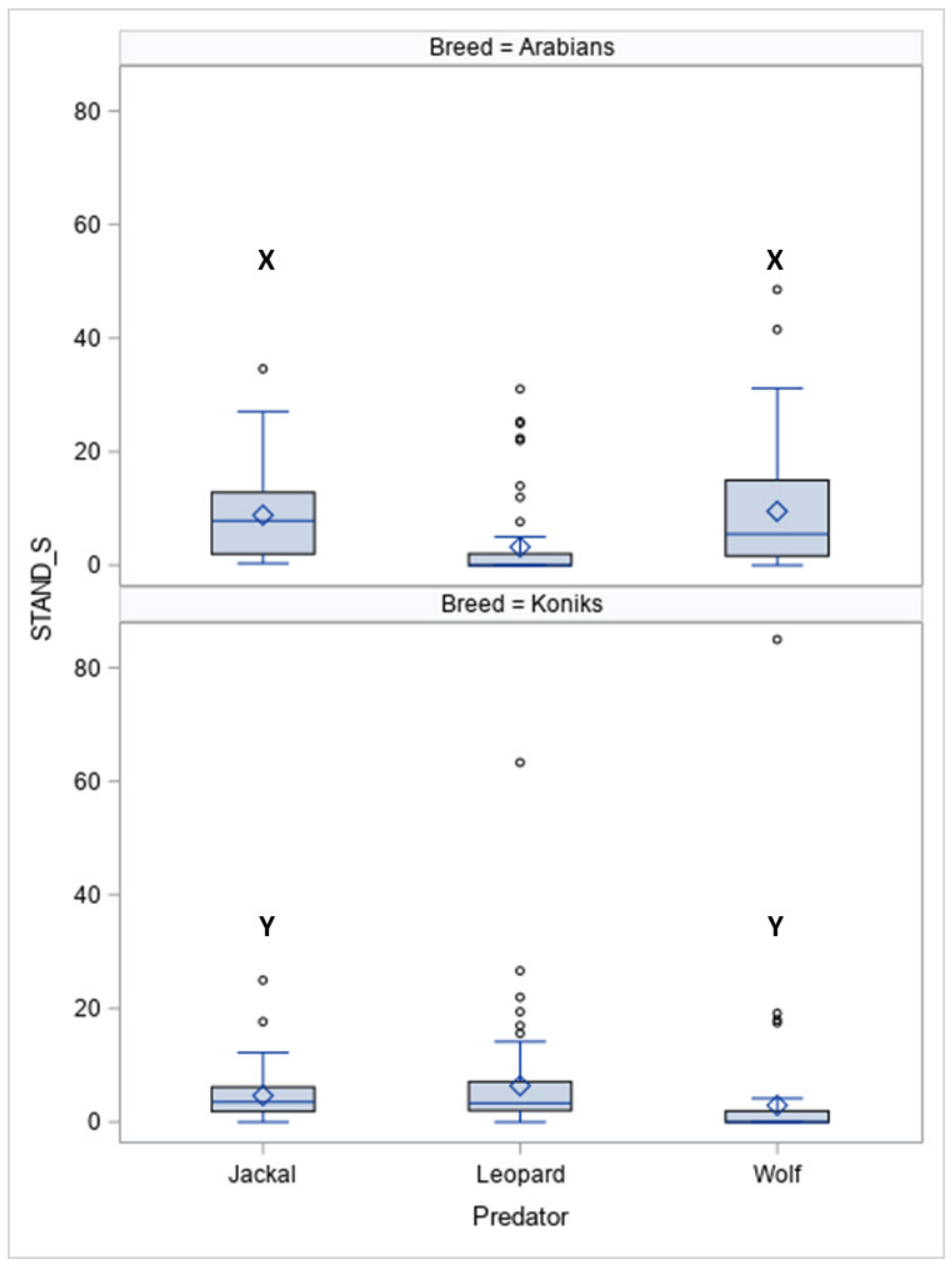
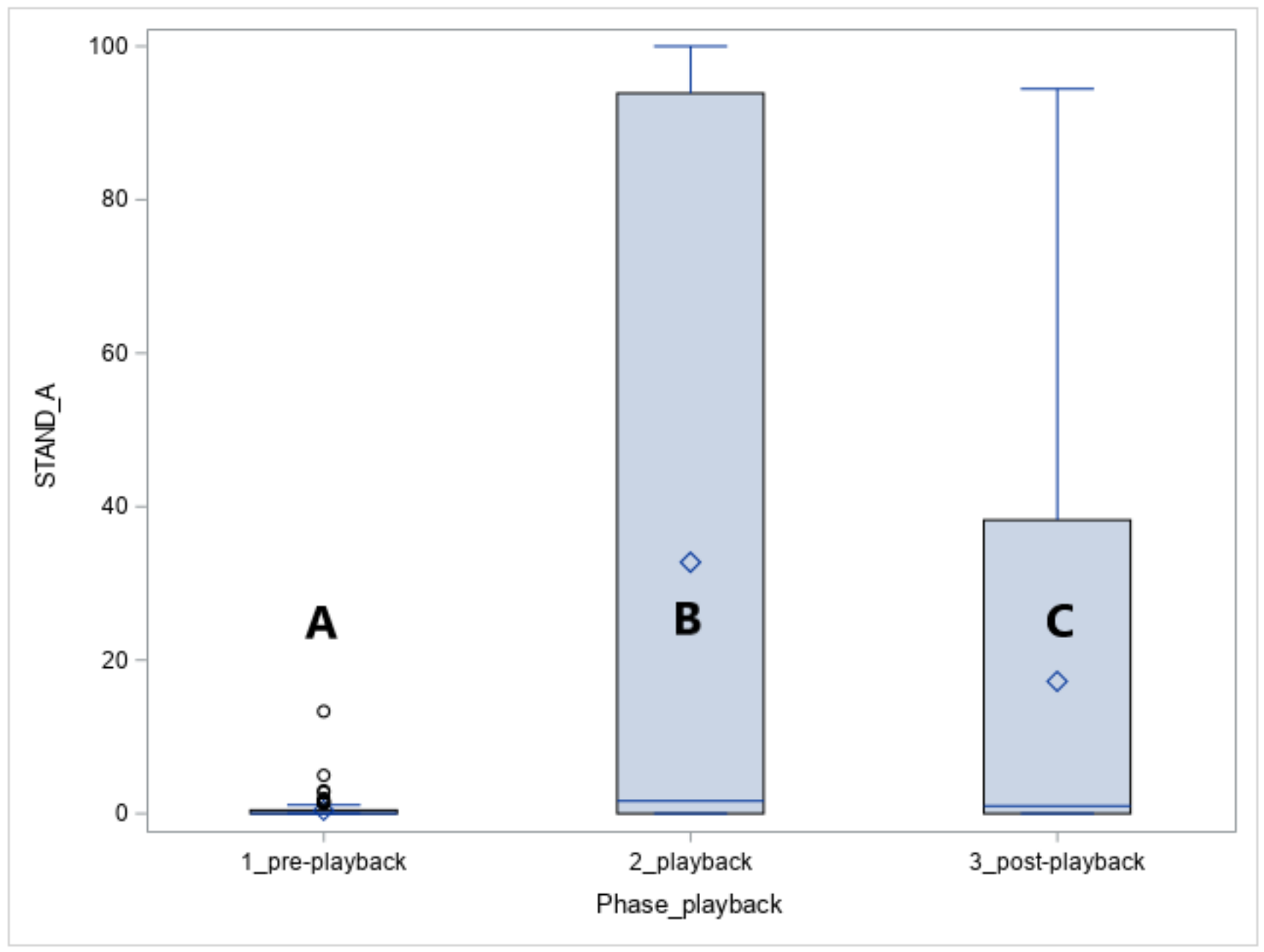
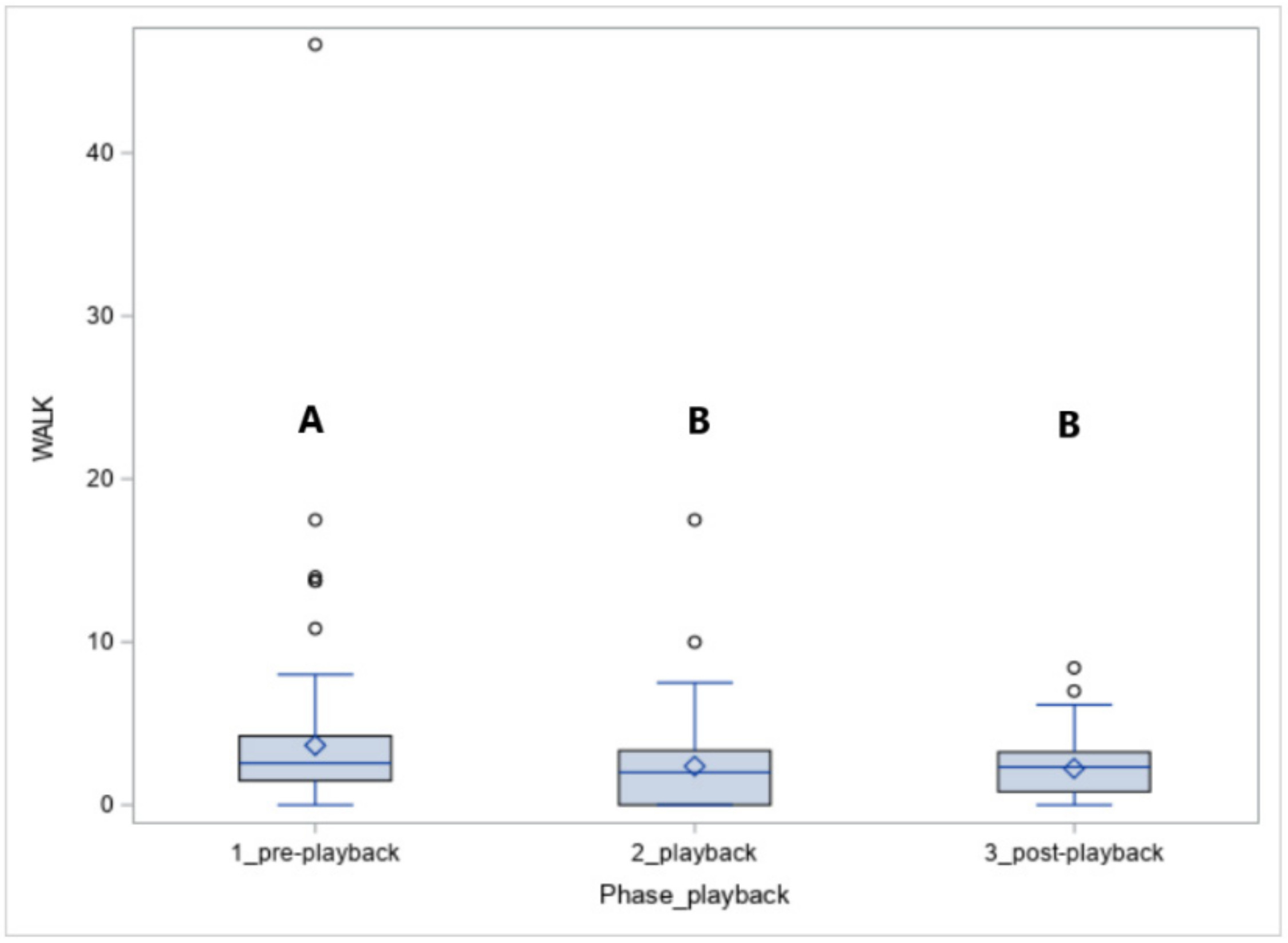
3.1. Behavioural Responses
3.2. Distance to Reference Horse and Loudspeaker(s)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

Appendix C

Appendix D

References
- Linnell, J.D.C.; Swenson, J.E.; Andersen, R. Predators and people: Conservation of large carnivores is possible at high human densities if management policy is favourable. Anim. Conserv. 2001, 4, 345–349. [Google Scholar] [CrossRef]
- Ruid, D.B.; Paul, W.J.; Roell, B.J.; Wydeven, A.P.; Wiliging, R.C.; Jurewicz, R.L.; Lonsway, D.H. Wolf—Human Conflicts and Management in Minnesota, Wisconsin, and Michigan. In Recovery of Gray Wolves in the Great Lakes Region of the United States; Heske, E., Deelen, T.R., Wydeven, A.P., Eds.; Springer: Madison, WI, USA, 2009; pp. 279–295. [Google Scholar] [CrossRef]
- Magrini, C. First data on canids depredation on livestock in an area of recent recolonization by wolf in Central Italy: Considerations on conflict survey and prevention methods. Ekol. Bratislava 2014, 33, 81–92. [Google Scholar] [CrossRef]
- Chetri, M.; Odden, M.; Devineau, O.; Wegge, P. Patterns of livestock depredation by snow leopards and other large carnivores in the Central Himalayas, Nepal. Glob. Ecol. Conserv. 2019, 17, e00536. [Google Scholar] [CrossRef]
- Linnell, J.D.C.; Nilsen, E.B.; Lande, U.S.; Herfindal, I.; Odden, J.; Skogen, K.; Andersen, R.; Breitenmoser, U. Zoning as a means of mitigating conflicts with large carnivores: Principles and reality. In People and Wildlife: Conflict or Coexistence? Woodroffe, R., Thirgood, S., Rabinowitz, A., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 162–175. [Google Scholar]
- Linnell, J.D.C.; Cretois, B. Research for AGRI Committee—The Revival of Wolves and Other Large Predators and Its Impact on Farmers and Their Livelihood in Rural Regions of Europe, European Parliament, Policy Department for Structural and Cohesion Policies, Brussels. 2018. Available online: https://www.europarl.europa.eu/cmsdata/191585/IPOL_STU(2018)617488_EN%20AGRI-original.pdf (accessed on 10 November 2020).
- Zimmermann, B.; Wabakken, P.; Dötterer, M. Human-carnivore interactions in Norway: How does the re-appearance of large carnivores affect people’s attitudes and levels of fear? For. Snow Landsc. Res. 2001, 76, 137–153. [Google Scholar]
- Røskaft, E.; Bjerke, T.; Kaltenborn, B.; Linnell, J.D.C.; Andersen, R. Patterns of self-reported fear towards large carnivores among the Norwegian public. Evol. Hum. Behav. 2003, 24, 184–198. [Google Scholar] [CrossRef]
- Linnell, J.D.C.; Alleau, J. Predators That Kill Humans: Myth, Reality, Context and the Politics of Wolf Attacks on People, In Problematic Wildlife; Angelici, F.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 357–371. [Google Scholar] [CrossRef]
- Woodroffe, R. Predators and people: Using human densities to interpret declines of large carnivores. Anim. Cons. 2000, 3, 165–173. [Google Scholar] [CrossRef]
- Spalton, J.A.; al Hikmani, H.M.; Willis, D.; Said, A.S.B. Critically Endangered Arabian leopards Panthera pardus nimr persist in the Jabal Samhan Nature Reserve, Oman. Oryx 2006, 40, 287–294. [Google Scholar] [CrossRef] [Green Version]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/118264161/163507876 (accessed on 10 November 2020).
- Lanszki, J.; Heltai, M.; Szabó, L. Feeding habits and trophic niche overlap between sympatric golden jackal (Canis aureus) and red fox (Vulpes vulpes) in the Pannonian ecoregion (Hungary). Can. J. Zool. 2006, 84, 1647–1656. [Google Scholar] [CrossRef]
- Arnold, J.; Humer, A.; Heltai, M.; Murariu, D.; Spassov, N.; Hackländer, K. Current status and distribution of golden jackals Canis aureus in Europe. Mammal Rev. 2012, 42, 1–11. [Google Scholar] [CrossRef]
- Bern Convention. Convention on the Conservation of European Wildlife and Natural Habitats. Available online: https://www.coe.int/en/web/bern-convention (accessed on 10 November 2020).
- Fabbri, E.; Velli, E.; D’Amico, F.; Galaverni, M.; Mastrogiuseppe, L.; Mattucci, F.; Caniglia, R. From predation to management: Monitoring wolf distribution and understanding depredation patterns from attacks on livestock. Hystrix 2018, 29, 101–110. [Google Scholar] [CrossRef]
- Pettorelli, N.; Hilborn, A.; Duncan, C.; Durant, S.M. Individual Variability: The Missing Component to Our Understanding of Predator–Prey Interactions. Adv. Ecol. Res. 2015, 52, 19–44. [Google Scholar] [CrossRef]
- Réale, D.; Festa-Bianchet, M. Predator-induced natural selection on temperament in bighorn ewes. Anim. Behav. 2003, 65, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Aanes, R.; Andersen, R. The effects of sex, time of birth, and habitat on the vulnerability of roe deer fawns to red fox predation. Can. J. Zool. 1996, 74, 1857–1865. [Google Scholar] [CrossRef]
- Laporte, I.; Muhly, T.B.; Pitt, J.A.; Alexander, M.; Musiani, M. Effects of Wolves on Elk and Cattle Behaviors: Implications for Livestock Production and Wolf Conservation. PLoS ONE 2010, 5, e11954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flörcke, C.; Grandin, T. Loss of anti-predator behaviors in cattle and the increased predation losses by wolves in the Northern Rocky Mountains. Open J. Anim. Sci. 2013, 3, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Hansen, I.; Christiansen, F.; Hansen, H.S.; Braastad, B.; Bakken, M. Variation in behavioural responses of ewes towards predator-related stimuli. Appl. Anim. Behav. Sci. 2001, 70, 227–237. [Google Scholar] [CrossRef]
- Dwyer, C.M. How has the risk of predation shaped the behavioural responses of sheep to fear and distress? Anim. Welfare 2004, 3, 269–281. [Google Scholar]
- Christensen, J.W.; Rundgren, M. Predator odour per se does not frighten domestic horses. Appl. Anim. Behav. Sci. 2008, 112, 136–145. [Google Scholar] [CrossRef]
- Ahmadinejad, M.; Hasani, A.; Kharazian, F. The responses of horses to predator stimuli. Int. J. Vet. Res. 2010, 4, 5–9. [Google Scholar]
- Shehzad, W.; Nawaz, M.A.; Pompanon, F.; Coissac, E.; Riaz, T.; Shah, S.S.; Taberlet, P. Forest without prey: Livestock sustain a leopard Panthera pardus population in Pakistan. Oryx 2014, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 2002, 418, 700–707. [Google Scholar] [CrossRef] [PubMed]
- König von Borstel, U. Assessing and influencing personality for improvement of animal welfare: A review of equine studies. CAB Rev. 2013, 8, 6. [Google Scholar] [CrossRef]
- Lansade, L.; Bouissou, M.-F.; Erhard, H.W. Fearfulness in horses: A temperament trait stable across time and situations. Appl. Anim. Behav. Sci. 2008, 115, 182–200. [Google Scholar] [CrossRef]
- Górecka-Bruzda, A.; Jastrzębska, E.; Sosnowska, Z.; Jaworski, Z.; Jezierski, T.; Chruszczewski, M. Reactivity to humans and fearfulness tests: Field validation in Polish Cold Blood Horses. Appl. Anim. Behav. Sci. 2011, 133, 207–215. [Google Scholar] [CrossRef]
- Gosling, S.D.; Bonnenburg, A.V. An integrative approach to personality research in anthrozoology: Ratings of six species of pets and their owners. Antrozoös 1998, 11, 148–156. [Google Scholar] [CrossRef]
- Roberts, M. The Man Who Listens to Horses; Hutchinson: London, UK, 1996; pp. 1–225. [Google Scholar]
- McGreevy, P.D.; Oddie, C.; Burton, F.L.; McLean, A.N. The horse–human dyad: Can we align horse training and handling activities with the equid social ethogram? Vet. J. 2009, 181, 12–18. [Google Scholar] [CrossRef]
- Janczarek, I.; Stachurska, A.; Kędzierski, W.; Wnuk–Pawlak, E.; Wilk, I.; Zyglewska, K.; Paszkowska, A.; Ryżak, M.; Wiśniewska, A. Heart rate variability in Polish Konik and Purebred Arabian horses in response to different predator vocalisations. Animal 2020. accepted. [Google Scholar]
- Janczarek, I.; Stachurska, A.; Kędzierski, W.; Wiśniewska, A.; Ryżak, M.; Kozioł, A. The intensity of physiological and behavioral responses of horses to predator vocalizations. BMC Vet Res. 2020, 16, 431. [Google Scholar] [CrossRef]
- Geist, V. On the Relationship of Social Evolution and Ecology in Ungulates. Am. Zool. 1974, 14, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Nowak, S.; Jędrzejewski, W.; Schmidt, K.; Theuerkauf, J.; Mysłajek, R.W.; Jędrzejewska, B. Howling activity of free-ranging wolves (Canis lupus) in the Białowieża Primeval Forest and the Western Beskidy Mountains (Poland). J. Ethol. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Bailey, I.; Myatt, J.P.; Wilson, A.M. Group hunting within the Carnivora: Physiological, cognitive and environmental influences on strategy and cooperation. Behav. Ecol. Sociobiol. 2013, 67, 1–17. [Google Scholar] [CrossRef]
- Visser, E.K.; van Reenen, C.G.; Hopster, H.; Schilder, M.H.B.; Knaap, J.H.; Barneveld, A.; Blokhuis, H.J. Quantifying aspects of young horses’ temperament: Consistency of behavioural variables. Appl. Anim. Behav. Sci. 2001, 74, 241–258. [Google Scholar] [CrossRef]
- Lescureux, N. Towards the necessity of a new interactive approach integrating ethnology, ecology and ethology in the study of the relationship between Kyrgyz stockbreeders and wolves. Soc. Sci. Inf. 2006, 45, 463–478. [Google Scholar] [CrossRef]
- Ersmark, E.; Klütsch, C.F.C.; Chan, Y.L.; Sinding, M.-H.S.; Fain, S.R.; Illarionova, N.A.; Oskarsson, M.; Uhlén, M.; Zhang, Y.-P.; Dalén, L.; et al. From the Past to the Present: Wolf Phylogeography and Demographic History Based on the Mitochondrial Control Region. Front. Ecol. Evol. 2016, 4, 134. [Google Scholar] [CrossRef] [Green Version]
- Kluever, B.M.; Howery, L.D.; Breck, S.W.; Bergman, D.L. Predator and heterospecific stimuli alter behaviour in cattle. Behav. Proc. 2009, 81, 85–91. [Google Scholar] [CrossRef]
- Pfefferle, D.; Fischer, J. Sounds and size: Identification of acoustic variables that reflect body size in hamadryas baboons, Papio hamadryas. Anim. Behav. 2006, 72, 43–51. [Google Scholar] [CrossRef]
- Vannoni, E.; McElligott, A.G. Low Frequency Groans Indicate Larger and More Dominant Fallow Deer (Dama dama) Males. PLoS ONE 2008, 3, e3113. [Google Scholar] [CrossRef] [Green Version]
- Sanvito, S.; Galimberti, F.; Miller, E.H. Vocal signalling of male southern elephant seals is honest but imprecise. Anim. Behav. 2007, 73, 287–299. [Google Scholar] [CrossRef]
- Pfefferle, D.; West, P.M.; Grinnell, J.; Packer, C.; Fischer, J. Do acoustic features of lion, Panthera leo, roars reflect sex and male condition? J. Acoust. Soc. Am. 2007, 121, 3947–3953. [Google Scholar] [CrossRef]
- Lemasson, A.; Boutin, A.; Boivin, S.; Blois-Heulin, C.; Hausberger, M. Horse (Equus caballus) whinnies: A source of social information. Anim. Cogn. 2009, 12, 693–704. [Google Scholar] [CrossRef]
- Carbyn, L.N.; Trottier, T. Responses of bison on their calving grounds to predation by wolves in Wood Buffalo National Park. Can. J. Zool. 1987, 65, 2072–2078. [Google Scholar] [CrossRef]
- Lloyd, A.S.; Martin, J.E.; Bornet-Gauci, H.L.I.; Wilkinson, R.G. Horse personality: Variation between breeds. Appl. Anim. Behav. Sci. 2008, 112, 369–383. [Google Scholar] [CrossRef]
- Andersson, M.; Ferguson, M.A.D. Movements and habitat use of muskoxen (Ovibos moschatus) on Bathurst, Cornwallis, and Devon islands, 2003–2006. In Status Report 2016-08; Nunavut Department of Environment, Wildlife Research Section: Igloolik, NU, Canada, 2016. Available online: https://gov.nu.ca/sites/default/files/movement_and_habitat_use_of_muskoxen_on_bathurst_cornwallis_and_devon_islands_2003-2006.pdf (accessed on 10 November 2020).
- Lingle, S.; Pellis, S.M. Fight or flight? Antipredator behavior and the escalation of coyote encounters with deer. Oecologia 2002, 131, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Creel, S.; Winnie, J.A. Responses of elk herd size to fine-scale spatial and temporal variation in the risk of predation by wolves. Anim. Behav. 2005, 69, 1181–1189. [Google Scholar] [CrossRef]
- Duranton, C.; Gaunet, F. Behavioural synchronization from an ethological perspective: Overview of its adaptive value. Adapt. Behav. 2016, 24, 181–191. [Google Scholar] [CrossRef]
- Deneubourg, J.L.; Goss, S. Collective patterns and decision making. Ethol. Ecol. Evol. 1989, 1, 295–311. [Google Scholar] [CrossRef]
- Jarman, P.J.; Jarman, M.V. The dynamics of ungulate social organization. In Serengeti: Dynamics of an Ecosystem; Sinclaire, A.R.E., Norton-Griffiths, M., Eds.; University of Chicago Press: Chicago, IL, USA, 1979; pp. 185–220. [Google Scholar]
- Górecka-Bruzda, A.; Jaworski, Z.; Jaworska, J.; Siemieniuch, M. Welfare of Free-Roaming Horses: 70 Years of Experience with Konik Polski Breeding in Poland. Animals 2020, 10, 1094. [Google Scholar] [CrossRef]
- Carbyn, L.N.; Trottier, T. Descriptions of Wolf Attacks on Bison Calves in Wood Buffalo National Park. Arctic 1988, 41, 297–302. [Google Scholar] [CrossRef]
- Pimenta, V.; Barroso, I.; Boitani, L.; Beja, P. Wolf predation on cattle in Portugal: Assessing the effects of husbandry systems. Biol. Cons. 2017, 207, 17–26. [Google Scholar] [CrossRef]

| Behaviour (Abbreviation) | Definition (Measure) |
|---|---|
| Grazing (GRAZ) | Feeding with grass; duration (minutes) |
| Standing still (STAND-S) | Standing motionless, including resting position; duration (minutes) |
| Standing alert (STAND-A) | Standing with high head position, looking around, ears either fixed in the forward position or frequently changing position; duration (minutes) |
| Walking (WALK) | Slow four-beat gait; the number of steps |
| Trotting, cantering (TROTCANT) | Medium-speed two-beat gait; high-speed three-beat gait; the number of steps in both gaits |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janczarek, I.; Wiśniewska, A.; Chruszczewski, M.H.; Tkaczyk, E.; Górecka-Bruzda, A. Social Behaviour of Horses in Response to Vocalisations of Predators. Animals 2020, 10, 2331. https://doi.org/10.3390/ani10122331
Janczarek I, Wiśniewska A, Chruszczewski MH, Tkaczyk E, Górecka-Bruzda A. Social Behaviour of Horses in Response to Vocalisations of Predators. Animals. 2020; 10(12):2331. https://doi.org/10.3390/ani10122331
Chicago/Turabian StyleJanczarek, Iwona, Anna Wiśniewska, Michael H. Chruszczewski, Ewelina Tkaczyk, and Aleksandra Górecka-Bruzda. 2020. "Social Behaviour of Horses in Response to Vocalisations of Predators" Animals 10, no. 12: 2331. https://doi.org/10.3390/ani10122331





