Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities
Abstract
1. Introduction
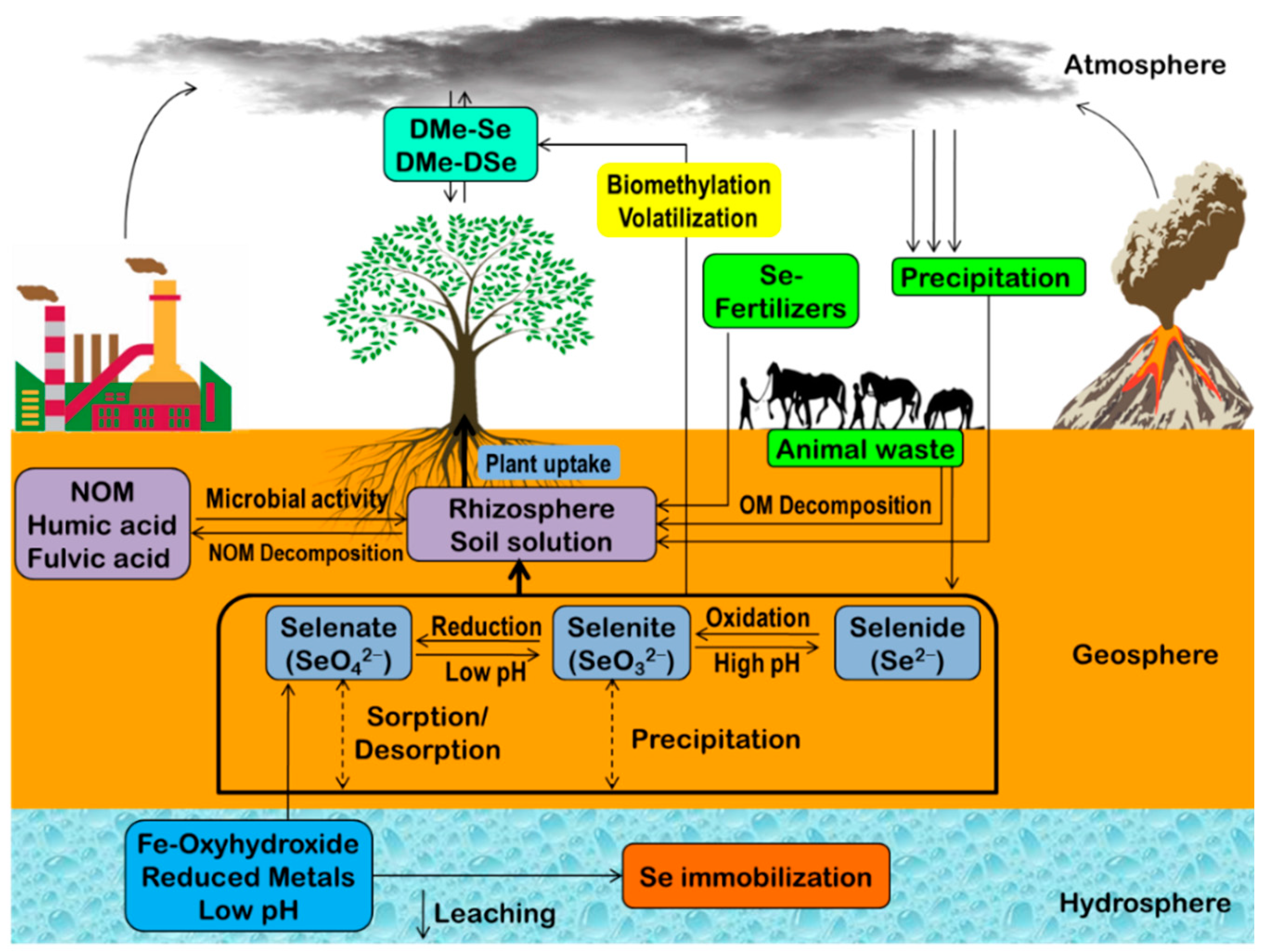
2. Selenium Biogeochemistry
2.1. Chemical Mechanisms Regulating Se Biogeochemistry
2.2. Physical Mechanisms Regulating Se Biogeochemistry
2.3. Biological Mechanisms Regulating Se Biogeochemistry
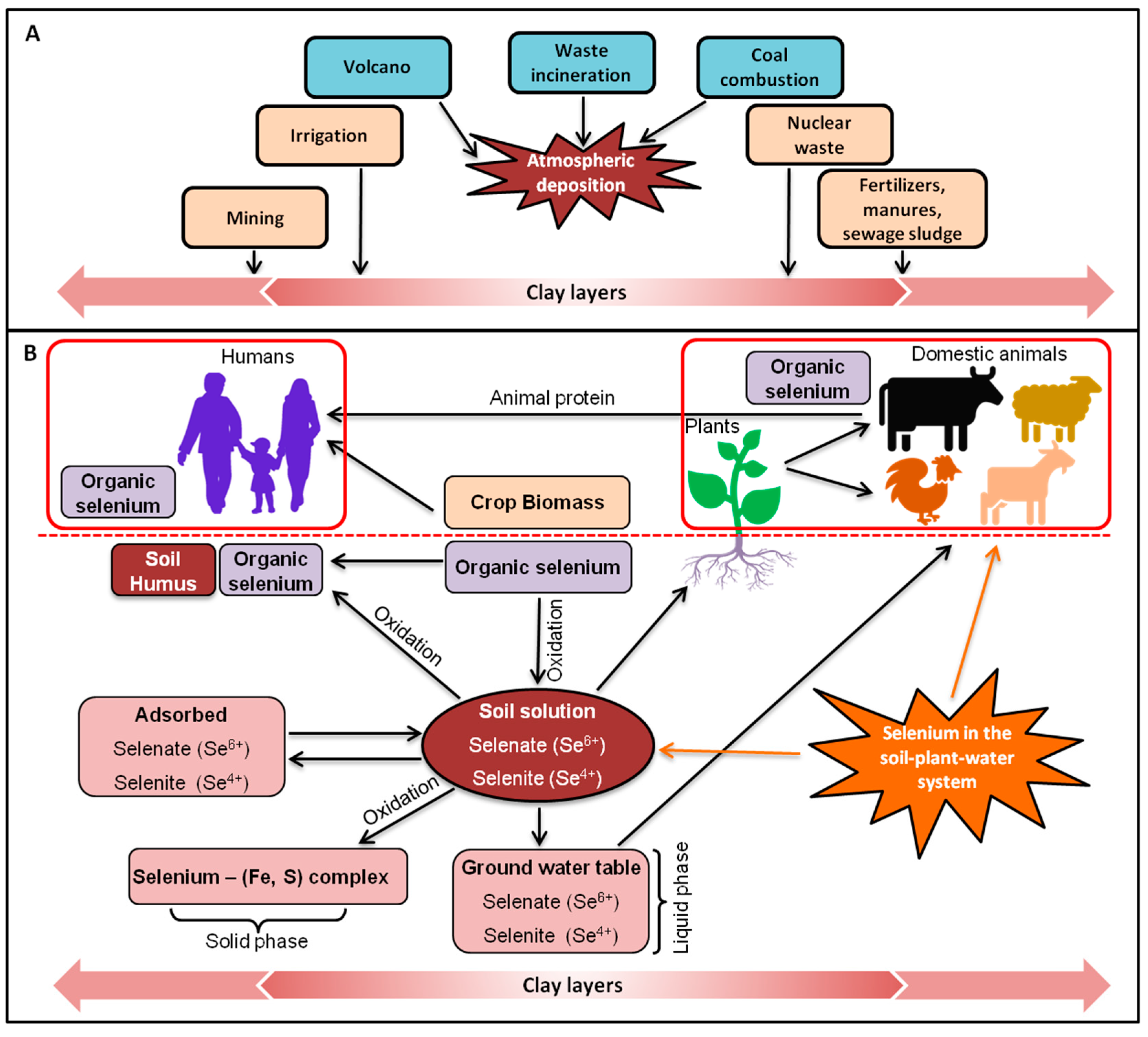
3. Selenium in the Environment
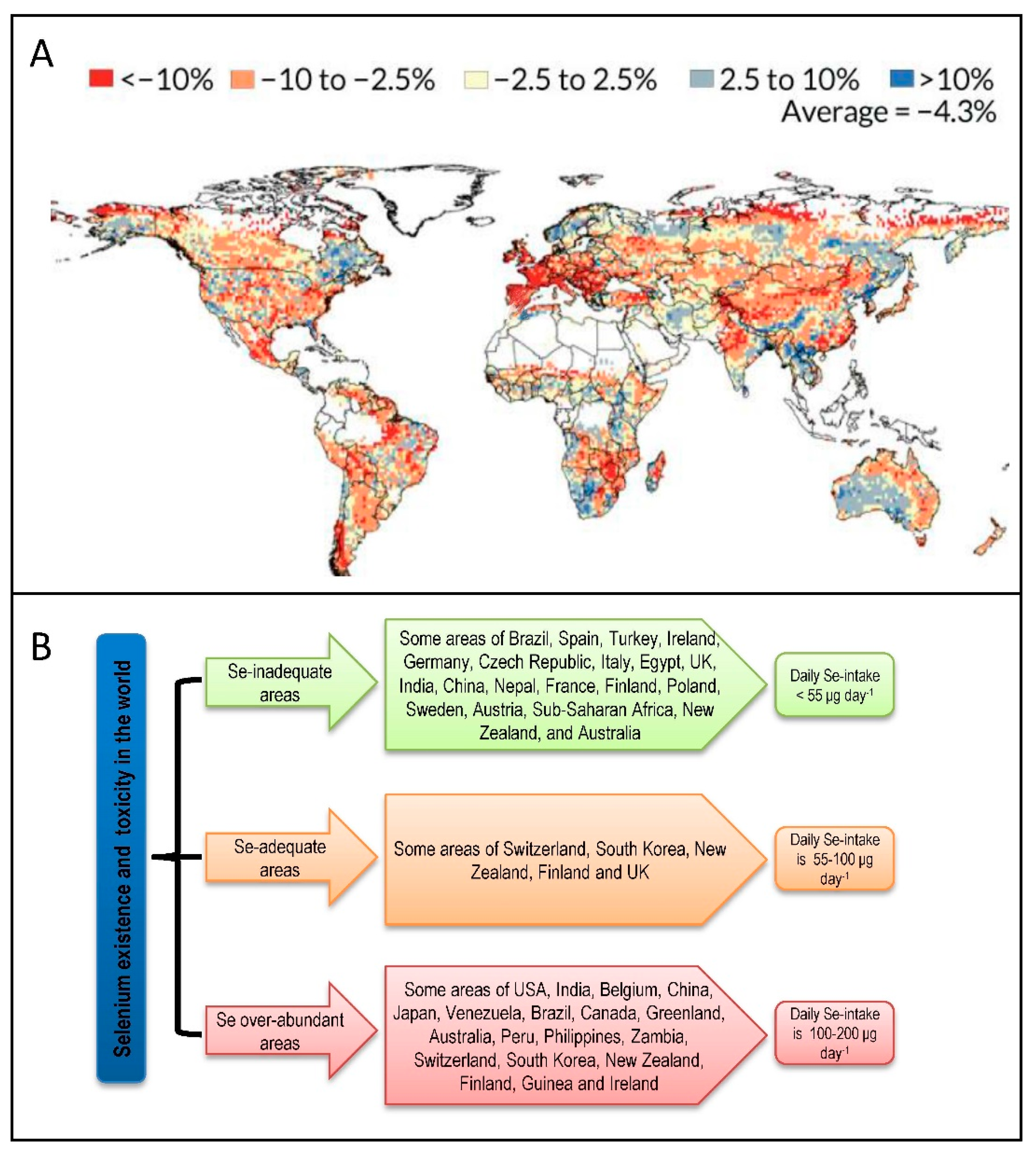
4. Selenium Abundance: A Global Distribution
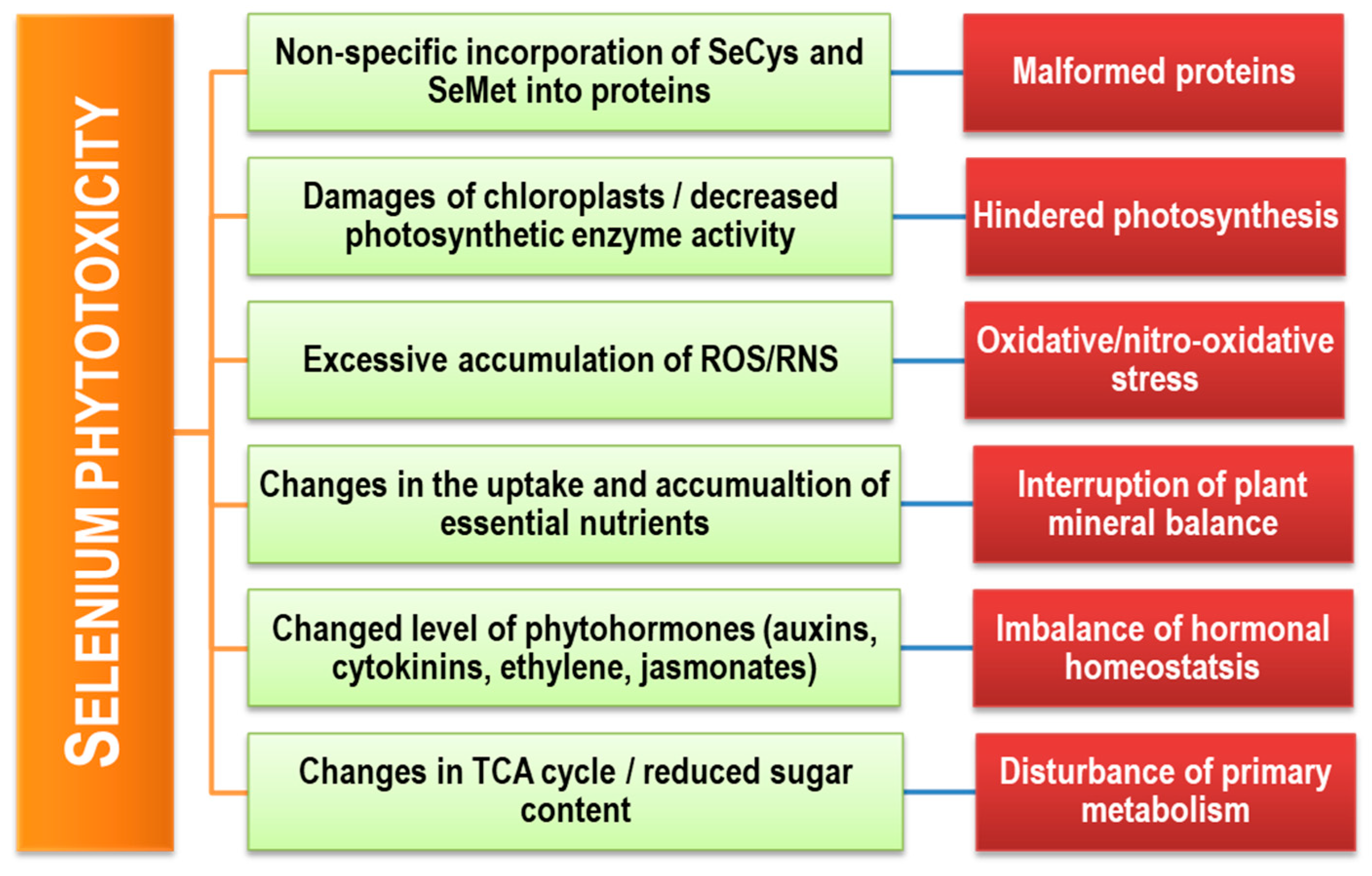
5. Selenium Toxicity in Plants
5.1. Toxic Effects on Plant Growth and Development
5.2. Toxic Effects on Physiological Processes
5.3. Selenium-Induced Oxidative Stress in Plants
6. Phytoremediation of Selenium-Contaminated Environments
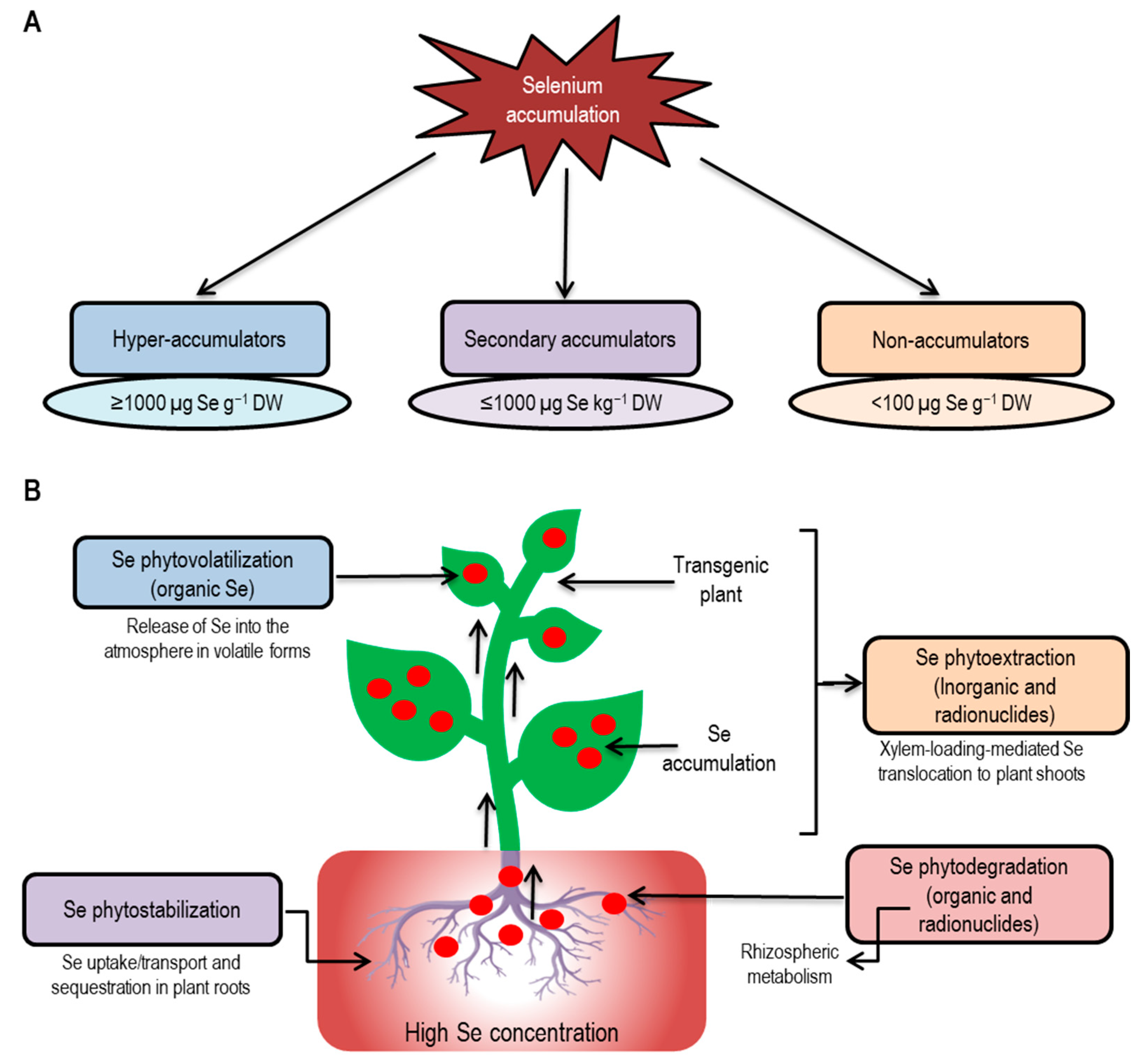
6.1. Selenium Hyperaccumulation
6.2. Phytoextraction
6.3. Phytovolatilization
6.4. Rhizofiltration
6.5. Genetic Engineering for Se Phytoremediation
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyd, R. Selenium stories. Nat. Chem. 2011, 3, 570. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, M.; Konieczka, P.; Namiesnik, J. The properties, functions, and use of selenium compounds in living organisms. J. Environ. Sci. Health Part C 2012, 30, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Floor, G.H.; Román-Ross, G. Selenium in volcanic environments: A review. Appl. Geochem. 2012, 27, 517–531. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, M.; Cui, Z.; Huang, J.; Chen, C.; Guo, L.; Liang, D. Assessment of bioavailability of selenium in different plant-soil systems by diffusive gradients in thin-films (DGT). Environ. Pollut. 2017, 225, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.; Dhankher, O.P.; Tripathi, R.D. Understanding selenium metabolism in plants and its role as a beneficial element. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Sobolev, O.I.; Gutyj, B.V.; Sobolieva, S.V.; Borshch, O.O.; Nedashkivsky, V.M.; Kachan, L.M.; Karkach, P.M.; Nedashkivska, N.V.; Poroshinska, O.A.; Stovbetska, L.S.; et al. Selenium in natural environment and food chains. A Review. Ukrainian J. Ecol. 2020, 4, 148–158. [Google Scholar]
- Trippe, R.C., 3rd; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2020, 404, 124178. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, K.; Esperanza, M.G.; Barrientos, E.Y.; Escobosa, A.R.C.; Wrobel, K. Different approaches in metabolomic analysis of plants exposed to selenium: A comprehensive review. Acta Physiol. Plant. 2020, 42, 1–20. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2020, in press. [Google Scholar] [CrossRef]
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Reeves, M.; Hoffmann, P. The human selenoproteome: Recent insights into functions and regulation. Cell. Mol. Life Sci. 2009, 66, 2457–2478. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Pilon-Smits, E.A.; Zhao, F.-J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Etteieb, S.; Magdouli, S.; Zolfaghari, M.; Brar, S. Monitoring and analysis of selenium as an emerging contaminant in mining industry: A critical review. Sci. Total Environ. 2020, 698, 134339. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. 2009. Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalHealth2009:Risks_report_annex.pdf (accessed on 5 March 2014).
- Malagoli, M.; Schiavon, M.; Pilon-Smits, E.A. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Winkel, L.H.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A. Selenium biofortification and phytoremediation phytotechnologies: A review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef]
- Ye, Y.; Qu, J.; Pu, Y.; Rao, S.; Xu, F.; Wu, C. Selenium Biofortification of Crop Food by Beneficial Microorganisms. J. Fungi 2020, 6, 59. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rehman, M.Z.; Rinklebe, J.; Tsang, D.C.W.; Tack, F.M.G.; Abbasi, G.H.; Hussain, A.; Igalavithana, A.D.; Lee, B.C.; et al. Effects of selenium on the uptake of toxic trace elements by crop plants: A review. Crit. Rev. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y.; Yu, Y.; Liu, H.; Rensing, C.; Wu, Z.; et al. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2020, 402, 123570. [Google Scholar] [CrossRef] [PubMed]
- Mroczek-Zdyrska, M.; Strubińska, J.; Hanaka, A. Selenium improves physiological parameters and alleviates oxidative stress in shoots of lead-exposed Vicia faba L. minor plants grown under phosphorus-deficient conditions. J. Plant Growth Regul. 2017, 36, 186–199. [Google Scholar] [CrossRef]
- Molnár, Á.; Kolbert, Z.; Kéri, K.; Feigl, G.; Ördög, A.; Szőllősi, R.; Erdei, L. Selenite-induced nitro-oxidative stress processes in Arabidopsis thaliana and Brassica juncea. Ecotoxicol. Environ. Saf. 2018, 148, 664–674. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Selenium in plant foods: Speciation analysis, bioavailability, and factors affecting composition. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press/Taylor & Francis Group ICC: Boca Raton, FL, USA, 2010. [Google Scholar]
- Hawrylak-Nowak, B. Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol. Trace Elem. Res. 2009, 132, 259–269. [Google Scholar] [CrossRef]
- Lehotai, N.; Lyubenova, L.; Schröder, P.; Feigl, G.; Ördög, A.; Szilágyi, K.; Erdei, L.; Kolbert, Z. Nitro-oxidative stress contributes to selenite toxicity in pea (Pisum sativum L). Plant Soil 2016, 400, 107–122. [Google Scholar] [CrossRef]
- Jain, M.; Panwar, M.; Gadre, R. Influence of selenium supplementation on δ-aminolevulinic acid formation in greening maize leaf segments. Res. J. Phytochem. 2017, 11, 111–117. [Google Scholar] [CrossRef][Green Version]
- Ali, W.; Zhang, H.; Junaid, M.; Mao, K.; Xu, N.; Chang, C.; Rasool, A.; Aslam, M.W.; Ali, J.; Yang, Z. Insights into the mechanisms of arsenic-selenium interactions and the associated toxicity in plants, animals, and humans: A critical review. Crit. Rev. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Ponton, D.E.; Graves, S.D.; Fortin, C.; Janz, D.; Amyot, M.; Schiavon, M. Selenium interactions with algae: Chemical processes at biological uptake sites, bioaccumulation, and intracellular metabolism. Plants 2020, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Banuelos, G.; Ajwa, H.; Mackey, B.; Wu, L.; Cook, C.; Akohoue, S.; Zambruzuski, S. Evaluation of different plant species used for phytoremediation of high soil selenium. J. Environ. Qual. 1997, 26, 639–646. [Google Scholar] [CrossRef]
- Yasin, M.; El-Mehdawi, A.F.; Anwar, A.; Pilon-Smits, E.A.; Faisal, M. Microbial-enhanced selenium and iron biofortification of wheat (Triticum aestivum L.)-applications in phytoremediation and biofortification. Int. J. Phytoremed. 2015, 17, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Duckart, E.; Waldron, L.; Donner, H. Selenium uptake and volatilization from plants growing in soil. Soil Sci. 1992, 153, 94–99. [Google Scholar] [CrossRef]
- Salhani, N.; Boulyga, S.; Stengel, E. Phytoremediation of selenium by two helophyte species in subsurface flow constructed wetland. Chemosphere 2003, 50, 967–973. [Google Scholar]
- Lin, Z.Q.; De Souza, M.; Pickering, I.; Terry, N. Evaluation of the macroalga, muskgrass, for the phytoremediation of selenium-contaminated agricultural drainage water by microcosms. J. Environ. Qual. 2002, 31, 2104–2110. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Can, H.; Dogan, I. Phytoremediation using genetically engineered plants to remove metals: A review. Environ. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Charya, L.S. Selenium pollution in the marine environment and marine bacteria in selenium bioremediation. In Marine Pollution and Microbial Remediation; Naik, M., Dubey, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 223–237. [Google Scholar]
- Wang, Z.; Becker, H. Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature 2013, 499, 328–331. [Google Scholar] [CrossRef]
- Fernández-Martínez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Biotechnol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.; Pettine, M.; Zboril, R. Biogeochemistry of selenium. A review. Environ. Chem. Lett. 2015, 13, 49–58. [Google Scholar] [CrossRef]
- Torres, J.; Pintos, V.; Domínguez, S.; Kremer, C.; Kremer, E. Selenite and selenate speciation in natural waters: Interaction with divalent metal ions. J. Sol. Chem. 2010, 39, 1–10. [Google Scholar] [CrossRef]
- Stroud, J.L.; McGrath, S.P.; Zhao, F.-J. Selenium speciation in soil extracts using LC-ICP-MS. Int. J. Environ. Anal. Chem. 2012, 92, 222–236. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, Y.; Peng, D.; Huang, T.; Sun, C. pH-Dependent Leaching Characteristics of Major and Toxic Elements from Red Mud. Int. J. Environ. Res. Public Health 2019, 16, 2046. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.-H.; Bang, S.; Kim, K.-W.; Kim, M.G.; Park, S.Y.; Choi, W.-K. Selenate removal by zero-valent iron in oxic condition: The role of Fe (II) and selenate removal mechanism. Environ. Sci. Pollut. Res. 2016, 23, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Wallschläger, D.; Feldmann, J. Formation, occurrence, significance, and analysis of organoselenium and organotellurium compounds in the environment. Metal Ions Life Sci. 2010, 7, 319–364. [Google Scholar]
- Maurer, F.; Christl, I.; Kretzschmar, R. Reduction and reoxidation of humic acid: Influence on spectroscopic properties and proton binding. Environ. Sci. Technol. 2010, 44, 5787–5792. [Google Scholar] [CrossRef] [PubMed]
- Nakamaru, Y.M.; Altansuvd, J. Speciation and bioavailability of selenium and antimony in non-flooded and wetland soils: A review. Chemosphere 2014, 111, 366–371. [Google Scholar] [CrossRef]
- Gennari, F.; Sharma, V.K.; Pettine, M.; Campanella, L.; Millero, F.J. Reduction of selenite by cysteine in ionic media. Geochim. Cosmochim. Acta 2014, 124, 98–108. [Google Scholar] [CrossRef]
- Vriens, B.; Lenz, M.; Charlet, L.; Berg, M.; Winkel, L.H. Natural wetland emissions of methylated trace elements. Nat. Commun. 2014, 5, 3035. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Lens, P. Ecology and biotechnology of selenium-respiring bacteria. Microbiol. Mol. Biol. Rev. 2015, 79, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Mal, J.; Nancharaiah, Y.; Van Hullebusch, E.; Lens, P. Effect of heavy metal co-contaminants on selenite bioreduction by anaerobic granular sludge. Bioresour. Technol. 2016, 206, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kagami, T.; Narita, T.; Kuroda, M.; Notaguchi, E.; Yamashita, M.; Sei, K.; Soda, S.; Ike, M. Effective selenium volatilization under aerobic conditions and recovery from the aqueous phase by Pseudomonas stutzeri NT-I. Water Res. 2013, 47, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Sager, M. Selenium in agriculture, food, and nutrition. Pure Appl. Chem. 2006, 78, 111–133. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Salah, E.-D.F.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium in higher plants: Physiological role, antioxidant metabolism and abiotic stress tolerance. J. Plant Sci. 2010, 5, 354–375. [Google Scholar]
- Rosenfeld, I.; Beath, O.A. Selenium: Geobotany, Biochemistry, Toxicity, and Nutrition; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Martens, D.A.; Suarez, D.L. Selenium speciation of soil/sediment determined with sequential extractions and hydride generation atomic absorption spectrophotometry. Environ. Sci. Technol. 1996, 31, 133–139. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Shalaby, T.A.; Prokisch, J.; Fári, M. Selenium in agriculture: Water, air, soil, plants, food, animals and nanoselenium. In CO2 Sequestration, Biofuels and Depollution; Eric, L., Jan, S., Didier, R., Eds.; Springer: Cham, Switzerland, 2015; pp. 153–232. [Google Scholar]
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elem. Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef]
- McNeal, J.M.; Balistrieri, L.S. Geochemistry and occurrence of selenium: An overview. In Selenium in Agriculture and the Environment; Jacobs, L.W., Ed.; ACSESS: Madison, WI, USA, 1989; pp. 1–13. [Google Scholar]
- Gupta, U.C.; Gupta, S.C. Selenium in soils and crops, its deficiencies in livestock and humans: Implications for management. Commun. Soil Sci. Plant Anal. 2000, 31, 1791–1807. [Google Scholar] [CrossRef]
- Beale, A.; Fasulo, D.; Craigmill, A. Effects of oral and parenteral selenium supplements on residues in meat, milk and eggs. In Reviews of Environmental Contamination and Toxicology; George, W.W., Ed.; Springer: New York, NY, USA, 1990; pp. 125–150. [Google Scholar]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Thomson, C.D.; Chisholm, A.; McLachlan, S.K.; Campbell, J.M. Brazil nuts: An effective way to improve selenium status. Am. J. Clin. Nutr. 2008, 87, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Martin, C.P.; Salt, D.E. Characterization of selenocysteine methyltransferases from Astragalus species with contrasting selenium accumulation capacity. Plant J. 2009, 59, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.W.; Ha, J.; Liang, S.X.; Kang, W.J. Protective role of selenium on garlic growth under cadmium stress. Commun. Soil Sci. Plant Anal. 2010, 41, 1195–1204. [Google Scholar] [CrossRef]
- Prins, C.N.; Hantzis, L.J.; Quinn, C.F.; Pilon-Smits, E.A. Effects of selenium accumulation on reproductive functions in Brassica juncea and Stanleya pinnata. J. Exp. Bot. 2011, 62, 5633–5640. [Google Scholar] [CrossRef]
- Bajaj, M.; Eiche, E.; Neumann, T.; Winter, J.; Gallert, C. Hazardous concentrations of selenium in soil and groundwater in North-West India. J. Hazard. Mater. 2011, 189, 640–646. [Google Scholar] [CrossRef]
- Thomson, C. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Qingyun, W.; Zhang, J.; Bingzi, Z.; Xiuli, X.; Xihai, D.; Zhang, H. Influence of long-term fertilization on selenium accumulation in soil and uptake by crops. Pedosphere 2016, 26, 120–129. [Google Scholar]
- Dos Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of selenium deficiency and toxicity worldwide: Affected areas, selenium-related health issues, and case studies. In Selenium in Plants; Pilon-Smits, E.A.H., Lenny, H.E.W., Lin, Z.-Q., Eds.; Springer: Cham, Switzerland, 2017; pp. 209–230. [Google Scholar]
- White, P.J. The genetics of selenium accumulation by plants. In Selenium in Plants; Elizabeth, A.H., Lenny, P.-S., Winkel, Z.-Q., Eds.; Springer: Cham, Switzerland, 2017; pp. 143–163. [Google Scholar]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar]
- Presser, T.S. The geologic origin and pathways of mobility of selenium from the California Coast Ranges to the west-central San Joaquin valley. In Publication of an Organization Other than the U.S. Geological Survey; Frankenberger, W.T., Benson, S., Eds.; Marcel Dekker: New York, NY, USA, 1994; pp. 139–156. [Google Scholar]
- Fessler, A.J.; Moller, G.; Talcott, P.; Exon, J. Selenium toxicity in sheep grazing reclaimed phosphate mining sites. Veter. Hum. Toxicol. 2003, 45, 294. [Google Scholar]
- Bullock, L.A.; Parnell, J. Selenium and molybdenum enrichment in uranium roll-front deposits of Wyoming and Colorado, USA. J. Geochem. Exp. 2017, 180, 101–112. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Gao, J.; Lin, Z.; Banuelos, G.S.; Yuan, L.; Yin, X. Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients 2013, 5, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhu, Y.; Lin, Z.-Q.; Banuelos, G.; Li, W.; Yin, X. A novel selenocystine-accumulating plant in selenium-mine drainage area in Enshi, China. PLoS ONE 2013, 8, e65615. [Google Scholar] [CrossRef]
- Jenkins, K.; Hidiroglou, M. A review of selenium/vitamin E responsive problems in livestock: A case for selenium as a feed additive in Canada. Can. J. Animal Sci. 1972, 52, 591–620. [Google Scholar] [CrossRef]
- Tinggi, U. Essentiality and toxicity of selenium and its status in Australia: A review. Toxicol. Lett. 2003, 137, 103–110. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, S.; Pramanik, A.; Chowdhury, S.; Ghosh, S. Selenium toxicosis in grazing buffaloes and its relationship with soil and plant of West Bengal. Ind. J. Animal Sci. 1993, 63, 557–560. [Google Scholar]
- Wadgaonkar, S.L.; Ferraro, A.; Race, M.; Nancharaiah, Y.V.; Dhillon, K.S.; Fabbricino, M.; Esposito, G.; Lens, P.N. Optimization of soil washing to reduce the selenium levels of seleniferous soil from Punjab, Northwestern India. J. Environ. Qual. 2018, 47, 1530–1537. [Google Scholar] [CrossRef]
- LSA Honors Physics. The Nutrition of Food Could Worsen with Climate Change. 2017. Available online: http://lasallehonorsphysics1617.blogspot.com/2017/03/the-nutrition-of-food-could-worsen-with.html (accessed on 14 August 2020).
- Yu, D.; Liang, D.; Lei, L.; Zhang, R.; Sun, X.; Lin, Z. Selenium geochemical distribution in the environment and predicted human daily dietary intake in northeastern Qinghai, China. Environ. Sci. Pollut. Res. 2015, 22, 11224–11235. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Schild, C.O.; Cantón, G.J.; Riet-Correa, F.; Armendano, J.I.; Caffarena, R.D.; Brambilla, E.C.; García, J.A.; Morrell, E.L.; Poppenga, R. White muscle disease in three selenium deficient beef and dairy calves in Argentina and Uruguay. Ciência Rural 2018, 48, e20170733. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Tomza-Marciniak, A.; Pilarczyk, R.; Marciniak, A.; Bąkowska, M.; Nowakowska, E. Selenium, Se. Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments; Elżbieta, K., Ed.; Springer: Cham, Switzerland, 2019; pp. 301–362. [Google Scholar]
- Yee, H.; Measures, C.; Edmond, J. Selenium in the tributaries of the Orinoco in Venezuela. Nature 1987, 326, 686–689. [Google Scholar] [CrossRef]
- Hansen, J.C.; Deutch, B.; Pedersen, H.S. Selenium status in Greenland Inuit. Sci. Total Environ. 2004, 331, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Koyama, H.; Sasada, Y.; Satoh, H.; Nojiri, M.; Suzuki, S. Dietary habits and selenium intake of residents in mountain and coastal communities in Japan. J. Nutr. Sci. Vitaminol. 2004, 50, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Orr, P.L.; Guiguer, K.R.; Russel, C.K. Food chain transfer of selenium in lentic and lotic habitats of a western Canadian watershed. Ecotoxicol. Environ. Saf. 2006, 63, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.D.; Mahmood, K.; Sultan, M.I.; Khan, A.S.; Xiong, Y.; Sagintayev, Z. Trace element geochemistry of groundwater from Quetta Valley, western Pakistan. Environ. Earth Sci. 2010, 60, 573–582. [Google Scholar] [CrossRef]
- Kagawa, M.; Ishizaka, Y.; Ohta, K. Sources of sulfate in winter aerosols over the Sea of Japan, as inferred from selenium composition. Atmos. Environ. 2003, 37, 1593–1600. [Google Scholar] [CrossRef]
- Junior, E.S.; Wadt, L.; Silva, K.; Lima, R.; Batista, K.; Guedes, M.; Carvalho, G.; Carvalho, T.; Reis, A.; Lopes, G. Natural variation of selenium in Brazil nuts and soils from the Amazon region. Chemosphere 2017, 188, 650–658. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Yin, X.; Lin, Z. Plant-based biofortification: From phytoremediation to Se-enriched agriculture products. In Green Chemistry for Environmental Sustainability; Sharma, S.K., Mudhoo, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 341–356. [Google Scholar]
- Li, Y.; Peng, T.; Yang, Y.; Niu, C.; Archard, L.; Zhang, H. High prevalence of enteroviral genomic sequences in myocardium from cases of endemic cardiomyopathy (Keshan disease) in China. Heart 2000, 83, 696–701. [Google Scholar] [CrossRef]
- Xia, Y.; Hill, K.E.; Byrne, D.W.; Xu, J.; Burk, R.F. Effectiveness of selenium supplements in a low-selenium area of China. Am. J. Clin. Nutr. 2005, 81, 829–834. [Google Scholar] [CrossRef]
- Zanetti, M.; Correa, L.; Netto, A.S.; Cunha, J.; Santana, R.; Cozzolino, S. Influence of canola oil, vitamin E and selenium on cattle meat quality and its effects on nutrition and health of humans. In Proceedings of the Global Advances in Selenium Research from Theory to Application: Proceedings of the 4th International Conference on Selenium in the Environment and Human Health; Banuelos, G.S., Lin, Z.-Q., Moraes, M.F., Guilherme, L.R.G., dos Reis, A.R., Eds.; CRS Press: London, UK, 2015; pp. 97–98. [Google Scholar]
- Gao, J.; Liu, Y.; Huang, Y.; Lin, Z.-q.; Bañuelos, G.S.; Lam, M.H.-W.; Yin, X. Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem. 2011, 126, 1088–1093. [Google Scholar] [CrossRef]
- Han, J.; Liang, H.; Yi, J.; Tan, W.; He, S.; Wu, X.; Shi, X.; Ma, J.; Guo, X. Selenium deficiency induced damages and altered expressions of metalloproteinases and their inhibitors (MMP1/3, TIMP1/3) in the kidneys of growing rats. J. Trace Elem. Med. Biol. 2016, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.A.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Morlon, H.; Fortin, C.; Floriani, M.; Adam, C.; Garnier-Laplace, J.; Boudou, A. Toxicity of selenite in the unicellular green alga Chlamydomonas reinhardtii: Comparison between effects at the population and sub-cellular level. Aquat. Toxicol. 2005, 73, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, L.; Gilbin, R.; Simon, O.; Floriani, M.; Adam, C.; Pradines, C.; Cournac, L.; Garnier-Laplace, J. Effect of selenate on growth and photosynthesis of Chlamydomonas reinhardtii. Aquat. Toxicol. 2007, 83, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Wang, X.F.; Eyal, Y.; She, Y.M.; Donald, L.J.; Standing, K.G.; Ben-Hayyim, G. A selenoprotein in the plant kingdom mass spectrometry confirms that an opal codon (UGA) encodes selenocysteine in Chlamydomonas reinhardtii glutathione peroxidase. J. Biol. Chem. 2002, 277, 25983–25991. [Google Scholar] [CrossRef]
- Lyons, G.H.; Stangoulis, J.C.; Graham, R.D. Tolerance of wheat (Triticum aestivum L.) to high soil and solution selenium levels. Plant Soil 2005, 270, 179–188. [Google Scholar] [CrossRef]
- Hopper, J.L.; Parker, D.R. Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 1999, 210, 199–207. [Google Scholar] [CrossRef]
- Moreno, O.D.; Acevedo Aguilar, F.J.; Yanez Barrientos, E. Selenium uptake and biotransformation and effect of selenium exposure on the essential and trace elements status: Comparative evaluation of four edible plants. J. Mex. Chem. Soc. 2018, 62, 247–258. [Google Scholar]
- Cartes, P.; Gianfreda, L.; Mora, M. Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant Soil 2005, 276, 359–367. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Łabanowska, M.; Filek, M.; Kościelniak, J.; Kurdziel, M.; Kuliś, E.; Hartikainen, H. The effects of short-term selenium stress on Polish and Finnish wheat seedlings—EPR, enzymatic and fluorescence studies. J. Plant Physiol. 2012, 169, 275–284. [Google Scholar] [CrossRef]
- Lehotai, N.; Kolbert, Z.; Pető, A.; Feigl, G.; Ördög, A.; Kumar, D.; Tari, I.; Erdei, L. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. J. Exp. Bot. 2012, 63, 5677–5687. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Schiavon, M.; Moro, I.; Pilon-Smits, E.A.; Matozzo, V.; Malagoli, M.; Dalla Vecchia, F. Accumulation of selenium in Ulva sp. and effects on morphology, ultrastructure and antioxidant enzymes and metabolites. Aquat. Toxicol. 2012, 122, 222–231. [Google Scholar] [CrossRef]
- Quinn, C.F.; Prins, C.N.; Freeman, J.L.; Gross, A.M.; Hantzis, L.J.; Reynolds, R.J.; in Yang, S.; Covey, P.A.; Bañuelos, G.S.; Pickering, I.J. Selenium accumulation in flowers and its effects on pollination. New Phytol. 2011, 192, 727–737. [Google Scholar] [CrossRef]
- Ramos, S.J.; Faquin, V.; Almeida, H.J.d.; Avila, F.W.; Guilherme, L.R.G.; Bastos, C.E.A.; Avila, P.A. Selenate and selenite on yield, mineral nutrition and biofortification with selenium in lettuce cultivars. Revista Brasileira de Ciência do Solo 2011, 35, 1347–1355. [Google Scholar] [CrossRef]
- Akbulut, M.; Çakır, S. The effects of Se phytotoxicity on the antioxidant systems of leaf tissues in barley (Hordeum vulgare L.) seedlings. Plant Physiol. Biochem. 2010, 48, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Tamaoki, M.; Stushnoff, C.; Quinn, C.F.; Cappa, J.J.; Devonshire, J.; Fakra, S.C.; Marcus, M.A.; McGrath, S.P.; Van Hoewyk, D. Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol. 2010, 153, 1630–1652. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S.; Wu, F. Effects of Se on the uptake of essential elements in Pteris vittata L. Plant Soil 2009, 325, 123–132. [Google Scholar] [CrossRef]
- Matraszek, R.; Hawrylak-Nowak, B. Macronutrients accumulation in useable parts of lettuce as affected by nickel and selenium concentrations in nutrient solution. Fres. Environ. Bullet. 2009, 18, 1059–1065. [Google Scholar]
- Hawrylak-Nowak, B. Changes in anthocyanin content as indicator of maize sensitivity to selenium. J. Plant Nutr. 2008, 31, 1232–1242. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Effect of selenium on selected macronutrients in maize plants. J. Elementol. 2008, 13, 513–519. [Google Scholar]
- El Kassis, E.; Cathala, N.; Rouached, H.; Fourcroy, P.; Berthomieu, P.; Terry, N.; Davidian, J.-C. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007, 143, 1231–1241. [Google Scholar] [CrossRef]
- Mazzafera, P. Growth and biochemical alterations in coffee due to selenite toxicity. Plant Soil 1998, 201, 189–196. [Google Scholar] [CrossRef]
- Konze, J.R.; Schilling, N.; Kende, H. Enhancement of ethylene formation by selenoamino acids. Plant Physiol. 1978, 62, 397–401. [Google Scholar] [CrossRef]
- Padmaja, K.; Somasekharaiah, B.; Prasad, A. Inhibition of chlorophyll synthesis by selenium: Involvement of lipoxygenase mediated lipid peroxidation and antioxidant enzymes. Photosynthetica 1995, 31, 1–7. [Google Scholar]
- Valkama, E.; Kivimäenpää, M.; Hartikainen, H.; Wulff, A. The combined effects of enhanced UV-B radiation and selenium on growth, chlorophyll fluorescence and ultrastructure in strawberry (Fragaria× ananassa) and barley (Hordeum vulgare) treated in the field. Agric. Forest Meteorol. 2003, 120, 267–278. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Randle, W.M.; Mills, H.A. Nutrient accumulation in leaf tissue of rapid-cycling Brassica oleracea responds to increasing sodium selenate concentrations. J. Plant Nutr. 2000, 23, 927–935. [Google Scholar] [CrossRef]
- Arvy, M.; Thiersault, M.; Doireau, P. Relationships between selenium, micronutrients, carbohydrates, and alkaloid accumulation in Catharanthus roseus cells. J. Plant Nutr. 1995, 18, 1535–1546. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.-Z. Selenium assimilation and nutrient element uptake in white clover and tall fescue under the influence of sulphate concentration and selenium tolerance of the plants. J. Exp. Bot. 1992, 43, 549–555. [Google Scholar] [CrossRef]
- Pazurkiewicz-Kocot, K.; Kita, A.; Pietruszka, M. Effect of selenium on magnesium, iron, manganese, copper, and zinc accumulation in corn treated by indole-3-acetic acid. Commun. Soil Sci. Plant Anal. 2008, 39, 2303–2318. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, M. Influence of selenium on uptake and toxicity of copper and cadmium in pea (Pisum sativum) and wheat (Triticum aestivum). Physiol. Plant. 1994, 90, 637–644. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, quality and antioxidant properties of Indian mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.; Waraich, E.; Khan, S. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Kinraide, T.B. The controlling influence of cell-surface electrical potential on the uptake and toxicity of selenate (SeO42–). Physiol. Plant. 2003, 117, 64–71. [Google Scholar] [CrossRef]
- Bailey, F.; Knight, A.; Ogle, R.; Klaine, S. Effect of sulfate level on selenium uptake by Ruppia maritima. Chemosphere 1995, 30, 579–591. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.; Spiby, R.; Meacham, M.; Mead, A.; Harriman, M.; Trueman, L. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium accumulation by plants. Annal. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Hondal, R.J.; Marino, S.M.; Gladyshev, V.N. Selenocysteine in thiol/disulfide-like exchange reactions. Antioxid. Redox Signal. 2013, 18, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Balk, J.; Pilon, M. Ancient and essential: The assembly of iron–sulfur clusters in plants. Trends Plant Sci. 2011, 16, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C.; George, G.N.; Prince, R.C.; Thorneley, R.N. Characterization of a modified nitrogenase Fe protein from Klebsiella pneumoniae in which the 4Fe4S cluster has been replaced by a 4Fe4Se cluster. JBIC J. Biol. Inorg. Chem. 2009, 14, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.; De Souza, M.; Tarun, A. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Châtelain, E.; Satour, P.; Laugier, E.; Vu, B.L.; Payet, N.; Rey, P.; Montrichard, F. Evidence for participation of the methionine sulfoxide reductase repair system in plant seed longevity. Proc. Natl. Acad. Sci. USA 2013, 110, 3633–3638. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Yang, X.; Xia, Z. Effects of sodium selenite and germination on the sprouting of chickpeas (Cicer arietinum L.) and its content of selenium, formononetin and biochanin A in the sprouts. Biol. Trace Elem. Res. 2012, 146, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, W.; Politycka, B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mo, H.-Z.; Hu, L.-B.; Li, Y.-Q.; Chen, J.; Yang, L.-F. The endogenous nitric oxide mediates selenium-induced phytotoxicity by promoting ROS generation in Brassica rapa. PLoS ONE 2014, 9, e110901. [Google Scholar] [CrossRef] [PubMed]
- Mroczek-Zdyrska, M.; Wójcik, M. The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol. Trace Elem. Res. 2012, 147, 320–328. [Google Scholar] [CrossRef]
- Sabbagh, M.; Van Hoewyk, D. Malformed selenoproteins are removed by the ubiquitin–proteasome pathway in Stanleya pinnata. Plant Cell Physiol. 2012, 53, 555–564. [Google Scholar] [CrossRef]
- Grant, K.; Carey, N.M.; Mendoza, M.; Schulze, J.; Pilon, M.; Pilon-Smits, E.A.; Van Hoewyk, D. Adenosine 5′-phosphosulfate reductase (APR2) mutation in Arabidopsis implicates glutathione deficiency in selenate toxicity. Biochem. J. 2011, 438, 325–335. [Google Scholar] [CrossRef]
- Hugouvieux, V.; Dutilleul, C.; Jourdain, A.; Reynaud, F.; Lopez, V.; Bourguignon, J. Arabidopsis putative selenium-binding protein1 expression is tightly linked to cellular sulfur demand and can reduce sensitivity to stresses requiring glutathione for tolerance. Plant Physiol. 2009, 151, 768–781. [Google Scholar] [CrossRef]
- Ríos, J.; Blasco, B.; Cervilla, L.; Rosales, M.; Sanchez-Rodriguez, E.; Romero, L.; Ruiz, J. Production and detoxification of H2O2 in lettuce plants exposed to selenium. Annal. Appl. Biol. 2009, 154, 107–116. [Google Scholar] [CrossRef]
- Spallholz, J.E. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 1994, 17, 45–64. [Google Scholar] [CrossRef]
- Tamaoki, M.; Freeman, J.L.; Pilon-Smits, E.A. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiol. 2008, 146, 1219–1230. [Google Scholar] [CrossRef]
- Dimkovikj, A.; Van Hoewyk, D. Selenite activates the alternative oxidase pathway and alters primary metabolism in Brassica napus roots: Evidence of a mitochondrial stress response. BMC Plant Biol. 2014, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Nowak, J.; Kaklewski, K.; Ligocki, M. Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol. Biochem. 2004, 36, 1553–1558. [Google Scholar] [CrossRef]
- de la Luz Mora, M.; Pinilla, L.; Rosas, A.; Cartes, P. Selenium uptake and its influence on the antioxidative system of white clover as affected by lime and phosphorus fertilization. Plant Soil 2008, 303, 139–149. [Google Scholar] [CrossRef]
- Aggarwal, M.; Sharma, S.; Kaur, N.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Kaur, R.; Singh, K.; Srivastava, A.; Nayyar, H. Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol. Trace Elem. Res. 2011, 140, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Junior, R.A.; Gratão, P.L.; Gaziola, S.A.; Mazzafera, P.; Lea, P.J.; Azevedo, R.A. Selenium-induced oxidative stress in coffee cell suspension cultures. Funct. Plant Biol. 2007, 34, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, G.; Zambrzuski, S.; Mackey, B. Phytoextraction of selenium from soils irrigated with selenium-laden effluent. Plant Soil 2000, 224, 251–258. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Arroyo, I.; Pickering, I.J.; Yang, S.I.; Freeman, J.L. Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem. 2015, 166, 603–608. [Google Scholar] [CrossRef]
- Parker, D.R.; Feist, L.J.; Varvel, T.W.; Thomason, D.N.; Zhang, Y. Selenium phytoremediation potential of Stanleya pinnata. Plant Soil 2003, 249, 157–165. [Google Scholar] [CrossRef]
- Esringü, A.; Turan, M. The roles of diethylenetriamine pentaacetate (DTPA) and ethylenediamine disuccinate (EDDS) in remediation of selenium from contaminated soil by Brussels sprouts (Brassica oleracea var. gemmifera). Water Air Soil Pollut. 2012, 223, 351–362. [Google Scholar] [CrossRef]
- Barceló, J.; Poschenrieder, C. Hyperaccumulation of trace elements: From uptake and tolerance mechanisms to litter decomposition; selenium as an example. Plant Soil 2011, 341, 31–35. [Google Scholar] [CrossRef]
- Dhillon, K.S.; Bañuelos, G.S. Overview and prospects of selenium phytoremediation approaches. In Selenium in Plants; Pilon-Smits, E.A.H., Lenny, H.E.W., Lin, Z.-Q., Eds.; Springer: Cham, Switzerland, 2017; pp. 277–321. [Google Scholar]
- Liu, J.; Xin, X.; Zhou, Q. Phytoremediation of contaminated soils using ornamental plants. Environ. Rev. 2018, 26, 43–54. [Google Scholar] [CrossRef]
- Beath, O.A.; Gilbert, C.S.; Eppson, H. The use of indicator plants in locating seleniferous areas in Western United States. I. General. Am. J. Bot. 1939, 26, 257–269. [Google Scholar] [CrossRef]
- Carey, A.-M.; Scheckel, K.G.; Lombi, E.; Newville, M.; Choi, Y.; Norton, G.J.; Price, A.H.; Meharg, A.A. Grain accumulation of selenium species in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 5557–5564. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Rai, J. The phytoextraction potential of water hyacinth (Eichchornia crassipes): Removal of selenium and copper. Chem. Ecol. 2010, 26, 163–172. [Google Scholar] [CrossRef]
- Bañuelos, G.; Shannon, M.; Ajwa, H.; Draper, J.; Jordahl, J.; Licht, J. Phytoextraction and accumulation of boron and selenium by poplar (Populus) hybrid clones. Int. J. Phytoremed. 1999, 1, 81–96. [Google Scholar] [CrossRef]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Landesman, L.; Fedler, C.; Duan, R. Plant nutrient phytoremediation using duckweed. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Gill, S.S., Lanza, G.R., Rast, W., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 341–354. [Google Scholar]
- Carvalho, K.M.; Gallardo, M.T.; McGettigan, M.J.; Martin, D.F. Remediation of selenium contamination by plants and microbes: An annotated bibliography. Fla. Sci. 2000, 63, 133–141. [Google Scholar]
- Jeke, N.N.; Zvomuya, F.; Cicek, N.; Ross, L.; Badiou, P. Biomass, nutrient, and trace element accumulation and partitioning in cattail (Typha latifolia L.) during wetland phytoremediation of municipal biosolids. J. Environ. Qual. 2015, 44, 1541–1549. [Google Scholar] [CrossRef]
- Sabogal, A.; Borkowski, D. Estado actual de la investigación sobre Ipomoea carnea: Toxicidad en ganado caprino. Revista Química 2007, 21, 29–35. [Google Scholar]
- Pilon-Smits, E.; De Souza, M.; Hong, G.; Amini, A.; Bravo, R.; Payabyab, S.; Terry, N. Selenium volatilization and accumulation by twenty aquatic plant species. J. Environ. Qual. 1999, 28, 1011–1018. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C. Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant. Plant Soil Environ. 2012, 58, 105–110. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Hwang, S.; Lytle, C.M.; Zhu, Y.; Tai, J.C.; Bravo, R.C.; Chen, Y.; Leustek, T.; Terry, N. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999, 119, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S.; Dhillon, K. Phytoremediation of selenium-contaminated soils: The efficiency of different cropping systems. Soil Use Manag. 2009, 25, 441–453. [Google Scholar] [CrossRef]
- Dhillon, K.; Dhillon, S.; Thind, H. Evaluation of different agroforestry tree species for their suitability in the phytoremediation of seleniferous soils. Soil Use Manag. 2008, 24, 208–216. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Marshall, B.; Broadley, M.R. Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’plants. Annal. Bot. 2007, 100, 111–118. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; LeDuc, D.L. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotechnol. 2009, 20, 207–212. [Google Scholar] [CrossRef]
- Cappa, J.J.; Cappa, P.J.; El Mehdawi, A.F.; McAleer, J.M.; Simmons, M.P.; Pilon-Smits, E.A. Characterization of selenium and sulfur accumulation across the genus Stanleya (Brassicaceae): A field survey and common-garden experiment. Am. J. Bot. 2014, 101, 830–839. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Paschke, M.W.; Pilon-Smits, E.A. Symphyotrichum ericoides populations from seleniferous and nonseleniferous soil display striking variation in selenium accumulation. New Phytol. 2015, 206, 231–242. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Reynolds, R.J.B.; Prins, C.N.; Lindblom, S.D.; Cappa, J.J.; Fakra, S.C.; Pilon-Smits, E.A. Analysis of selenium accumulation, speciation and tolerance of potential selenium hyperaccumulator Symphyotrichum ericoides. Physiol. Plant. 2014, 152, 70–83. [Google Scholar] [CrossRef]
- Cabannes, E.; Buchner, P.; Broadley, M.R.; Hawkesford, M.J. A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiol. 2011, 157, 2227–2239. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D. A tale of two toxicities: Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Annal. Bot. 2013, 112, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, J.; Berkelaar, E. Plant uptake and translocation of inorganic and organic forms of selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Van Hoewyk, D.; Garifullina, G.F.; Ackley, A.R.; Abdel-Ghany, S.E.; Marcus, M.A.; Fakra, S.; Ishiyama, K.; Inoue, E.; Pilon, M.; Takahashi, H. Overexpression of AtCpNifS enhances selenium tolerance and accumulation in Arabidopsis. Plant Physiol. 2005, 139, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 2012, 149, 248–261. [Google Scholar] [CrossRef]
- Malik, J.A.; Goel, S.; Kaur, N.; Sharma, S.; Singh, I.; Nayyar, H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012, 77, 242–248. [Google Scholar] [CrossRef]
- Bañuelos, G.S. Phytoremediation of selenium contaminated soil, and water produces biofortified products and new agricultural byproducts. In Development and Uses of Biofortified Agricultural Products; Bañuelos, G.S., Lin, Z.Q., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 57–70. [Google Scholar]
- Bhargava, A.; Carmona, F.F.; Bhargava, M.; Srivastava, S. Approaches for enhanced phytoextraction of heavy metals. J. Environ. Manag. 2012, 105, 103–120. [Google Scholar] [CrossRef]
- Chaney, R. Plant uptake of inorganic waste. In Land Treatment of Hazardous Wastes; Parr, J.E., Marsh, P.B., Kla, J.M., Eds.; Noyes Data Corp.: Park Ridge, IL, USA, 1983; pp. 50–67. [Google Scholar]
- Schmidt, U. Enhancing phytoextraction: The effect of chemical soil manipulation on mobility, plant accumulation, and leaching of heavy metals. J. Environ. Qual. 2003, 32, 1939–1954. [Google Scholar] [CrossRef]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-enhanced phytoremediation of heavy metals: A review. Soil Sediment Contam. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Robinson, B.; Fernández, J.-E.; Madejón, P.; Marañón, T.; Murillo, J.M.; Green, S.; Clothier, B. Phytoextraction: An assessment of biogeochemical and economic viability. Plant Soil 2003, 249, 117–125. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, Z.; Ouyang, Y. Phytoextraction of metal-contaminated soil by Sedum alfredii H: Effects of chelator and co-planting. Water Air Soil Pollut. 2007, 180, 131–139. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Lucchini, P.; Dickinson, N.M.; Mosca, G. Long-term phytomanagement of metal-contaminated land with field crops: Integrated remediation and biofortification. Eur. J. Agron. 2014, 53, 56–66. [Google Scholar] [CrossRef]
- Johnsson, L. Selenium uptake by plants as a function of soil type, organic matter content and pH. Plant Soil 1991, 133, 57–64. [Google Scholar] [CrossRef]
- Dhillon, K.; Dhillon, S.; Dogra, R. Selenium accumulation by forage and grain crops and volatilization from seleniferous soils amended with different organic materials. Chemosphere 2010, 78, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Gupta, S.; Prakash, R.; Spallholz, J.; Prakash, N. Selenium uptake by Allium cepa grown in Se-spiked soils. Am.-Eur. J. Agric. Environ. Sci. 2007, 2, 80–84. [Google Scholar]
- Limmer, M.; Burken, J. Phytovolatilization of organic contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef]
- Evans, C.S.; Asher, C.; Johnson, C. Isolation of dimethyl diselenide and other volatile selenium compounds from Astragalus racemosus (Pursh.). Aust. J. Biol. Sci. 1968, 21, 13–20. [Google Scholar] [CrossRef]
- Lewis, B.; Johnson, C.; Broyer, T. Cleavage of Se-ethylselenomethionine selenonium salt by a cabbage leaf enzyme fraction. Biochim. Biophy. Acta 1971, 237, 603–605. [Google Scholar] [CrossRef]
- Bañuelos, G.; Leduc, D.L.; Pilon-Smits, E.A.; Terry, N. Transgenic Indian mustard overexpressing selenocysteine lyase or selenocysteine methyltransferase exhibit enhanced potential for selenium phytoremediation under field conditions. Environ. Sci. Technol. 2007, 41, 599–605. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M. Selenium Volatilization by Plants; Marcel Dekker: New York, NY, USA, 1994; Volume 343. [Google Scholar]
- Lin, Z.-Q.; Terry, N.; Gao, S.; Mohamed, S.; Ye, Z. Vegetation changes and partitioning of selenium in 4-year-old constructed wetlands treating agricultural drainage. Int. J. Phytoremed. 2010, 12, 255–267. [Google Scholar] [CrossRef]
- Krishna, R.; Fulekar, M.; Patakg, B. Rhizofiltration: A green technology for remediation of heavy metals. Int. J. Innov. Bio-Sci. 2012, 2, 193–199. [Google Scholar]
- Ornes, W.; Sajwan, K.; Dosskey, M.; Adriano, D. Bioaccumulation of selenium by floating aquatic plants. Water Air Soil Pollut. 1991, 57, 53–57. [Google Scholar] [CrossRef]
- Miranda, A.; Muradov, N.; Gujar, A.; Stevenson, T.; Nugegoda, D.; Ball, A.; Mouradov, A. Application of aquatic plants for the treatment of selenium-rich mining wastewater and production of renewable fuels and petrochemicals. J. Sustain. Bioenergy Syst. 2014, 4, 97–112. [Google Scholar] [CrossRef][Green Version]
- Nattrass, M.; McGrew, N.R.; Morrison, J.I.; Baldwin, B.S. Phytoremediation of selenium-impacted water by aquatic macrophytes1. J. Amer. Soc. Min. Reclam. 2019, 8, 69–79. [Google Scholar] [CrossRef]
- Lin, Z.-Q.; Terry, N. Selenium removal by constructed wetlands: Quantitative importance of biological volatilization in the treatment of selenium-laden agricultural drainage water. Environ. Sci. Technol. 2003, 37, 606–615. [Google Scholar] [CrossRef]
- Ohlbaum, M.; Wadgaonkar, S.L.; van Bruggen, J.J.; Nancharaiah, Y.V.; Lens, P.N. Phytoremediation of seleniferous soil leachate using the aquatic plants Lemna minor and Egeria densa. Ecol. Eng. 2018, 120, 321–328. [Google Scholar] [CrossRef]
- Carvalho, K.M.; Martin, D.F. Removal of aqueous selenium by four aquatic plants. J. Aquat. Plant Manag. 2001, 39, 33–36. [Google Scholar]
- Visioli, G.; D’Egidio, S.; Sanangelantoni, A.M. The bacterial rhizobiome of hyperaccumulators: Future perspectives based on omics analysis and advanced microscopy. Front. Plant Sci. 2015, 5, 752. [Google Scholar] [CrossRef]
- Huysen, T.; Terry, N.; Pilon-Smits, E. Exploring the selenium phytoremediation potential of transgenic Indian mustard overexpressing ATP sulfurylase or cystathionine-γ-synthase. Int. J. Phytoremed. 2004, 6, 111–118. [Google Scholar] [CrossRef]
- Pilon, M.; Owen, J.D.; Garifullina, G.F.; Kurihara, T.; Mihara, H.; Esaki, N.; Pilon-Smits, E.A. Enhanced selenium tolerance and accumulation in transgenic Arabidopsis expressing a mouse selenocysteine lyase. Plant Physiol. 2003, 131, 1250–1257. [Google Scholar] [CrossRef]
- Garifullina, G.F.; Owen, J.D.; Lindblom, S.D.; Tufan, H.; Pilon, M.; Pilon-Smits, E.A. Expression of a mouse selenocysteine lyase in Brassica juncea chloroplasts affects selenium tolerance and accumulation. Physiol. Plant. 2003, 118, 538–544. [Google Scholar] [CrossRef]
- LeDuc, D.L.; Tarun, A.S.; Montes-Bayon, M.; Meija, J.; Malit, M.F.; Wu, C.P.; AbdelSamie, M.; Chiang, C.-Y.; Tagmount, A.; de Souza, M. Overexpression of selenocysteine methyltransferase in Arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiol. 2004, 135, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.R.; Sors, T.G.; Brunk, D.G.; Albrecht, C.; Orser, C.; Lahner, B.; Wood, K.V.; Harris, H.H.; Pickering, I.J.; Salt, D.E. Production of Se-methylselenocysteine in transgenic plants expressing selenocysteine methyltransferase. BMC Plant Biol. 2004, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, G.; Terry, N.; LeDuc, D.L.; Pilon-Smits, E.A.; Mackey, B. Field trial of transgenic Indian mustard plants shows enhanced phytoremediation of selenium-contaminated sediment. Environ. Sci. Technol. 2005, 39, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, D.L.; AbdelSamie, M.; Móntes-Bayon, M.; Wu, C.P.; Reisinger, S.J.; Terry, N. Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances selenium phytoremediation traits in Indian mustard. Environ. Pollut. 2006, 144, 70–76. [Google Scholar] [CrossRef] [PubMed]
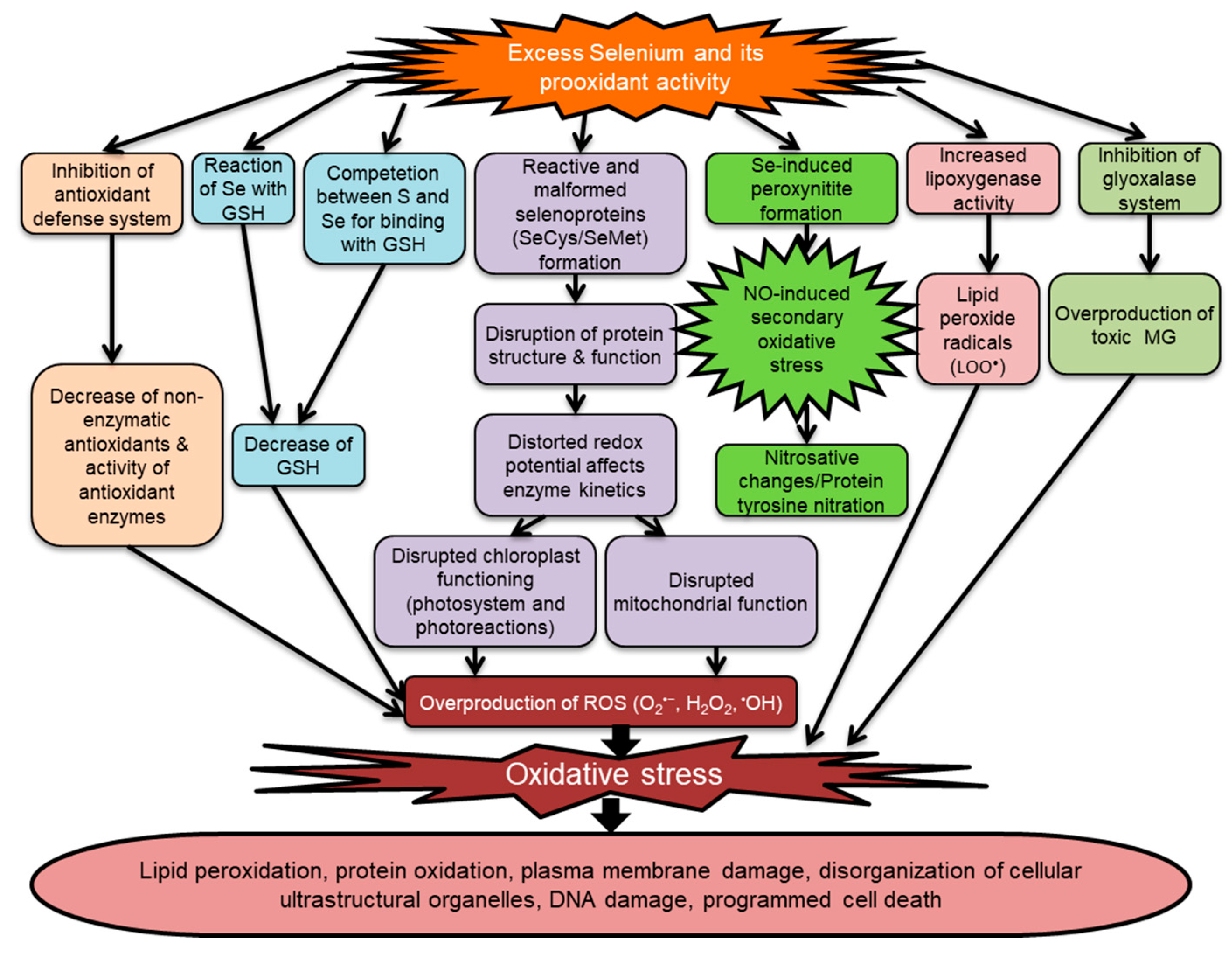
| Plant Species | Form and Dose of Se | Negative Impact on Growth and Physiology | Reference |
|---|---|---|---|
| Arabidopsis thaliana | SeO32–; 50 or 100 μM | Se-induced secondary nitrooxidative stress. Decreased root growth and biomass (FW and DW). Reduced cell viability. Modified cell wall structure by modifying the pectin and callose. Decreased stomatal density and impaired stomatal regulations sensitive varieties were affected more than the tolerant. | [26] |
| Raphanus sativus, Helianthus annuus, Medicago sativa Beta vulgaris var. cicla | SeO32–; 5 or 10 mg Se L–1 | Growth inhibition. | [115] |
| Pisum sativum cv. Petit Provençal | SeO32–; 50 or 100 μM | Altered vegetative and reproductive development. Shoot and root length and FW decreased. Chl a, chl b, chl a/b, total chl, total carotenoids content decreased. | [30] |
| Cucumis sativus cv. Polan F1 | SeO42–; 80 µM SeO32–; 20 µM | Decreased shoot root growth, biomass and leaf area. Impaired nutrient content. Reduced photosynthetic pigments accumulation and chl fluorescence. Increased lipid peroxidation. | [117] |
| Oryza sativa | SeO32–; 100 g Se ha−1 | Increased Se content in root and shoot. Reduced photosynthesis and transpiration rate, and intercellular [CO2]. Impaired PSII quantum yield and diminished potential photosynthetic capacity. Reduced grain yield. | [118] |
| Lactuca sativa var. capitata cv. Justyna | SeO42–; 20 µM SeO32–; 15 µM | High accumulation of Se and S. Decreased biomass and leaf area. Reduced concentrations of photosynthetic pigments. Increased lipid peroxidation and H2O2 accumulation. | [119] |
| Triticum aestivum | SeO42–; 100 μM | Reduction of PSII and PSI activities. | [120] |
| A. thaliana | SeO42–; 20 or 40 μM | Root growth inhibition. Loss of root apex cell viability and malformed root architecture. Reduction of primary root growth, an increase of lateral root growth. Decreased meristem cell activities. Hormonal imbalance. | [121] |
| Spinacia oleracea cv. Missouri | SeO32–; 6 mg L−1 | Increased Se accumulation. Decreased growth parameters, e.g., shoot and root length, and FW and DW. Increased Na and Ca content, but decreased K content. | [122] |
| Ulva sp. | SeO42–; 100 μM | Decreased level of chl and carotenoids. | [123] |
| Brassica juncea | SeO42–; 80 μM | Augmented Se and S concentration in different floral parts. Increased floral Se accumulation and impaired pollen germination. | [124] |
| Lactuca sativa | SeO32– and SeO42–; 20 µM | Increased shoot Se concentration. Decreased P, S, Mg, Mn, and Fe concentrations. A slight reduction in shoot DW and yield. | [125] |
| Hordeum vulgare | SeO42–; 2, 4, 8, or 16 ppm | Decreased plant height. Reduced chl concentrations. | [126] |
| Stanleya albescens | SeO42–, 20 μM | Reduced growth. Chlorosis and impaired photosynthesis. Accumulation of the free amino acid selenocystathionine, a carbon-Se-carbon compounds (presumably selenocystathionine) together with some selenocysteine and selenate. | [127] |
| Pteris vittata | SeO42–; 50 and 100 mg kg−1 in soil. | Suppressed uptake of Mg, K, P, Fe, Cu, and Zn. | [128] |
| Lactuca sativa var. capitata | SeO32–; 20 μM | Decreased productivity. Declined macronutrients accumulation in leaves. | [129] |
| Zea mays | SeO32–; 50 and 100 µmol L−1 | Decreased DW accumulation. Root tolerance index severely decreased. | [130] |
| Z. mays | SeO42– or selenomethionine (C5H11NO2Se); 100 µM | High Se accumulation in root and shoot. Reduction in root and shoot FW. Altered anthocyanin level. Reduced chl level. | [131] |
| Chlamydomonas reinhardtii | SeO32– and SeO42–; 4.5 ± 0.2 µM | Photosynthesis disorders. Ultrastructural damage. Inhibition and interruption of the photosynthetic electron transport chain. Growth inhibition. | [111] |
| Plant Species | Form and Concentration of Se | Indicators of Oxidative Stress and Changes in Antioxidant Enzymes Activities under Se Exposure | Reference |
|---|---|---|---|
| Arabidopsis thaliana | SeO32–; 50 or 100 μM | Distinct oxidative stress. Nitrosative modifications. Callose accumulation. Pectin accumulation. | [26] |
| Pisum sativum | SeO32–; 50 or 100 μM | Increased H2O2 concentration in leaves and roots. Increased content of thiobarbituric acid reactive substances (TBARS). Altered GSH content, APX and CAT activities. Increased nitric oxide level in shoot and root. Nitric oxide-induced nitrooxidative stress by increasing peroxynitrite formation, as well as tyrosine nitration. | [30] |
| Brassica rapa | SeO32–; 0.03–0.46 mM | Increased endogenous total ROS, O2•−, and enhanced lipid peroxidation. Loss of plasma membrane integrity in the roots. | [157] |
| Triticum aestivum | SeO42–; 100 μM | Altered carbohydrates (soluble and starch) level. AsA and GSH contents were modified. Suppressed activities of SOD, APX, and GR. Higher generation of ROS. Augmented lipid peroxidation. Repressed PSII and PSI system activities. Modified redox status connected with Mn(II)/Mn(III), and semiquinone/quinone ratios. | [120] |
| A. thaliana | SeO42–; 20 and 40 μM | Decreased NO content. Increased H2O2 content. Reduced cell viability. | [121] |
| Vicia faba | SeO42–; 6 μM | Elevated lipid peroxidation and total -SH (T-SH) content. Increased GPX activity. Decreased guaiacol peroxidase (GPOX) activity. Increased O2•− production in the roots. Cell membrane injury and reduced cell viability. | [158] |
| Stanleya pinnata | SeO42–; 40 and 80 μM | Oxidized proteins. Malformed or misfolded selenoproteins. | [159] |
| Ulva sp. | SeO42–; 100 μM | Increased accumulation of H2O2. The activity of antioxidant enzymes such as SOD, CAT increased. Antioxidant metabolites including phenols, flavonoids, carotenoids, and gallic acid increased. | [123] |
| A. thaliana | SeO42–; 20 μM | The cad2-1 mutant was recognized with a flawed GSH synthetic pathway that showed decreased root length, in contrast to the wild type. In the apr2-1 mutant, GSH depletion and ROS accretion were prominent. | [160] |
| Hordeum vulgare | SeO42–; 4, 8 and 16 ppm | Increased membrane lipid peroxidation. Higher proline accumulation. Stimulated CAT, APX, GR, and glutathione-S-transferase (GST) activities. | [126] |
| Stanleya albescens | SeO42–; 20 μM | Increased O2•− and H2O2 levels. Reduced AsA and GSH content. Declined radical-scavenging capacity. | [127] |
| A. thaliana | SeO42–; 50 mM | Decreased GSH level. | [161] |
| Plant Species | Family | References |
|---|---|---|
| Brassica oleracea var. capitata, B. oleracea var. italica, B. oleracea var. botrytis, B. juncea, B. napus, Stanleya pinnata | Brassicaceae | [35,171,172,173,174] |
| Gaillardia aristata and Calendula officinalis | Asteraceae | [175,176,177] |
| Astragalus bisulcatus | Fabaceae | [171,178] |
| Arundo donax, Triticum aestivum, and Oryza sativa | Poaceae | [36,153,179] |
| Eichchornia crassipes | Pontederiaceae | [180] |
| Populus spp. | Salicaceae | [181] |
| Lemnoideae spp. | Lemnaceae | [182,183] |
| Hippuris vulgaris L. | Plantaginaceae | [184] |
| Typha latifolia | Typhaceae | [185] |
| Ipomoea purpurea | Convolvulaceae | [186] |
| Azolla caroliniana | Salviniaceae | [187] |
| Pteris vittata | Pteridaceae | [188] |
| Juncus xiphioides | Juncaceae | [189] |
| Bolboschoenus maritimus | Cyperaceae | [189] |
| Chara spp. | Characeae | [38,39] |
| Corchorus capsularis | Malvaceae | [190] |
| Eucalyptus globulus | Myrtaceae | [191] |
| Transgenic Species | Gene Transferred | Effects | Reference |
|---|---|---|---|
| Brassica juncea | Cystathionine-γ-synthase (CgS) | Increased Se volatilization | [228] |
| A. thaliana | Selenocysteine lyase (SL) | Enhanced Se accumulation | [229] |
| B. juncea | SL | Enhanced Se accumulation | [230] |
| A. thaliana | Selenocysteine methyltransferase (SMT) | Enhanced Se accumulation and volatilization | [231] |
| B. juncea | SMT | Enhanced Se accumulation and tolerance | [232] |
| B. juncea | APS | Three-fold increased Se accumulation in leaves | [233] |
| B. juncea | γ Glutamyl-cysteine synthetase (ECS) | Improved Se accumulation | [233] |
| B. juncea | APS×SMT | Increased Se accumulation under both SeO42− and SeO32− exposure | [217] |
| B. juncea | SL×SMT | Enhanced Se accumulation | [217] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Nahar, K.; Fujita, M. Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities. Plants 2020, 9, 1711. https://doi.org/10.3390/plants9121711
Hasanuzzaman M, Bhuyan MHMB, Raza A, Hawrylak-Nowak B, Matraszek-Gawron R, Nahar K, Fujita M. Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities. Plants. 2020; 9(12):1711. https://doi.org/10.3390/plants9121711
Chicago/Turabian StyleHasanuzzaman, Mirza, M. H. M. Borhannuddin Bhuyan, Ali Raza, Barbara Hawrylak-Nowak, Renata Matraszek-Gawron, Kamrun Nahar, and Masayuki Fujita. 2020. "Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities" Plants 9, no. 12: 1711. https://doi.org/10.3390/plants9121711
APA StyleHasanuzzaman, M., Bhuyan, M. H. M. B., Raza, A., Hawrylak-Nowak, B., Matraszek-Gawron, R., Nahar, K., & Fujita, M. (2020). Selenium Toxicity in Plants and Environment: Biogeochemistry and Remediation Possibilities. Plants, 9(12), 1711. https://doi.org/10.3390/plants9121711










