Multi-Mycotoxin Occurrence in Dairy Cattle and Poultry Feeds and Feed Ingredients from Machakos Town, Kenya
Abstract
:1. Introduction
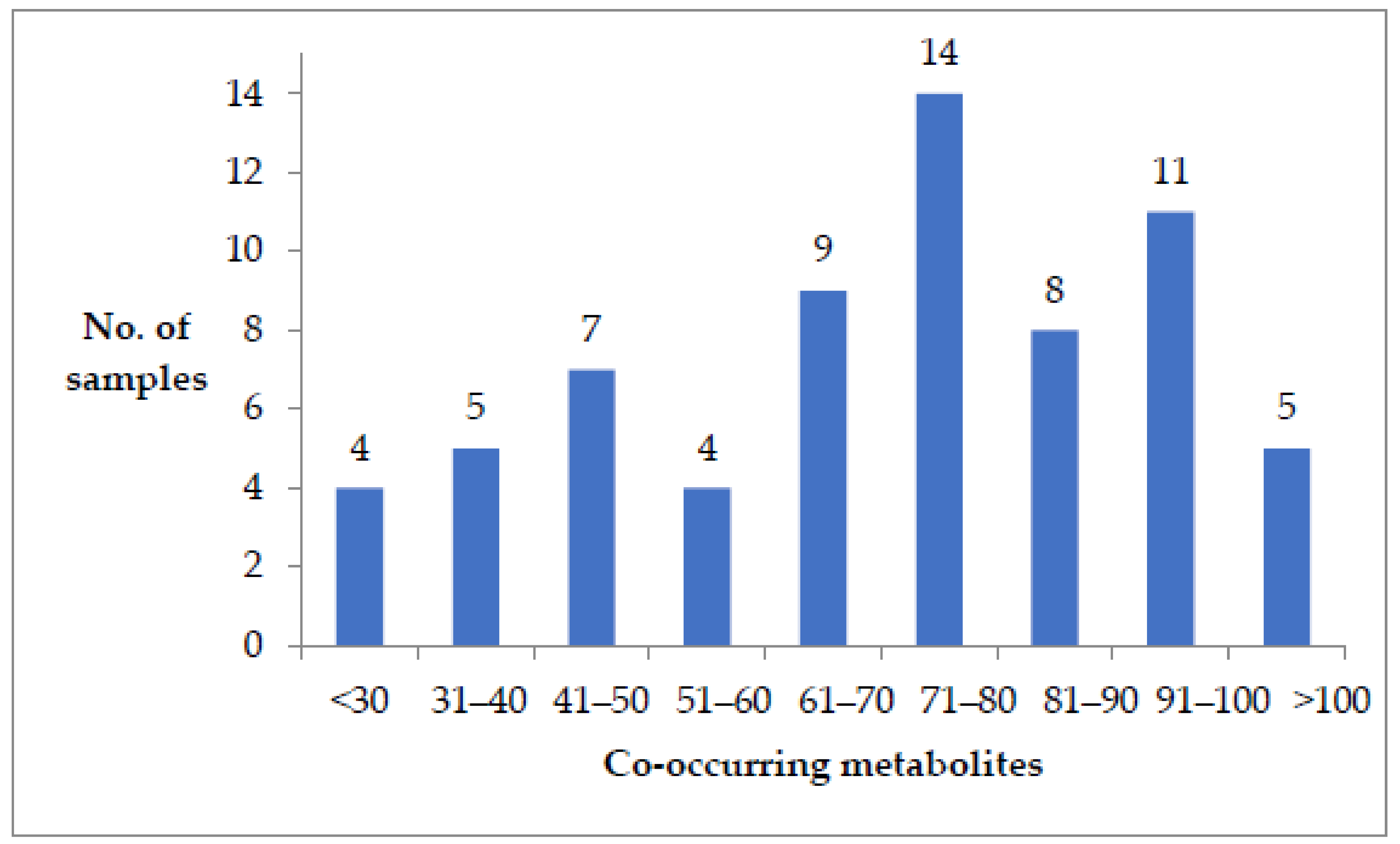
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Site
5.2. Sample Preparation
5.3. Analytical Method
5.4. Data Management and Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonçalves, B.; Corassin, C.; Oliveira, C. Mycotoxicoses in Dairy Cattle: A Review. Asian J. Anim. Vet. Adv. 2015, 10, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Gbashi, S.; Madala, N.E.; de Saeger, S.; de Boevre, M.; Adekoya, I.; Adebo, O.A.; Njobeh, P.B. The Socio-Economic Impact of Mycotoxin Contamination in Africa. In Mycotoxins-Impact and Management Strategies; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/online-first/the-socio-economic-impact-of-mycotoxin-contamination-in-africa (accessed on 23 September 2020). [CrossRef] [Green Version]
- Njobeh, P.B.; Dutton, M.F.; Aberg, A.T.; Haggblom, P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 2012, 4, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.I.; El-Zubeir, I.; Fadel Elseed, A.M.A. Aflatoxin M1 in raw and imported powdered milk sold in Khartoum state, Sudan. Food Addit. Contam. Part B 2014, 7, 208–212. [Google Scholar] [CrossRef]
- Gizachew, D.; Szonyi, B.; Tegegne, A.; Hanson, J.; Grace, D. Aflatoxin contamination of milk and dairy feeds in the Greater Addis Ababa milk shed, Ethiopia. Food Control 2016, 59, 773–779. [Google Scholar] [CrossRef]
- Kuilman, M.E.M. Bovine hepatic metabolism of aflatoxin B1. J. Agric. Food Chem. 1998, 46, 2707–2713. [Google Scholar] [CrossRef]
- Lindahl, J.F.; Kagera, I.N.; Grace, D. Aflatoxin M1 levels in different marketed milk products in Nairobi, Kenya. Mycotoxin Res. 2018, 34, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribelin, W.E.; Fukushima, K.; Still, P.E. The toxicity of ochratoxin to ruminants. Can. J. Comp. Med. Rev. Can. Med. Comp. 1978, 42, 172–176. [Google Scholar]
- World Health Organization; International Agency for Research on Cancer. Aflatoxins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; WHO: Geneva, Switzerland; IARC: Lyon, France, 2002. [Google Scholar]
- Kang’ethe, E.K.; Lang’a, K.A. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr. Health Sci. 2009, 9, 218–226. [Google Scholar] [PubMed]
- Makau, C.M.; Matofari, J.W.; Muliro, P.S.; Bebe, B.O. Aflatoxin B1 and Deoxynivalenol contamination of dairy feeds and presence of Aflatoxin M1 contamination in milk from smallholder dairy systems in Nakuru, Kenya. Int. J. Food Contam. 2016, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Okoth, S.A.; Kola, M.A. Market samples as a source of chronic aflatoxin exposure in Kenya. Afr. J. Health Sci. 2012, 20, 56–61. [Google Scholar]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food Addit. Contam. Part B 2011, 4, 168–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirma, A.; Daniel, S.; Lindahl, J.; Makita, K.; Kang’ethe, E.; Grace, D. Aflatoxin M1 survey in dairy households in Kenya. In Proceedings of the Food Africa Midterm Seminar, Helsinki, Finland, 16 June 2014. [Google Scholar]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A Review of the Impact of Mycotoxins on Dairy Cattle Health: Challenges for Food Safety and Dairy Production in Sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria Mycotoxins in Food and Feed: An Overview. J. Food Qual. 2017, 2017, 1569748. [Google Scholar] [CrossRef] [Green Version]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of Mycotoxins in Food and Feed from Burkina Faso and Mozambique Using a Modern LC-MS/MS Multitoxin Method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef] [Green Version]
- Coufal-Majewski, S.; Stanford, K.; McAllister, T.; Blakley, B.; McKinnon, J.; Chaves, A.V.; Wang, Y. Impacts of Cereal Ergot in Food Animal Production. Front. Vet. Sci. 2016, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Ezekiel, C.N.; Bandyopadhyay, R.; Sulyok, M.; Warth, B.; Krska, R. Fungal and bacterial metabolites in commercial poultry feed from Nigeria. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1288–1299. [Google Scholar] [CrossRef]
- Jefferson, W.N.; Patisaul, H.B.; Williams, C.J. Reproductive consequences of developmental phytoestrogen exposure. Reprod. Camb. Engl. 2012, 143, 247–260. [Google Scholar] [CrossRef]
- Hessenberger, S.; Botzi, K.; Degrassi, C.; Kovalsky, P.; Schwab, C.; Schatzmayr, D.; Schatzmayr, G.; Fink-Gremmels, J. Interactions between plant-derived oestrogenic substances and the mycoestrogen zearalenone in a bioassay with MCF-7 cells. Pol. J. Vet. Sci. 2017, 20, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [Green Version]
- Miazzo, R.; Peralta, M.F.; Magnoli, C.; Salvano, M.; Ferrero, S.; Chiacchiera, S.; Carvalho, E.C.Q.; Rosa, C.A.R.; Dalcero, A.M. Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poult. Sci. 2005, 84, 1–8. [Google Scholar] [CrossRef]
- Dänicke, S.; Winkler, J. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals andtransfer of its residues into edible tissues (carry over). Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 84, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Changwa, R.; Abia, W.; Msagati, T.; Nyoni, H.; Ndleve, K.; Njobeh, P. Multi-Mycotoxin Occurrence in Dairy Cattle Feeds from the Gauteng Province of South Africa: A Pilot Study Using UHPLC-QTOF-MS/MS. Toxins 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyangi, C. Aflatoxins and fumonisin contamination of marketed maize, maize bran and maize used as animal feed in Northern Tanzania. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11054–11065. [Google Scholar] [CrossRef]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Hagler, W.M.J.; Swanson, S.P.; Phillips, T.D.; Creger, C.R. Individual and combined effects of aflatoxin and deoxynivalenol (DON, vomitoxin) in broiler chickens. Poult. Sci. 1986, 65, 1291–1298. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Ledoux, D.R.; Naehrer, K.; Berthiller, F.; Applegate, T.J.; Grenier, B.; Phillips, T.D.; Schatzmayr, G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015, 94, 1298–1315. [Google Scholar] [CrossRef]
- Broekaert, N.; Devreese, M.; van Bergen, T.; Schauvliege, S.; De Boevre, M.; De Saeger, S.; Vanhaecke, L.; Berthiller, F.; Michlmayr, H.; Malachová, A.; et al. In vivo contribution of deoxynivalenol-3-β-d-glucoside to deoxynivalenol exposure in broiler chickens and pigs: Oral bioavailability, hydrolysis and toxicokinetics. Arch. Toxicol. 2017, 91, 699–712. [Google Scholar] [CrossRef] [Green Version]
- Senerwa, D.M.; Mtimet, N.; Sirma, A.J.; Nzuma, J.; Kang’ethe, E.K.; Lindahl, J.F.; Grace, D. Direct market costs of aflatoxins in Kenyan dairy value chain. In Proceedings of the ANH Academy Week, Addis Ababa, Ethiopia, 20–24 June 2016. [Google Scholar]
- Mohammed, S.; Munissi, J.J.E.; Nyandoro, S.S. Aflatoxin M1 in raw milk and aflatoxin B1 in feed from household cows in Singida, Tanzania. Food Addit. Contam. Part B Surveill. 2016, 9, 85–90. [Google Scholar] [CrossRef]
- Kirino, Y.; Makita, K.; Grace, D.; Lindahl, J. Survey of informal milk retailers in Nairobi, Kenya and prevalence of aflatoxin M1 in marketed milk. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11022–11038. [Google Scholar] [CrossRef]
- Mulunda, F. Determination and Quantification of Aflatoxin M1 in Fresh Milk Samples Obtained in Goats and Cattle in Selected Rural Areas of the Limpopo Province, South Africa. J. Hum. Ecol. 2016, 56, 183–187. [Google Scholar] [CrossRef]
- Kagera, I.; Kahenya, P.; Mutua, F.; Anyango, G.; Kyallo, F.; Grace, D.; Lindahl, J. Status of aflatoxin contamination in cow milk produced in smallholder dairy farms in urban and peri-urban areas of Nairobi County: A case study of Kasarani sub county, Kenya. Infect. Ecol. Epidemiol. 2018, 9, 1547095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluwafemi, F.; Badmos, A.; Kareem, S.; Ademuyiwa, O.; Kolapo, A. Survey of aflatoxin M1 in cows’ milk from free-grazing cows in Abeokuta, Nigeria. Mycotoxin Res. 2014, 30. [Google Scholar] [CrossRef] [PubMed]
- Kuboka, M.M.; Imungi, J.K.; Njue, L.; Mutua, F.; Grace, D.; Lindahl, J.F. Occurrence of aflatoxin M1 in raw milk traded in peri-urban Nairobi, and the effect of boiling and fermentation. Infect. Ecol. Epidemiol. 2019, 9, 1625703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumantri, I.; Murti, T.W.; van der Poel, A.F.B.; Boehm, J.; Agus, A. Carry-over of aflatoxin B1-feed into aflatoxin M1-milk in dairy cows treated with natural sources of aflatoxin and bentonite. J. Indones. Trop. Anim. Agric. 2012, 37, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Coppock, R.W.; Christian, R.R.G.; Jacobsen, B.J. Aflatoxins. In Veterinary Toxicology: Basic and Clinical Principles; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Herzallah, S. Aflatoxin B1 residues in eggs and flesh of laying hens fed aflatoxin B1 contaminated diet. Am. J. Agric. Biol. Sci. 2013, 8, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.Z.; Nisar, S.; Asi, M.R.; Jinap, S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control 2014, 43, 98–103. [Google Scholar] [CrossRef]
- Sirma, A.J.; Lindahl, J.F.; Makita, K.; Senerwa, D.; Mtimet, N.; Kang’ethe, E.K.; Grace, D. The impacts of aflatoxin standards on health and nutrition in sub-Saharan Africa: The case of Kenya. Glob. Food Secur. 2018, 18, 57–61. [Google Scholar] [CrossRef]
- Bailey, C.A.; Fazzino, J.J.J.; Ziehr, M.S.; Sattar, M.; Haq, A.U.; Odvody, G. Evaluation of sorghum ergot toxicity in broilers. Poult. Sci. 1999, 78, 1391–1397. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.K.J.; Koreissi Dembélé, Y.; Eyangoh, S.; Verger, P.; et al. Regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria Reveals the Presence of 164 Mycotoxins and Other Secondary Metabolites in Foods. Toxins 2019, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- European Commission (EC). Setting Maximum Levels for Certain Contaminants in Foodstuffs; EC: Brussels, Belgium, 2006. [Google Scholar]
- Dvorska, J.E.; Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Effect of the mycotoxin aurofusarin on the antioxidant composition and fatty acid profile of quail eggs. Br. Poult. Sci. 2001, 42, 643–649. [Google Scholar] [CrossRef]
- Broomhead, J.N.; Ledoux, D.R.; Bermudez, A.J.; Rottinghaus, G.E. Chronic Effects of Moniliformin in Broilers and Turkeys Fed Dietary Treatments to Market Age. Avian Dis. 2002, 46, 901–908. [Google Scholar] [CrossRef]
- Harvey, R.; Edrington, T.; Kubena, L.; Rottinghaus, G.; Turk, J.; Genovese, K.; Nisbet, D. Toxicity of moniliformin from Fusarium fujikuroi culture material to growing barrows. J. Food Prot. 2001, 64, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, S.C.; Marasas, W.F.O.; Thiel, P.G.; Schneider, D.J.; Knox-Davies, P.S. Incidence and toxigenicity of seedborne Fusarium species from annual Medicago species in South Africa. Phytopathology 1986, 76, 1040–1042. [Google Scholar] [CrossRef]
- Kubena, L.; Harvey, R.; Buckley, S.; Edrington, T.; Rottinghaus, G. Individual and combined effects of moniliformin present in Fusarium fujikuroi culture material and aflatoxin in broiler chicks. Poult. Sci. 1997, 76, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.B.; Kubena, L.F.; Rottinghaus, G.E.; Turk, J.R.; Casper, H.H.; Buckley, S.A. Moniliformin from Fusarium fujikuroi culture material and deoxynivalenol from naturally contaminated wheat incorporated into diets of broiler chicks. Avian Dis. 1997, 41, 957–963. [Google Scholar] [CrossRef]
- Harvey, B.; Edrington, T.S.; Kubena, L.F.; Rottinghaus, G.E.; Turk, J.R.; Genovese, K.J.; Ziprin, R.L.; Nisbet, D.J. Toxicity of fumonisin from Fusarium verticillioides culture material and moniliformin from Fusarium fujikuroi culture material when fed singly and in combination to growing barrows. J. Food Prot. 2002, 65, 373–377. [Google Scholar] [CrossRef]
- Jestoi, M. Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Ducatelle, R.; Rychlik, M.; Antonissen, G. Chronic Dietary Intake of Enniatin B in Broiler Chickens Has Low Impact on Intestinal Morphometry and Hepatic Histology, and Shows Limited Transfer to Liver Tissue. Toxins 2018, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.M.; Cole, R.J.; Kirksey, J.W. Emodin, a toxic metabolite of Aspergillus wentii isolated from weevil-damaged chestnuts. Appl. Microbiol. 1975, 30, 26–28. [Google Scholar] [CrossRef] [Green Version]
- Grenier, B.; Oswald, I. Mycotoxin co-contamination of food and feed: Meta-Analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Klaric, M.S.; Rasic, D.; Peraica, M. Deleterious effects of mycotoxin combinations involving ochratoxin A. Toxins 2013, 5, 1965–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoev, S.D.; Paskalev, M.; MacDonald, S.; Mantle, P.G. Experimental one year ochratoxin a toxicosis in pigs. Exp. Toxicol. Pathol. Off. J. Ges. Toxikol. Pathol. 2002, 53, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Kang’ethe, E.K.; Korhonen, H.; Marimba, K.A.; Nduhiu, G.; Mungatu, J.K.; Okoth, S.A.; Joutsjoki, V.; Wamae, L.W.; Shalo, P. Management and mitigation of health risks associated with the occurrence of mycotoxins along the maize value chain in two counties in Kenya. Food Qual. Saf. 2017, 1, 268–274. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-Based Dilute-and-Shoot Approach for the Quantification of > 500 Mycotoxins and Other Secondary Metabolites in Food Crops: Challenges and Solutions. Anal. and Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef] [Green Version]

| All Feed and Feed Ingredients (n = 67) | Dairy Feed (n = 16) | Poultry Feed (n = 27) | Feed Ingredients (Cottonseed, Soybean Meal, Maize) (n = 24) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD (µg/kg) | % POSITIVE | GEOMEAN (µg/kg) | RANGE (µg/kg) | MEAN ± SD (µg/kg) | % POSITIVE | GEOMEAN (µg/kg) | RANGE (µg/kg) | MEAN ± SD (µg/kg) | % POSITIVE | GEOMEAN (µg/kg) | RANGE (µg/kg) | MEAN ± SD (µg/kg) | % POSITIVE | GEOMEAN (µg/kg) | RANGE (µg/kg) | MEAN ± SD (µg/kg) | |
| AFB1 | 0.2 | 69 | 2.3 | 0.5–134 | 18.3 ± 23.7 | 94 | 13.5 | 1.3–134 | 31.2 ± 34 | 93 | 4.7 | 0.5–38.8 | 10.2 ± 10 | 25 | 0.3 | 0.9–49.8 | 19.7 ± 17.2 |
| AFB2 | 0.06 | 45 | 0.2 | 0.4–22.1 | 3.4 ± 4.3 | 81 | 1.4 | 0.91–22.1 | 5.1 ± 5.8 | 48 | 0.2 | 0.4–4.4 | 1.7 ± 1 | 17 | 0.1 | 1.2–7 | 3.4 ± 2.1 |
| AFG1 | 0.2 | 58 | 1.1 | 0.2–123 | 13.7 ± 20.9 | 88 | 5.6 | 0.2–123 | 21.7 ± 29.8 | 70 | 1.2 | 0.6–41.7 | 6.7 ± 9.8 | 25 | 0.3 | 0.2–34.9 | 17.1 ± 11.8 |
| AFG2 | 0.5 | 31 | 0.6 | 0.5–28.5 | 5.1 ± 6 | 44 | 1 | 2.7–28.5 | 8.8 ± 8.6 | 33 | 0.5 | 0.5–6.4 | 2.5 ± 2 | 21 | 0.4 | 1.6–9.6 | 4.6 ± 2.9 |
| AFM1 | 0.1 | 22 | 0.1 | 0.4–11 | 2.6 ± 2.8 | 38 | 0.2 | 1.6–11 | 3.7 ± 3.3 | 15 | 0.1 | 0.4–0.6 | 0.5 ± 0.1 | 21 | 0.1 | 0.5–6.9 | 2.9 ± 2.3 |
| AFs | 0.1 | 70 | 2.3 | 0.2–318.5 | 34.5 ± 51.5 | 94 | 20.4 | 1.5–318.5 | 61.5 ± 76.6 | 93 | 6.2 | 0.5–89 | 17.2 ± 20.5 | 29 | 0.2 | 0.2–99.4 | 38.9 ± 33.1 |
| DON | 0.4 | 82 | 64.4 | 22.2–1037 | 317.5 ± 224.9 | 94 | 195.4 | 66.1–567 | 359.4 ± 159.1 | 100 | 271.3 | 28.2–1037 | 329.1 ± 203.2 | 54 | 6.1 | 22.2–996.1 | 244.9 ± 302.5 |
| DON-3-gluc | 1 | 72 | 5.7 | 2.0–63.4 | 18.5 ± 13.7 | 88 | 11.6 | 8.1–61.7 | 22.1 ± 14.8 | 100 | 13.9 | 3.8–45.7 | 16.4 ± 9.7 | 29 | 1.2 | 2–63.4 | 19.4 ± 21 |
| NIV | 0.02 | 70 | 0.9 | 0.4–285.7 | 26.1 ± 29.2 | 94 | 32.8 | 15.9–102.1 | 51.1 ± 26.8 | 96 | 31.9 | 12.1–105.5 | 43.2 ± 22.5 | 33 | 1.9 | 10–144 | 50.2 ± 46 |
| FA1 | 0.6 | 54 | 2.7 | 1.6–280.4 | 35.3 ± 51.2 | 38 | 1.7 | 13.8–83.2 | 39 ± 23.8 | 52 | 2 | 3.3–29.2 | 14.2 ± 8.1 | 67 | 5.3 | 1.6–280.4 | 52.2 ± 70.4 |
| FA2 | 0.6 | 66 | 4.7 | 2.4–175.6 | 31.2 ± 34.2 | 75 | 7.7 | 4.7–87.2 | 31.9 ± 24.9 | 74 | 5.9 | 2.4–103.1 | 24.5 ± 26 | 50 | 2.6 | 5.7–175.6 | 41.6 ± 48.6 |
| FB1 | 2 | 90 | 198.5 | 32.4–8345.6 | 742 ± 1223.6 | 100 | 311.2 | 52.4–1494 | 487.9 ± 407.1 | 100 | 305.5 | 38.4–1926 | 431.4 ± 387.2 | 71 | 90.5 | 32.4–8345.6 | 1474.4 ± 2034.7 |
| FB2 | 2 | 85 | 72.8 | 16.7–3313.1 | 325.4 ± 554.4 | 94 | 91 | 26.6–677.3 | 175.5 ± 158.3 | 96 | 102.2 | 23.5–728.8 | 172.9 ± 156.7 | 67 | 42.9 | 16.7–3313.1 | 713.8 ± 906.5 |
| FB3 | 6 | 73 | 32.6 | 10.3–948.3 | 136.3 ± 197.1 | 63 | 22.1 | 26.4–124.3 | 79.8 ± 30.6 | 85 | 36.7 | 20.4–243 | 70.8 ± 51.8 | 67 | 37 | 10.3–948.3 | 265.8 ± 299.4 |
| FB4 | 6 | 78 | 55.8 | 5.1–1283.4 | 127.2 ± 212.6 | 75 | 38.8 | 6.7–124.8 | 54.2 ± 34.9 | 89 | 41.3 | 5.5–387.8 | 73.7 ± 95.1 | 67 | 115.1 | 5.1–1283.4 | 262.3 ± 325.4 |
| FUM | 0.6 | 90 | 264.6 | 32.4–11, 658.7 | 1051.1 ± 1722.4 | 100 | 414.9 | 52.4–2171.3 | 652.4 ± 559.8 | 100 | 420 | 63.7–2684.8 | 597.9 ± 541.5 | 71 | 116.6 | 32.4–11,658.7 | 2146.2 ± 2612.9 |
| OTA | 1 | 3 | 0.55 | 11.9–13.8 | 12.9 ± 5.6 | 56 | 1.2 | 2–24.3 | 5.6 ± 6.8 | 19 | 0.4 | 2.5–10.6 | 4.8 ± 3 | 8 | 0.3 | 0.2–1.1 | 0.6 ± 0.4 |
| Ergot | 0.4 | 73 | 11.9 | 9.9–144 | 46.8 ± 49.9 | 63 | 1.7 | 0.6–285.7 | 56.9 ± 88 | 81 | 3.1 | 1.1–113.2 | 26 ± 32.4 | 63 | 0.2 | 0.4–24.8 | 5.9 ± 7.4 |
| HT-2 | 0.5 | 24 | 0.5 | 1.1–24.3 | 4.8 ± 1 | 6 | 0.6 | 11.9–11.9 | 11.9 ± 0 | 4 | 0.6 | 13.8–13.8 | 13.8 ± 0 | ND | ND | ND | ND |
| T-2 | 0.7 | 4 | 0.4 | 2.7–5.2 | 4.1 ± 1 | 13 | 0.5 | 2.7–4.4 | 3.5 ± 0.8 | 4 | 0.4 | 5.2–5.2 | 5.2 ± 0 | ND | ND | ND | ND |
| ZEN | 0.2 | 94 | 18.1 | 0.3–910.4 | 81.3 ± 165.7 | 100 | 19.9 | 3.9–140.2 | 35.2 ± 40.7 | 100 | 56.1 | 5.2–873.4 | 103.4 ± 178.6 | 83 | 4.8 | 0.3–910.4 | 71.3 ± 196.7 |
| February 2019 (n = 47) | August 2019 (n = 20) | |||||
|---|---|---|---|---|---|---|
| % POSITIVE | GEOMEAN (µg/kg) | RANGE (µg/kg) | % POSITIVE | GEOMEAN (µg/kg) | RANGE (µg/kg) | |
| AFB1 | 60 | 1.4 | 0.5–134 | 90 | 7.5 | 3.4–38.8 |
| AFB2 | 38 | 0.2 | 0.4–22.1 | 60 | 0.4 | 0.9–5.3 |
| AFG1 | 47 | 0.7 | 0.2–123 | 85 | 3.2 | 1.6–41.7 |
| AFG2 | 34 | 0.6 | 0.5–28.5 | 25 | 0.5 | 0.6–12.5 |
| AFM1 | 32 | 0.2 | 0.4–11 | ND | ND | ND |
| AFs | 62 | 1.2 | 0.2–318.5 | 90 | 10 | 3.4–89.0 |
| DON | 77 | 41.6 | 22.2–1037 | 95 | 179.9 | 28.2–743.3 |
| DON-3-gluc | 62 | 3.7 | 2–63.4 | 95 | 14.5 | 3.8–61.9 |
| NIV | 66 | 7.9 | 9.9–144 | 95 | 30.2 | 15.9–102.1 |
| FA1 | 72 | 5.7 | 1.6–280.4 | 10 | 0.5 | 21.7–43.9 |
| FA2 | 57 | 3.2 | 5.3–175.6 | 85 | 11.8 | 2.4–103.1 |
| FB1 | 85 | 145.9 | 32.4–8345.6 | 100 | 409 | 69.7–1926 |
| FB2 | 81 | 56.5 | 16.7–3313.1 | 95 | 132.3 | 28.3–728.8 |
| FB3 | 75 | 35.3 | 10.3–948.3 | 70 | 27 | 20.5–172.7 |
| FB4 | 81 | 49.5 | 5.1–1283.4 | 70 | 77.3 | 7.6–387.8 |
| FUM | 85 | 192 | 32.4–11,658.7 | 100 | 562.4 | 98–2654.8 |
| OTA | 6 | 0.3 | 0.2–24.3 | 65 | 1.4 | 1.9–10.6 |
| Ergot | 77 | 1.1 | 0.4–154.5 | 55 | 0.5 | 0.6–285.7 |
| HT-2 | ND | ND | ND | 10 | 0.7 | 11.9–13.8 |
| T-2 | 2 | 0.4 | 2.7 | 10 | 0.5 | 4.4–5.2 |
| ZEN | 92 | 14.4 | 0.3–910.4 | 100 | 30.8 | 4.2–131 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemboi, D.C.; Ochieng, P.E.; Antonissen, G.; Croubels, S.; Scippo, M.-L.; Okoth, S.; Kangethe, E.K.; Faas, J.; Doupovec, B.; Lindahl, J.F.; et al. Multi-Mycotoxin Occurrence in Dairy Cattle and Poultry Feeds and Feed Ingredients from Machakos Town, Kenya. Toxins 2020, 12, 762. https://doi.org/10.3390/toxins12120762
Kemboi DC, Ochieng PE, Antonissen G, Croubels S, Scippo M-L, Okoth S, Kangethe EK, Faas J, Doupovec B, Lindahl JF, et al. Multi-Mycotoxin Occurrence in Dairy Cattle and Poultry Feeds and Feed Ingredients from Machakos Town, Kenya. Toxins. 2020; 12(12):762. https://doi.org/10.3390/toxins12120762
Chicago/Turabian StyleKemboi, David Chebutia, Phillis E. Ochieng, Gunther Antonissen, Siska Croubels, Marie-Louise Scippo, Sheila Okoth, Erastus K. Kangethe, Johannes Faas, Barbara Doupovec, Johanna F. Lindahl, and et al. 2020. "Multi-Mycotoxin Occurrence in Dairy Cattle and Poultry Feeds and Feed Ingredients from Machakos Town, Kenya" Toxins 12, no. 12: 762. https://doi.org/10.3390/toxins12120762










