The Genomic Regions That Contain Ochratoxin A Biosynthetic Genes Widely Differ in Aspergillus Section Circumdati Species
Abstract
1. Introduction
2. Results
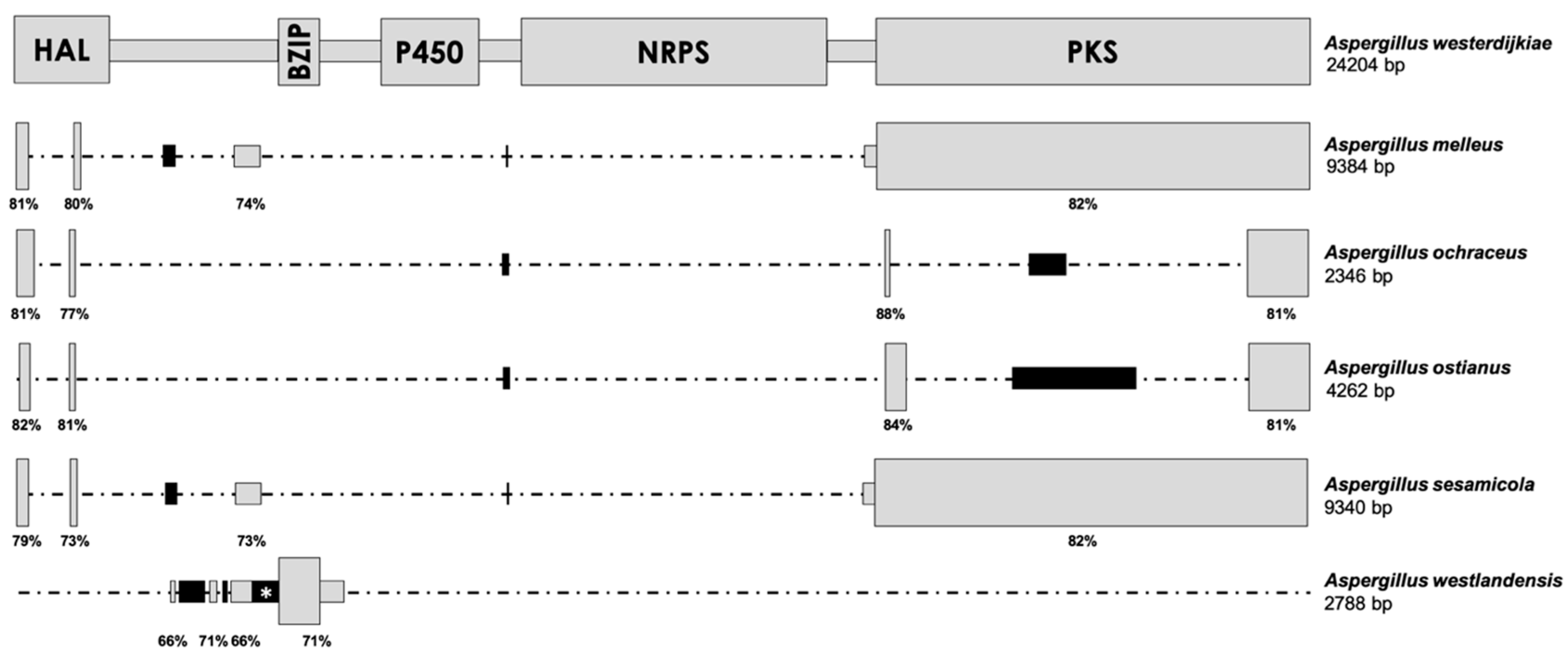
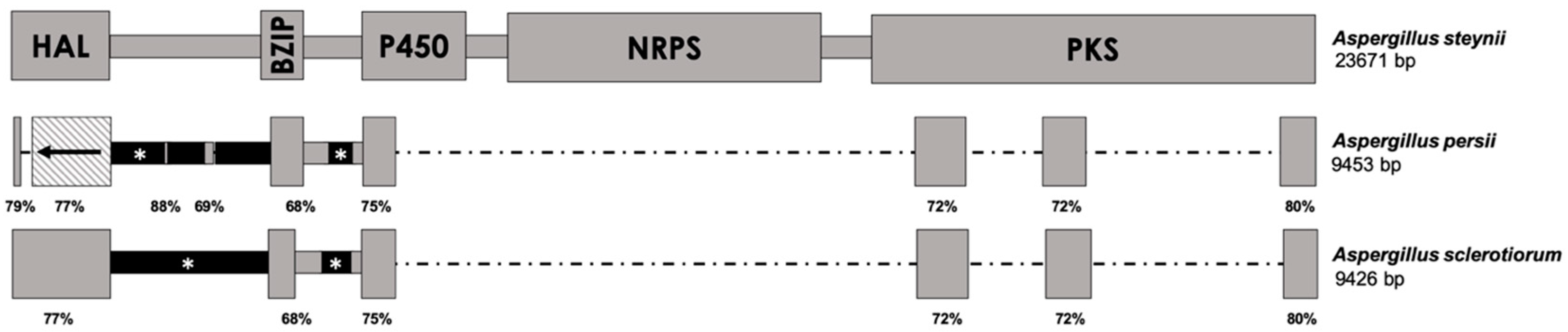
2.1. Analysis of the OTA Biosynthetic Cluster in Aspergillus Section Circumdati Species Containing the Complete Cluster
2.2. Analysis of the OTA Biosynthetic Cluster in Aspergillus Section Circumdati Species Containing the Truncated Cluster
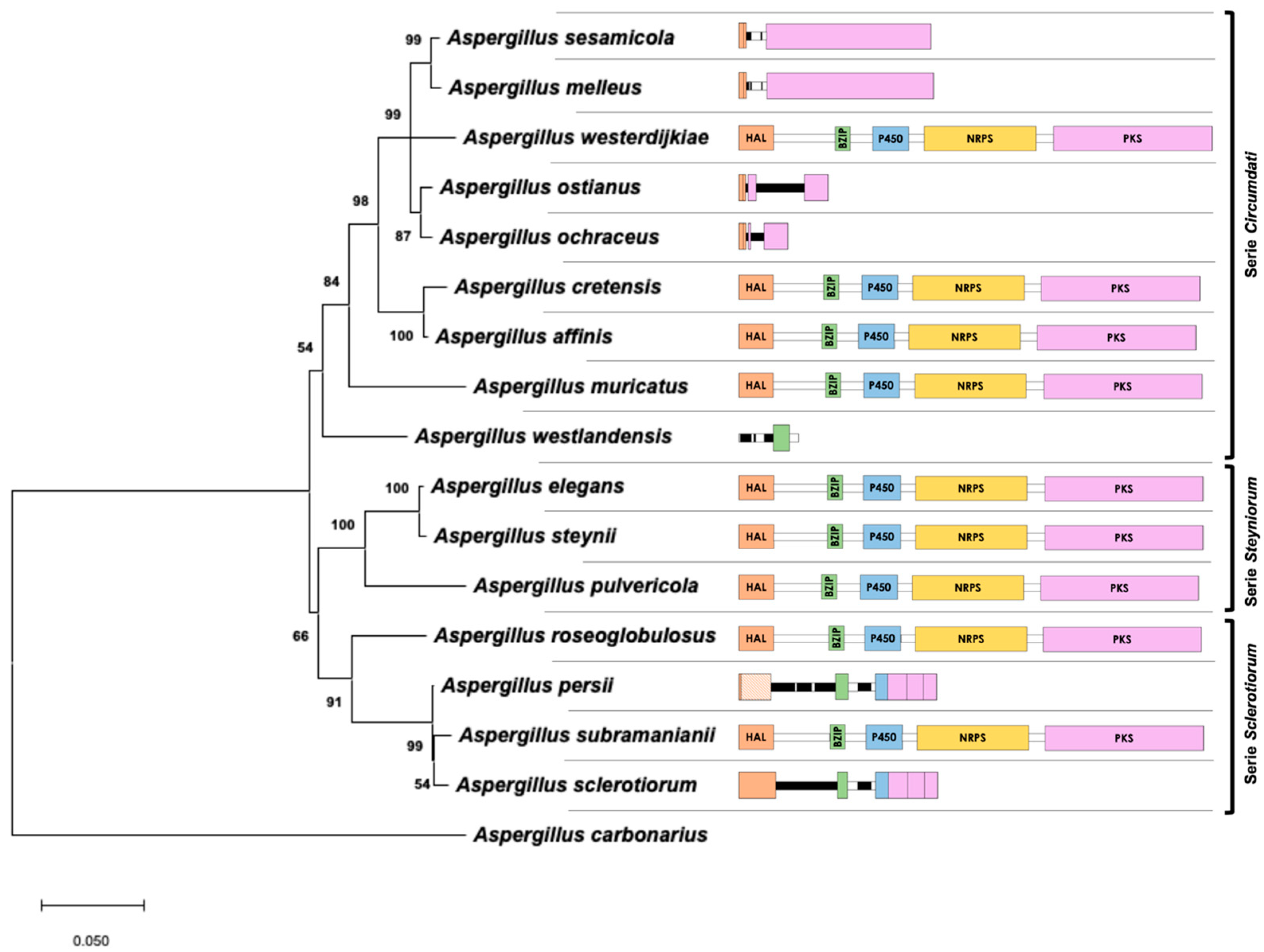
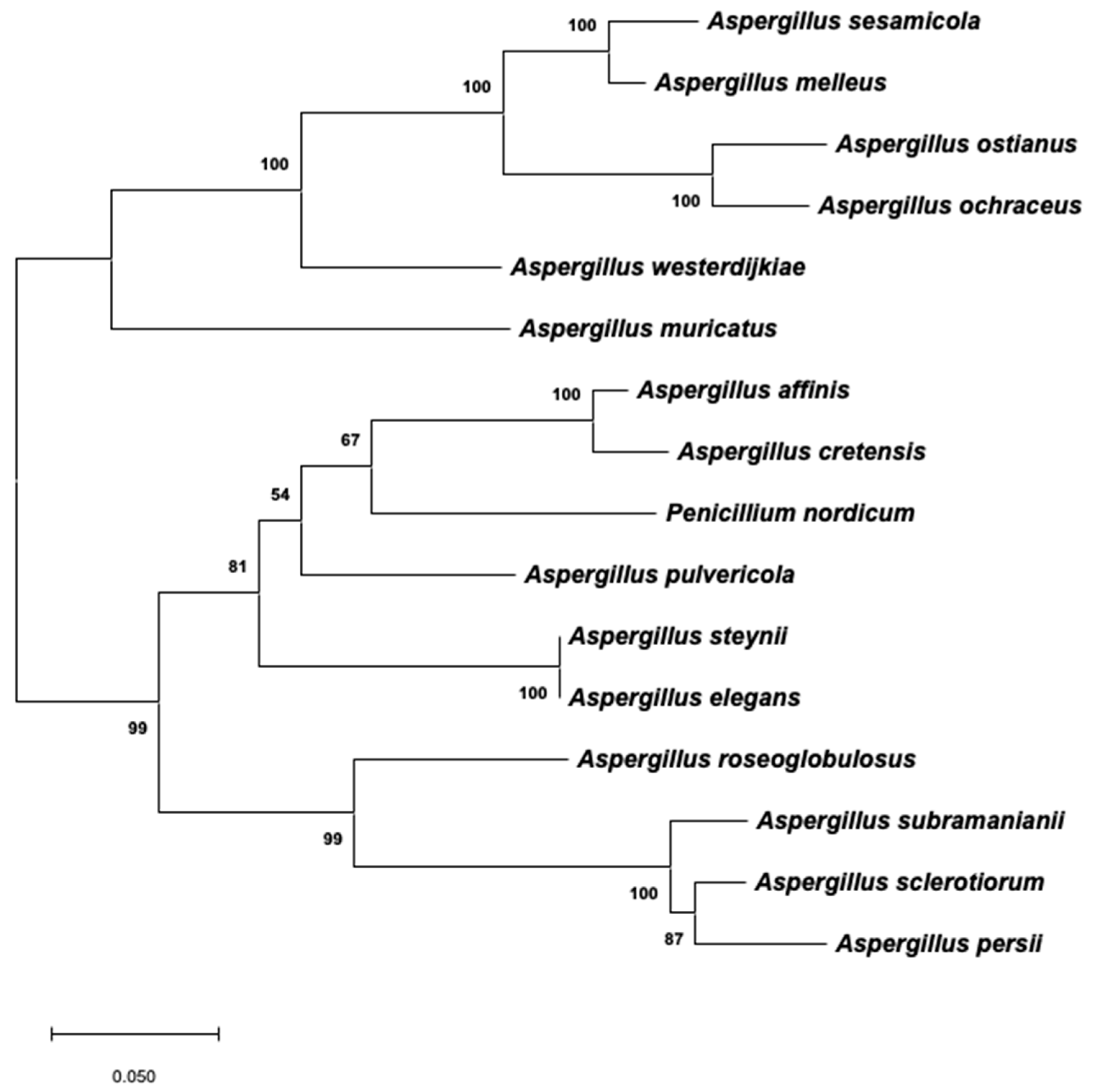
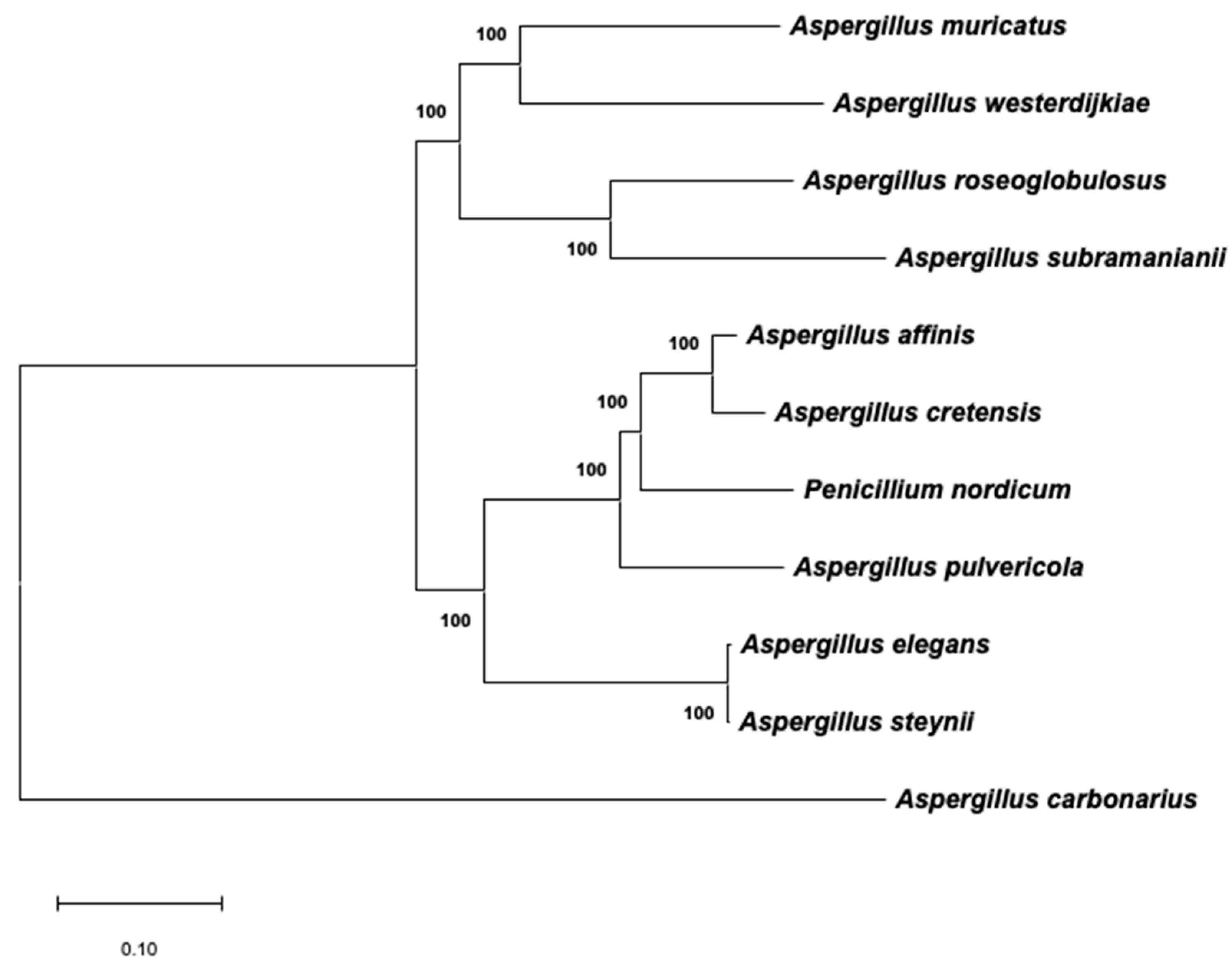
2.3. Phylogenetic Analysis of Aspergillus Section Circumdati Related to the Presence of the OTA Biosynthetic Cluster
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Putative OTA Cluster Determination in Aspergillus ochraceus Strains
5.2. Genome Sequencing of Aspergillus melleus and Aspergillus elegans
5.3. Sequence Analysis and Putative Cluster Identification in Other Aspergillus Section Circumdati Species
5.4. Bioinformatics Analysis for Ochratoxin A Biosynthetic Cluster Comparisons
5.5. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 years of research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.C.; Pena, A.; Lino, C.M. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010, 27, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Wine contamination with ochratoxins: A review. Beverages 2018, 4, 6. [Google Scholar] [CrossRef]
- Leitao, A.L. Occurrence of ochratoxin A in coffee: Threads and solutions—A mini-review. Beverages 2019, 5, 36. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Risk assessment of ochratoxin A in food. EFSA J. 2020, 18, 6113. [Google Scholar]
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Frank, J.M.; Houbraken, J.A.M.P.; Kuijpers, F.A.; Samson, R.A. New ochratoxin A producing species of Aspergillus section Circumdati. Stud. Mycol. 2004, 50, 23–43. [Google Scholar]
- Gil-Serna, J.; Vázquez, C.; Sardiñas, N.; González-Jaén, M.T.; Patiño, B. Discrimination of the main ochratoxin A-producing species in Aspergillus section Circumdati by specific PCR assays. Int. J. Food Microbiol. 2009, 136, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Vázquez, C.; Sardiñas, N.; González-Jaén, M.T.; Patiño, B. Revision of ochratoxin A production capacity by the main species of Aspergillus section Circumdati. Aspergillus steynii revealed as the main risk of OTA contamination. Food Control 2011, 22, 343–345. [Google Scholar] [CrossRef]
- Visagie, C.M.; Varga, J.; Houbraken, J.; Meijer, M.; Kocsubé, S.; Yilmaz, N.; Fotedar, R.; Seifert, K.A.; Frisvad, J.C.; Samson, R.A. Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati). Stud. Mycol. 2014, 78, 1–61. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Perrone, G.; Gallo, A. Aspergillus species and their associated mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Springer: New York, NY, USA, 2017; pp. 33–49. [Google Scholar]
- Cabañes, F.J.; Bragulat, M.R. Black aspergilli and ochratoxin A-producing species in foods. Curr. Opin. Food Sci. 2018, 23, 1–10. [Google Scholar] [CrossRef]
- Geisen, R.; Schmidt-Heydt, M.; Stoll, D.; Touhami, N. Aspects of the occurrence, genetics, and regulation of biosynthesis of the three food relevant Penicillium mycotoxins: Ochratoxin A, citrinin and patulin. In The Mycota XV. Physiology and Genetics, 2nd ed.; Anke, T., Schüffler, A., Eds.; Springer: New York, NY, USA, 2018; pp. 413–433. [Google Scholar]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.-B.; Nováková, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenár, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Ferrara, M.; Perrone, G. Recent advances on the molecular aspects of ochratoxin A biosynthesis. Curr. Opin. Food Sci. 2017, 17, 49–56. [Google Scholar] [CrossRef]
- El Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Gil-Serna, J.; García-Díaz, M.; González-Jaén, M.T.; Vázquez, C.; Patiño, B. Description of an orthologous cluster of ochratoxin A biosynthetic genes in Aspergillus and Penicillium species. A comparative analysis. Int. J. Food Microbiol. 2018, 268, 35–43. [Google Scholar] [CrossRef]
- Susca, A.; Proctor, R.H.; Morelli, M.; Haidukowski, M.; Gallo, A.; Logrieco, A.F.; Moretti, A. Variation in fumonisin and ochratoxin production with differences in biosynthetic gene content in Aspergillus niger and A. welwitschiae isolates from multiple crop and geographic origins. Front. Microbiol. 2016, 7, 1412. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Genetic regulation of aflatoxin, ochratoxin A, trichothecene, and fumonisin biosynthesis: A review. Int. Microbiol. 2020, 23, 89–96. [Google Scholar] [CrossRef]
- Peterson, S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 2008, 100, 205–226. [Google Scholar] [CrossRef]
- Kusumoto, K.I.; Nogata, Y.; Ohta, H. Directed deletions in the aflatoxin biosynthesis gene homolog cluster of Aspergillus oryzae. Curr. Genet. 2000, 37, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.N.; Bandyopadhyay, R.; Cotty, P.J. Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 2016, 6, 62. [Google Scholar] [CrossRef]
- Gil-Serna, J.; García-Díaz, M.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Significance of Aspergillus niger aggregate species as contaminants of food products in Spain regarding their occurrence and their ability to produce mycotoxins. Food Microbiol. 2019, 82, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.A.; Staats, M.; van Kan, J.A.L.; Rokas, A.; Slot, J.C. Repeated loss of an anciently horizontally transferred gene cluster in Botrytis. Mycologia 2013, 105, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Fernane, F.; Cano-Sancho, G.; Sanchis, V.; Marín, S.; Ramos, A.J. Aflatoxins and ochratoxin A in pistachios sampled in Spain: Occurrence and presence of mycotoxigenic fungi. Food Addit. Contam. Part B Surveill. 2010, 3, 185–192. [Google Scholar] [CrossRef]
- Jeswal, P.; Kumar, D. Mycofloral association and co-occurrence of ochratoxin A and citrinin in aromatic herbs and spices from Bihar state (India) detected by ELISA and LC-MS/MS. Am. J. Biol. Chem. Pharm. Sci. 2013, 1, 96–105. [Google Scholar]
- Priyanka, S.R.; Venkataramana, M.; Kumar, G.P.; Rao, V.K.; Murali, H.C.S.; Batra, H.V. Occurrence and molecular detection of toxigenic Aspergillus species in food grain samples from India. J. Sci. Food Agric. 2014, 94, 537–543. [Google Scholar] [CrossRef]
- Jeswal, P.; Kumar, D. Mycobiota and natural incidence of aflatoxins, ochratoxin A, and citrinin in Indian spices confirmed by LC-MS/MS. Int. J. Microbiol. 2015, 242486. [Google Scholar] [CrossRef]
- Nyongesa, B.W.; Okoth, S.; Ayugi, V. Identification key for Aspergillus species isolated from maize and soil of Nandi County, Kenia. Adv. Microbiol. 2015, 5, 55282. [Google Scholar] [CrossRef]
- Pandit, P.; Panta, O.; Karki, T. Isolation of Aspergillus ochraceus and production of ochratoxin in coffee samples. Nepal J. Sci. Technol. 2015, 15, 133–138. [Google Scholar] [CrossRef]
- Orole, O.O. Ochratoxin A production by cocoa infested Aspergillus species in Ondo State, Nigeria. Biotechnol. J. Int. 2016, 12, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.M.; Zhao, Y.; Xing, F.; Yin, W.-B.; et al. A consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genome analysis. App. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shi, J.; Jiang, C.; Liu, Y. Inhibition of the growth and ochratoxin A production by Aspergillus carbonarius and Aspergillus ochraceus in vitro and in vivo through antagonistic yeasts. Food Control 2015, 50, 125–132. [Google Scholar] [CrossRef]
- Storari, M.; Bigler, L.; Gessler, C.; Broggini, G.A.L. Assessment of the ochratoxin A production ability of Aspergillus tubingensis. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk. Assess. 2012, 29, 1450–1454. [Google Scholar] [CrossRef]
- Stajich, J.E. Fungal genomes and insights into the evolution of the kingdom. Microbiol. Spectr. 2018, 5, 1–25. [Google Scholar]
- Gillot, G.; Jany, J.L.; Domínguez-Santos, R.; Poirier, E.; Debaets, S.; Hidalgo, P.I.; Ullán, R.V.; Coton, E.; Coton, M. Genetic basis for mycophenolic acid production and strain-dependent production variability in Penicillium roqueforti. Food Microbiol. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Evolutionary formation of gene clusters by reorganization: The meleagrin/roquefortine paradigm in different fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1579–1587. [Google Scholar] [CrossRef]
- Frías-De-León, M.G.; Espinosa-Hernández, V.M.; Bonifaz, A.; Martínez-Herrera, E. Onychomycosis due to Aspergillus spp.: A current review. Curr. Fungal Infect. Rep. 2018, 12, 112–119. [Google Scholar] [CrossRef]
- Oetari, A.; Susetyo-Salim, T.; Sjamsuridzal, W.; Suherman, E.A.; Monica, M.; Wongso, R.; Fitri, R.; Nurlaili, D.G.; Ayu, D.C.; Teja, T.P. Occurrence of fungi on deteriorated old dluwang manuscripts from Indonesia. Int. Biodeterior. Biodegrad. 2016, 114, 94–103. [Google Scholar] [CrossRef]
- Davolos, D.; Persiani, A.M.; Pietrangeli, B.; Ricelli, A.; Maggi, O. Aspergillus affinis sp. nov., a novel ochratoxin A-producing Aspergillus species (section Circumdati) isolated from decomposing leaves. Int. J. Syst. Evol. Microbiol. 2012, 62, 1007–1015. [Google Scholar] [CrossRef]
- Nováková, A.; Hubka, V.; Valinová, S.; Kolarík, M.; Hillebrand-Voiculescu, A.M. Cultivable microscopic fungi from an underground chemosynthesis-based ecosystem: A preliminary study. Folia Microbiol. 2018, 63, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Castellá, G.; Bragulat, M.R.; Puig, L.; Sanseverino, W.; Cabañes, F.J. Genomic diversity in ochratoxigenic and non ochratoxigenic strains of Aspergillus carbonarius. Sci. Rep. 2018, 8, 5439. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lan, F.; Yang, W.; Zhang, F.; Yang, K.; Li, Z.; Gao, P.; Wang, S. sRNA profiling in Aspergillus flavus reveals differentially expressed miRNA-like RNAs response to water activity and temperature. Fungal Genet. Biol. 2015, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Chitara, W.; Siciliano, I.; Gaiotti, F.; Ciuffo, M.; Forgia, M.; Varese, G.C.; Turina, M. Mycoviruses mediate mycotoxin regulation in Aspergillus ochraceus. Environ. Microbiol. 2018, 21, 1957–1968. [Google Scholar] [CrossRef]
- Varga, J.; Baranyi, N.; Chandrasekaran, M.; Vágvölgyi, C.; Kocsubé, S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015, 59, 151–167. [Google Scholar]
- Abdel-Azeem, A.M.; Abdel-Azeem, M.A.; Abdul-Hadi, S.Y.; Darwish, A.G. Aspergillus: Biodiversity, ecological significances and industrial applications. In Recent Advancement in White Biotechnology through Fungi; Yadav, A.N., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: New York, NY, USA, 2019; pp. 121–179. [Google Scholar]
- Magan, N.; Aldred, D. Why do fungi produce mycotoxins? In Food Mycology. A Multifaceted Approach to Fungi and Food; Dijksterhuis, J., Samson, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 121–133. [Google Scholar]
- Querol, A.; Barrio, E.; Huerta, T.; Ramón, D. Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl. Environ. Microbiol. 1992, 58, 2948–2953. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; the UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knya, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
| Species | Cluster Length | Accession Number | Identity with OTA Cluster | |
|---|---|---|---|---|
| A. steynii | A. westerdijkiae | |||
| A. affinis | 23,387 | MT955635 | 75% | 67% |
| A. cretensis | 23,586 | MT955636 | 73% | 66% |
| A. elegans | 23,680 | MT904985 | 99% | 67% |
| A. muricatus | 23,696 | MT955638 | 70% | 70% |
| A. pulvericola | 23,409 | MT904986 | 74% | 66% |
| A. roseoglobulosus | 23,599 | MT955637 | 70% | 67% |
| A. subramanianii | 23,730 | MT955639 | 68% | 65% |
| Species | β-Tubulin | ITS1-5.8S-ITS2 | Calmodulin |
|---|---|---|---|
| Aspergillus affininis CBS 129190 | GU721092 | JN882309 | GU721091 |
| Aspergillus cretensis CBS 112802 | AY819977 | FJ491572 | FJ491534 |
| Aspergillus elegans CBS 102.14 | EF661349 | EF661414 | EF661390 |
| Aspergillus melleus CBS 546.65 | EF661326 | EF661425 | EF661391 |
| Aspergillus muricatus CBS 112808 | EF661356 | EF661434 | EF661377 |
| Aspergillus ochraceus CECT 2093 | EF661322 | EF661419 | EF661381 |
| Aspergillus ostianus CBS 103.07 | EF661324 | EF661421 | EF661385 |
| Aspergillus persii CBS 112795 | AY819988 | FJ491580 | FJ491559 |
| Aspergillus pulvericola CBS 137327 | KJ775055 | KJ775440 | KJ775236 |
| Aspergillus roseoglobulosus CBS 112800 | AY819984 | FJ491583 | FJ491555 |
| Aspergillus sclerotiorum CBS 549.65 | EF661337 | EF661400 | EF661384 |
| Aspergillus sesamicola CBS 137324 | KJ775063 | KJ775437 | KJ775233 |
| Aspergillus steynii CBS 112.812 | EF661347 | EF661416 | EF661378 |
| Aspergillus subramanianii CBS 138230 | EF661339 | EF661403 | EF661397 |
| Aspergillus westerdijkiae CECT 2948 | EF661329 | EF661427 | EF661360 |
| Aspergillus westlandensis CBS 123905 | KJ775065 | KJ775433 | KJ775229 |
| Aspergillus carbonarius NRRL 4849 | EF661100 | EF661205 | EF661168 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Serna, J.; Vázquez, C.; Patiño, B. The Genomic Regions That Contain Ochratoxin A Biosynthetic Genes Widely Differ in Aspergillus Section Circumdati Species. Toxins 2020, 12, 754. https://doi.org/10.3390/toxins12120754
Gil-Serna J, Vázquez C, Patiño B. The Genomic Regions That Contain Ochratoxin A Biosynthetic Genes Widely Differ in Aspergillus Section Circumdati Species. Toxins. 2020; 12(12):754. https://doi.org/10.3390/toxins12120754
Chicago/Turabian StyleGil-Serna, Jéssica, Covadonga Vázquez, and Belén Patiño. 2020. "The Genomic Regions That Contain Ochratoxin A Biosynthetic Genes Widely Differ in Aspergillus Section Circumdati Species" Toxins 12, no. 12: 754. https://doi.org/10.3390/toxins12120754
APA StyleGil-Serna, J., Vázquez, C., & Patiño, B. (2020). The Genomic Regions That Contain Ochratoxin A Biosynthetic Genes Widely Differ in Aspergillus Section Circumdati Species. Toxins, 12(12), 754. https://doi.org/10.3390/toxins12120754








