Abstract
Petroleum hydrocarbon (PHC) contamination of soil is a widespread global environmental concern due to the persistence and recalcitrant nature of PHCs. The PHCs are highly toxic and their removal from the terrestrial ecosystem is necessary to maintain soil as well as human health. Here, a pot experiment was performed to examine the impact of Enterobacter sp. MN17 and biochar addition on the growth of mungbean plants and PHCs removal from diesel-polluted soil. For this purpose, soil was contaminated artificially with diesel to achieve a final concentration of 5000 mg kg−1. Untreated and Enterobacter sp. MN17 treated mungbean seeds were sown in pots. Sugarcane bagasse biochar was applied as an amendment in respective pots along with the recommended levels of essential nutrients. Results showed that PHCs significantly suppressed the seedling emergence as well as agronomic and physiological attributes of mungbean as compared to un-contaminated controls. However, the co-application of Enterobacter sp. MN17 and biochar significantly reduced the phytotoxicity of PHCs to mungbean plants and effectively increased the seedling emergence, shoot and root length, shoot fresh and dry biomass, root fresh and dry biomass of plants up to 24%, 54%, 52%, 52%, 54%, 55% and 60%, respectively as compared to controls. Similarly, 30%, 57%, 64%, 36% and 57% increase in chlorophylls contents, transpiration rate, stomatal conductance, sub-stomatal conductance, and photosynthetic rate, respectively were observed in their combined application as compared to respective controls. Furthermore, the co-addition of biochar and Enterobacter sp. MN17 could remove 69% and 85% higher PHCs from unplanted and planted pots, respectively, than that of their respective controls. Our results suggest that the co-application of biochar and Enterobacter sp. MN17 may be useful in enhancing plant growth and eliminating PHCs from contaminated soil.
1. Introduction
To overcome the energy demands of the fast-growing industry and human population, the daily use of petroleum hydrocarbons (PHCs) is increasing alarmingly [1]. PHCs are a mixture of different compounds comprising aliphatic, aromatic, resins, and asphaltenes that are highly toxic to living beings [2,3]. The contamination of PHCs in the soil mostly occurs due to accidental spills, underground storage malfunction, extraction and transportation losses and causes a serious environmental threat to all living communities due to recalcitrance and the xenobiotic nature of PHCs [4,5]. The soil contamination with PHCs results in infertile soil because of hydrophobic conditions that ultimately lead to low plant and microbial growth [6]. PHCs affect physico-chemical properties of soils such as pH, electrical conductivity (EC), total exchangeable cation, soil moisture and oxygen contents that disrupt micro-climate around the soil and impairs plant growth by reducing soil porosity [7,8].
Among different PHC remediation techniques, bioremediation—particularly microbe-assisted phytoremediation—is considered the most efficient and environmentally friendly remediation approach [9]. PHCs removal through microbe-assisted phytoremediation in soil is currently a promising strategy to reduce oil pollution [10]. The removal efficiency of PHCs can be enhanced by strengthening the plant rhizosphere [11]. Plant growth and development is directly improved by the microbial actions as siderophores production, phosphorus solubilization, nitrogen-fixation and ACC-deaminase activities [12,13]. In return, plant roots excrete enormous amounts of vitamins, organic acids, hormones, amino acids, mucilage, sugars and other substances that stimulate microbial activities in the rhizosphere [14,15]. Most of these root exudates have structural similarities with PHC aromatic fractions [16,17]. Thus, microbes use PHCs as a carbon source to increase their biomass and subsequently remove PHCs from soil [18].
Although biochar addition to agricultural soils may pose potential risks to food safety due to the release of polycyclic aromatic hydrocarbons from the biochar [19], its addition to contaminated soils improves the properties of soil such as fertility, nutrient status, porosity, pH, water holding capacity and oxygen supply [20]. Biochar also minimizes the extent of pollutant’s toxicity by precipitation, surface adsorption, sequestration and partitioning [21]. Furthermore, the addition of biochar also promotes microbial growth and their enzymatic activities by providing substrates and shelter for microbiota in the soil [22,23]. Hence, biochar and microbe addition could be helpful in minimizing pollutant toxicity, boosting plant growth and improving the removal of PHCs from the contaminated soil [23].
Legumes can easily grow in different habitats including nutrient-deficient soils due to their nitrogen-fixing abilities [24]. Nitrogen fixation makes legume plants suitable for the restoration of soil health and fertility status of poor or contaminated lands [25]. Mungbean is an important legume crop and is ranked second among pulses in Pakistan [26]. The total area under mungbean cultivation in Pakistan is about 0.25 million hectares, with 178 tons of total production (grains) and with an estimated yield of 515 kg ha−1 [27]. Although mungbean plants have the ability to grow in contaminated soils [28], studies reporting the effects of biochar and microbe addition on mungbean growth and PHC removal from diesel contaminated soil are still limited [22]. Therefore, the aim of this study was to examine the effects of biochar and Enterobacter sp. MN17 addition on the removal of PHCs and growth of mungbean in diesel-polluted soil.
2. Materials and Methods
2.1. Experimental Design
A pot trial was carried out at the research area of the University of Agriculture, Faisalabad (UAF) to examine the impact of biochar and Enterobacter sp. MN17 on physiology and growth of mungbean plants in artificially contaminated soil with diesel. The diesel was purchased from Shell petrol filling station, Faisalabad, Pakistan. Clean soil was collected from the research area of UAF and sieved through a 2-mm sieve. Each pot was filled with 8 kg of soil and then pots were placed according to completely randomized design (CRD). The calculated amount of diesel was applied to the pots to get a final concentration of 5000 mg kg−1 (this concentration was selected on the basis of the results of our initial experiments on PHC phytotoxicity to mungbean germination and growth). Acetone was used as a carrier solvent for spiking. After spiking, the soil was thoroughly mixed and left for one month to enable the biochemical characteristics of the soil and diesel to become stable [29].
Mungbean (Vigna radiata L.), variety AZRI-2006 was selected as a test plant due to its widespread nature, root morphology, fast growth and adaptation characteristics [27]. The selected pots were amended with 1% biochar (w/w) [30]. Previously isolated and identified endophytic bacterium Enterobacter sp. MN17 (deposited in National Center for Biotechnology Information, with accession number KT375575) was obtained from the Laboratory of Soil and Environmental Microbiology, ISES, UAF [31]. The Enterobacter sp. MN17 was grown in Erlenmeyer flask (250 mL) carrying a mineral salt medium (MSM) [32] at 28 ± 2 °C and 180 rpm for 72 h. The culture optical density (OD600) was determined using a spectrophotometer (Thermo Electron Corporation, Evolution-300LC, Warwickshire, UK) and OD600 was adjusted to 0.5 to get a constant count of bacterial cells (108–109 CFU mL−1) for use [33]. Mungbean seeds were surface sterilized by soaking them in a 5% NaClO solution for 120 s and then soaked them in 70% C2H5OH solution for another 120 s, then rinsed them thoroughly with autoclaved distilled water [22]. Mungbean seeds were dipped for 30 min in bacterial culture. While, for controls, seeds were soaked in sterilized MSM for 30 min.
Six mungbean seeds (treated or un-treated) were sown in the respective pots containing diesel contaminated or uncontaminated soil. After seedling establishment, only three seedlings were maintained in each pot. The suggested amount of N, P, and K fertilizers (i.e., 22.5 kg N ha−1 as urea, 57.5 kg P ha−1 as super-phosphate, and 30 kg K ha-1 as sulfate of potash) were applied to each pot [34]. The experiment was performed in triplicate with nine different treatments, including two levels of PHCs (0 and 5000 mg kg−1) in different combinations with biochar and Enterobacter MN17, with a total twenty-seven experimental units (see Table S1 for detail). The relative humidity was 67.4% at the start of experiment, and remained in the range of 64.4–65.5% during plant growth till harvesting. All twenty-seven pots were harvested after 90 d. Agronomic and physiological attributes of plants and remaining diesel contents in soil were determined.
2.2. Physicochemical Properties of Soil and Biochar
Before experiment, various physicochemical characteristics of soil and biochar were determined and are shown in Table S2. Biochar was prepared from sugarcane bagasse at 400 °C in a laboratory setup muffle furnace. The hydrometer was used to determine the texture of the soil, and electrical conductivity, pH and organic matter were determined following the methods described in [33]. While contents of N, K, and P were determined using standard protocols via the Kjeldahl apparatus, a flame photometer (FP7, Jenway, Essex, UK) and a spectrophotometer (T60 UV-Visible Spectrophotometer-PG Instruments Limited, Leicestershire, UK), respectively [35,36].
2.3. Seedling Emergence, Physiological and Growth Attributes of Mungbean Plants
Seedling emergence was monitored every day until there was a constant count. Root and shoot lengths were taken by measuring stick, and dry and fresh weights of shoots and roots were recorded using weighing balance after 90 d of sowing. For dry plant biomass, shoots and roots were dried in an oven at 65 °C till constant values. The SPAD meter (SPAD-502 Konica, Minolta, Japan) was used to take the SPAD value (relative chlorophyll content) after 45 d of sowing, while the rate of transpiration, stomatal conductance, sub-stomatal conductance and photosynthetic rate were determined using an infrared gas analyzer (IRGA) (LCi Bio Scientific Ltd., Herts, UK) [37].
2.4. Petroleum Hydrocarbons Analysis of Soil
PHCs analysis was carried out following the standard protocol. Briefly, after thoroughly mixing the soil in each pot, soil sample was collected from each pot at the end of experiment for PHCs analysis. The PHCs analysis for soil was performed using TPH analyzer (PHA-100 plus, PETROSENSE, San Diego, CA, USA) following instructions provided on the training manual of PetroSense® (please see detail on www.petrosense.com, www.equipcoservices.com), with slight modifications. Briefly, 50 g of soil was added to the glass jar and mixed with deionized water until a small layer formed on the top of the soil. Finally, a probe was inserted in the jar to take measurement [38]. Before taking the next measurement, the probe was allowed to sit for five minutes in room air.
2.5. Statistical Analysis
The Statistix 8.1 software was used to check the significant relationship between the treatments by applying F-test and least significant difference (LSD) test was used to compare the means 5% level of significance [39]. To estimate the variation from means (n = 3) values by the standard deviation, the analysis of variance (ANOVA) was done on given data. The relationship among all the studied parameters of mungbean plants was determined by Spearman’s correlation analysis performed in R software (Version 2.3.1). The principal component analysis (PCA) was also performed in R software to compare the response of Enterobacter sp. MN17 and biochar to augment remediation of petroleum hydrocarbons and growth of mungbean in diesel contaminated soil.
3. Results
3.1. Seedling Emergence and Agronomic Parameters of Mungbean Plants
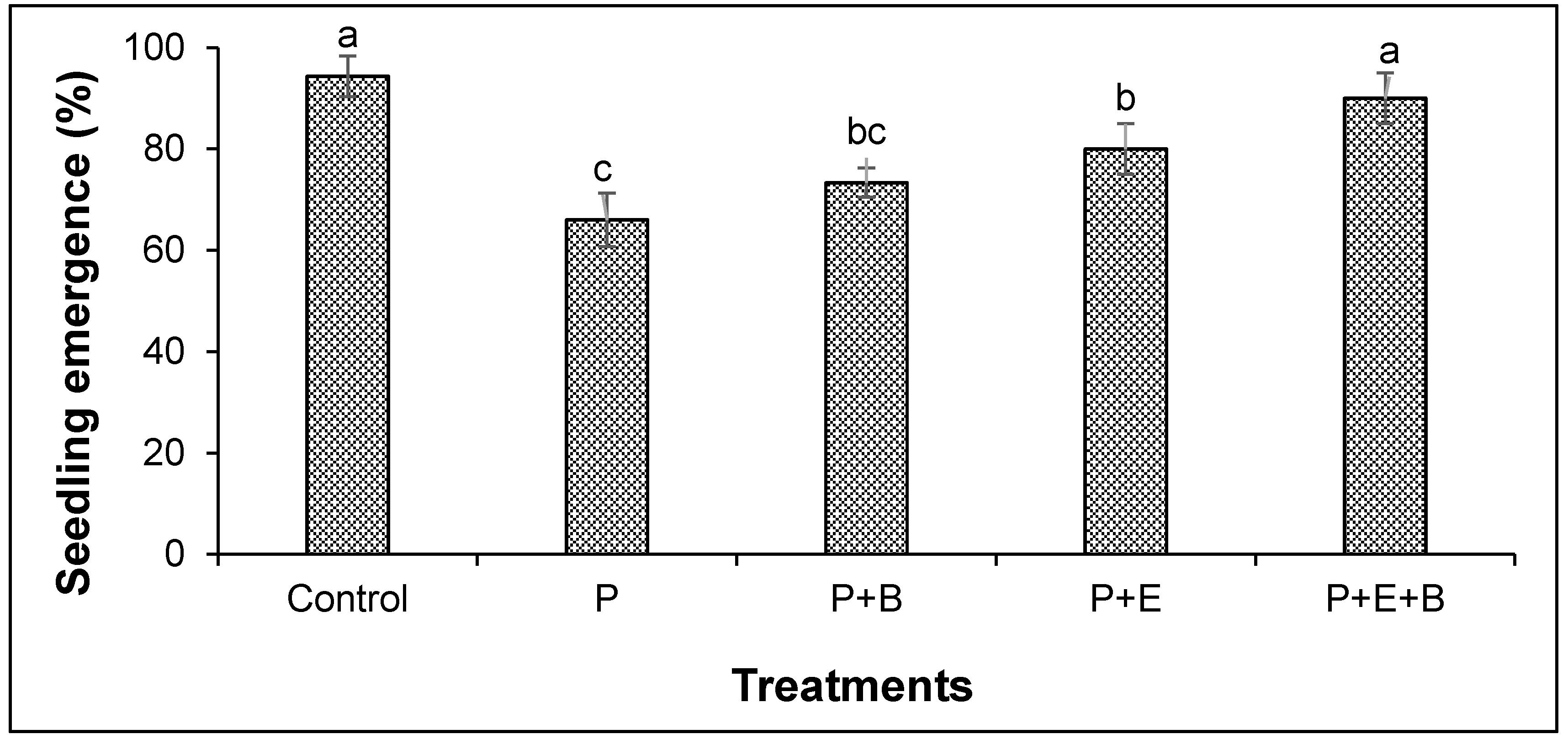
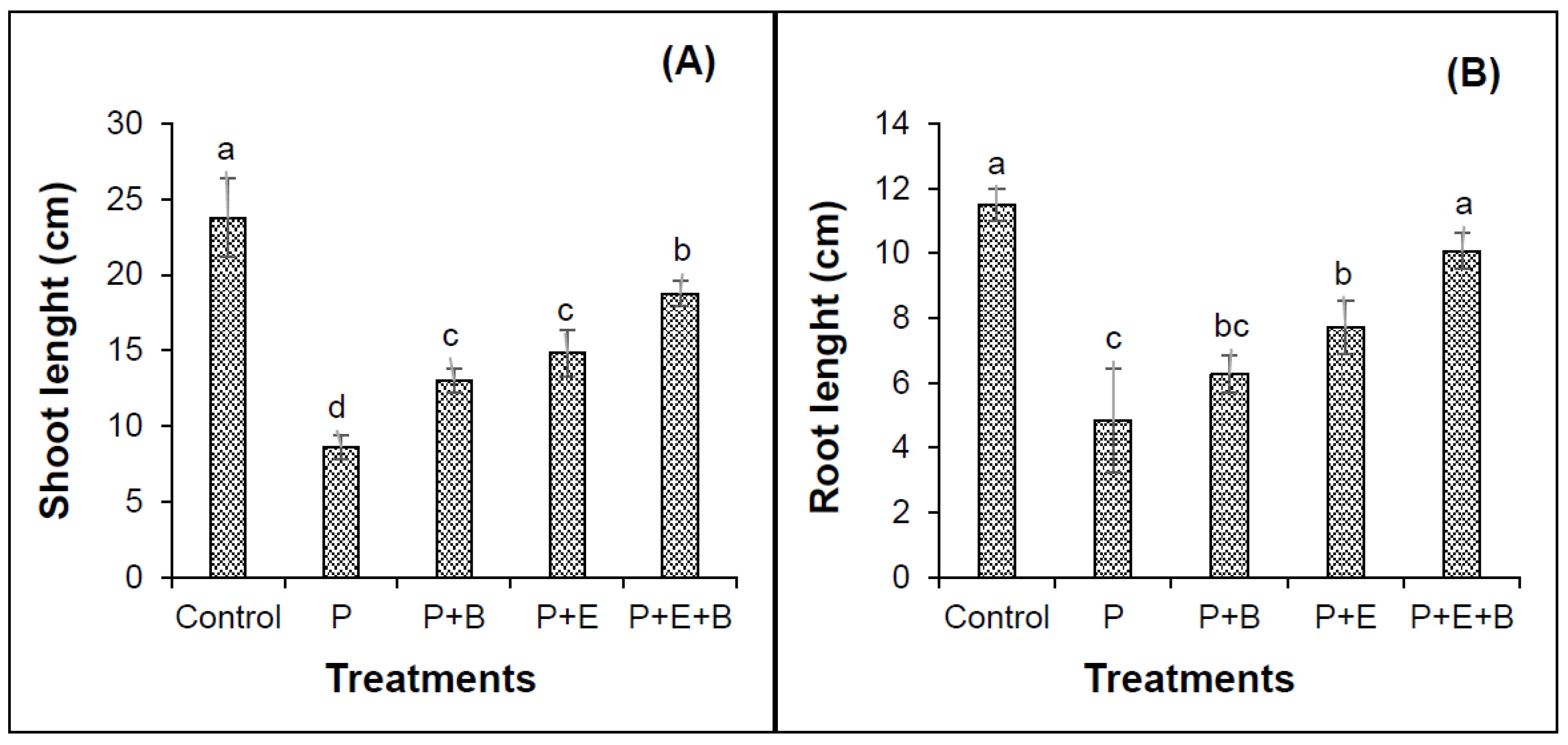
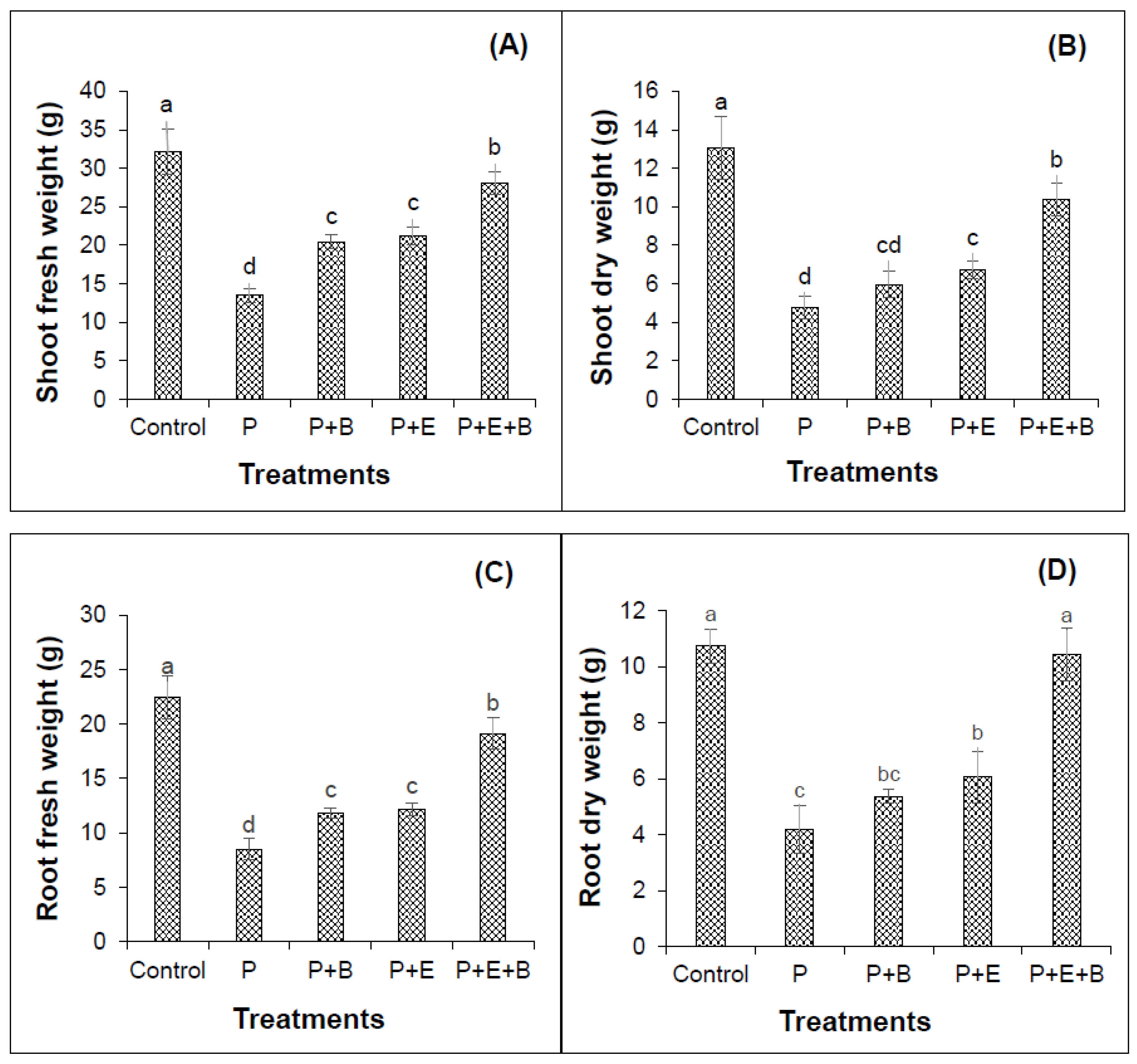
Our results indicated that PHC contamination substantially reduced the seedling emergence (Figure 1). Furthermore, irrespective of treatments, seedlings started to emerge after four days of sowing in most of the pots with only a few exceptions. Interestingly, the co-addition of biochar and Enterobacter MN17 showed significant improvement in seedling emergence when compared with planted control or their individual application (Figure 1), indicating a significant effect of biochar and strain MN17 application on the emergence of mungbean seedlings. The PHCs also showed significant phytotoxicity to mungbean plants and significantly (p ≤ 0.05) reduced the root and shoot lengths, and fresh and dry biomass (Figure 2 and Figure 3). About 138% and 175% reduction in shoot and root lengths, respectively, were observed in the treatment with PHCs (5000 mg kg−1) compared only to the planted control (Figure 2). Moreover, when compared with control (with plants but without diesel or any amendments), 138%, 173%, 163% and 156% reduction in shoot fresh weights, shoot dry weight, root fresh weight and root dry weight, respectively, were observed in the treatment with PHCs only (Figure 3). Furthermore, a decrease in root-to-shoot ratios of mungbean plants in contaminated soil indicated a more negative impact of PHCs on plant roots than shoots (Table 1). Results revealed that PHC phytotoxicity resulted in a significant decrease in the overall growth of mungbean plants.
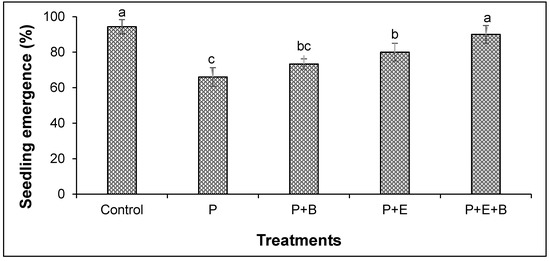
Figure 1.
Effects of Enterobacter sp. MN17 and biochar on seedling emergence of mungbean grown in PHC-contaminated soil. Columns and bars represent means and standard deviation, respectively, of triplicate values. Means with different letters differ significantly according to the least significant difference (LSD) test at p ≤ 0.05. (Control, Uncontaminated soil with plants; P, Contaminated soil with plants; P+B, Contaminated soil with plants and biochar; P+E, Contaminated soil with plants and Enterobacter sp. MN17; P+E+B, Contaminated soil with plants, Enterobacter sp. MN17 and biochar).
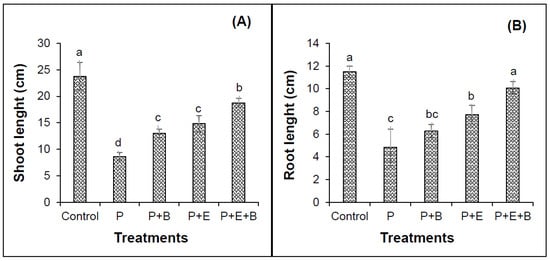
Figure 2.
Effects of Enterobacter sp. MN17 and biochar on shoot length (A), root length (B) of mungbean grown in PHC-contaminated soil. Shoot and root lengths were taken at 90 d after sowing. Columns and bars represent means and standard deviation, respectively, of triplicate values. Means with different letters differ significantly according to the least significant difference (LSD) test at p ≤ 0.05. (Control, Uncontaminated soil with plants; P, Contaminated soil with plants; P+B, Contaminated soil with plants and biochar; P+E, Contaminated soil with plants and Enterobacter sp. MN17; P+E+B, Contaminated soil with plants, Enterobacter sp. MN17 and biochar).
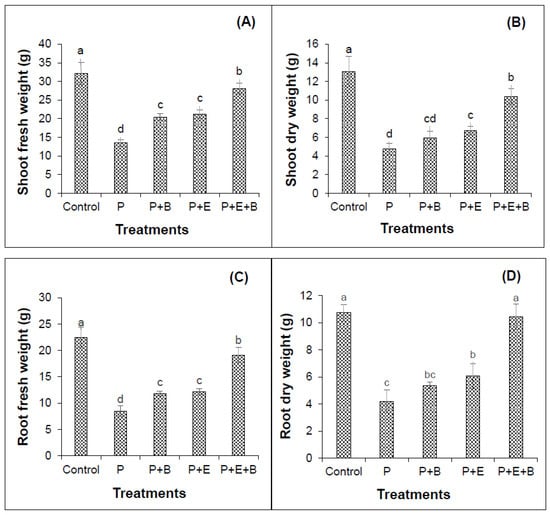
Figure 3.
Effects of Enterobacter sp. MN17 and biochar on shoot fresh weight (A), shoot dry weight (B) root fresh weight (C) and root dry weight (D) of mungbean grown in PHC-contaminated soil. Shoot and root fresh and dry weights were taken at 90 d after sowing. Columns and bars represent means and standard deviation, respectively, of triplicate values. Means with different letters differ significantly according to the least significant difference (LSD) test at p ≤ 0.05. (Control, Uncontaminated soil with plants; P, Contaminated soil with plants; P+B, Contaminated soil with plants and biochar; P+E, Contaminated soil with plants and Enterobacter sp. MN17; P+E+B, Contaminated soil with plants, Enterobacter sp. MN17 and biochar).

Table 1.
Root–shoot ratio and specific root length of mungbean plants after 90 days of sowing.
Individual or co-addition of strain MN17 and biochar had positive impacts on the root and shoot lengths, and dry and fresh weights of mungbean plants in PHC contamination (Figure 2 and Figure 3). Remarkably, 33.6% and 41.8% more lengths of the shoots were obtained with the sole application of biochar and strain MN17, respectively, in comparison with the planted control treatment (Figure 2A). Likewise, with the co-addition of biochar and strain MN17, 54% showed higher shoot lengths than that of planted control treatments. Similar observations were made in the case of root length, indicating a nearly 52% increase in root length in their combined application than that of their planted controls (Figure 2B). Additionally, an increase in root-to-shoot ratios of mungbean plants in contaminated soil indicated a more negative impact of PHCs on plant shoots than roots (Table 1). Moreover, in the co-addition of strain MN17 and biochar; 52%, 54%, 55% and 59% higher shoot fresh, shoot dry, root fresh and root dry weights, respectively, were obtained than that of their respective control treatments (Figure 3). Overall, the co-application of Enterobacter sp. MN17 and biochar showed higher growth attributes of mungbean plants under PHC contamination as compared to individual treatment.
3.2. Physiological Attributes of Mungbean Plants
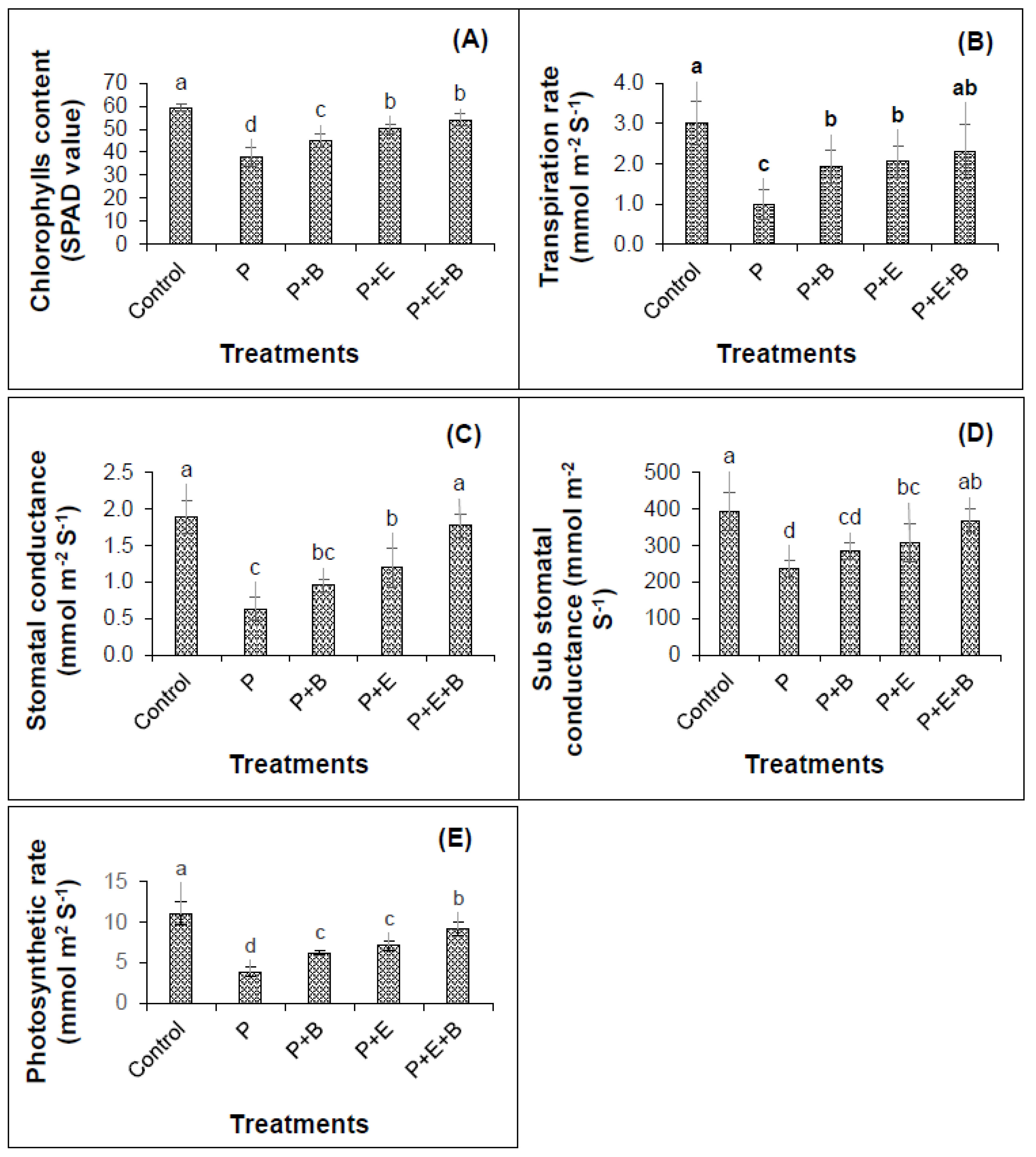
Results revealed that PHCs (at 5000 mg kg−1) induced significant decrease (p ≤ 0.05) in physiological attributes of mungbean showing 57%, 205%, 199%, 67% and 185% reduction in chlorophyll contents (SPAD value), transpiration rate, stomatal and sub-stomatal conductances and photosynthetic rate of mungbean plants as compared to their respective controls (Figure 4). Generally, the PHCs showed higher toxicity to the physiological attributes of mungbean plants.
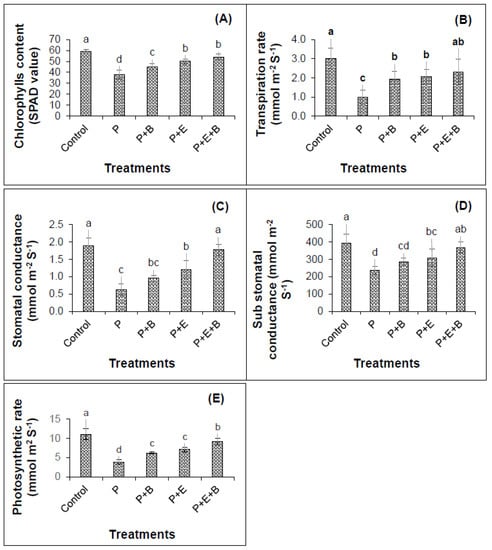
Figure 4.
Effects of Enterobacter sp. MN17 and biochar on SPAD chlorophyll contents (A), transpiration rate (B), stomatal conductance (C) sub-stomatal conductance (D) and photosynthetic rate (E) of mungbean plants grown in PHC-contaminated soil. Values shown here were taken after 45 d of sowing. Columns and bars represent means and standard deviation, respectively of triplicate values. Means withdifferent letters differ significantly according to the least significant difference (LSD) test at p ≤ 0.05. (Control, Uncontaminated soil with plants; P, Contaminated soil with plants; P+B, Contaminated soil with plants and biochar; P+E, Contaminated soil with plants and Enterobacter sp. MN17; P+E+B, Contaminated soil with plants, Enterobacter sp. MN17 and biochar).
In the sole application of biochar and Enterobacter sp. MN17, when compared with controls, a 16% and 24% increase in chlorophyll content (SPAD values), and a 49% and 52% rise in transpiration rates, respectively, were observed (Figure 4). Interestingly, the co-application of strain MN17 and biochar significantly enhanced SPAD value, transpiration rate, stomatal, sub-stomatal conductance and photosynthetic rate that are 30%, 57%, 64%, 36% and 57%, respectively higher than that of respective controls (Figure 4). Notably, the individual application of strain MN17 and biochar could significantly increase the values of these parameters by minimizing the toxic impacts of PHCs on plant physiology. However, the co-application of strain MN17 and biochar could further improve this impact on the physiology of mungbean plants in PHC stress (Figure 4).
3.3. Nutrient Uptake by Mungbean Plants
Results showed that on applying biochar and Enterobacter sp. MN17 alone, 15.9% and 17.7% increase in N, 24.6% and 26.7% increase in P, and 15.9% and 24.4% increase in K contents in plants, respectively, were observed than that of controls (Figure S1). Interestingly, the combined usage of strain MN17 and biochar significantly improved the N, P, and K contents in plants that are 30.2%, 40.8%, and 30.1% respectively higher than that of their respective controls (Figure S1). Interestingly, the use of strain MN17 and biochar could significantly improve nutrients uptake by reducing the toxic effects of PHCs on mungbean plants. However, the co-application of strain MN17 and biochar could further improve this impact on mungbean plants under PHC stress.
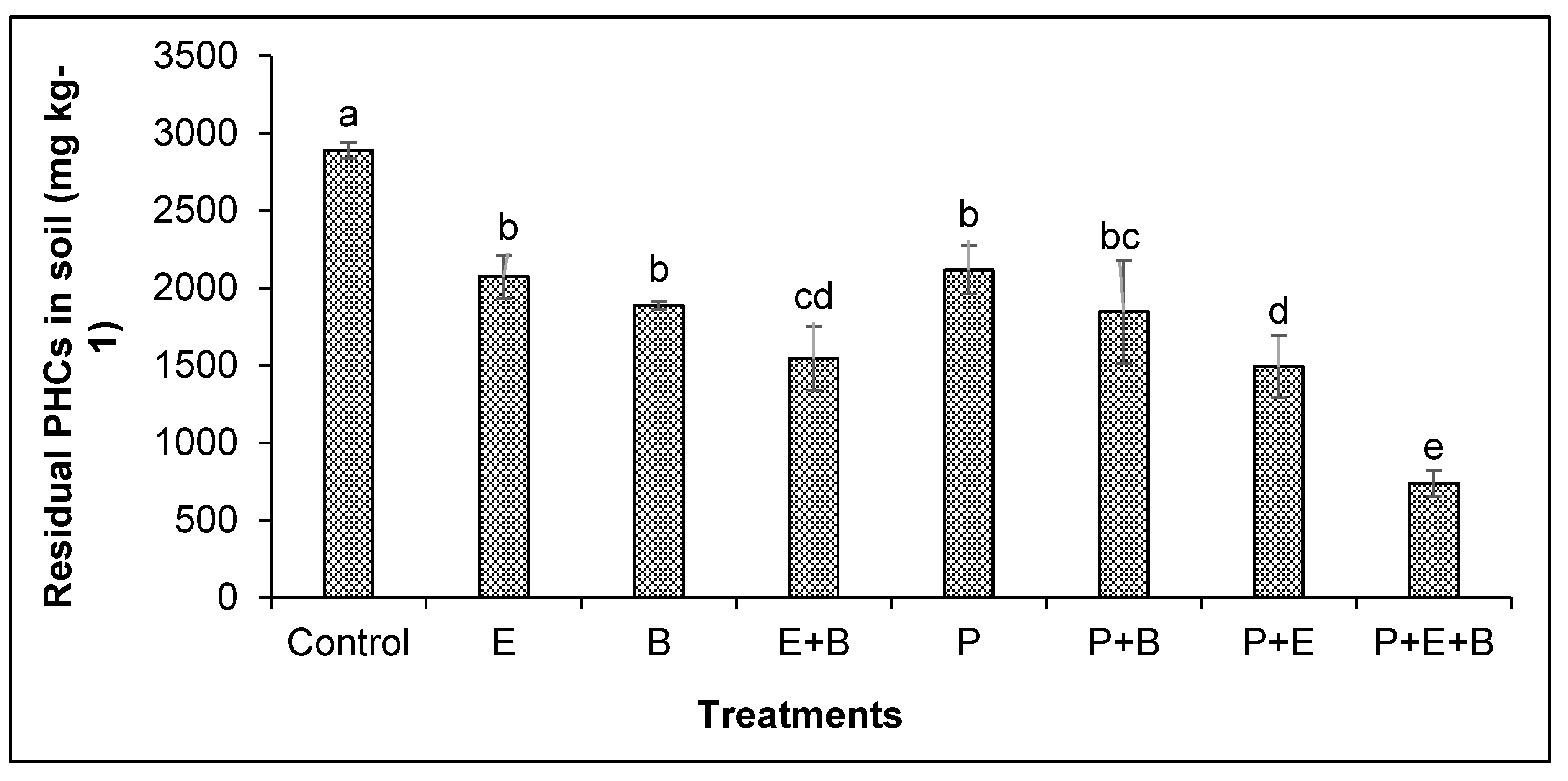
3.4. PHCs Removal and Degradation in Soil
The remaining concentration of PHCs was examined after 90 days of experiment and it showed that in the control treatment (the soil with 5000 mg kg−1 initial concentration of PHCs but without plants or any amendments) 42.2% reduction in PHCs was observed as compared to the initial concentration of PHCs (i.e., 5000 mg kg−1) (Figure 5). The individual application of biochar and Enterobacter sp. MN17 and their combination in the absence of plants could remove 27.9%, 32.3% and 38.9% higher amounts of PHCs than their respective unplanted control. Meanwhile, mungbean plants could remove 26.8% higher amounts of PHCs than their respective unplanted controls (without any amendments). Similarly, plants in the presence of biochar or strain MN17 could remove 33.1% and 39.9% higher PHCs than the control. Interestingly, plants in the presence of both biochar and strain MN17 could remove substantial amounts (50.5%) of PHCs that were higher than control as well as any other treatments (Figure 5), suggesting that the co-application of biochar and Enterobacter sp. MN17 could significantly improve the phytoremediation potential of mungbean plants for PHCs.
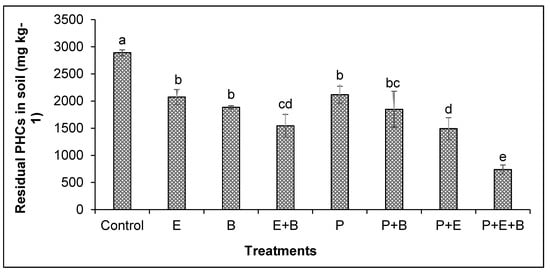
Figure 5.
Effects of Enterobacter sp. MN17 and biochar on the removal of PHCs by mungbean grown in PHC-contaminated soil. Values shown here were taken after 90 d of sowing. Columns and bars represent means and standard deviation, respectively, of triplicate values. Means with different letters differ significantly according to the LSD test at p ≤ 0.05. (Control, Uncontaminated soil with plants; E, Contaminated soil with Enterobacter sp. MN17; B, Contaminated soil with biochar; E+B, Contaminated soil with Enterobacter sp. MN17 and biochar; P, Contaminated soil with plants; P+B, Contaminated soil with plants and biochar; P+E, Contaminated soil with plants and Enterobacter sp. MN17; P+E+B, Contaminated soil with plants, Enterobacter sp. MN17 and biochar).
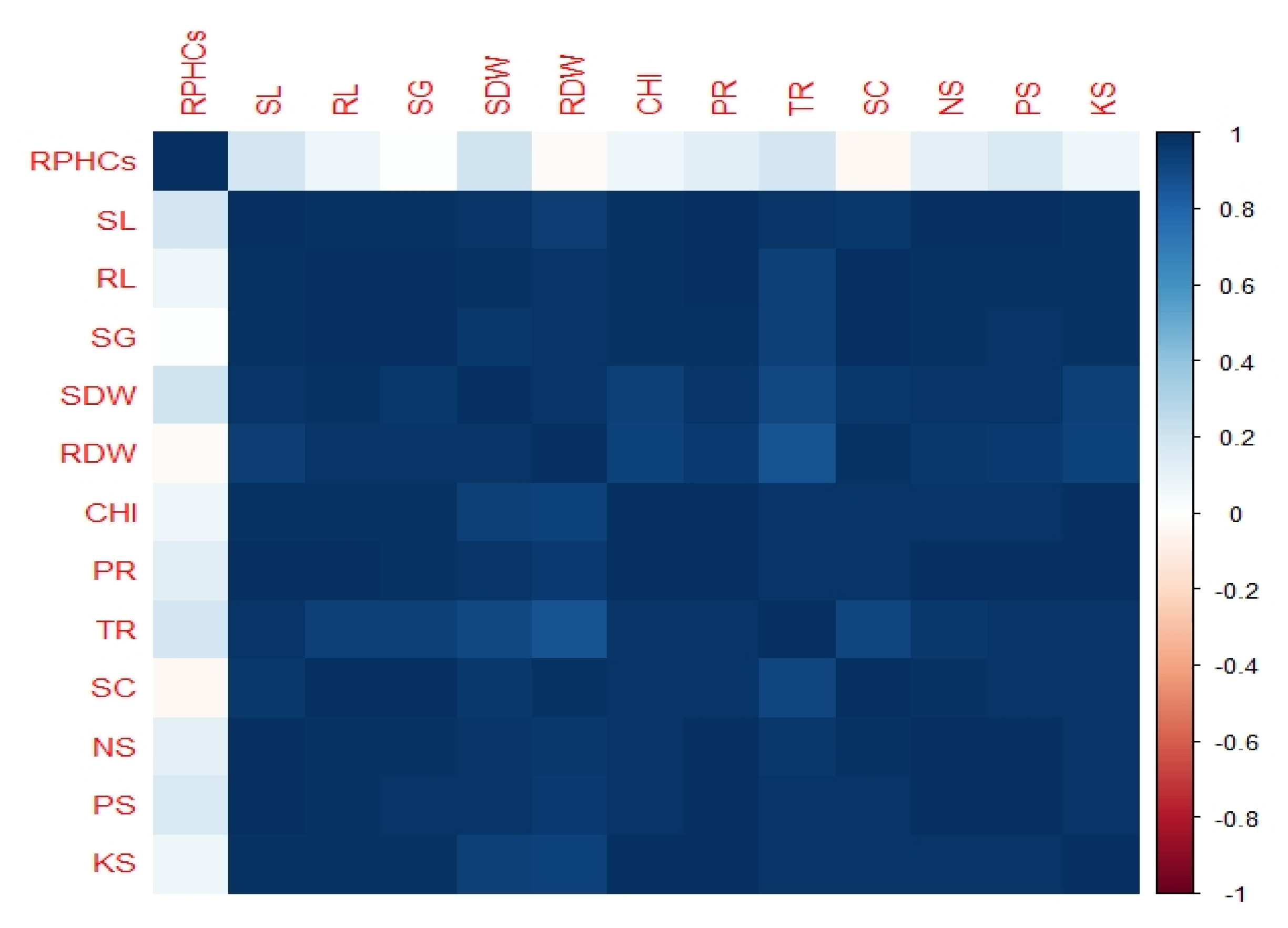
3.5. Correlation among Different Attributes of Mungbean
Correlation analysis showed that there was a highly significant relationship among all the growth, physiological and nutrient parameters of mungbean. Figure 6 revealed a correlation matrix graphically by corrplot. A highly significant association was observed in growth physiological and nutrient parameters of mungbean while residual of PHCs in soil has a less significant relationship with all other measured attributes.

Figure 6.
Corrplot (Correlation plot) represents correlation matrix among different attributes of mungbean followed by treatments as (1) Control, Uncontaminated soil with plants (2) P, Contaminated soil with plants; (3) P+B, Contaminated soil with plants and biochar (4) P+E, Contaminated soil with plants and Enterobacter sp. MN17 (5) P+E+B, Contaminated soil with plants, Enterobacter sp. MN17 and biochar. The dark blue color shows a high positive correlation while light blue and sky blue represent less association among measured parameters. The color legend on the right-hand side of corrplot shows the correlation coefficient and corresponding colors. The abbreviations are as Shoot length (SL), Root length (RL), Shoot dry weight (SDW), Root dry weight (RDW), Chlorophyll content (CHl), Photosynthetic rate (PR), Transpiration rate (TR), Stomatal conductance (SC), Seed emergence (SG), Nitrogen in shoot (NS), Phosphorus in shoot (PS), Potassium in shoot (KS) Residual PHCs in soil (RPHCs).
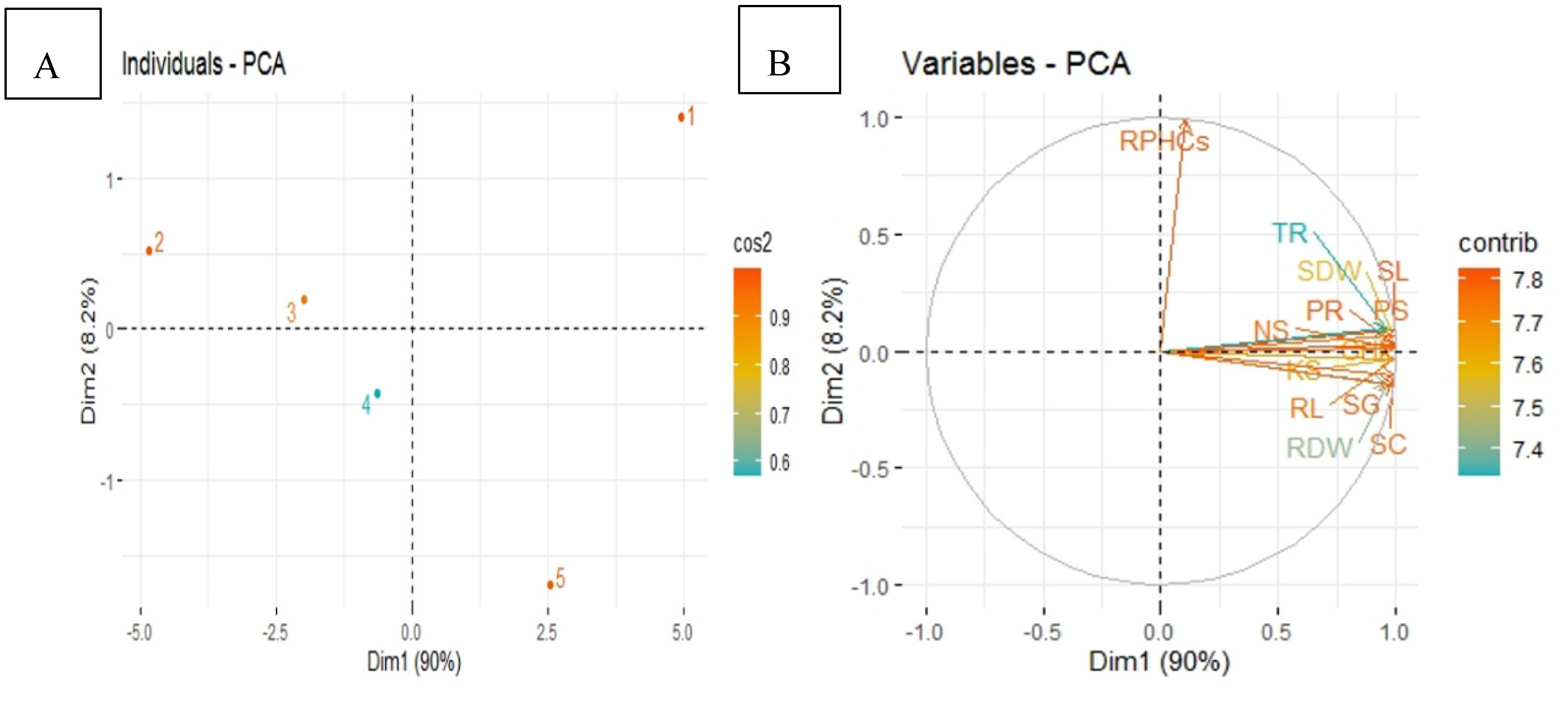
3.6. Principal Component Analysis
The principal component analysis (PCA) showed the distribution of different treatments in mungbean crop in diesel contaminated soil as presented in the score plot (Figure 7A). Significant results were obtained from the score plot of PCA performed for two factors (Cumulative variance 98.2%), the first represents 90.0% of variation while 8.2% of the difference is explained by the second factor. Hence, there is great variation among all treatments in mungbean plants. The loading plot (Figure 7B) shows a better visualization of the relationship and variation among all studied parameters of mungbean.
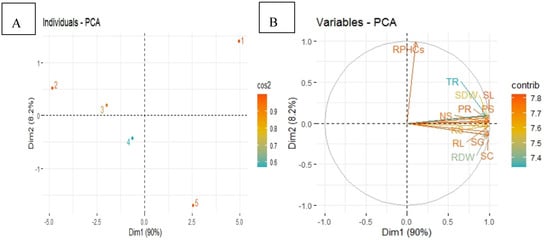
Figure 7.
Principal component analysis (PCA) showing score plots (A) and loading plots (B) of different attributes of mungbean plants in diesel contaminated soil. Score plots (A) represent separation of treatments as (1) Control, Uncontaminated soil with plants (2) P, Contaminated soil with plants; (3) P+B, Contaminated soil with plants and biochar (4) P+E, Contaminated soil with plants and Enterobacter sp. MN17 (5) P+E+B, Contaminated soil with plants, Enterobacter sp. MN17 and biochar. Loading plots (B) shows loading of each studied attribute (arrow) and arrow lengths approximate their variance whereas the angles between them represent their correlation The abbreviations are as Shoot length (SL), Root length (RL), Shoot dry weight (SDW), Root dry weight (RDW), Chlorophyll content (CHl), Photosynthetic rate (PR), Transpiration rate (TR), Stomatal conductance (SC), Seed emergence (SG), Nitrogen in shoot (NS), Phosphorus in shoot (PS), Potassium in shoot (KS) Residual PHCs in soil (RPHCs) Dimensions (Dim), Square cosine (Cos2) Contribution (Contrib).
4. Discussion
4.1. Emergence of Seedlings and Agronomic Attributes of Mungbean Plants
Seed germination is an important process that influences crop yield and quality. Decreased seedling emergence observed under PHCs toxicity in this study was found to be strongly in agreement with those of [40,41], who observed a significant decrease in seed germination of different plant species (i.e., Festuca arundinacea, Lolium multiflorum, and Lotus corniculatus) sown in PHC-contaminated soil. Inhibition in the germination of seeds in this study might be due to the presence of PHCs and volatile fractions that penetrate the seed coat and cause the death of the embryo by reducing the seed imbibition due to the coating of oil around the seeds that alters physiological processes inside the seeds as well as hinder the supply of water and oxygen to the seeds [42,43,44]. Results of PHC phytotoxicity to mungbean agree with findings of earlier studies indicating that PHCs and other contaminants could induce shoot and root length reductions and plant growth [27]. Similarly, shoot and root weights (both dry and fresh) of mungbean plants were decreased in treatments where PHCs were applied [41] and this might be due to the residual PHCs or metabolites of PHCs that cause toxicity and reduce nutrients and water uptake by plant roots [45]. Other reasons for the phytotoxic effects of PHCs on mungbean growth would be attributed to changes in cell membrane permeability, development of flavonoids, and enzymatic disruptions [42,46,47].
Our findings on the increased emergence of seedlings and enhanced growth and physiology of mungbean plants by strain MN17 endorse the observations of [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] who reported that the Enterobacter sp. MN17 is capable of enhancing plant growth by the production of siderophores and auxins, solubilization of phosphate, and ACC-deaminase activity. Improved growth may be attributed to plant growth-enhancing hormones such as gibberellins and indole-3-acetic acid secreted by the bacteria [49] and this may assist plants in strengthening their resistance to PHC stress [1]. In addition, our findings on the use of biochar and strain MN17 to enhance the growth and resistance of mungbean even under PHC stress are consistent with the results of previous studies [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. These enhanced impacts could be justified by the fact that the addition of Enterobacter sp. MN17 together with biochar could improve mungbean growth due to siderophores and auxins production, phosphate solubilization and ACC-deaminase activities of strain MN17 [31].
4.2. Physiological Attributes of Mungbean Plants
In this study, the physiology of mungbean plants was also adversely influenced by PHCs. These findings support the previous studies concluding that PHCs toxicity affects chlorophyll contents by reducing their capacity to capture the solar energy needed for photosynthesis [51]. PHC stress in the soil inhibits photosynthesis due to the accumulation of PHCs in chloroplast and also causes cell injuries [52]. Inhibition of intracellular enzymatic activities due to PHCs can disrupt the uptake of essential nutrients leading towards the reduction of chlorophyll production [46]. In addition, PHC-induced stress can also affect biomembranes due to the accumulation of reactive oxygen species [53,54]. Moreover, due to PHC toxicity stomatal, sub-stomatal conductance (gaseous exchange) and transpiration rate were also adversely affected. The hydrophobic nature of PHCs causes a higher transpiration rate and clog the stomatal openings [55]. Our findings endorse the observations made by [41] suggesting the negative effects of PHCs on the physiology of Lolium multiflorum and Lotus corniculatus. In general, the physiology of mungbean plants was found to be adversely affected by PHCs stress [41].
Application of Enterobacter sp. MN17 and biochar alone or in combination were observed to be effective in mitigating the PHCs toxicity to mungbean plants. It is shown that the bacterial strain, which has ACC-deaminase activity under stress conditions, can reduce the ethylene concentration by ACC hydrolysis [56]. Another explanation could be that the biochar could sorb PHCs and other pollutants due to the presence of functional sites and pore spaces for microbial growth [22] and plays an important role in enhancing soil physical health through immobilizing and removing PHCs present in the soil [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. The integrated use of Enterobacter sp. MN17 and biochar demonstrated that their co-addition is more useful in promoting physiology and plant growth under PHCs stress.
4.3. Nutrient Uptake by Mungbean Plants
In the present study, the nutrients uptake by mungbean plants was also negatively affected by PHCs. In PHC-contaminated soil, it is often observed that N, P, and K become less available for plant uptake, while their uptake increases by the co-addition of bacteria and biochar when compared with their controls or individual use [58]. Similarly, it has been found that rhizospheric microbes have the ability to enhance nutrient uptake by plants in hydrocarbons contaminated soil [59]. These findings also support the previous studies describing that the phytotoxicity of PHCs could disturb nutrients balance by clogging the pores, reducing aeration, and increasing hydrophobicity [60]. In fact, individual application of biochar was not effective in improving nutrient uptake by plants. This could be explained by the sorption of nutrients to the biochar which reduces the availability of nutrients to plants [61]. Unlike biochar, the introduction of microbial inoculum could increase the release of nutrients and thus their availability to plants [62]. However, the integrated use of Enterobacter sp. MN17 and biochar could be more effective in enhancing the availability and uptake of nutrients by plants. This is because biochar could play a role as a carrier or a stimulating agent for microbes, eventually improving nutrient availability [63].
4.4. PHCs Removal and Degradation in Soil
Although plants face toxicity when pollutants are above a certain level, they have the ability to remove a certain amount of organic pollutants from soil through different mechanisms [64,65]. In this study, individual use of biochar, mungbean plants and Enterobacter sp. MN17 could remove a substantial amount of PHCs from soil. However, mungbean plants in the presence of microbes and biochar could further improve the removal of PHCs from soil [1]. This is because a beneficial microbe can influence the PHCs availability to the plants through a number of processes including the formation of organic acids, chelation, protonation, chemical transformation of chemicals, and solubilization of phosphate [66]. Another mechanism involved in the reduction of PHC-concentration in soil amended with biochar might be the adsorption of PHCs on the surface of biochar [63,64,65,66,67]. This because it has been reported that biochar can sorb PHCs due to the presence of diverse functional groups and provide micro-pores for aeration and microbial growth that further improve the removal or degradation of PHCs [4,22,68].
To the best of our knowledge, this is the first report on the interactive effects of Enterobacter sp. MN17 and biochar on mungbean growth and PHCs remediation from contaminated soil. Findings from the present study enhance our knowledge on plant–microbe interactions in PHC-contaminated soil and provide new information on the usage of beneficial microbes and biochar in improving PHCs remediation and crop productivity under PHC stress and open new areas for studying the significance of biological interactions in hydrocarbon-polluted environments.
5. Conclusions
Here, we examined the effects of Enterobacter sp. MN17 and biochar addition on mungbean growth and removal of PHCs from diesel contaminated soil. PHCs showed significant phytotoxicity to the mungbean plants. However, strain MN17 or biochar applications were shown to be helpful in alleviating the toxic effects of PHCs on mungbean plants, thus enhancing the growth of plants in soil contaminated with PHCs. Moreover, it was found that the co-application of strain MN17 and biochar was very promising for the remediation of PHCs from the soil. Our results indicate that the co-application of Enterobacter sp. MN17 and biochar would be supportive in boosting the growth and productivity of plants in soil contaminated with PHCs and could act as a suitable choice for rhizoremediation investigations.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/23/8548/s1, Figure S1: Effects of Enterobacter sp. MN17 and biochar on nitrogen (A), phosphorus (B), and potassium (C) in mungbean plants grown in PHCs contaminated soil, Table S1: Detail of treatments used in this study, Table S2: Physicochemical properties of the soil used in the study.
Author Contributions
Conceptualization, M.T.S. and M.I.K.; data curation, M.H.A. and M.I.K.; formal analysis, M.T.S., M.R.; investigation, M.N. and M.I.K.; methodology, M.T.S. and M.H.A.; project administration, M.N., M.I.K., S.A.; resources, S.A.; software, M.R., M.H.A.; supervision, M.I.K.; validation, M.H.S.; visualization, M.N.; writing—original draft, M.T.S. and M.H.A.; writing—review and editing, M.I.K., S.A., M.N., and M.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project number (RSP-2020/194), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2020/194), King Saud University, Riyadh, Saudi Arabia. The authors would like further to extend their gratefulness to Higher Education Commission of Pakistan and Institute of Soil and Environmental Sciences, UAF for technical assistance and support.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Nahrgang, J. Characterizing crude oil toxicity to early-life stage fish based on a complex mixture: Are we making unsupported assumptions. Environ. Sci. Technol. 2019, 53, 11080–11092. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.D.; Mattam, A.J.; Jose, S.; Ramachandrarao, B.; Velankar, H.R. Heavy hydrocarbons as selective substrates for isolation of asphaltene degraders: A substrate-based bacterial isolation strategy for petroleum hydrocarbon biodegradation. Environ. Technol. Innov. 2020, 19, 100832. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Tanveer, S.; Anees, M.; Muhammad, Y.S.; Iqbal, M.; Yousaf, S. Role of nutrients and illuminance in predicting the fate of fungal mediated petroleum hydrocarbon degradation and biomass production. J. Environ. Manag. 2016, 176, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Balseiro-Romero, M.; Monterroso, C.; Casares, J.J. Environmental fate of petroleum hydrocarbons in soil: Review of multiphase transport, mass transfer, and natural attenuation processes. Pedosphere 2018, 28, 833–847. [Google Scholar] [CrossRef]
- Adipah, S. Introduction of petroleum hydrocarbons contaminants and its human effects. J. Environ. Sci. Public Health 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Shahzad, A.; Saddiqui, S.; Bano, A. The response of maize (Zea mays L.) plant assisted with bacterial consortium and fertilizer under oily sludge. Int. J. Phytopathol. 2016, 18, 521–526. [Google Scholar]
- Errington, I.; King, C.K.; Houlahan, S.; George, S.C.; Michie, A.; Hose, G.C. The influence of vegetation and soil properties on springtail communities in a diesel-contaminated soil. Sci. Total Environ. 2018, 619, 1098–1104. [Google Scholar] [CrossRef]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: Application, mechanisms, challenges and opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- Fatima, K.; Imran, A.; Amin, I.; Khan, Q.M.; Afzal, M. Successful phytoremediation of crude-oil contaminated soil at an oil exploration and production company by plants-bacterial synergism. Int. J. Phytoremed. 2018, 20, 675–681. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xu, J.; Xie, W.; Yao, Z.; Yang, H.; Sun, C.; Li, X. Pseudomonas aeruginosa L10: A hydrocarbon-degrading, biosurfactant-producing, and plant-growth-promoting endophytic bacterium isolated from a reed (Phragmites australis). Front. Microbiol. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Arshad, M.; Karthikeyan, R.; Gentry, T.J.; Rashid, J.; Ahmed, I.; Schwab, A.P. Diesel degrading bacterial endophytes with plant growth promoting potential isolated from a petroleum storage facility. Biotechnology 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Madhukar, S.M.; Raha, P.; Singh, R.K. Identification of amino acids and sugars in root exudate of mungbean (Vigna radiata L.). J. Pharma. Phytochem. 2018, 7, 1676–1680. [Google Scholar]
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Guo, M.; Gong, Z.; Miao, R.; Su, D.; Li, X.; Jia, C.; Zhuang, J. The influence of root exudates of maize and soybean on polycyclic aromatic hydrocarbons degradation and soil bacterial community structure. Ecol. Eng. 2017, 99, 22–30. [Google Scholar] [CrossRef]
- Garrido-Sanz, D.; Redondo-Nieto, M.; Guirado, M.; Pindado Jiménez, O.; Millán, R.; Martin, M.; Rivilla, R. Metagenomic insights into the bacterial functions of a diesel-degrading consortium for the rhizoremediation of diesel-polluted soil. Genes 2019, 10, 456. [Google Scholar] [CrossRef]
- Wu, M.; Dick, A.L.W.; Wang, X.; Yang, Q.; Wan, T.; Chen, L. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int. Biodeterior. Biodegr. 2016, 107, 158–164. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Lee, Y.B.; Naidu, R. Isolation and characterization of polycyclic aromatic hydrocarbons (PAHs) degrading, pH tolerant, N-fixing and P-solubilizing novel bacteria from manufactured gas plant (MGP) site soils. Environ. Technol. Innov. 2016, 6, 204–219. [Google Scholar] [CrossRef]
- Dos-santos, J.J.; Maranho, L.T. Rhizospheric microorganisms as a solution for the recovery of soils contaminated by petroleum: A review. J. Environ. Manag. 2018, 210, 104–113. [Google Scholar] [CrossRef]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237, 105–116. [Google Scholar] [CrossRef]
- Hussain, F.; Hussain, I.; Khan, A.H.A.; Muhammad, Y.S.; Iqbal, M.; Soja, G.; Yousaf, S. Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ. Exp. Bot. 2018, 153, 80–88. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, Z.; Wang, Z.; Li, C.; Wei, H. Significance of soil microbe in microbial-assisted phytoremediation: An effective way to enhance phytoremediation of contaminated soil. Int. J. Environ. Sci. Technol. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Gartler, J.; Wimmer, B.; Soja, G.; Reichenauer, T.G. Effects of rapeseed oil on the rhizodegradation of polyaromatic hydrocarbons in contaminated soil. Int. J. Phytoremed. 2014, 16, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Sinkko, H.; Penttine, P.; Lindström, K. Characterization of successional changes in bacterial community composition during bioremediation of used motor oil-contaminated soil in a boreal climate. Sci. Total. Environ. 2016, 542, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.M.R.; Akhtar, M.J.; Ahmad, I.; Khalid, M. Synergistic use of rhizobium, compost and nitrogen to improve growth and yield of mungbean (Vigna radiata). Pak. J. Agri. Sci. 2014, 51, 383–388. [Google Scholar]
- Ali, Q.; Javed, M.T.; Noman, A.; Haider, M.Z.; Waseem, M.; Iqbal, N.; Perveen, R. Assessment of drought tolerance in mung bean cultivars/lines as depicted by the activities of germination enzymes, seedling’s antioxidative potential and nutrient acquisition. Arch. Agron. Soil. Sci. 2018, 64, 84–102. [Google Scholar] [CrossRef]
- Chouychai, W.; Paemsom, T.; Pobsuwan, C.; Somtrakoon, K.; Lee, H. Effect of Indole-3-Acetic acid-producing bacteria on phytoremediation of soil contaminated with phenanthrene and anthracene by mungbean. Environ. Asia. 2016, 9, 128–133. [Google Scholar]
- Alaboudi, K.; Ahmed, A.B.; Brodie, G. Effect of biochar on Pb, Cd and Cr availability and maize growth in artificial contaminated soil. Ann. Agric. Sci. 2019, 64, 95–102. [Google Scholar] [CrossRef]
- Barati, M.; Bakhtiari, F.; Mowla, D.; Safarzadeh, S. Total petroleum hydrocarbon degradation in contaminated soil as affected by plants growth and biochar. Environ. Earth Sci. 2017, 76, 688. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Yousaf, S.; Pastar, M.; Afzal, M.; Sessitsch, A. The endophyte Enterobacter sp. MN17: A maize growth enhancer selected based on rigorous testing of plant beneficial traits and colonization characteristics. Biol. Fertil. Soils. 2014, 50, 249–262. [Google Scholar] [CrossRef]
- Suja, F.; Rahim, F.; Taha, M.R.; Hambali, N.; Razali, M.R.; Khalid, A.; Hamzah, A. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int. Biodeterior Biodegr. 2014, 90, 115–122. [Google Scholar] [CrossRef]
- Afzal, M.J.; Khan, M.I.; Cheema, S.A.; Hussain, S.; Anwar-ul-Haq, M.; Ali, M.H.; Naveed, M. Combined application of Bacillus sp. MN-54 and phosphorus improved growth and reduced lead uptake by maize in the lead-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, R.A.; Ashraf, M.; Javaid, M.M. Response of foliar-applied nutrient solution with and without soil-applied fertilizers on growth and yield of mungbean. J. Plant Nutr. 2018, 41, 1083–1093. [Google Scholar] [CrossRef]
- Buondonno, A.; Rashad, A.A.; Coppola, E. Comparing tests for soil fertility. II. The hydrogen peroxide/sulfuric acid treatment as an alternative to the copper/selenium catalyzed digestion process for routine determination of soil nitrogen-kjeldahl. Commun. Soil Sci. Plant Anal. 1995, 26, 1607–1619. [Google Scholar] [CrossRef]
- McLean, E.O.; Watson, M.E. Soil Measurements of Plant Available Potassium. In Potassium in Agriculture, ASA, CSSA, and SSSA, Madison; Munson, R.D., Ed.; Scintific Research Publishing Inc.: Wuhan, China, 1985; pp. 277–308. [Google Scholar]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef]
- Takahashi, A. Development of TPH analyzer for oil contaminated soil. J. Jpn. Petrol Inst. 2013, 56, 176–179. [Google Scholar] [CrossRef][Green Version]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods 1989; Iowa State College Press: Ames, IA, USA, 1980. [Google Scholar]
- Zarinkamar, F.; Reypour, F.; Soleimanpour, S. Effect of diesel fuel contaminated soil on the germination and the growth of Festuca arundinacea. Res. J. Chem. Environ. Sci. 2013, 1, 37–41. [Google Scholar]
- Hussain, I.; Puschenreiter, M.; Gerhard, S.; Sani, S.G.A.S.; Reichenauer, T.G. Differentiation between physical and chemical effects of oil presence in freshly spiked soil during rhizoremediation trial. Environ. Sci. Pollut. Res. 2019, 26, 18451–18464. [Google Scholar] [CrossRef]
- Sharifi, M.; Sadegh, Y.; Akbarpour, M. Germination and growth of six plant species on contaminated soil with spent oil. Int. J. Environ. Sci. Technol. 2007, 4, 463–470. [Google Scholar] [CrossRef]
- Adenipekun, C.O.; Oyetunji, O.J.; Kassim, L.Q. Screening of Abelmoschus esculentus L. moench for tolerance to spent engine. J. Appl. Biosci. 2009, 20, 1131–1137. [Google Scholar]
- Dutta, T.; Kwon, E.; Bhattacharya, S.S.; Jeon, B.H.; Deep, A.; Uchimiya, M.; Kim, K.H. Polycyclic aromatic hydrocarbons and volatile organic compounds in biochar and biochar-amended soil: A review. GCB Bioenergy 2017, 9, 990–1004. [Google Scholar] [CrossRef]
- Graj, W.; Lisiecki, P.; Szulc, A.; Chrzanowski, Ł.; Wojtera-Kwiczor, J. Bioaugmentation with petroleum-degrading consortia has a selective growth-promoting impact on crop plants germinated in diesel oil-contaminated soil. Water Air Soil Pollut. 2013, 224, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- Bona, C.; Rezende, I.M.D.; Santos, G.D.O.; Souza, L.A.D. Effect of soil contaminated by diesel oil on the germination of seeds and the growth of Schinus terebinthifolius Raddi (Anacardiaceae) seedlings. Braz. Arch. Biol. Technol. 2011, 54, 1379–1387. [Google Scholar] [CrossRef]
- Khan, M.A.I.; Biswas, B.; Smith, E.; Naidu, R.; Megharaj, M. Toxicity assessment of fresh and weathered petroleum hydrocarbons in contaminated soil: A review. Chemosphere 2018, 212, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770–781. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Rahman, I.U.; Iqbal, A.; Hamayun, M. IAA producing fungal endophyte Penicillium roqueforti thom enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS ONE 2018, 13, 0208150. [Google Scholar] [CrossRef]
- Abhijit, S.R.; Reshita, B.; Maina, B.; Anil, K.S.; Hari, P.D.B.; Neelima, S.; Manab, D.; Nipu, D.; Tarun, C.B. Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int. Biodeterior. Biodegrad. 2014, 94, 79–89. [Google Scholar]
- Castro-Mancilla, Y.V.; de la Rosa-Manzano, E.; Castro-Nava, S.; Martínez-Avalos, J.G. Physiological responses of Quercus oleoides (Schltdl & Cham) to soils contaminated by diesel. Plant Prod. Sci. 2019, 22, 519–529. [Google Scholar]
- Arellano, P.; Tensey, K.; Balzter, H.; Boyd, D.S. Detecting the effects of hydrocarbon pollution in the Amazon forest using hyperspectral satellite images. Environ. Pollut. 2015, 205, 225–239. [Google Scholar] [CrossRef]
- Shukry, W.M.; Al-Hawas, G.H.S.; Al-Moaikal, R.M.S.; El-Bendary, M.A. Effect of petroleum crude oil on mineral nutrient elements, soil properties and bacterial biomass of the rhizosphere of jojoba. Br. J. Environ. Clim. Chang. 2013, 3, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Achuba, F.I. Petroleum products in soil mediated oxidative stress in cowpea (Vigna unguiculata) and maize (Zea mays) seedlings. Open J. Soil. Sci. 2014, 4, 417. [Google Scholar] [CrossRef]
- Baker, J.M. The effects of oil on plants. Environ. Pollut. 1970, 1, 27–44. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Zafar-ul-Hye, M.; Sajja, S.; Naveed, M. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for coinoculation with Rhizobium leguminosarum to improve growth, nodulation, and yield of lentil. Biol. Fertil. Soils. 2011, 47, 457–465. [Google Scholar] [CrossRef]
- Hussain, I.; Aleti, G.; Naidu, R.; Puschenreiter, M.; Mahmood, Q.; Rahman, M.M.; Reichenauer, T.G. Microbe and plant assisted-remediation of organic xenobiotics and its enhancement by genetically modified organisms and recombinant technology: A review. Sci. Total Environ. 2018, 628, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Ducan, H. Influence of diesel on seed germination. Environ. Pollut. 2002, 120, 363–367. [Google Scholar] [CrossRef]
- Kotoky, R.; Rajkumari, J.; Pandey, P. The rhizosphere microbiome: Significance in rhizoremediation of polyaromatic hydrocarbon contaminated soil. J. Environ. Manag. 2018, 217, 858–870. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.; Zhou, Q.; Zhang, Z.; Chen, C. Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnol. Bioeng. 2012, 109, 426–433. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil–concepts and mechanisms. Plant Soil. 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Scala, F. Biochar as plant growth promoter: Better off alone or mixed with organic amendments. Front. Plant Sci. 2017, 8, 1570. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, J.; Wang, L.; Liu, J.; Gurav, R.G.; Sun, K. A novel bioremediation strategy for petroleum hydrocarbon pollutants using salt tolerant Corynebacterium variabile HRJ4 and biochar. J. Environ. Sci. 2016, 47, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhou, Q.; Cai, Z.; Zhang, Z. Phytoremediation of petroleum contaminated soils by Mirabilis jalapa L. in a greenhouse plot experiment. J. Hazard Mater. 2009, 168, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Mucha, A.P.; Almeida, C.M.R.; Bordalo, A.A. Potential of phytoremediation for the removal of petroleum hydrocarbons in contaminated salt marsh sediments. J. Environ. Manag. 2014, 137, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Y.; Zhang, W.; Wang, F.; Bian, Y.; Boughner, L.A.; Jiang, X. Novel biochar-plant tandem approach for remediating hexachlorobenzene contaminated soils: Proof-of-concept and new insight into the rhizosphere. J. Agric. Food Chem. 2016, 64, 5464–5471. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; Jehmlich, N.; Lima, K.; Morris, B.E.L.; Richnow, H.H.; Hernández, T.; García, C. The ecological and physiological responses of the microbial community from a semiarid soil to hydrocarbon contamination and its bioremediation using compost amendment. J. Proteom. 2016, 135, 162–169. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).