Protective Effects of Fucoxanthin on High Glucose- and 4-Hydroxynonenal (4-HNE)-Induced Injury in Human Retinal Pigment Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Human RPE Cell Culture
2.3. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.4. Cell Counting and Propidium Iodide (PI) Staining
2.5. Protein Extraction and Western Blot
2.6. Immunofluorescence
2.7. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.8. Antioxidant Ability Measurement
2.9. Statistical Analysis
3. Results
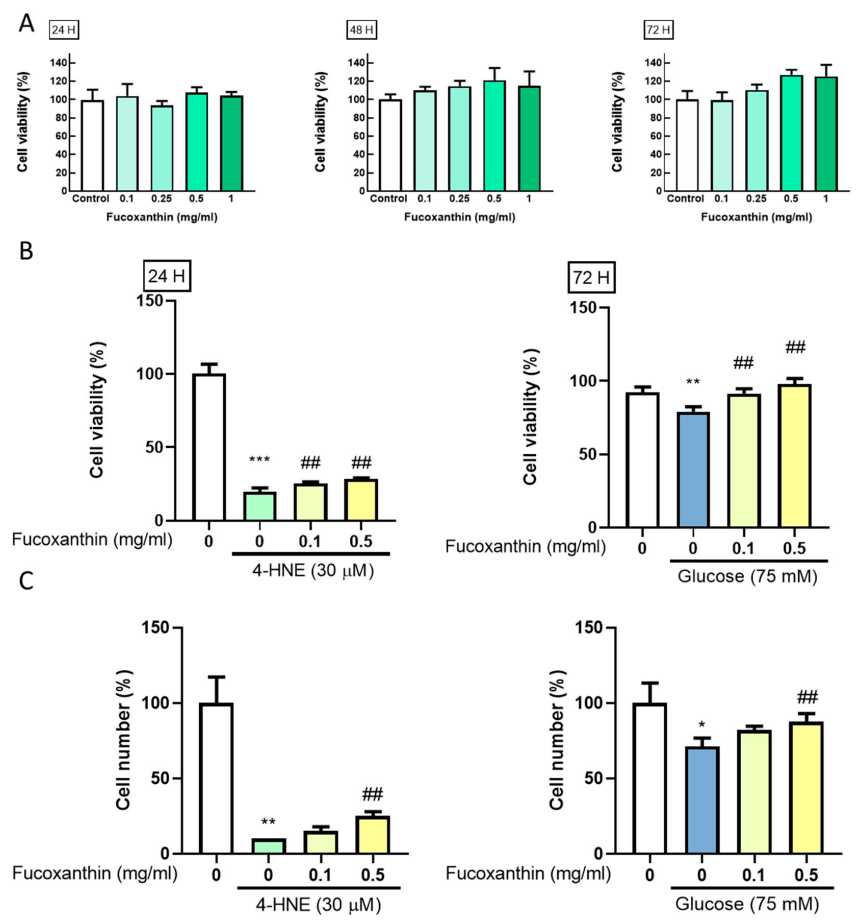
3.1. Fucoxanthin Prevents 4-HNE- and High Glucose-Mediated Suppression of ARPE-19 Cell Viability
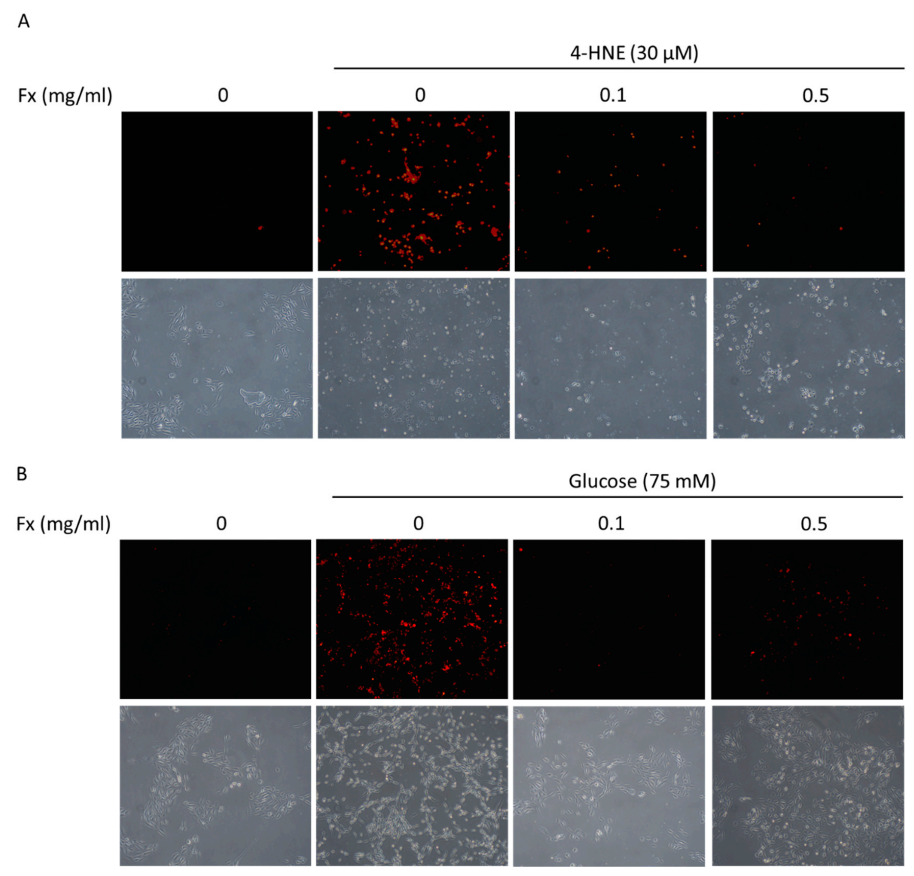
3.2. Fucoxanthin Prevented 4-HNE- and High Glucose-Mediated ARPE-19 Cell Morphology Changes and DNA Damage
3.3. Fucoxanthin Decreased the Apoptosis-Related Protein Expression
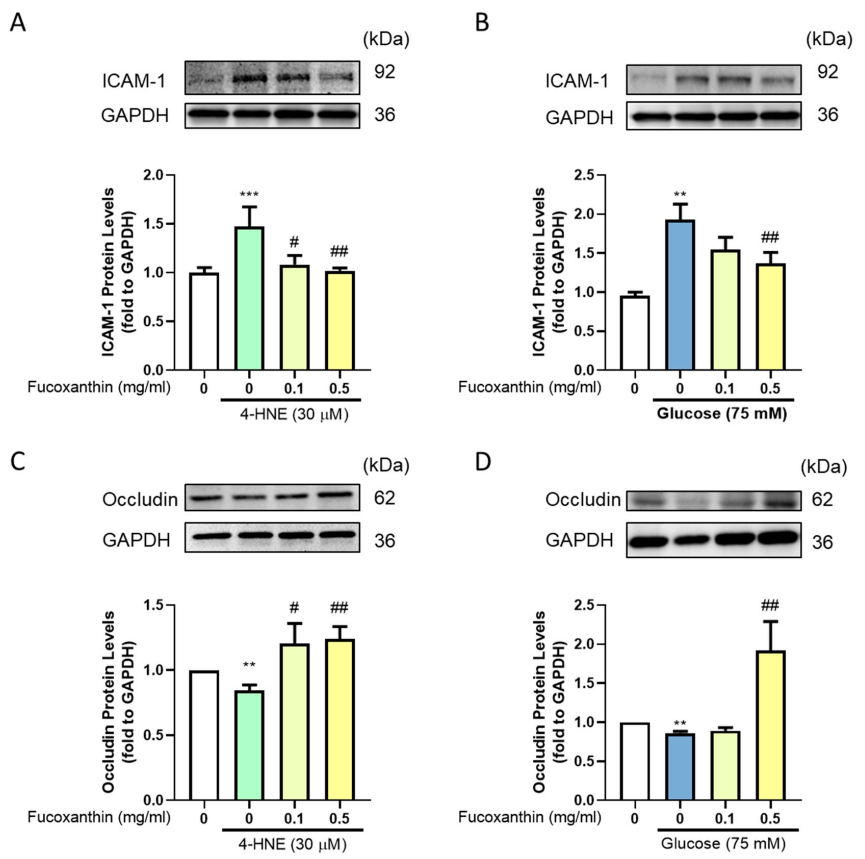
3.4. Effect of Fucoxanthin on the Protection of the Endothelial Function and Completeness in the Blood–Retinal Barrier (BRB)
3.5. The Protective Effect of Fucoxanthin on Tight Junction Connections
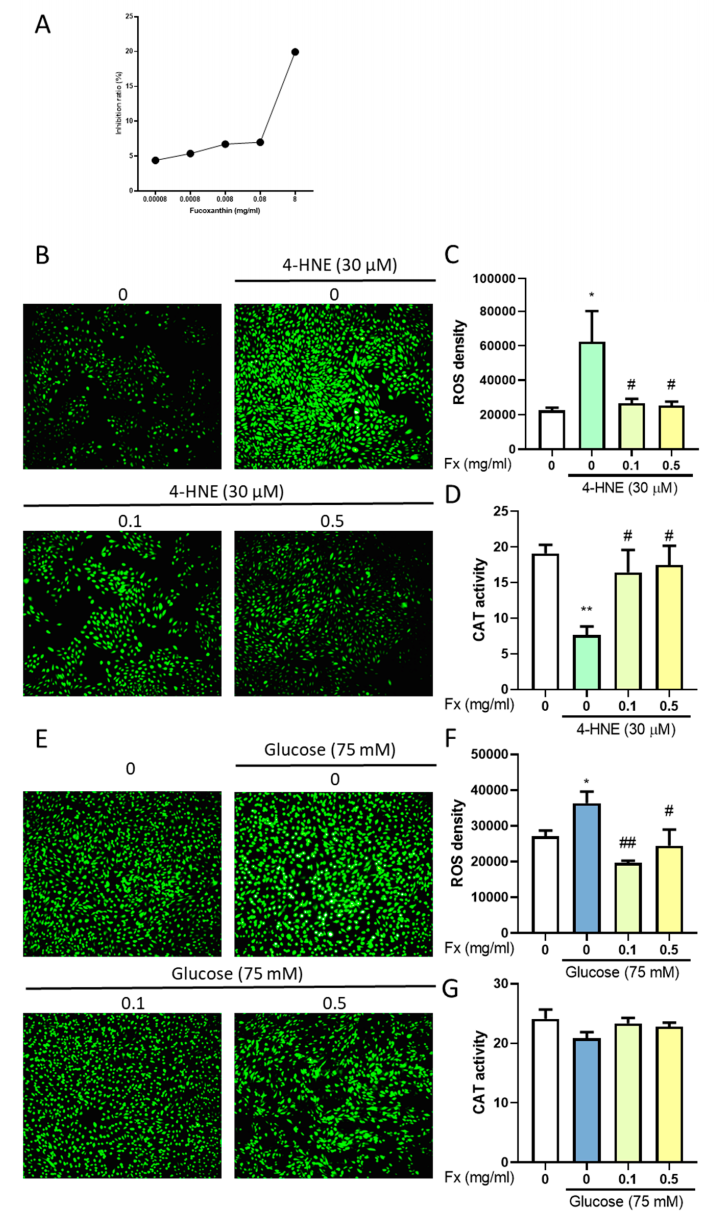
3.6. The Antioxidant Ability of Fucoxanthin in 4-HNE- or High Glucose-Induced Diabetes Retinopathy
4. Discussion
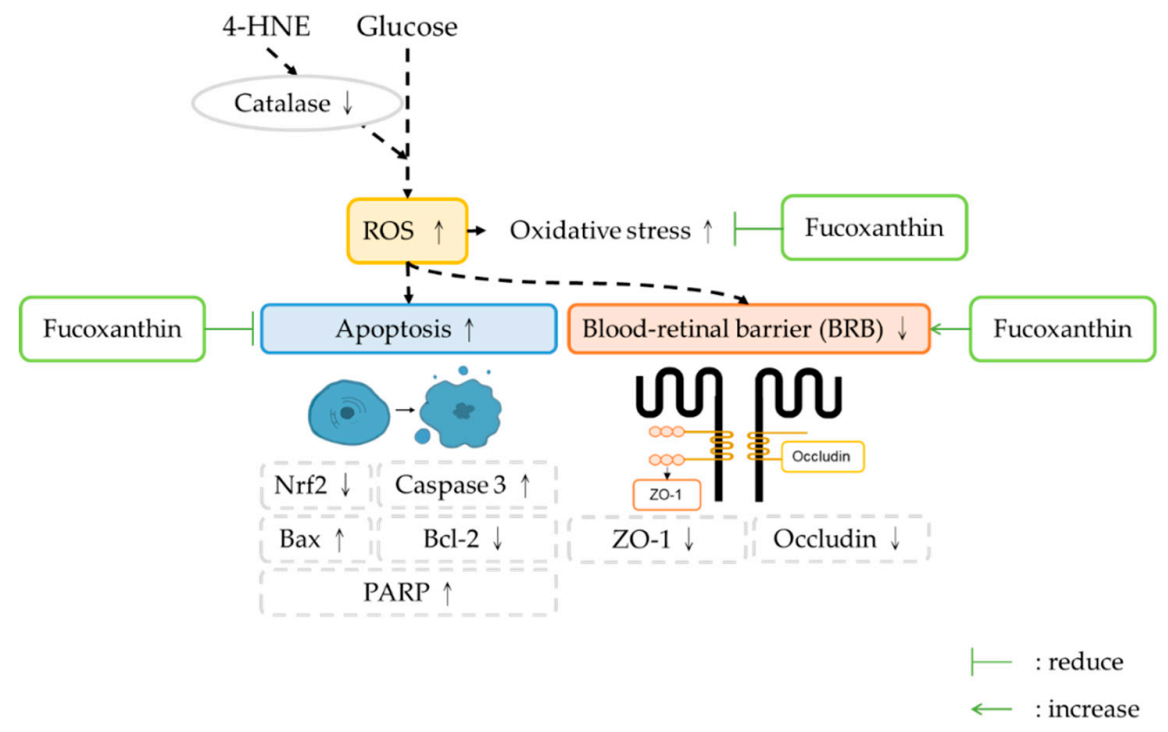
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ram, C.; Jha, A.K.; Ghosh, A.; Gairola, S.; Syed, A.M.; Murty, U.S.; Naidu, V.G.M.; Sahu, B.D. Targeting NLRP3 inflammasome as a promising approach for treatment of diabetic nephropathy: Preclinical evidences with therapeutic approaches. Eur. J Pharmacol. 2020, 885, 173503. [Google Scholar] [CrossRef] [PubMed]
- Shukla, U.V.; Tripathy, K. Diabetic Retinopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Zhang, D.; Ren, Q.; Su, X.; Sun, Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: A community based cross-sectional study. Medicine 2020, 99, e19236. [Google Scholar] [CrossRef] [PubMed]
- Munz, I.V.; Direev, A.O.; Gusarevitch, O.G.; Scherbakova, L.V.; Mazdorova, E.V.; Malyutina, S.K. Prevalence of ophthalmic diseases in the population older than 50 years. Vestn. Oftalmol. 2020, 136, 106–115. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, L.; Xin, G.; Li, S.; Ma, L.; Xu, Y.; Zhuang, M.; Xiong, Q.; Wei, Z.; Xing, Z.; et al. Oxidative stress mediated by lipid metabolism contributes to high glucose-induced senescence in retinal pigment epithelium. Free Radic. Biol. Med. 2019, 130, 48–58. [Google Scholar] [CrossRef]
- Cohen, G.; Riahi, Y.; Sunda, V.; Deplano, S.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic. Biol. Med. 2013, 65, 978–987. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Simó, R.; Hernández, C. Circulating Biomarkers of Diabetic Retinopathy: An Overview Based on Physiopathology. J. Diabetes Res. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.; Green, C.R.; Rupenthal, I.D.; Mugisho, O.O. Connexin43 hemichannel block protects against retinal pigment epithelial cell barrier breakdown. Acta Diabetol. 2020, 57, 13–22. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Lazzara, F.; Fidilio, A.; Fresta, C.G.; Conti, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Blood-retinal barrier protection against high glucose damage: The role of P2X7 receptor. Biochem. Pharmacol. 2019, 168, 249–258. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Zhang, X.; Chen, Q.; Chen, H.; Sun, L.; Liu, G. Protective Effect of Fucoxanthin Isolated from Laminaria japonica against Visible Light-Induced Retinal Damage Both in Vitro and in Vivo. J. Agric. Food Chem. 2016, 64, 416–424. [Google Scholar] [CrossRef]
- Genç, Y.; Bardakci, H.; Yücel, Ç.; Karatoprak, G.; Küpeli-Akkol, E.; Hakan-Barak, T.; Sobarzo-Sánchez, E. Oxidative Stress and Marine Carotenoids: Application by Using Nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Widjaja-Adhi, M.A.K.; Koide, A.; Kaga, T.; Nakano, S.; Beppu, F.; Hosokawa, M.; Miyashita, K. In Vivo Antioxidant Activity of Fucoxanthin on Obese/Diabetes KK- Ay Mice. Food Nutr. Sci. 2012, 3, 1491–1499. [Google Scholar]
- Silva, P.; Fernandes, C.; Barros, L.; Ferreira, I.C.F.R.; Pereira, L.; Gonçalves, T. The antifungal activity of extracts of Osmundea pinnatifida, an edible seaweed, indicates its usage as a safe environmental fungicide or as a food additive preventing post-harvest fungal food contamination. Food Funct. 2018, 9, 6187–6195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a Marine Carotenoid, Attenuates β-Amyloid Oligomer-Induced Neurotoxicity Possibly via Regulating the PI3K/Akt and the ERK Pathways in SH-SY5Y Cells. Oxid Med. Cell Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasedya, E.S.; Martyasari, N.W.R.; Abidin, A.S.; Pebriani, S.A.; Ilhami, B.T.K.; Frediansyah, A.; Sunarwidhi, A.L.; Widyastuti, S.; Sunarpi, H. Macroalgae Sargassum cristaefolium Extract Inhibits Proinflammatory Cytokine Expression in BALB/C Mice. Scientifica 2020, 2020, 9769454. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.-T.; Chen, H.-Y.; Lin, P.-H.; Hsia, S.-M. Preventive Effects of Fucoidan and Fucoxanthin on Hyperuricemic Rats Induced by Potassium Oxonate. Mar. Drugs 2019, 17, 343. [Google Scholar] [CrossRef] [Green Version]
- Le Goff, M.; Le Ferrec, E.; Mayer, C.; Mimouni, V.; Lagadic-Gossmann, D.; Schoefs, B.; Ulmann, L. Microalgal carotenoids and phytosterols regulate biochemical mechanisms involved in human health and disease prevention. Biochimie 2019, 167, 106–118. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Hung, H.-C.; Chen, H.-Y.; Huang, K.-C.; Lin, P.-H.; Chang, J.-Y.; Huang, T.-C.; Hsia, S.-M. The Inhibitory Effect of Extra Virgin Olive Oil and Its Active Compound Oleocanthal on Prostaglandin-Induced Uterine Hypercontraction and Pain—Ex Vivo and In Vivo Study. Nutrients 2020, 12, 3012. [Google Scholar] [CrossRef]
- Dumitrescu, A.G.; Istrate, S.L.; Iancu, R.C.; Guta, O.M.; Ciuluvica, R.; Voinea, L. Retinal changes in diabetic patients without diabetic retinopathy. Rom. J. Ophthalmol. 2017, 61, 249–255. [Google Scholar] [CrossRef]
- Han, F.; Zhang, J.; Li, K.; Wang, W.; Dai, D. Triptolide protects human retinal pigment epithelial ARPE-19 cells against high glucose-induced cell injury by regulation of miR-29b/PTEN. Arch. Physiol. Biochem. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lu, H.; Xu, J.; Yu, H.; Wang, X.; Zhang, X. Protective roles of autophagy in retinal pigment epithelium under high glucose condition via regulating PINK1/Parkin pathway and BNIP3L. Biol. Res. 2018, 51, 22. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Rong, H. Pterostilbene impact on retinal endothelial cells under high glucose environment. Int. J. Clin. Experim. Pathol. 2015, 8, 12589–12594. [Google Scholar]
- Wang, P.; Chen, F.; Wang, W.; Zhang, X.D. Hydrogen Sulfide Attenuates High Glucose-Induced Human Retinal Pigment Epithelial Cell Inflammation by Inhibiting ROS Formation and NLRP3 Inflammasome Activation. Mediators Inflamm. 2019, 2019, 8908960. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Robredo, P.; González-Zamora, J.; Recalde, S.; Bilbao-Malavé, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020, 9, 838. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, L.; Chen, B. Oxidative Stress: Implications for the Development of Diabetic Retinopathy and Antioxidant Therapeutic Perspectives. Oxid. Med. Cell. Longev. 2014, 2014, 752387. [Google Scholar] [CrossRef] [Green Version]
- Shivarudrappa, A.H.; Ponesakki, G. Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. J. Cell Commun. Signal 2020, 14, 207–221. [Google Scholar] [CrossRef]
- Borras, C.; Canonica, J.; Jorieux, S.; Abache, T.; El Sanharawi, M.; Klein, C.; Delaunay, K.; Jonet, L.; Salvodelli, M.; Naud, M.C.; et al. CFH exerts anti-oxidant effects on retinal pigment epithelial cells independently from protecting against membrane attack complex. Sci. Rep. 2019, 9, 13873. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xie, H.; Yang, Q.; Yang, Y.; Li, W.; Tian, H.; Lu, L.; Wang, F.; Xu, J.Y.; Gao, F.; et al. Erythropoietin protects outer blood-retinal barrier in experimental diabetic retinopathy by up-regulating ZO-1 and occludin. Clin. Exp. Ophthalmol. 2019, 47, 1182–1197. [Google Scholar] [CrossRef]
- Wang, S.; Du, S.; Wu, Q.; Hu, J.; Li, T. Decorin Prevents Retinal Pigment Epithelial Barrier Breakdown Under Diabetic Conditions by Suppressing p38 MAPK Activation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2971–2979. [Google Scholar] [CrossRef] [Green Version]
- Woodby, B.; Schiavone, M.L.; Pambianchi, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Pecorelli, A.; Valacchi, G. Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model. Int. J. Environ. Res. Public Health 2020, 17, 3234. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.J.; Chen, D.; Mott, R.; Yu, Q.; Ma, J.X.; Zhang, S.X. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp. Eye Res. 2009, 89, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Du, J.; Li, R.; Zhao, L.; Luo, N.; Zhai, J.Y.; Long, L. Association between ICAM-1 level and diabetic retinopathy: A review and meta-analysis. Postgrad. J. 2019, 95, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Seo, Y.J.; Pan, C.H.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Fucoxanthin Suppresses Lipid Accumulation and ROS Production During Differentiation in 3T3-L1 Adipocytes. Phytother. Res. 2016, 30, 1802–1808. [Google Scholar] [CrossRef]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Ngoc-Hieu, B.T.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [Green Version]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, Y.-F.; Chen, H.-Y.; Chang, Y.-J.; Shih, Y.-H.; Shieh, T.-M.; Wang, K.-L.; Hsia, S.-M. Protective Effects of Fucoxanthin on High Glucose- and 4-Hydroxynonenal (4-HNE)-Induced Injury in Human Retinal Pigment Epithelial Cells. Antioxidants 2020, 9, 1176. https://doi.org/10.3390/antiox9121176
Chiang Y-F, Chen H-Y, Chang Y-J, Shih Y-H, Shieh T-M, Wang K-L, Hsia S-M. Protective Effects of Fucoxanthin on High Glucose- and 4-Hydroxynonenal (4-HNE)-Induced Injury in Human Retinal Pigment Epithelial Cells. Antioxidants. 2020; 9(12):1176. https://doi.org/10.3390/antiox9121176
Chicago/Turabian StyleChiang, Yi-Fen, Hsin-Yuan Chen, Yen-Jui Chang, Yin-Hwa Shih, Tzong-Ming Shieh, Kai-Lee Wang, and Shih-Min Hsia. 2020. "Protective Effects of Fucoxanthin on High Glucose- and 4-Hydroxynonenal (4-HNE)-Induced Injury in Human Retinal Pigment Epithelial Cells" Antioxidants 9, no. 12: 1176. https://doi.org/10.3390/antiox9121176





