1. Introduction
Hydrogen sulfide (H
2S), which was first documented as a highly hazardous chemical with a distinct smell of a rotten egg roughly 300 years ago, is a well-established gaseous molecule with numerous biological activities [
1]. To date, studies have reported H
2S participation in a variety of biological processes, including antioxidative stress response [
2,
3], protection against ischemia-reperfusion injury [
4], and the development/progression of neurodegenerative diseases [
5], under physiological and pathological conditions. It has been reported that H
2S is endogenously generated in mammalian cells and tissues from cysteine or cysteine derivatives via reactions catalyzed by cystathionine β-synthase, cystathionine γ-lyase, and 3-mercaptopyruvate sulfurtransferase [
1,
6,
7], as well as by degradation of persulfides/polysulfides [
8,
9,
10]. H
2S has been shown to function as a gaseous signaling compound [
1] with cytoprotective capabilities. For example, Kakinohana et al. reported that inhaling H
2S at 80 ppm after ischemia/reperfusion could prevent motor neuron degeneration in the ventral horn of the lumbar spinal cord via persulfidation of nuclear factor-κB (NF-κB) and p65 [
11]. Indeed, H
2S level in biological fluids, such as plasma and serum, is correlated with the severity of various diseases [
12,
13,
14,
15,
16]. However, the chemical mechanisms associated with the biological activities of H
2S are not fully elucidated.
H
2S is soluble in water and hence in biological fluids, such as plasma and extracellular matrix. The percentage composition of H
2S in biological fluids depends on temperature and pH. Approximately 20% and 80% of H
2S exist in the gaseous and deprotonated (hydrogen sulfide anion, HS
−) forms, respectively, while a very negligible amount is found as sulfide anions (S
2−), in a physiological environment (pH 7.4, 37 °C). However, 40% of H
2S exists in the gaseous form at pH 7.4 and 25 °C [
17]. Several methods are available for H
2S detection, from the classic colorimetric assay using methylene blue [
18,
19] to the more recent techniques, such as gas chromatography [
20], high-performance liquid chromatography (HPLC) [
21], and mass spectrometry (MS) [
22]. Fluorescent, electrochemical, and chemiluminescent methods with multiple chemical probes to visualize intracellular H
2S levels in living cells have also been developed [
23]. In these methods, H
2S is often derivatized to form bis-S-adduct using several alkylating agents, such as monobromobimane (MBB), β-(4-hydroxyphenyl)ethyl iodoacetamide (HPE-IAM), and maleimide-based compounds, including
N-ethylmaleimide (NEM) and
N-(1-pyrene) maleimide [
14,
16,
17]. Although these recently developed techniques are highly specific and sensitive with much lower detection limits, they require costly instruments, such as a mass spectrometer.
From the viewpoint of disease prevention and diagnosis, it is important to evaluate H2S levels in biological fluids, and a simple and highly selective H2S measurement method is required. Colorimetric immunological detection methods, such as enzyme-linked immunosorbent assay (ELISA), are easy to handle, widely used, and require a microplate reader, which is a relatively low-cost piece of equipment compared with other pieces of equipment used in recently developed methods, such as gaseous or liquid chromatographs and mass spectrometers. Therefore, the present study aimed to generate a specific monoclonal antibody to detect H2S using NEM as the H2S detection agent, by immunizing rabbit and rat with a protein conjugated with bis-S-adduct of NEM (NEM-S-NEM) as immunogen, and to develop a simple colorimetric immunoassay for detection of H2S in biological samples.
2. Materials and Methods
2.1. Materials
NEM and bovine serum albumin (BSA) were obtained from Nacalai Tesque (Kyoto, Japan). Furthermore, 3-maleimidopropionic acid (MPA) and water soluble carbodiimide (WSC) were obtained from Tokyo Chemical Industry (Tokyo, Japan). All other chemicals and reagents were procured from common suppliers and were of the highest grade commercially available.
2.2. Preparation of NEM-S-Adducts
To prepare NEM-S-NEM and NEM-S-MPA, a bis-S-heteroadduct of NEM and MPA, 50 mM NEM was reacted with 50 mM sodium hydrosulfide (NaHS) in 500 mM sodium phosphate buffer (pH 7.4) containing 50% methanol with or without 50 mM MPA with continuous stirring at room temperature (25–28 °C) for 1 h. The NEM-S-NEM and NEM-S-MPA adducts were purified by HPLC (JASCO Corporation, Tokyo, Japan) using a C18 reverse-phase column (CAPCELL PAK C18 UG80 20 × 250 mm; Shiseido, Tokyo, Japan) and a linear gradient of solvent A (water containing 0.1% formic acid [FA]) and solvent B (100% methanol) (0% B at 0 min; 60% B at 70 min) at a flow rate of 6 mL/min. The elution was monitored by detecting absorbance at 220 nm. The purified NEM-S-NEM and NEM-S-MPA were confirmed by HPLC with a C18 reverse-phase column (Mightysil RP-18 GP 3.0 × 75 mm; Kanto Chemical, Tokyo, Japan) using a linear gradient of solvent A (water containing 0.1% FA) and solvent B (100% methanol) (0% B at 0 min; 70% B at 10 min) at a flow rate of 1 mL/min.
To produce NEM-adduct of cysteine (NEM-Cys), which was used as a competitor in competitive ELISA, 500 mM NEM was incubated with 50 mM cysteine in 100 mM sodium phosphate buffer (pH 7.4) containing 50% methanol with continuous stirring at room temperature (25–28 °C) for 1 h. N-acetyl cysteine (NAC) was also incubated with NEM to generate NEM-NAC that was used to prepare an affinity column for removal of putative contaminants from the rabbit antiserum, which included antibodies recognizing NEM-conjugated thiols, such as cysteine, glutathione, and protein cysteine residues. The produced NEM-Cys and NEM-NAC were purified by HPLC as described above. The purified NEM-NAC was conjugated to TOYOPEARL AF-Amino-650M beads (particle size, 65 μm; Tosoh, Kyoto, Japan) according to the manufacturer’s instructions.
The chemical structures of NEM-S-adducts were characterized by liquid chromatography-electrospray ionization-tandem MS (LC-ESI-MS/MS), which was performed on a triple quadrupole mass spectrometer (Xevo TQD; Waters, Milford, MA) coupled with an Alliance e2695 system (Waters). NEM-S-adducts were separated in an Alliance e2695 system using a C18 reverse-phase column (Mightysil RP-18 GP 2.0 × 50 mm; Kanto Chemical) with a linear gradient of solvent A (water containing 0.1% FA) and solvent B (100% methanol) (1% B at 0 min; 99% B at 5 min) at a flow rate of 0.3 mL/min. The mass spectrometer was operated in positive mode under the following conditions: capillary voltage, 1000 V; desolvation gas, nitrogen, at 1000 L/h at 500 °C. NEM-S-adducts were identified by multiple reaction monitoring (MRM). The MRM parameters are provided in
Supplementary Table S1. Chemical structures of the NEM-adducts are depicted in
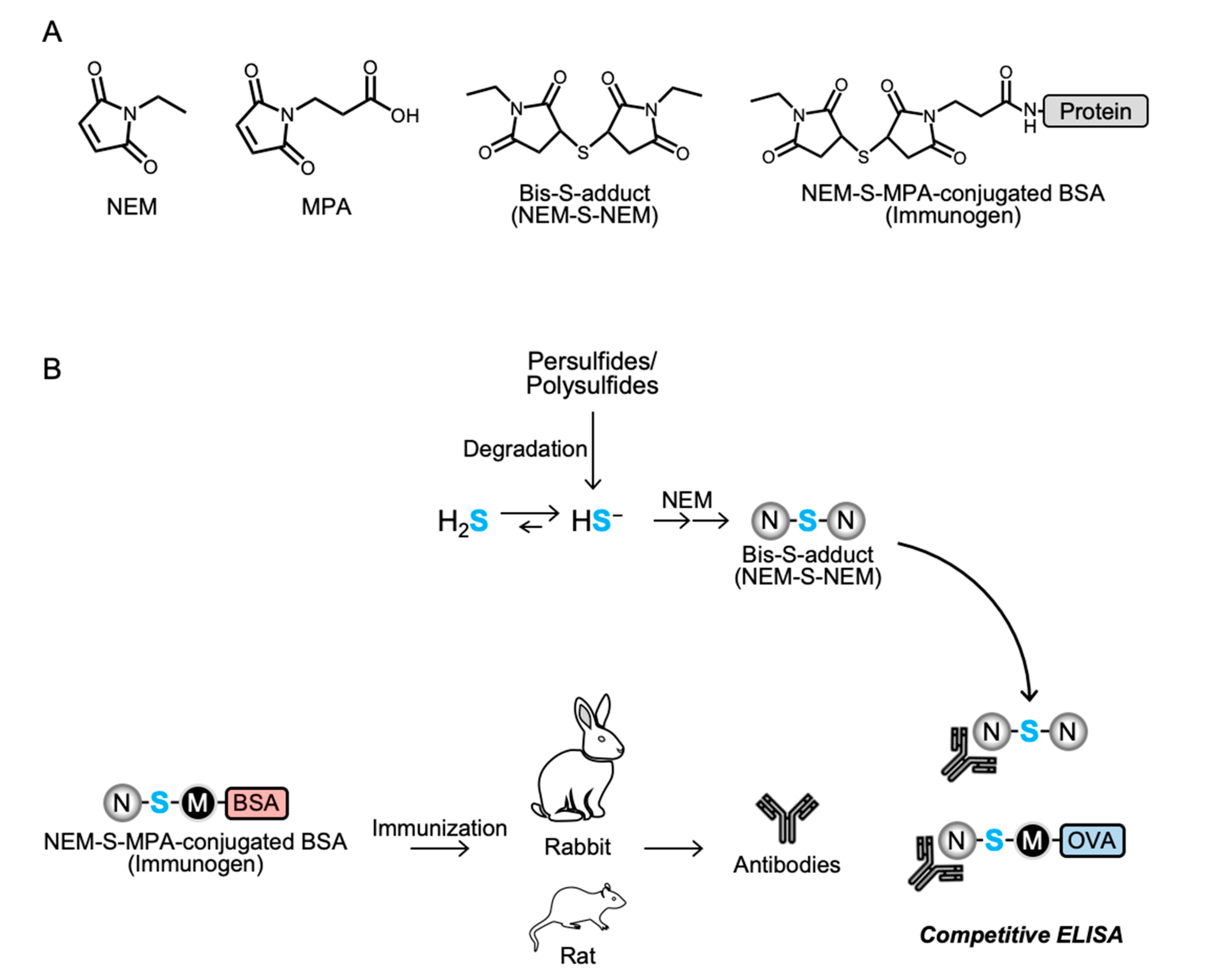
Figure 1A and
Supplementary Figures S1 and S2.
2.3. Conjugation of NEM-S-MPA to Protein
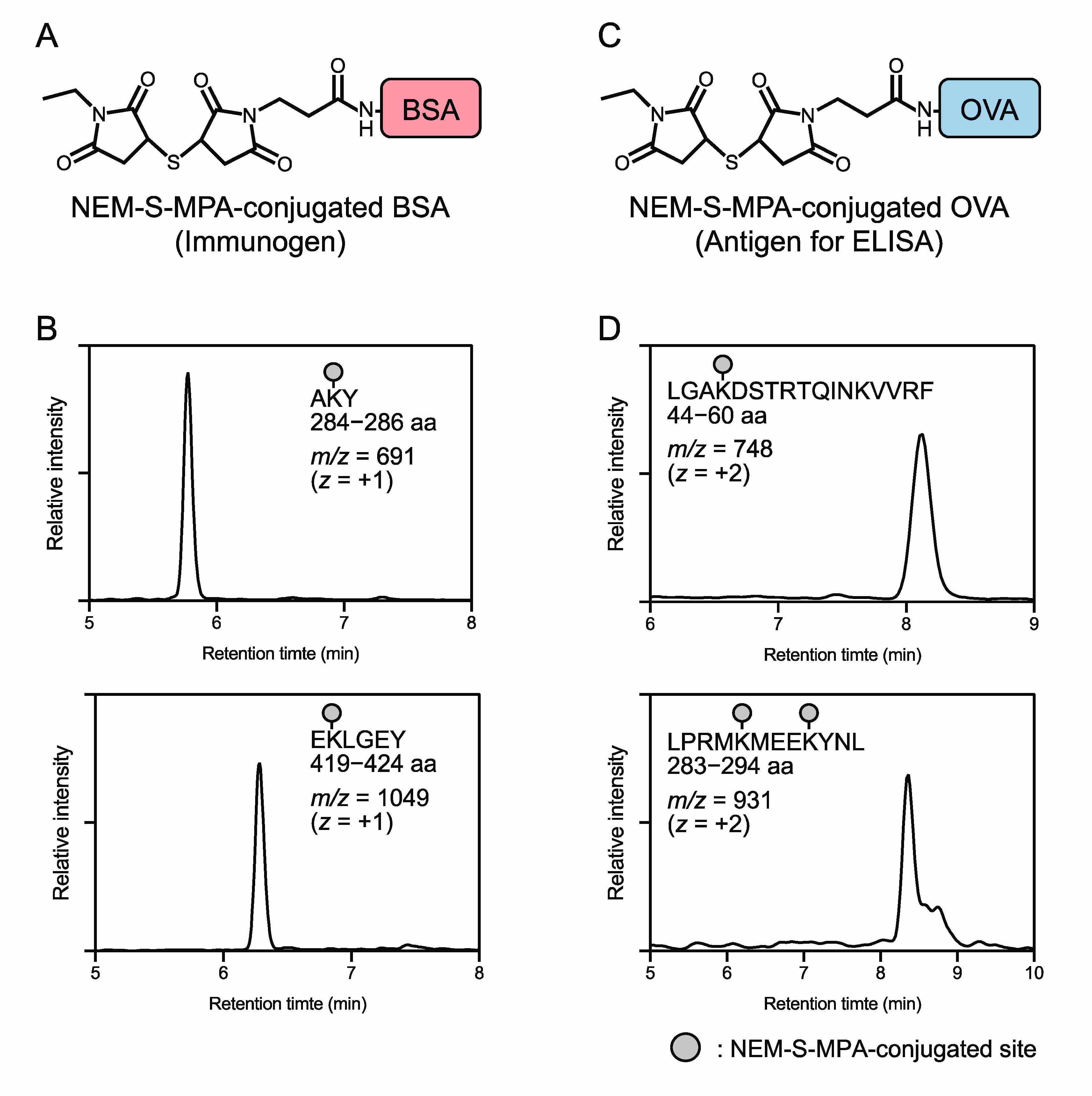
To prepare an immunogen, NEM-S-MPA was conjugated to BSA via a coupling reaction of the propionic acid moiety of NEM-S-MPA and the primary amine moiety (lysine residue) of BSA using WSC. In brief, NEM-S-MPA was incubated with 3.3 mg/mL BSA and 30 mM WSC in 0.1 M 2-(N-morpholino)ethanesulfonic acid buffer (pH 5.5) with continuous stirring at room temperature (25–28 °C) for 12 h. After centrifugation at 750× g, 4 °C for 5 min, the resultant supernatant was transferred into a desalting column (PD-10, GE Healthcare, Little Chalfont, England), pre-equilibrated with ice-cold phospho-buffered saline (PBS), to remove free WSC and NEM-S-MPA. Protein fractions were collected with ice-cold PBS, and the protein concentration was determined by the Bradford method with BSA as a standard. To prepare an antigen for ELISA, ovalbumin (OVA; CalbioChem, San Diego, CA, USA) was used instead of BSA.
To verify NEM-S-MPA conjugation onto BSA and OVA proteins, NEM-S-MPA-conjugated BSA and OVA proteins were digested by chymotrypsin and the generated NEM-S-MPA-containing peptide fragments were detected by LC-ESI-MS. Chymotryptic digestion of NEM-S-MPA-conjugated BSA and OVA proteins was carried out by the general protocol. In brief, the protein was denatured in 100 mM Tris-HCl (pH 8.0) containing 6 M urea at 37 °C for 1 h, reduced in the presence of 20 mM dithiothreitol at 37 °C for 30 min, and then incubated with 50 mM iodoacetamide at 37 °C for 30 min. The reaction mixture was diluted 1.6 times with 100 mM Tris-HCl (pH 8.0) containing 10 mM CaCl
2 and 6.25 μg/mL chymotrypsin, and further incubated at 37 °C for 12 h. The chymotryptic digest was enriched by a C18 column (Discovery
® DSC-18 100 mg, Sigma-Aldrich, St. Louis, MO, USA), and the eluate was concentrated
in vacuo, and then the NEM-S-MPA-containing peptide fragments in the solution were analyzed by LC-ESI-MS analysis. LC-ESI-MS analysis was performed by the same LC-MS system described above. Peptide fragments were separated in an Alliance e2695 system using a C18 reverse-phase column (Mightysil RP-18 GP 2.0 × 50 mm) with a linear gradient of solvent A (water containing 0.1% FA) and solvent B (100% methanol) (1% B at 0 min; 99% B at 10 min) at a flow rate of 0.3 mL/min. The MS setting parameters were the same as described above. NEM-S-MPA-containing peptide fragments, the predicted mass of which was calculated by ProteinProspector (version 6.2.2) developed by the University of California San Francisco Mass Spectrometry Faculty (
http://prospector.ucsf.edu), were identified by selected ion monitoring (SIM). The SIM parameters are provided in
Supplementary Table S2.
2.4. Preparation of Anti-NEM-S-NEM Antibody
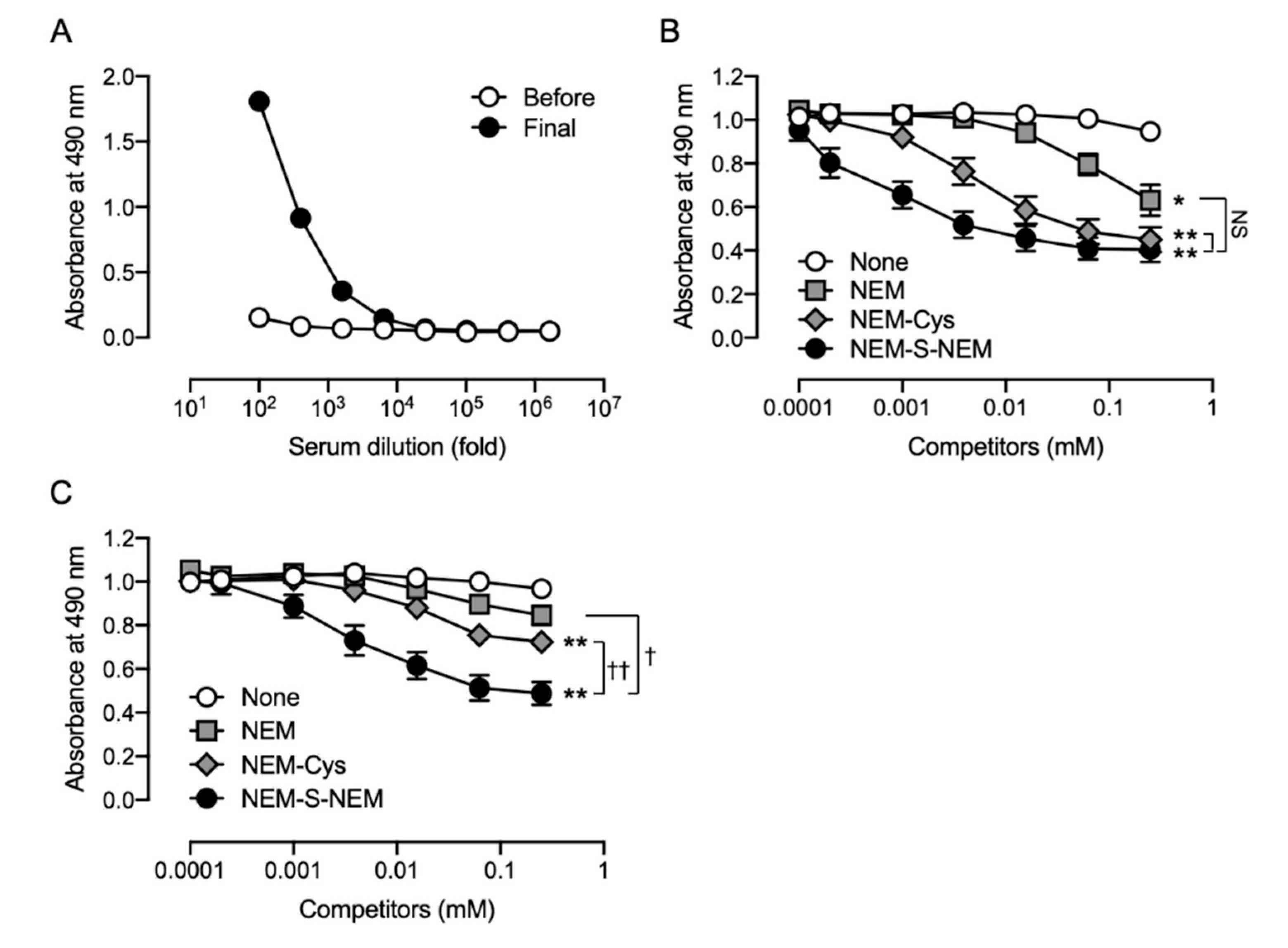
This study was performed in accordance with the Guidelines for Animal Experimentation of Osaka Prefecture University, Osaka, Japan. All animal experiments were approved by the Animal Ethical Committee of Osaka Prefecture University. To obtain the anti-NEM-S-NEM rabbit polyclonal antibody (pAb), a 10 week-old male Japanese white rabbit (Kiwa Laboratory Animals, Wakayama, Japan) was immunized by subcutaneous administration of NEM-S-MPA-conjugated BSA with Freund’s complete adjuvant (Difco Laboratories, Detroit, MI, USA). Booster doses of the same immunogen plus Freund’s incomplete adjuvant were administered five times every 2 weeks. Three days after the last booster injection, the animal was anesthetized with isoflurane and sacrificed by collection of whole blood volume followed by serum separation. To eliminate putative contamination with antibodies recognizing NEM-conjugated thiols, including cysteine, glutathione, and protein cysteine residues, in the rabbit antiserum, the serum was subjected into an NEM-NAC-conjugated TOYOPEARL AF-Amino-650 M beads affinity column, and the flow-through fraction, collected in a new tube, was used as rabbit pAb. Antibody specificity was confirmed by competitive ELISA using NEM, NEM-Cys, and NEM-S-NEM (detailed procedure is described in
Section 2.5).
The rat monoclonal antibody (mAb) specific for NEM-S-NEM was prepared as described previously [
24], with slight modifications. A murine myeloma cell line (P3X63-Ag653) was used for cell fusion. In brief, a 10 week-old male Wister rat (Kiwa Laboratory Animals) was subcutaneously administrated NEM-S-MPA-conjugated BSA plus Freund’s complete adjuvant. Booster doses of the same immunogen plus Freund’s incomplete adjuvant were administered three times every 2 weeks. Three days after the last booster dose, the immunized rat was anesthetized with isoflurane and sacrificed by collection of whole blood volume, and the spleen tissue was harvested. After washing the spleen cells twice with ice-cold Dulbecco’s modified Eagle’s medium (DMEM, Wako Pure Chemical, Osaka, Japan) without serum, cells were mixed with the myeloma cells at a ratio of 4:1. After centrifugation, the resultant supernatant was removed, and the cell pellets were resuspended in DMEM containing 50% (
w/
v) polyethylene glycol 4000 (Merck Millipore, Darmstadt, Germany), which was added dropwise. After washing cells with DMEM containing 15% fetal bovine serum (FBS, Cytosystems, Castle Hill, NSW, Australia), cells were resuspended in GIT medium (Wako Pure Chemical) supplemented with 10% FBS, 1% penicillin-streptomycin, and 1× HAT media supplement (Sigma-Aldrich, St. Louis), and then seeded into four 96-well plates (Iwaki, Tokyo, Japan). On day 7 after cell fusion, the culture supernatant of the growing hybridoma cells was tested by competitive ELISA, and positive hybridoma clones were screened. Twenty-four positive hybridoma clones were successfully obtained. Finally, two stable hybridoma cell lines (1C6 and 2D7) producing anti-NEM-S-NEM mAb were selected and used for further studies.
2.5. Competitive ELISA
Specificity of the rabbit and rat antisera was examined by competitive ELISA; the culture supernatants of hybridoma cells were also screened for production of anti-NEM-S-NEM rat mAb by competitive ELISA. In brief, ELISA plates (Iwaki) were coated with NEM-S-MPA-conjugated OVA (0.2 μg/well in PBS) at 4 °C overnight. The plates were washed three times with Tris-buffered saline containing 0.05% Tween 20 (TBST) and then blocked with 5 mg/mL gelatin for 30 min at 37 °C. The plates were washed three times with TBST, and a mixture of antibodies (the rabbit/rat antisera, rabbit pAb, or culture supernatant of hybridoma cells) with 2-fold dilution series of competitors (NEM, NEM-Cys, and NEM-S-NEM; 0.24 μM–1 mM) pre-incubated at 37 °C for 30 min was added to each well. After 30 min incubation at 37 °C, the plates were washed three times with TBST, and horse radish peroxidase-conjugated anti-rabbit immunoglobulin g (IgG) or anti-rat IgG antibody (1:5000 dilution; Santa Cruz Biotechnology, Dallas, TX, USA) was added to each well. After 30 min incubation at 37 °C, the plates were washed three times with TBST followed by addition of 0.5 mg/mL o-phenylenediamine (Nacalai Tesque) in 0.1 M citrate-phosphate buffer (pH 5.0) containing 0.024% hydrogen peroxide to each well and further incubated at room temperature (25–28 °C) for 30 min. The colorimetric reaction was terminated by the addition of 2 M H2SO4. Absorbance was measured at 490 nm using a microplate reader (Bio-Rad model 450; Hercules, CA, USA).
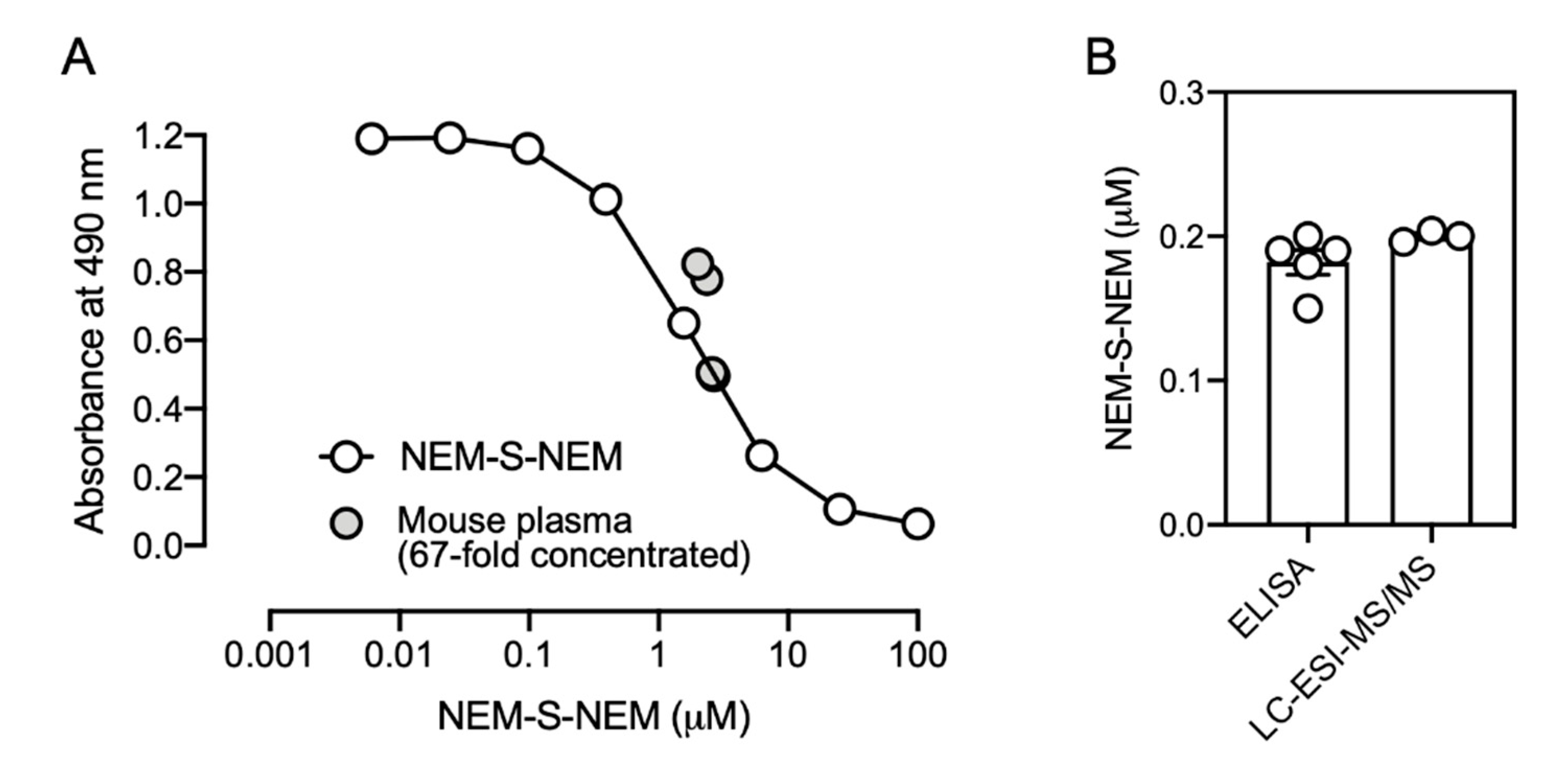
In a model experiment for biological samples, a reaction mixture containing 1 mM NEM and various concentrations of NaHS (6.3, 25, and 100 μM), incubated in PBS at 37 °C for 1 h, was used as a competitor for competitive ELISA with the rat mAb (1C6).
To evaluate whether the immunoassay was useful for analyzing biological samples, mouse plasma obtained from 20-week-old male C57BL/6J mice (Kiwa Laboratory Animals) was utilized. The mice (
n = 5) were sacrificed by exsanguination under general anesthesia; plasma was prepared, flash frozen in liquid nitrogen, and stored at −80 °C until further procedures. Plasma was reacted with 10 mM NEM in PBS containing 50% methanol at 37 °C for 1 h. After centrifugation, the supernatant was collected, dried
in vacuo, and then dissolved in 0.1% FA, an aliquot of which was analyzed by LC-ESI-MS/MS for quantification of the formed NEM-S-NEM as described previously [
8]. For detecting the formed NEM-S-NEM by competitive ELISA to enrich the formed NEM-S-NEM, samples were loaded onto a C18 column (Discovery
® DSC-18 500 mg, Sigma-Aldrich), the column was washed with 0.1% FA containing 25% methanol, then NEM-S-NEM was collected with 0.1% FA containing 50% methanol. Eluates were dried in vacuo, and then dissolved in PBS. The final solutions, 67-fold concentrated compared with the original plasma, were utilized as competitors for competitive ELISA as described above. Mouse plasma samples were mixed with rat mAb at a ratio of 1:4. The concentration in the original plasma was calculated by the following formula: the original concentration = the concentration determined by competitive ELISA × 5 (dilution factor) ÷ 67 (concentration factor). The exact values of original mouse plasma determined by competitive ELISA and LC-ESI-MS/MS analysis are described in
Supplementary Table S3.
2.6. Statistical Analyses
Data are presented as mean ± standard error of the mean (SEM) of at least three independent experiments unless otherwise specified. For statistical comparisons, we utilized two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. All analyses were performed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). p < 0.05 was considered statistically significant.
4. Discussion
In the present study, we developed a strategy to generate antibodies for detection of H
2S that involved the following steps: (i) derivatization of H
2S by NEM, (ii) preparation of NEM-S-MPA-conjugated BSA and OVA, (iii) immunization of animal models and isolation of antibodies. H
2S is derivatized by several alkylating agents, such as MBB, HPE-IAM, and maleimide-based compounds including NEM, to form a stable bis-S-adduct. In this study, we used NEM to derivatize H
2S because NEM is a well-known thiol alkylating agent that is cost-effective, easily available, and easy to handle. Furthermore, because several maleimide compounds are commercially available, heterogenic bis-S-adduct analogous to NEM-S-NEM, such as NEM-S-MPA, was successfully generated; this could conjugate protein via a coupling reaction between the propionic acid moiety of MPA and the amino moiety of protein. Next, we prepared a protein-conjugated bis-S-adduct NEM-S-MPA as the immunogen and antigen. Since the structure of NEM-S-MPA is analogous to that of NEM-S-NEM, NEM-S-MPA-conjugated protein was expected to function as an immunogen for the antibodies recognizing NEM-S-NEM. Moreover, we employed two different animal species: rabbit for pAb and rat for mAb generation because it is known that rabbits produce high titers of high-affinity antibodies, even against antigens that are not immunogenic in mice [
28,
29,
30]. The antibody titer in antiserum obtained from both immunized animals markedly increased after the administration of NEM-S-MPA-conjugated BSA as immunogen. Competitive ELISA showed that rabbit antiserum contained antibodies recognizing NEM-S-NEM, indicating that our strategy for generation of NEM-S-NEM-recognizing antibody was appropriate. However, since the rabbit pAb also showed high-affinity to NEM-labeled thiols and NEM, the rabbit pAb was not suitable for the detection of H
2S in biological samples containing large amounts of thiols, such as cysteine, glutathione, and protein cysteine residue. Further, we fused spleen cells obtained from the immunized rat and murine myeloma cells to generate hybridoma cell lines. After several screening processes, we finally obtained two stable hybridoma cell lines (1C6 and 2D7) producing rat mAbs that were highly specific for NEM-S-NEM but not NEM-Cys and NEM. Furthermore, we stoichiometrically detected NEM-S-NEM in a reaction mixture of NEM-S adducts by competitive ELISA with the generated rat mAb (clone 1C6). Thus, we successfully generated two mAbs highly specific for NEM-S-NEM, the bis-S-adduct of NEM. The mAbs prepared herein may be used to detect derivatized H
2S, and hence H
2S levels, in biological fluids, such as plasma, serum, and cerebrospinal fluid.
Early studies have documented the concentration of H
2S in plasma as 20–80 μM; although recent reports indicate it to be lower (submicromolar), a large labile pool of reactive persulfides/polysulfides exist that may degrade into H
2S in response to biological stimuli [
31]. In fact, it was recently reported that reactive persulfides and polysulfides, such as glutathione persulfide, are endogenously produced at significant levels in various organisms, including mammals [
8,
32,
33]. Persulfides/polysulfides exist not only as low-molecular-weight compounds but also as high-molecular-weight compounds, including proteins [
8,
32,
34]. Accumulating evidence indicates that persulfides/polysulfides play important roles in multiple cellular functions, such as antioxidative stress response, anti-inflammation, regulation of redox signal transduction, and mitochondrial biogenesis, via their unique chemical properties [
8,
32,
35,
36]. Polysulfides, which are also considered as bound sulfide, can release sulfides from their bound form in acidic environments [
37]. Recently, Ikeda et al. reported a novel combined assay by modifying sulfide antioxidant buffer (SAOB), a strong redox buffer, composed of 0.5 M sodium salicylate, 0.12 M ascorbic acid, and 2.2 M NaOH, to produce an Elimination Method of Sulfide from Polysulfide (EMSP) treatment solution that liberates sulfides [
38]. The sulfides released from polysulfides via reduction under alkaline conditions can be measured by H
2S detection techniques, and hence polysulfides are considered as a pool of H
2S. Therefore, the concentration of H
2S in biological samples reported previously might have included degradation products of reactive persulfides/polysulfides. With regard to the yield of NEM-S-NEM formation in biological samples, the derivatization of H
2S to NEM-S-NEM in the study was carried out by 1 h incubation in PBS containing 5 mM NEM at 37 °C, conditions at which we expected that a section of the sulfides was converted to NEM-S-NEM because some types of polysulfides are relatively stable at neuronal pH. Therefore, it is required to develop a derivatization procedure to degrade polysulfides to sulfides, which can definitely be derivatized by NEM to form NEM-S-NEM. To avoid this and enable precise measurement of H
2S in biological samples containing persulfides/polysulfides, it is necessary to carefully control the pH and temperature of the experimental conditions.
Our previous studies with LC-ESI-MS/MS analysis revealed that oxidized glutathione tetrasulfide, a biological polysulfide, can be decomposed by an electrophilic reaction with alkylating compounds, such as iodoacetamide, MBB, and NEM, especially at a higher pH (pH 8.0) [
8,
9,
10]. Furthermore, we recently established that polysulfides can be degraded by incubating them with reductants, such as dithiothreitol, at alkaline pH (unpublished data), releasing sulfides that can be detected by subsequent incubation with NEM to form NEM-S-NEM. Thus, it will be possible to distinguish sulfide that is already present in the sample from sulfide derived from polysulfides by comparison of the values obtained from samples prepared by two different derivatization procedures, i.e., one is direct derivatization of sulfides at lower pH for detection of sulfide that is already present in the sample and the other one is decomposition of polysulfides to sulfides at higher pH in the presence of reductants and then derivatization of sulfides for detection of the whole sulfides, including polysulfides. However, it has been reported that alkylating agents can enhance alkaline hydrolysis of polysulfides [
8,
9,
10], and thus, when NEM is utilized to derivatize sulfides, the results should be carefully considered. Combined with such procedures to distinguish derivatized sulfides, the anti-NEM-S-NEM rat mAb generated herein might be a useful tool for comprehensive measurement of polysulfides in biological samples, including cells, tissues, and various fluids.
5. Conclusions
In the present study, we employed NEM for derivatization of H2S, prepared NEM-S-MPA-conjugated protein as an antigen, and immunized rabbit and rat to generate polyclonal and monoclonal antibodies, respectively. Finally, we successfully generated two stable hybridoma cell lines (1C6 and 2D7) producing mAbs highly specific for NEM-S-NEM but not NEM-Cys and NEM. In fact, we determined H2S concentration in mouse plasma by competitive ELISA with the rat mAb developed herein, and the value was identical to that detected by LC-ESI-MS/MS analysis. However, presently, several important issues, such as time-consuming preparation steps for preparing biological samples and improvement of sensitivity and reproducibility, remain to be addressed. In future, in order to improve sensitivity, specificity, and reproducibility, and to more easily detect H2S in biological samples, conditions for pretreatment of biological samples, preparation of antigen proteins, and alternative detection methods, such as fluorescence and luminescence, need to be further optimized. To our knowledge, this is the first study to report antibody-mediated H2S detection. The mAb generated herein may be used to develop a convenient method to measure H2S levels as well as H2S pools (persulfides/polysulfides) in biological samples.