Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy
Abstract
1. Introduction
2. Materials and Methods
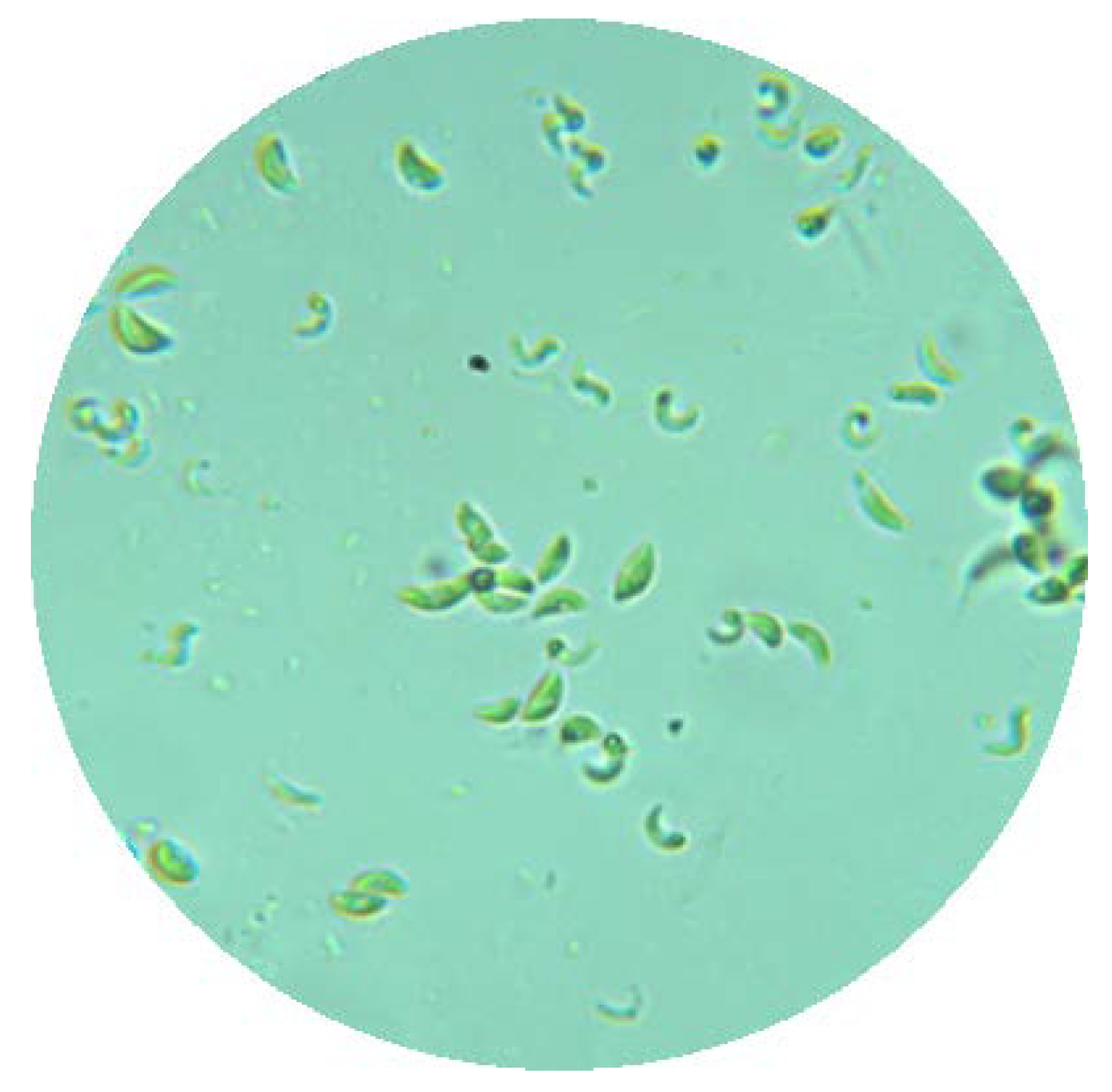
2.1. Microalgae and Preculture Conditions
2.2. Aquaculture Wastewater
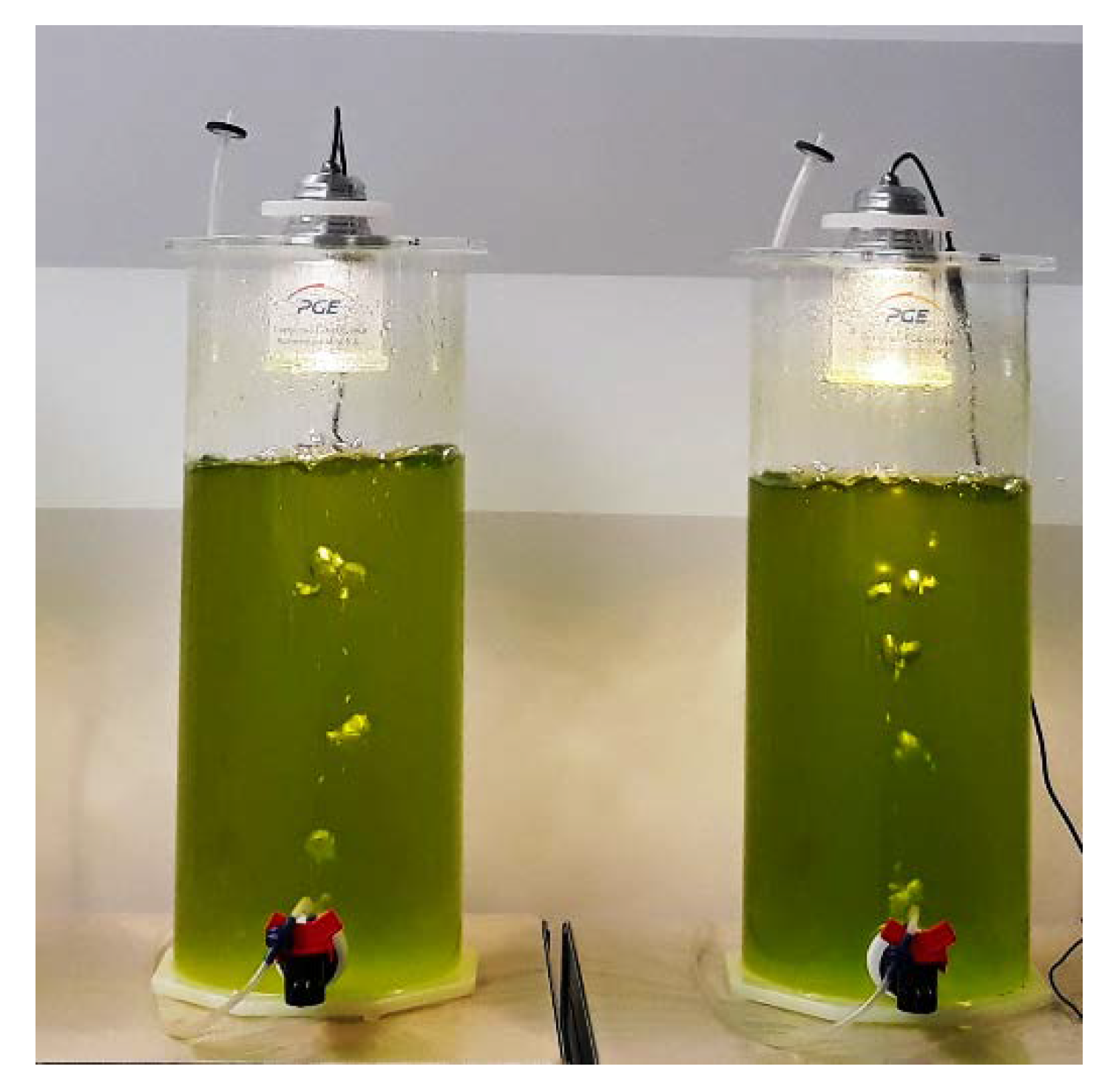
2.3. Experimental Setup
2.4. Analytical Methods
2.4.1. Determination of Microalgal Growth and Biomass Production
2.4.2. Determination of Lipid and Ash Content
2.4.3. Determination of Nutrient Concentration
2.4.4. Removal Efficiency and Ratio of Consumed Nutrients
2.4.5. Statistical Analysis
3. Results and Discussion
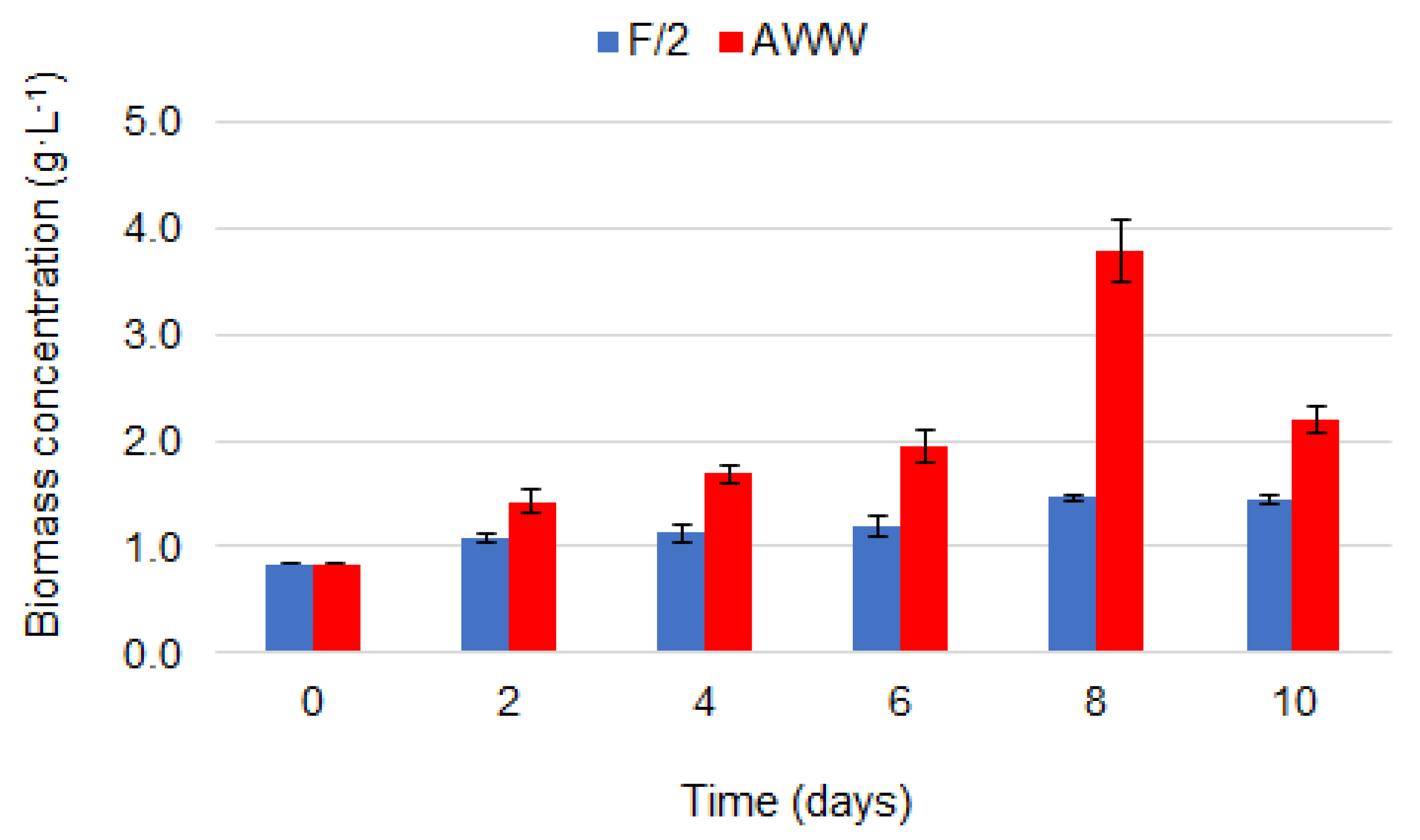
3.1. Microalgal Growth and Biomass Production
3.2. Lipid Production and Ash Content
3.3. Nutrient Removal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shuba, E.S.S.; Kifle, D. Microalgae to biofuels: Promising alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Shirvani, T.; Yan, X.; Inderwildi, O.R.; Edwards, P.P.; King, D.A. Life cycle energy and greenhouse gas analysis for algae-derived biodiesel. Energy Environ. Sci. 2011, 4, 3773–3778. [Google Scholar] [CrossRef]
- Beig, A.R.; Muyeen, S.M. Wind energy. In Electric Renewable Energy Systems; Rashid, M.H., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 60–77. [Google Scholar]
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Develop. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Driver, T.; Bajhaiya, A.; Pittman, J.K. Potential of Bioenergy Production from Microalgae. Curr. Sustain. Renew. Energy Rep. 2014, 1, 94–103. [Google Scholar] [CrossRef]
- IRENA. REmap 2030, A Renewable Energy Roadmap; International Renewable Energy Agency: Abu Dhabi, UAE, 2014. [Google Scholar]
- Perea-Moreno, M.-A.; Samerón-Manzano, E.; Perea-Moreno, A.-J. Biomass as Renewable Energy: Worldwide Research Trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef]
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–577. [Google Scholar] [CrossRef]
- IRENA. Global Energy Transformation: A Roadmap to 2050 (2019 Edition); International Renewable Energy Agency: Abu Dhabi, UAE, 2019. [Google Scholar]
- Goldemberg, J.; Coelho, S.T. Renewable energy—Traditional biomass vs. modern biomass. Energy Policy 2004, 32, 711–714. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Hoyer, J.; Cotta, F.; Diete, A.; Großmann, J. Bioenergy from Microalgae—Vision or Reality? ChemBioEng Rev. 2018, 5, 207–216. [Google Scholar] [CrossRef]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.; Grivalský, T.; Kopecký, J.; Masojídek, J. Bioethanol production from microalgae polysaccharides. Folia Microbiol. (Praha) 2019, 64, 627–644. [Google Scholar] [CrossRef]
- Wu, N.; Moreira, C.; Zhang, Y.; Doan, N.; Yang, S.; Phlips, E.; Svoronos, S.; Pullammanappallil, P. Techno-economic analysis of biogas production from microalgae through anaerobic digestion. In Anaerobic Digestion; Banu, J.R., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Singh, H.; Das, D. Biofuels from Microalgae: Biohydrogen in Energy from Microalgae. In Green Energy and Technology; Jacob-Lopes, E., Queiroz Zepka, L., Queiroz, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 201–228. [Google Scholar]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. The potential of microalgae in biodiesel production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Soomro, R.R.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Lin, L.; Danquah, M.K. Development of a two-stage microalgae dewatering process—A life cycle assessment approach. Front. Plant. Sci. 2016, 7, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Wang, Z.; Yuan, Z. Generation and harvesting of microalgae biomass for biofuel production. In Prospects and Challenges in Algal Biotechnology; Tripathi, B.N., Kumar, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 89–111. [Google Scholar]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Singh, M.; Das, K.C. Low Cost Nutrients for Algae Cultivation. In Starch Overproduction by Means of Algae; Bajpai, R., Prokop, A., Zappi, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–82. [Google Scholar]
- Do, J.-M.; Jo, S.-W.; Kim, I.-S.; Na, H.; Lee, J.H.; Kim, H.S.; Yoon, H.-S. A Feasibility Study of Wastewater Treatment Using Domestic Microalgae and Analysis of Biomass for Potential Applications. Water 2019, 11, 2294. [Google Scholar] [CrossRef]
- Whitton, R.; Ometto, F.; Pidou, M.; Jarvis, P.; Villa, R.; Jefferson, B. Microalgae for municipal wastewater nutrient remediation: Mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 2015, 4, 133–148. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.; Sun, Y.; Shu, Q.; Feng, P.; Zhu, L.; Xu, J.; Yuan, Z. Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ. Sci. Pollut. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Kumar, S.M. Microalgae biomass from swine wastewater and its conversion to bioenergy. Bioresour. Technol. 2019, 275, 109–122. [Google Scholar] [CrossRef]
- Gałczyńska, M.; Mańkowska, N.; Milke, J.; Buśko, M. Possibilities and limitations of using Lemna minor, Hydrocharis morsus-ranae and Ceratophyllum demersum in removing metals with contaminated water. J. Water Land Dev. 2019, 40, 161–173. [Google Scholar] [CrossRef][Green Version]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M.; Zając, G.; Szyszlak-Bargłowicz, J. Production of Microalgal Biomass using Aquaculture Wastewater as Growth Medium. Water 2020, 12, 106. [Google Scholar] [CrossRef]
- Xiaoning, L.; Guangyao, C.; Yi, T.; Jun, W. Application of effluent from WWTP in cultivation of four microalgae for nutrients removal and lipid production under the supply of CO2. Renew. Energy 2020, 149, 708–715. [Google Scholar]
- Moreno García, L.; Gariépy, Y.; Barnabé, S.; Raghavan, V. Biorefinery of microalgae biomass cultivated in wastewaters. In Refining Biomass Residues for Sustainable Energy and Bioproducts Technology, Advances, Life Cycle Assessment, and Economics; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 7; pp. 149–180. [Google Scholar]
- Luangpipat, T.; Chisti, Y. Biomass and oil production by Chlorella vulgaris and four other microalgae—Effects of salinity and other factors. J. Biotechnol. 2017, 257, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.J. Studies of marine planktonic diatoms in Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Polish Standards PN-EN 1189:2000. Water quality. In Determination of Phosphorus. Ammonium Molybdate Spectrometric Method; 2000; Available online: https://sklep.pkn.pl/catalogsearch/advanced (accessed on 15 November 2020).
- Polish Standards PN-ISO 7150-1:2002. Water and sewage quality. In Determination of Content of Compounds of Ammonium Nitrogen by Indophenol Blue Method; 2002; Available online: https://sklep.pkn.pl/pn-iso-7150-1-2002p.html (accessed on 15 November 2020). (In Polish)
- Polish Standards PN-73/C-04576.06. Water and sewage quality. In Determination of Content of Compounds of Nitrite Nitrogen with Sulphanilic Acid and 1-Naphthylamine; 1973; Available online: https://sklep.pkn.pl/pn-c-04576-06-1973p.html (accessed on 15 November 2020). (In Polish)
- Polish Standards PN-82/C-04576.08. Water and sewage quality. In Determination of Nitrate Nitrogen by Colorimetric Method with Sodium Salicylate; 1982; Available online: https://sklep.pkn.pl/pn-c-04576-08-1982p.html (accessed on 15 November 2020). (In Polish)
- Liu, Y.; Lv, J.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Treatment of real aquaculture wastewater from a fishery utilizing phytoremediation with microalgae. J. Chem. Technol. Biotechnol. 2019, 94, 900–910. [Google Scholar] [CrossRef]
- Guiza-Franco, L.; Orozco-Rojas, L.G.; Sánchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Production of Chlorella vulgaris Biomass on UV-treated Wastewater as an Alternative for Environmental Sustainability on High-Mountain Fisheries. Chem. Eng. Trans. 2018, 64. [Google Scholar] [CrossRef]
- Halfhide, T.; Åkerstrøm, A.; Lekang, O.I.; Gislerød, H.R.; Ergas, S.J. Production of algal biomass, chlorophyll, starch and lipids using aquaculture wastewater under axenic and non-axenic conditions. Algal Res. 2014, 6, 152–159. [Google Scholar] [CrossRef]
- Nogueira, S.M.S.; Junior, J.S.; Maia, H.D.; Saboya, J.P.S.; Farias, W.R.L. Use of Spirulina platensis in treatment of fish farming wastewater. Rev. Ciênc. Agron. 2018, 49, 599–606. [Google Scholar] [CrossRef]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.-M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated utilization of microalgae cultured in aquaculture wastewater: Wastewater treatment and production of valuable fatty acids and tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef]
- Kuo, C.; Jian, J.; Lin, T.; Chang, Y.; Wan, X.; Lai, J.; Lin, C. Simultaneous microalgal biomass production and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour. Technol. 2016, 221, 241–250. [Google Scholar] [CrossRef]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S.; Anyanwu, C.N. Algal biomass as a global source of transport fuels: Overview and development perspectives. Prog. Nat. Sci. Mater. Int. 2014, 24, 329–339. [Google Scholar]
- Yu, H.; Kim, J.; Lee, C. Nutrient removal and microalgal biomass production from different anaerobic digestion effluents with Chlorella species. Sci. Rep. 2019, 9, 6123. [Google Scholar] [PubMed]
- Diaz, G.C.; Cruz, Y.R.; Carliz, R.G.; Paula, R.C.; Aranda, D.A.; Dario, M.A.; Marass, G.S.; Furtado, N.C. Cultivation of Microalgae Monoraphidium sp., in the Plant Pilot the Grand Valle Bio Energy, for Biodiesel Production. Nat. Sci. 2015, 7, 370–378. [Google Scholar]
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [PubMed]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar]
- Aratboni, H.A.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar]
- Sacristán de Alva, M.; Luna Pabello, V.M.; Orta Ledesma, M.T.; Cruz Gómez, M.J. Carbon, nitrogen, and phosphorus removal, and lipid production by three saline microalgae grown in synthetic wastewater irradiated with different photon fluxes. Algal Res. 2018, 34, 97–103. [Google Scholar]
- Dhup, S.; Kannan, D.C.; Dhawan, V. Growth, lipid productivity and cellular mechanism of lipid accumulation in microalgae Monoraphidium sp. following different phosphorous concentrations for biofuel production. Curr. Sci. 2017, 112, 539–548. [Google Scholar]
- Bi, Z.; He, B.B. Characterization of microalgae for the purpose of biofuel production. Trans. ASABE 2013, 56, 1529–1539. [Google Scholar]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Comparison of Growth Rate and Nutrient Content of Five Microalgae Species Cultivated in Greenhouses. Plants 2019, 8, 279. [Google Scholar]
- Liu, J.; Pan, Y.; Yao, C.; Wang, H.; Cao, X.; Xue, S. Determination of ash content and concomitant acquisition of cell compositions in microalgae via thermogravimetric (TG) analysis. Algal Res. 2015, 12, 149–155. [Google Scholar]
- Roostaei, J.; Zhang, Y.; Gopalakrishnan, K.; Ochocki, A.J. Mixotrophic Microalgae Biofilm: A Novel Algae Cultivation Strategy for Improved Productivity and Cost-efficiency of Biofuel Feedstock Production. Sci. Rep. 2018, 8, 12528. [Google Scholar] [PubMed]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal Biofuels: Current Status and Key Challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Romanelli, A.; Soto, D.X.; Matiatos, I.; Martínez, D.E.; Esquiusa, S. A biological and nitrate isotopic assessment framework to understand eutrophication in aquatic ecosystems. Sci. Total Environ. 2020, 715, 136909. [Google Scholar] [CrossRef] [PubMed]
- Rasoul-Amini, S.; Montazeri-Najafabady, N.; Shaker, S.; Safari, A.; Kazemi, A.; Mousavi, P.; Mobasher, M.A.; Ghasemi, Y. Removal of nitrogen and phosphorus from wastewater using microalgae free cells in bath culture system. Biocatal. Agric. Biotechnol. 2014, 3, 126–131. [Google Scholar]
- Jordaan, E.; Roux-van der Merwe, P.; Badenhorst, J.; Knothe, G.; Botha, B.M. Evaluating the usability of 19 effluents for heterotrophic cultivation of microalgal consortia as biodiesel feedstock. J. Appl. Phycol. 2018, 30, 1533–1547. [Google Scholar]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.-M.; Feng, L.-J.; Liu, J.-Z.; Liu, M.; Cai, H.W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Carré, E.; Cocaud, E.; Beelen, V.; Boon, N.; Vervaeren, H. Treatment of industrial wastewaters by microalgal bacterial flocs in sequencing batch reactors. Bioresour. Technol. 2014, 161, 245–254. [Google Scholar]
- Jiang, L.; Pei, H.; Hu, W.; Hou, Q.; Han, F.; Nie, C. Biomass production and nutrient assimilation by a novel microalga, Monoraphidium spp. SDEC-17, cultivated in a high-ammonia wastewater. Energy Convers. Manag. 2016, 123, 423–430. [Google Scholar]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production. Rev. Energ. 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Wu, Z.; Shi, X. Phosphate assimilation by Chlorella and adjustment of phosphate concentration in basal medium for its cultivation. Biotechnol. Lett. 2008, 30, 1735. [Google Scholar] [CrossRef]
- W, Y.-H.; Yu, Y.; Hu, H.-Y. Potential biomass yield per phosphorus and lipid accumulation property of seven microalgal species. Bioresour. Technol. 2013, 130, 599–602. [Google Scholar]
- Kumar, K.; Nag Dasgupta, C.; Das, D. Cell growth kinetics of Chlorella sorokiniana and nutritional values of its biomass. Bioresour. Technol. 2014, 167, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
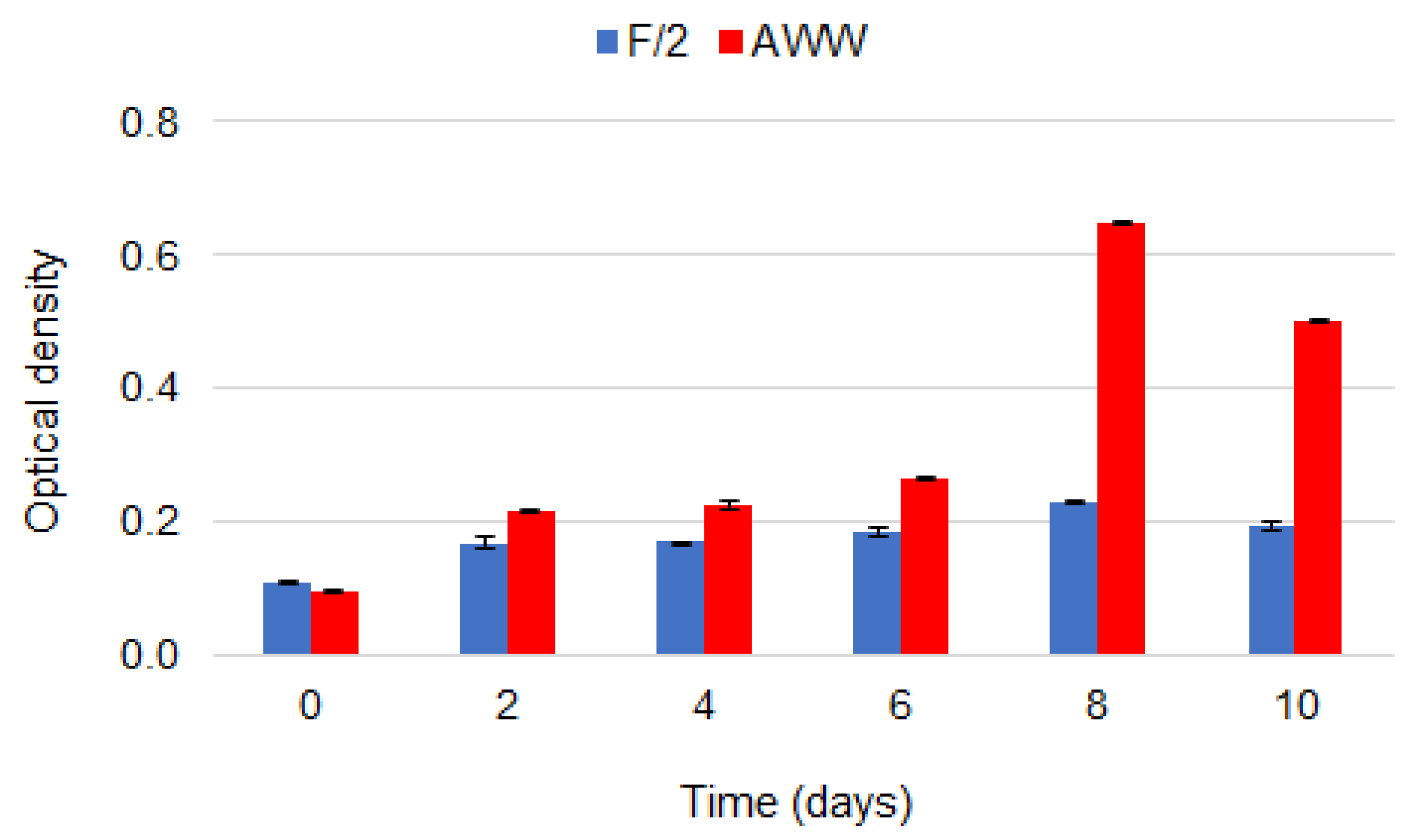

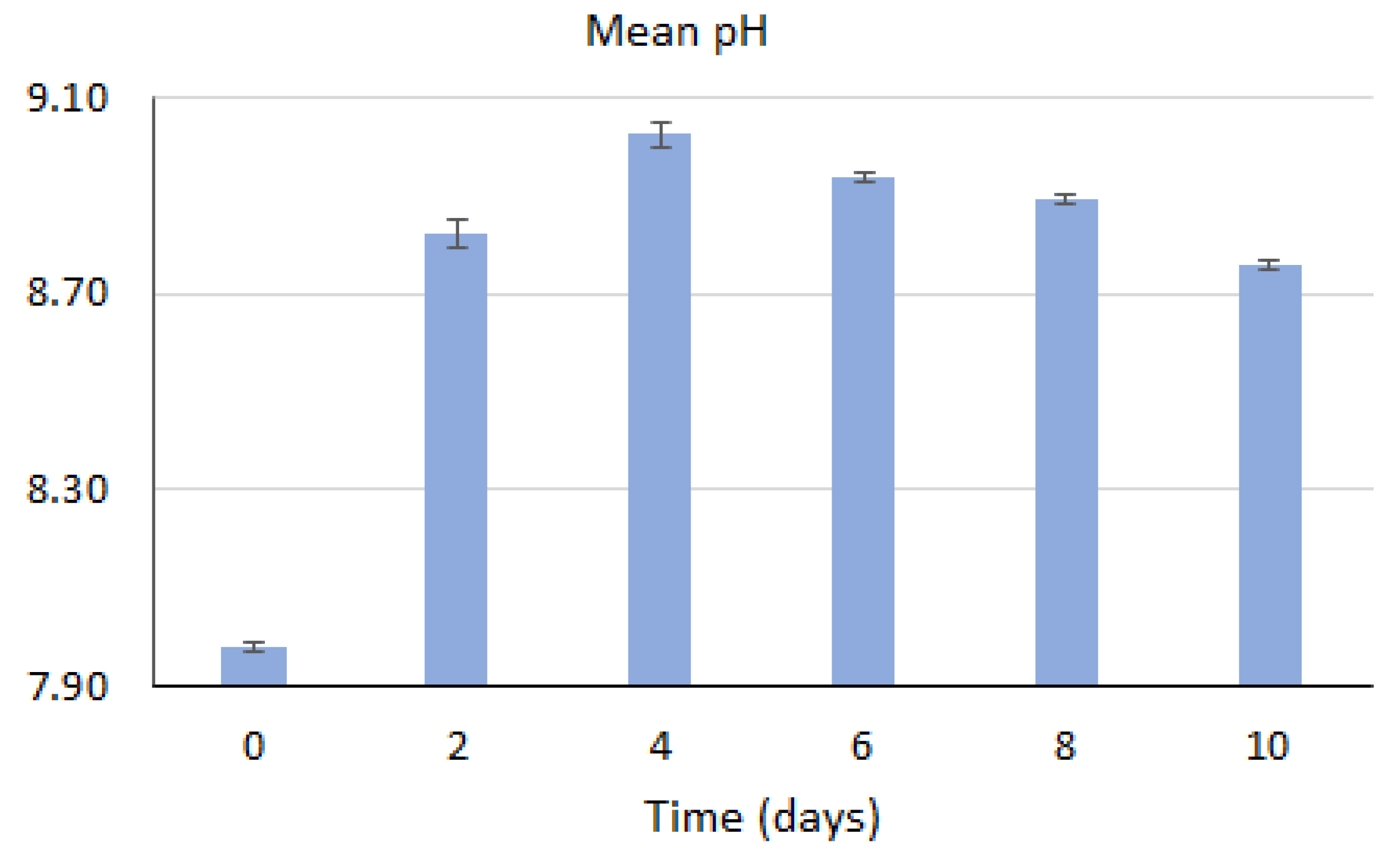
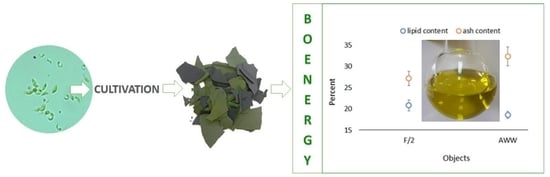
| Content % | Objects | |
|---|---|---|
| F/2 | AWW | |
| Lipid | 20.80 ± 1.25 | 18.53 ± 0.83 |
| Ash | 27.15 ± 1.66 | 32.34 ± 2.25 |
| Total N mg·L−1 | Total P mg·L−1 | N/P | Reference |
|---|---|---|---|
| 24.10–23.70 | 3.50–3.60 | 6.64 | Tossavainen et al. [40] |
| 31.53–32.13 | 1.08–1.12 | 28.94 | Present study |
| TN Concentration | TP Concentration | |
|---|---|---|
| Nutrient removal efficiency (%) | 82.62 | 99.06 |
| Initial mg·L−1 | 31.83 ± 0.31 | 1.10 ± 0.02 |
| Final mg·L−1 | 5.53 ± 0.06 | 0.01 ± 0.00 |
| Term (Days) | Nutrient Concentration, Mean ± SD (mg·L−1) | |||
|---|---|---|---|---|
| N-NO3− | N-NH4+ | N-NO2− | PO43− | |
| 0 | 27.67 ± 0.69 a,* | 0.23 ± 0.02 | 1.53 ± 0.02 a | 1.29 ± 0.09 a |
| 2 | 24.50 ± 1.04 b | 0.00 | 0.55 ± 0.01 d | 0.52 ± 0.07 b |
| 4 | 19.14 ± 1.58 c | 0.00 | 0.63 ± 0.00 b | 0.07 ± 0.01 c |
| 6 | 13.46 ± 1.68 d | 0.00 | 0.59 ± 0.01 c | 0.00 |
| 8 | 12.04 ± 0.20 d | 0.00 | 0.38 ± 0.01 e | 0.00 |
| 10 | 4.05 ± 0.15 e | 0.00 | 0.33 ± 0.01 f | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M. Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy. Energies 2020, 13, 5975. https://doi.org/10.3390/en13225975
Hawrot-Paw M, Koniuszy A, Gałczyńska M. Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy. Energies. 2020; 13(22):5975. https://doi.org/10.3390/en13225975
Chicago/Turabian StyleHawrot-Paw, Małgorzata, Adam Koniuszy, and Małgorzata Gałczyńska. 2020. "Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy" Energies 13, no. 22: 5975. https://doi.org/10.3390/en13225975
APA StyleHawrot-Paw, M., Koniuszy, A., & Gałczyńska, M. (2020). Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy. Energies, 13(22), 5975. https://doi.org/10.3390/en13225975