Astaxanthin Counteracts Vascular Calcification In Vitro Through an Early Up-Regulation of SOD2 Based on a Transcriptomic Approach
Abstract
:1. Introduction
2. Results
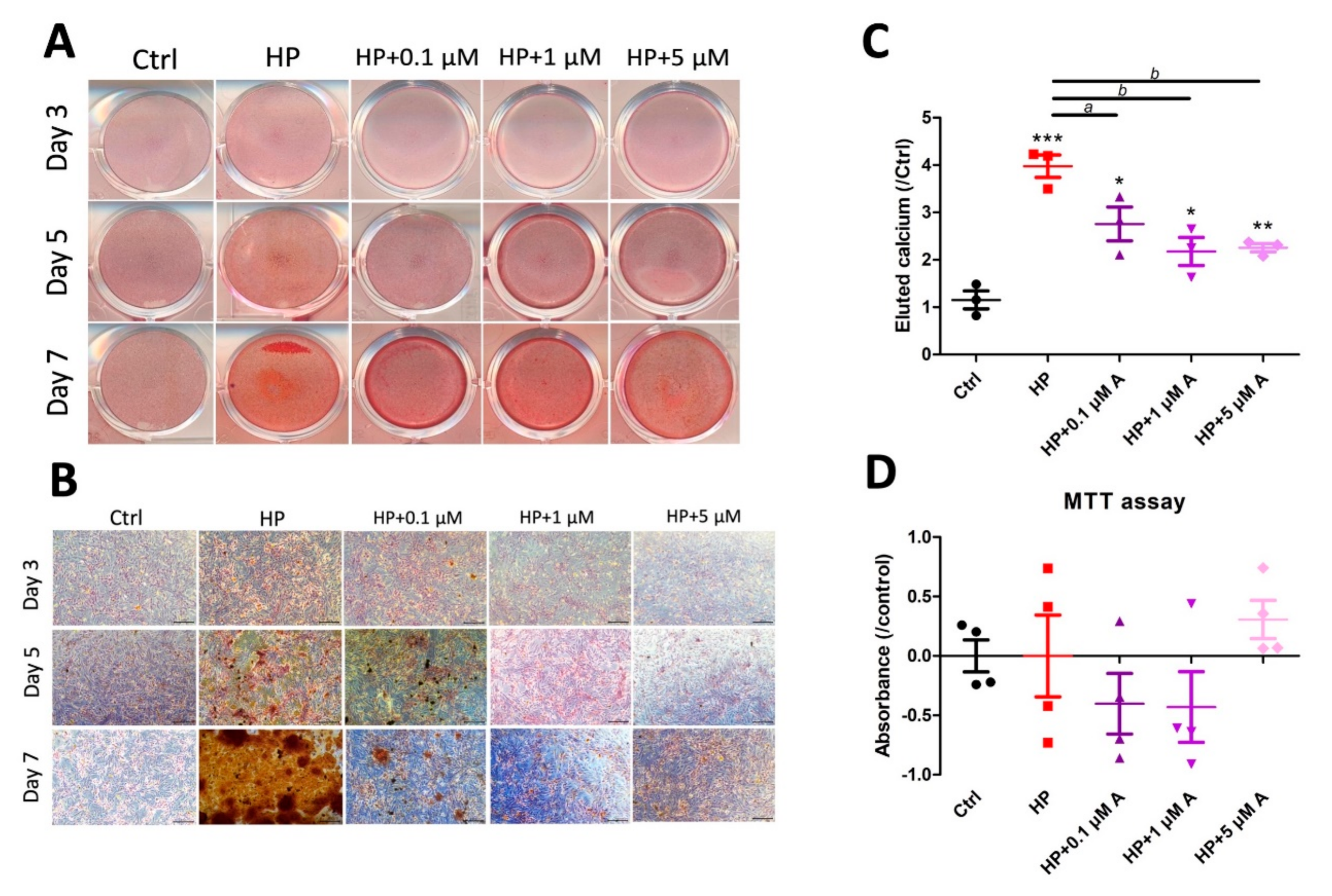
2.1. Evaluating the Effect of Astaxanthin in the In Vitro VC Model
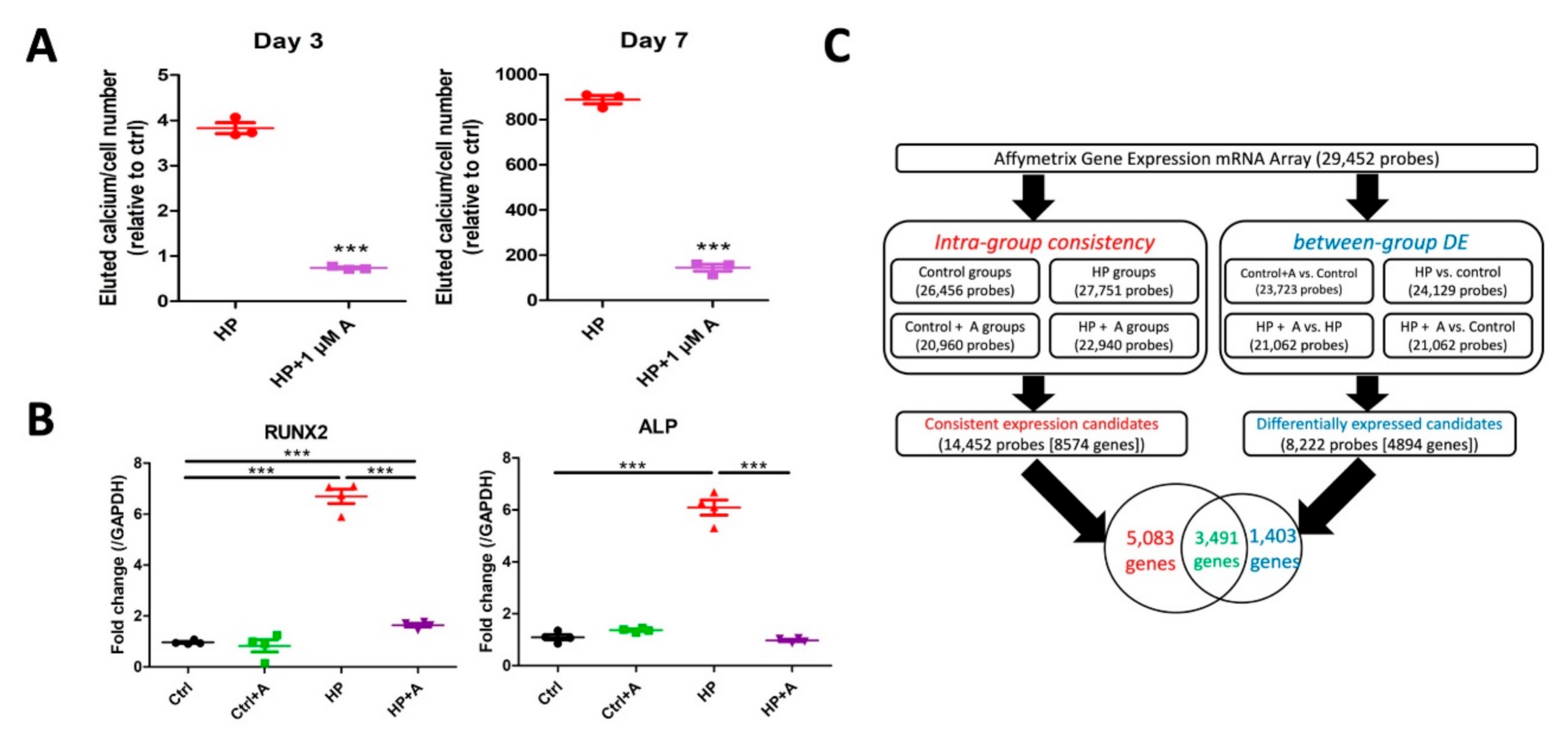
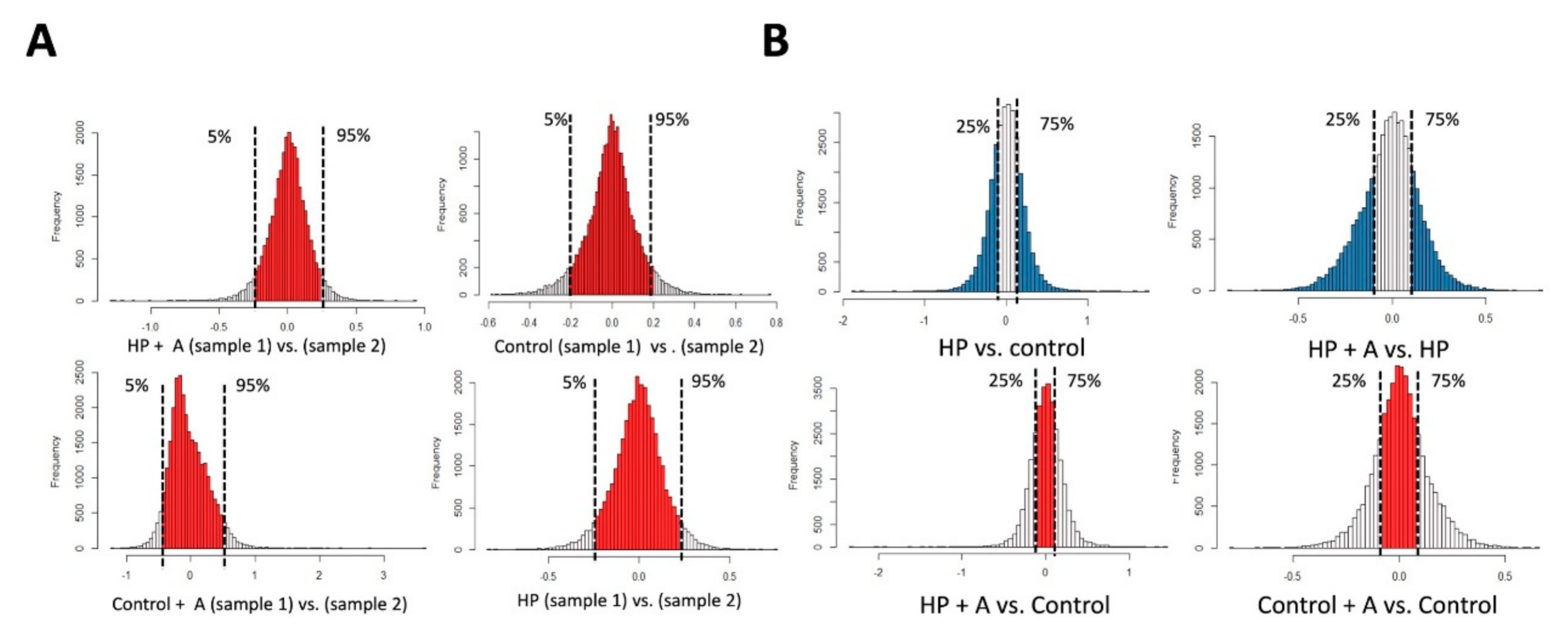
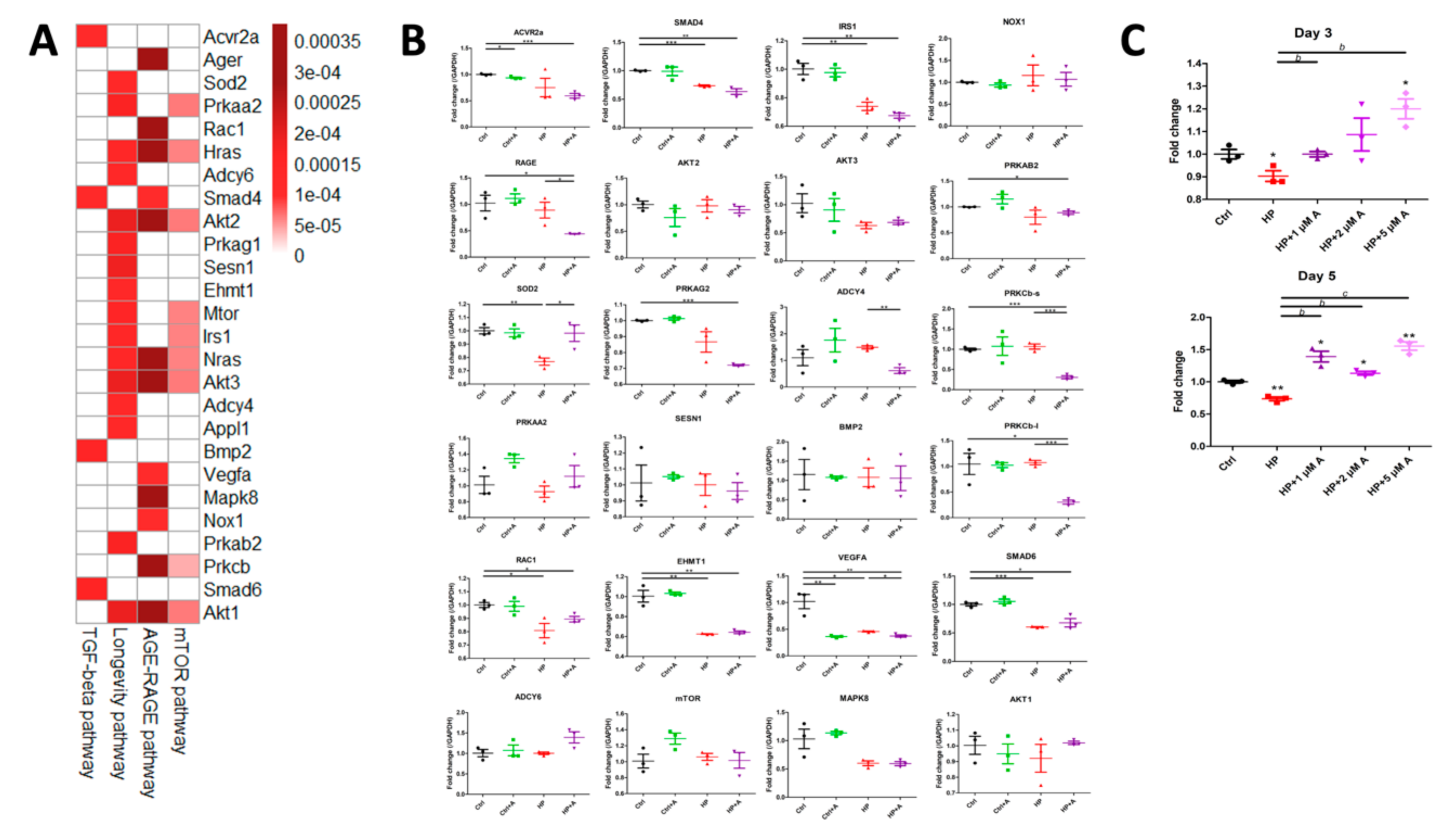
2.2. Microarray Experiments for Investigating Mechanisms Behind the Astaxanthin Effect
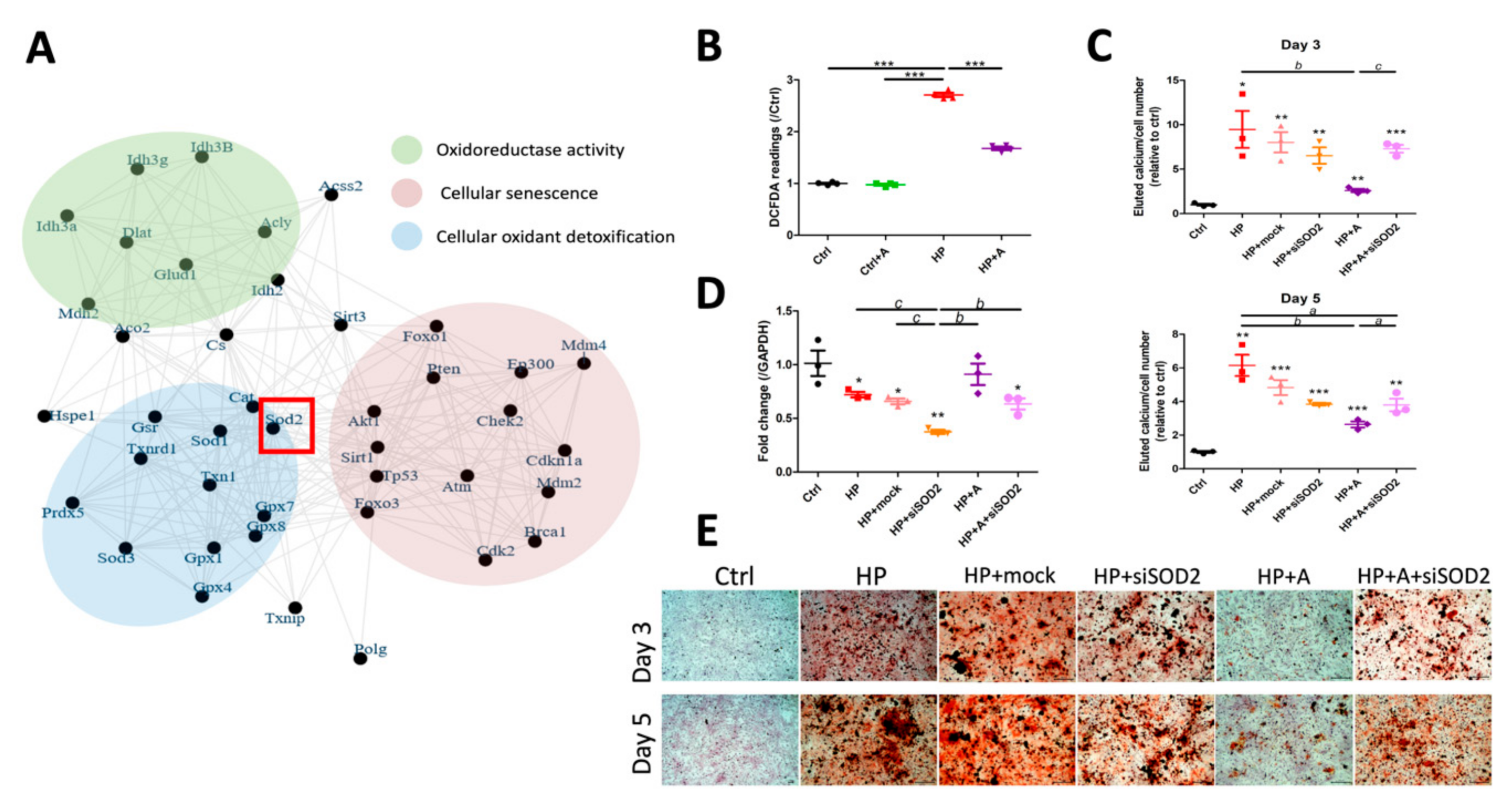
2.3. Validation of Potential Early Hub Gene In Vitro
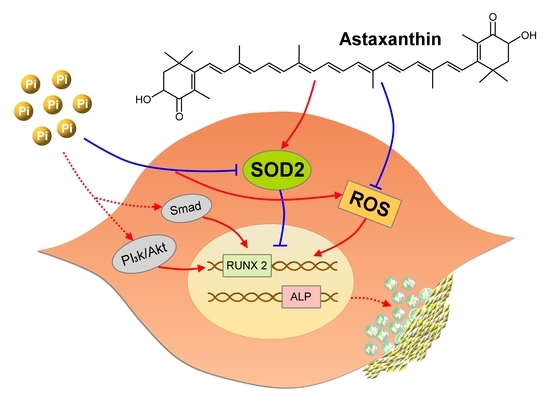
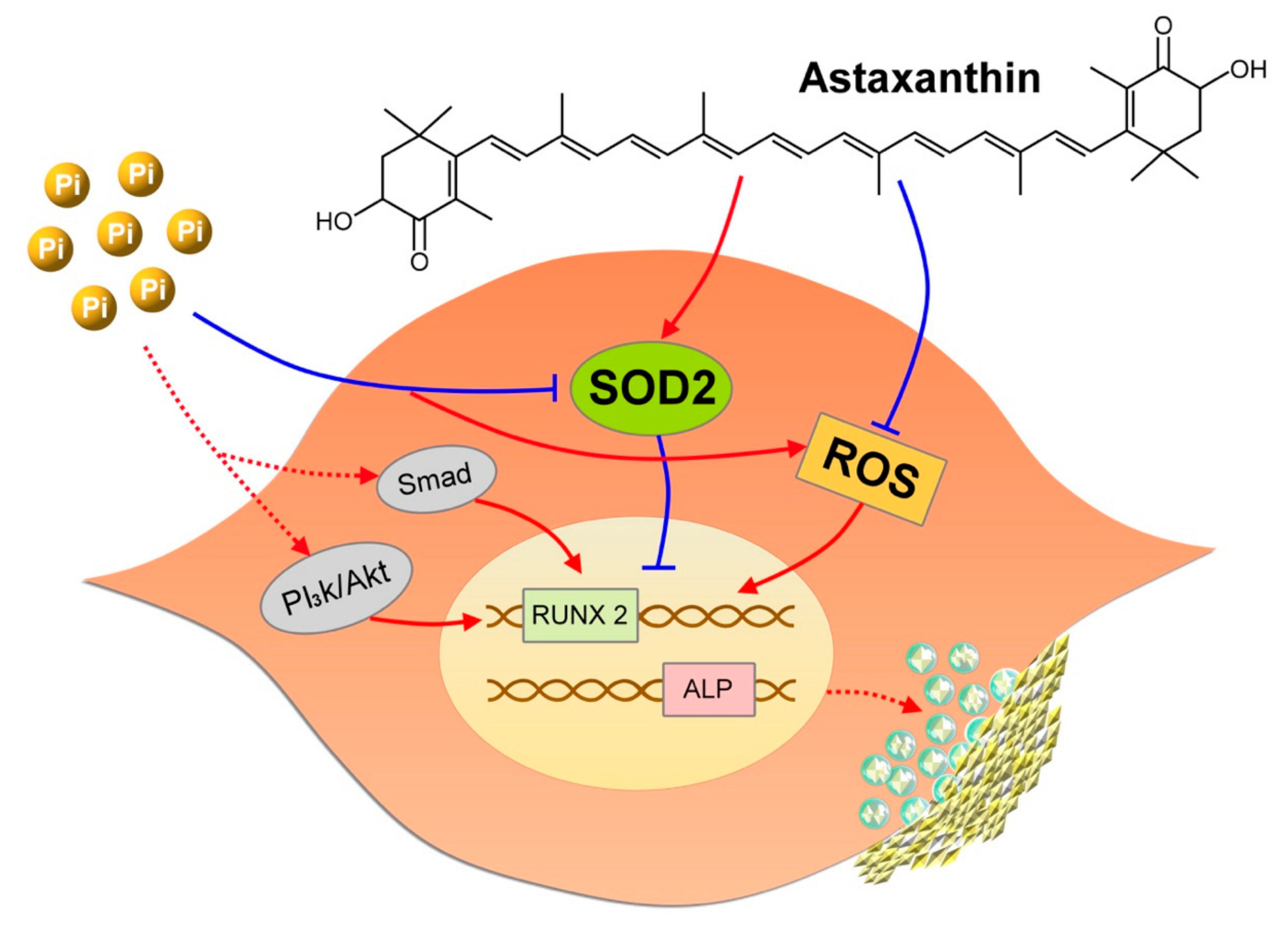
2.4. Functional Characterization of SOD2 During VC
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Reagents, and Experimental Utilities
4.2. VC Model Construction and Calcification Phenotype Assay
4.3. Cell Viability Assay
4.4. Microarray Experiments and Bioinformatic Analyses for Mechanistic Investigations
4.5. Functional Pathway Enrichment
4.6. Network Closeness and Measurement
4.7. Extended Protein Interaction Network
4.8. Functional Evaluation of Hub Genes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | advanced glycation endproduct |
| ALP | alkaline phosphatase |
| ATCC | American Type Culture Collection |
| AOPP | advanced oxidized protein product |
| CEL | cell intensity file |
| CKD | chronic kidney disease |
| DM | diabetes mellitus |
| EMA | European Medical Agency |
| HP | high phosphate |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| mTOR | mammalian target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PPI | protein-protein interaction |
| RAGE | receptor of advanced glycation endproduct |
| RIN RNA | integrity number |
| ROS | reactive oxygen species |
| RT-PCR | real-time polymerase chain reaction |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| TGF-β | transforming growth factor-β |
| VC | vascular calcification |
| VSMC | vascular smooth muscle cell |
References
- Nelson, A.J.; Raggi, P.; Wolf, M.; Gold, A.M.; Chertow, G.M.; Roe, M.T. Targeting Vascular Calcification in Chronic Kidney Disease. JACC Basic Transl. Sci. 2020, 5, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chao, C.T.; Huang, J.W.; Huang, K.C. Vascular Calcification as an Underrecognized Risk Factor for Frailty in 1783 Community-Dwelling Elderly Individuals. J. Am. Heart Assoc. 2020, 9, e017308. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Miedema, M.D.; Dardari, Z.A.; Nasir, K.; Blankstein, R.; Knickelbine, T.; Oberembt, S.; Shaw, L.; Rumberger, J.; Michos, E.D.; Rozanski, A.; et al. Association of Coronary Artery Calcium With Long-term, Cause-Specific Mortality Among Young Adults. JAMA Netw. Open 2019, 2, e197440. [Google Scholar] [CrossRef] [PubMed]
- van der Toorn, J.E.; Engelkes, S.R.; Ikram, M.K.; Ikram, M.A.; Vernooij, M.W.; Kavousi, M.; Bos, D. Vertebrobasilar artery calcification: Prevalence and risk factors in the general population. Atherosclerosis 2019, 286, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Covic, A.; Vervloet, M.; Massy, Z.A.; Torres, P.U.; Goldsmith, D.; Brandenburg, V.; Mazzaferro, S.; Evenepoel, P.; Bover, J.; Apetril, M.; et al. Bone and mineral disorders in chronic kidney disease: Implications for cardiovascular health and ageing in the general population. Lancet. Diabetes. Endocrinol. 2018, 6, 319–331. [Google Scholar] [CrossRef]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trend. Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Byon, C.H.; Javed, A.; Dai, Q.; Kappes, J.C.; Clemens, T.L.; Darley-Usmar, V.M.; McDonald, J.M.; Chen, Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008, 283, 15319–15327. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.S.; Cheng, S.L.; Sadhu, J.; Towler Dwight, A. Inflammation and the Osteogenic Regulation of Vascular Calcification. Hypertension 2010, 55, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.T.; Yeh, H.Y.; Tsai, Y.T.; Chuang, P.H.; Yuan, T.H.; Huang, J.W.; Chen, H.W. Natural and non-natural antioxidative compounds: Potential candidates for treatment of vascular calcification. Cell Death Discov. 2019, 5, 145. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. Synthesis of Extracellular Pyrophosphate Increases in Vascular Smooth Muscle Cells During Phosphate-Induced Calcification. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2137–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Zhang, Y.; Zhang, M.; Guo, L.; Wang, J.; Zeng, F.; Xu, D.; Yin, Z.; Xu, Y.; Wang, D.; et al. Interleukin-1β-Induced Senescence Promotes Osteoblastic Transition of Vascular Smooth Muscle Cells. Kidney Blood Press. Res. 2020, 45, 314–330. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Pescatore Luciana, A.; Gamarra Lionel, F.; Liberman, M. Multifaceted Mechanisms of Vascular Calcification in Aging. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1307–1316. [Google Scholar] [CrossRef]
- Lu, C.L.; Liao, M.T.; Hou, Y.C.; Fang, Y.W.; Zheng, C.M.; Liu, W.C.; Chao, C.T.; Lu, K.C.; Ng, Y.Y. Sirtuin-1 and Its Relevance in Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 1593. [Google Scholar] [CrossRef] [Green Version]
- Che, M.; Wang, R.; Li, X.; Wang, H.Y.; Zheng, X.F.S. Expanding roles of superoxide dismutases in cell regulation and cancer. Drug Discov. Today 2016, 21, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Campoio, T.R.; Oliveira, F.A.; Otton, R. Oxidative stress in human lymphocytes treated with fatty acid mixture: Role of carotenoid astaxanthin. Toxicol. In Vitro 2011, 25, 1448–1456. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Kim, Y.J.; Lee, E.K.; Park, S.W.; Yu, H.G. Antioxidative Effects of Ascorbic Acid and Astaxanthin on ARPE-19 Cells in an Oxidative Stress Model. Antioxidants 2020, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Fu, K.; Zhao, X.; Zhang, Y.; Yuan, Y.; Zhang, S.; Gu, X.; Guo, H. Protective effects of astaxanthin against ischemia/reperfusion induced renal injury in mice. J. Transl. Med. 2015, 13, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, X.L.; Han, X.D.; Li, Y.; Chu, X.F.; Miao, W.M.; Zhang, J.L.; Fan, S.J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Lim, J.W.; Kim, H. Astaxanthin Prevents Decreases in Superoxide Dismutase 2 Level and Superoxide Dismutase Activity in Helicobacter pylori-infected Gastric Epithelial Cells. J. Cancer Prev. 2019, 24, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arefin, S.; Buchanan, S.; Hobson, S.; Steinmetz, J.; Alsalhi, S.; Shiels, P.G.; Kublickiene, K.; Stenvinkel, P. Nrf2 in early vascular ageing: Calcification, senescence and therapy. Clin. Chim. Acta 2020, 505, 108–118. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, T.; Yang, L.; Xu, Y.; Ota, T.; Fu, Z. Protective effects of astaxanthin on a combination of D-galactose and jet lag-induced aging model in mice. Endocr. J. 2018, 65, 569–578. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Association. Opinion of the Scientific Panel on additives and products or substances used in animal feed (FEEDAP) on the safety of use of colouring agents in animal nutrition, PART I. General Principles and Astaxanthin. EFSA J. 2005, 3, 291. [Google Scholar] [CrossRef]
- Kim, R.E.; Shin, C.Y.; Han, S.H.; Kwon, K.J. Astaxanthin suppresses PM2.5-induced neuroinflammation by regulating Akt phosphorylation in BV-2 microglial cells. Int. J. Mol. Sci. 2020, 21, 7227. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, Y.M.; Hong, S. Astaxanthin suppresses the metastasis of colon cancer by inhibiting the MYC-mediated downregulation of microRNA-29a-3p and microRNA-200a. Sci. Rep. 2019, 9, 9457. [Google Scholar] [CrossRef]
- Kowshik, J.; Nivetha, R.; Ranjani, S.; Venkatesan, P.; Selvamuthukumar, S.; Veeravarmal, V.; Nagini, S. Astaxanthin inhibits hallmarks of cancer by targeting the PI3k/NF-kB/STAT3 signalling axis in oral squamous cell carcinoma models. IUBMB Life 2019, 71, 1595–1610. [Google Scholar] [CrossRef]
- Chao, C.T.; Liu, Y.P.; Su, S.F.; Yeh, H.Y.; Chen, H.Y.; Lee, P.J.; Chen, W.J.; Lee, Y.M.; Huang, J.W.; Chiang, C.K.; et al. Circulating MicroRNA-125b Predicts the Presence and Progression of Uremic Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1402–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.T.; Yuan, T.H.; Yeh, H.Y.; Chen, H.Y.; Huang, J.W.; Chen, H.W. Risk Factors Associated With Altered Circulating Micro RNA -125b and Their Influences on Uremic Vascular Calcification Among Patients With End-Stage Renal Disease. J. Am. Heart Assoc. 2019, 8, e010805. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Yeh, H.-Y.; Tsai, Y.-T.; Chiang, C.-K.; Chen, H.-W. A combined microRNA and target protein-based panel for predicting the probability and severity of uremic vascular calcification: A translational study. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequences |
|---|---|
| ACVR2a | F: AAGTCACACAGCCCACATCA R: GTCCAGGGTCCTGAGTAGGAA |
| ADCY4 | F: GAGCCTAGCTTTGCTACGAG R: GATCGCGCAGAGCACAATG |
| ADCY6 | F: GGCGCAGCAGCATGTCA R: GTGAGGCTGCTCTGGTTCAT |
| AKT1 | F: TACCTGAAGCTACTGGGCAAGGG R: CGGTCGTGGGTCTGGAATGAG |
| AKT2 | F: CTCTGTAGCAGAATGCCAGC R: ATGGAAGGTCCTCTCGATGA |
| AKT3 | F: CCCCCGAACACTCTCTTCAG R: AGTCTTCCTGAAATTGAACCAGAT |
| ALP | F: TTGGTCTGGCTCCCATGGTG R: GCAAAGACCGCCACATCTTCC |
| BMP2 | F: CACGAGAATGGACGTGCCC R: GCTTCAGGCCAAACATGCTG |
| EHMT1 | F: GGTCAAGTATGAGCTGATGCG R: GTCACTGTGGAAGTCGTGTCTG |
| GAPDH | F: AACGGCACAGTCAAGGCTGA R: ACGCCAGTAGACTCCACGACAT |
| IRS1 | F: TTCGTGCAGACACTCAAGGC R: TTTAGGTCTTTCATTCTGCCTGT |
| MAPK8 | F: AGCCGGCCATTTCAGAATCA R: CTTGTAGCCCATGCCGAGAA |
| NOX1 | F: CGAAGTGGCTGTACTGGTTG R: AAAGGCACCCGTCTCTCTAC |
| mTOR | F: CCTCGGCACATCACTCCCTT R: GCTCCTACATTTCAGCACCCACT |
| PRKAA2 | F: GATCGGACACTACGTGCTGG R: CTTCCACCTCTTCAACCCTCC |
| PRKAB2 | F: TGATTCTGTGAAGCCCACCC R: TGACTGGTAACCACAGGCTCT |
| PRKAG2 | F: CAGCACCGGTCACACTACTT R: CAGACGGTGAACCTCTGCTT |
| PRKCb-s | F: CATCCACCAGTCCTAACACCT R: TGTTGAGCTTGAATGACAGAAAT |
| PRKCb-l | F: GAACTGACTCCCACTGACAAACT R: CACCATGAATCCTGGAAGACT |
| RAC1 | F: CCTACCCGCAAACAGACGTG R: ACACTGTCTTGAGTCCTCGC |
| RAGE | F: GTCTGGGCTGGGAACGCTAG R: TGAAGGAGACAGGGTTTGCC |
| RUNX2 | F: TCTTCCCAAAGCCACAGCG R: TGCCATTCGAGGTGGTCG |
| SMAD4 | F: TTCACTGCTCAGCCAGCTAC R: TCATTGTGAACCGGCCAGTAA |
| SMAD6 | F: CCGGGTGAATTCTCAGATGCC R: TGCTGACAGCCTGGTCATAC |
| SESN1 | F: AGGAAGTGCCTTGGTGAGTGA R: TGGAACATGCATCCCATGCC |
| SOD2 | F: ACGCGACCTACGTGAACAAT R: TAACATCTCCCTTGGCCAGC |
| VEGFA | F: GCAGAAAGCCCATGAAGTGGT R: TGCTGCAGGAAGCTCATCTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-T.; Yeh, H.-Y.; Tsai, Y.-T.; Yuan, T.-H.; Liao, M.-T.; Huang, J.-W.; Chen, H.-W. Astaxanthin Counteracts Vascular Calcification In Vitro Through an Early Up-Regulation of SOD2 Based on a Transcriptomic Approach. Int. J. Mol. Sci. 2020, 21, 8530. https://doi.org/10.3390/ijms21228530
Chao C-T, Yeh H-Y, Tsai Y-T, Yuan T-H, Liao M-T, Huang J-W, Chen H-W. Astaxanthin Counteracts Vascular Calcification In Vitro Through an Early Up-Regulation of SOD2 Based on a Transcriptomic Approach. International Journal of Molecular Sciences. 2020; 21(22):8530. https://doi.org/10.3390/ijms21228530
Chicago/Turabian StyleChao, Chia-Ter, Hsiang-Yuan Yeh, You-Tien Tsai, Tzu-Hang Yuan, Min-Tser Liao, Jenq-Wen Huang, and Huei-Wen Chen. 2020. "Astaxanthin Counteracts Vascular Calcification In Vitro Through an Early Up-Regulation of SOD2 Based on a Transcriptomic Approach" International Journal of Molecular Sciences 21, no. 22: 8530. https://doi.org/10.3390/ijms21228530