Micropropagation of Mountain Mulberry (Morus bombycis Koidz.) ‘Kenmochi’ on Cytokinin-Free Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Initiation of In Vitro Cultures
2.2. Multiplication Stage (Main Experiments)
2.3. Ex Vitro Rooting of Shoots (Additional Experiment)
2.4. Statistical Analyses
3. Results
3.1. Initiation of In Vitro Cultures
3.2. Multiplication Stage (Main Experiments)
3.3. Ex Vitro Rooting of Shoots (Additional Experiment)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Statements
Abbreviations
| BA | 6-Benzylaminopurine |
| IBA | Indole-3-butyric acid |
| MS | Murashige and Skoog medium [14] |
| WPM | Woody Plant Medium [15] |
References
- Sharma, K.K.; Thorpe, T.A. In vitro propagation of mulberry (Morus alba L.) through nodal segments. Sci. Hortic. 1990, 42, 307–320. [Google Scholar] [CrossRef]
- Sharma, S.; Madan, M. Potential of mulberry (Morus alba L.) biomass. J. Sci. Ind. Res. 1994, 53, 710–714. [Google Scholar]
- Bajaj, P.S.; Ivanicka, J.; Ueda, S. Morus Species (Mulberry): In Vitro Culture, Micropropagation, and the Formation of Mulberrofuran, Kuwanol, and Other Secondary Metabolites. In Biotechnology in Agriculture and Forestry, Medicinal and Aromatic Plants; Bajaj, P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 41, pp. 261–285. [Google Scholar]
- Zaki, M.; Kaloo, Z.A.; Sofi, M.S. Micropropagation of Morus nigra L. from nodal segments with axillary buds. World J. Agric. Sci. 2011, 7, 496–503. [Google Scholar]
- Vijayan, K.; Raju, P.J.; Tikader, A.; Saratchnadra, B. Biotechnology of mulberry (Morus L.)—A review. Emir. J. Food Agric. 2014, 26, 472–496. [Google Scholar] [CrossRef]
- Machii, H.; Koyama, A.; Yamanouchi, H. Mulberry Breeding, Cultivation and Utilization in Japan. Available online: http://www.fao.org/3/x9895e05.htm (accessed on 30 October 2020).
- Alipanah, M.; Abedian, Z.; Nasiri, A.; Sarjamei, F. Nutritional Effects of Three Mulberry Varieties on Silkworms in Torbat Heydarieh. Psyche J. Entomol. 2020. [Google Scholar] [CrossRef]
- Tomczyk, M.; Miłek, M.; Sidor, E.; Kapusta, I.; Litwińczuk, W.; Puchalski Cz Dżugan, M. The Effect of Adding the Leaves and Fruits of Morus alba to Rape Honey on Its Antioxidant Properties, Polyphenolic Profile, and Amylase Activity. Molecules 2020, 25, 84. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Patel, R.; Thorpe, T.A. Regeneration of mulberry plantlets through tissue culture. Bot. Gaz. 1985, 146, 335–340. [Google Scholar] [CrossRef]
- Oka, S.; Ohyama, K. Mulberry (Morus alba L.). In Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; Volume 1, pp. 384–394. [Google Scholar]
- Jain, A.K.; Dandin, S.B.; Sengupta, K. In vitro propagation through axillary bud multiplication in different mulberry genotypes. Plant Cell Rep. 1990, 8, 737–740. [Google Scholar] [CrossRef]
- Marcotrigiano, M.; McGlew, S.P. A two-stage micropropagation system for cranberries. J. Am. Soc. Hortic. Sci. 1991, 116, 911–916. [Google Scholar] [CrossRef] [Green Version]
- George, E.F. Plant Propagation by Tissue Culture. Part 2. Micropropagation in Practice, 2nd ed.; Exegetics Ltd.: Edington, UK, 1996; pp. 1026–1029. [Google Scholar]
- Litwińczuk, W.; Wadas, M. Auxin-depended development and habituation of highbush blueberry (Vaccinium × covilleanum But. et Pl.) ‘Herbert’ in vitro shoot cultures. Sci. Hortic. 2008, 119, 41–48. [Google Scholar] [CrossRef]
- Litwińczuk, W. Micropropagation of Vaccinium sp. by in vitro axillary shoot proliferation. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Methods in Molecular Biology 11013, Springer Protocols; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 63–76. [Google Scholar]
- Boxus, P. Micropropagation of strawberry via axillary shoot proliferation. In Plant Cell Culture Protocols; Methods in Molecular Biology. Part III. Plant Propagation In Vitro; Humana Press: Totowa, NJ, USA, 1999; pp. 103–114. [Google Scholar]
- Siekierzynska, A.; Litwińczuk, W. Micropropagation of strawberry (Fragaria x ananassa Duch.) on chemically sterilized media. Acta Sci. Pol. Hortorum Cultus 2018, 17, 147–156. [Google Scholar] [CrossRef]
- De Klerk, G.J. How to measure somaclonal variation. Acta Bot. Neerl. 1990, 39, 129–144. [Google Scholar] [CrossRef]
- Mohamed, M.A.H.; Alsadon, A.A. Influence of ventilation and sucrose on growth and leaf anatomy of micropropagated potato plantlets. Sci. Hortic. 2010, 123, 295–300. [Google Scholar] [CrossRef]
- Ohyama, K. Tissue culture in mulberry tree. Jpn. Agric. Res. Q. 1970, 5, 30–34. [Google Scholar]
- Enomoto, S. Preservation of genetic resource of mulberry by means of tissue culture. Jpn. Agric. Res. Q. 1987, 21, 205–210. [Google Scholar]
- Ivanička, J. In vitro propagation of mulberry, Morus nigra L. Sci. Hortic. 1987, 32, 33–39. [Google Scholar] [CrossRef]
- Litwińczuk, W.; Borkowska, B.; Szczerba, J. Morwy (Morus sp.)—Zastosowanie, rozmnażanie w kulturach in vitro. [Mulberries (Morus sp.)—Utilisation, propagation through in vitro cultures]. Zeszyty Problemowe Postępów Nauk Rolniczych 1999, 468, 359–370. (In Polish) [Google Scholar]
- Anis, M.; Faisal, M.; Singh, S.K. Micropropagation of mulberry (Morus alba L.) through in vitro culture of shoot tip and nodal explants. Plant Tissue Cult. 2003, 13, 47–51. [Google Scholar]
- Akram, M.; Aftab, F. Efficient micropropagation and rooting of king white mulberry (Morus macroura Miq.) var. laevigata from nodal explants of mature tree. Pak. J. Bot. 2012, 44, 285–289. [Google Scholar]
- Kavyashree, R. A repeatable protocol for in vitro micropropagation of mulberry variety S54. Indian J. Biotechnol. 2007, 6, 385–388. [Google Scholar]
- Choudhary, R.; Chaudhury, R.; Malik, S.K. Development of an efficient regeneration and rapid clonal multiplication protocol for three different Morus species using dormant buds as explants. J. Hortic. Sci. Biotechnol. 2015, 90, 245–253. [Google Scholar] [CrossRef]
- Attia, A.O.; Sdessoky, E.; El-Hallous, E.; Shaaban, H. Micropropagation of Mulberry (Morus alba L.) cv. Al-Taify. Int. J. Bio Technol. Res. 2014, 4, 15–22. [Google Scholar]
- Chiancone, B.; Germana, P.; Germana, M.A. In vitro response of two Sicilian genotypes of Morus (L.) through axillary bud culture. Caryologia 2007, 60, 178–181. [Google Scholar]
- Pattnaik, S.K.; Chand, P.K. Rapid clonal propagation of three mulberries, Morus cathayana Hemsl., M. lhou Koiz. and M. serrata Roxb., through in vitro culture of apical shoot buds and nodal explants from mature trees. Plant Cell Rep. 1997, 16, 503–508. [Google Scholar] [PubMed]
- Chitra, D.S.V.; Padmaja, G. Seasonal influence on axillary bud sprouting and micropropagation of elite cultivars of mulberry. Sci. Hort. 2002, 92, 55–68. [Google Scholar] [CrossRef]
- Bhau, B.S.; Wakhlu, A.K. Rapid micropropagation of five cultivars of mulberry. Biol. Plant. 2003, 46, 349–355. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for the rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–496. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propagators Soc. 1981, 30, 421–427. [Google Scholar]
- Narayan, P.; Chakraborty, S.; Rao, G.S. Regeneration of plantlets from the callus of stem segments of mature plants of Morus alba L. Proc. Indian Nat. Sci. Acad. 1989, 55, 469–472. [Google Scholar]
- Vijayan, K.; Chakraborti, S.P.; Roy, B.N. Plant regeneration form leaf explants of mulberry: Influence of sugar, genotype and 6-benzyladenine. Indian J. Exp. Biol. 2000, 38, 504–508. [Google Scholar]
- Bhatnagar, S.; Kapur, A.; Khurana, P. TDZ mediated differentiation in commercially valuable Indian mulberry, Morus indica cultivars K2 and DD. Plant Biotechnol. 2001, 18, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Chitra, D.V.; Padmaja, G. Shoot regeneration via direct organogenesis from in vitro derived leaves of mulberry using thidiazuron and 6-benzylaminopurine. Sci. Hort. 2005, 106, 593–602. [Google Scholar] [CrossRef]
- Raghunath, M.K.; Nataraja, K.N.; Meghana, J.S.; Sajeevan, R.S.; Rajan, M.V.; Qadri, S.M.H. In vitro plant regeneration of Morus indica L cv. V-1 using leaf explants. Am. J. Plant Sci. 2013, 4, 2001–2005. [Google Scholar] [CrossRef] [Green Version]
- Pattnaik, S.K.; Sahoo, Y.; Chand, P.K. Efficient plant retrieval from alginate-encapsulated vegetative buds of mature mulberry trees. Sci. Hortic. 1995, 61, 227–239. [Google Scholar] [CrossRef]







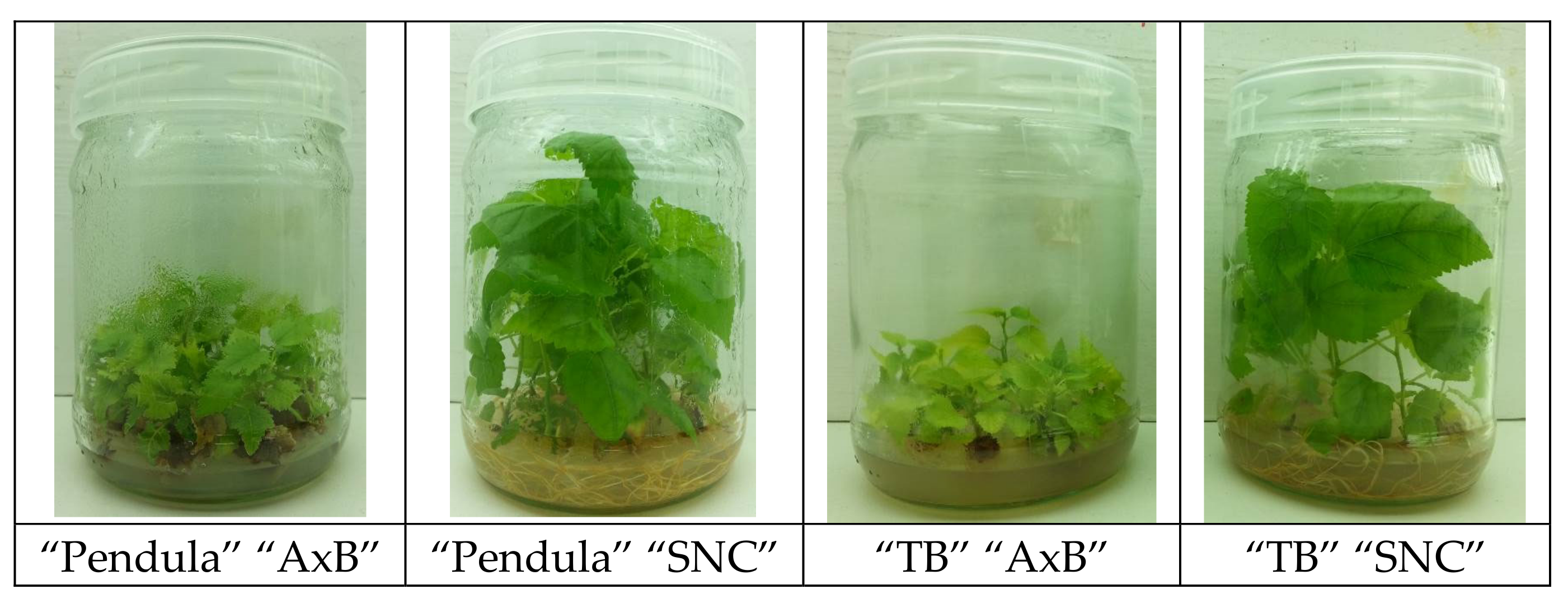
| Media Constituents | “AxB” Medium (Standard) | “SNC” Medium |
|---|---|---|
| Macronutrients | 50% MS 1 | |
| N salts | 100% MS | |
| K salts | 100% MS | |
| P salts | 100% MS | |
| Ca salts | 125% MS | |
| Mg salts | 125% MS | |
| Micronutrients | 100% MS | 50% MS |
| Vitamins | 100% WPM2 | 100% WPM |
| Myo-inositol | 100 mg L−1 | 100 mg L−1 |
| Sucrose | 30 g L−1 | 15 g L−1 |
| BA | 1.5 mg L-1 | - |
| IBA | - | 0.05 mg L−1 |
| Agar | 7 g L−1 | 7 g L−1 |
| pH | 5.8 | 5.8 |
| Source of Explants | No of Survived Explants (S) [%] | No of Explants which Started to Grow (G) [%] | Efficiency of Initiation (S x G) [%] | |||
|---|---|---|---|---|---|---|
| “SNC” | “AxB” | “SNC” | “AxB” | “SNC” | “AxB” | |
| field tree | 52 a,A | 100 b,B | 38 a,A | 100 b,B | 21 a,A | 100 b,B |
| nursery plants | 100 b,B | 92 a,A | 64 a,B | 82 a,A | 64 a,B | 75 a,A |
| Medium/ No of Subculture | Explant Type | No of Growing Cultures [%] | Number of Shoots [pcs] | Length of the Longest Shoot [cm] | Total Length of Shoots [cm] | Callus Size [cm] |
|---|---|---|---|---|---|---|
| SNC/1 | ST | 91.7 a | 1.0 a | 5.9 b | 6.0 a | 0.0 a |
| AxB/1 | ST | 91.7 a | 1.8 b* | 3.2 a | 4.9 a | 2.3 b |
| SNC/2 | 1N | 96.9 a | 1.0 a | 7.4 b | 7.5 b | 0.0 a |
| AxB/2 | xN | 100 a | 1.6 b | 2.0 a | 2.6 a | 1.6 b |
| SNC/3 | 1N | 100 a | 1.0 a | 5.2 a | 5.2 a | 0.0 a |
| AxB/3 | xN | 100 a | 1.7 b | 5.0 a | 7.0 b | 1.1 b |
| SNC/4 | 1N | 86.3 a | 1.0 a | 5.9 b | 6.1 b | 0.0 a |
| AxB/4 | xN | 100 a | 1.6 b | 1.9 a | 2.6 a | 1.5 b |
| SNC/5 | 1N | 100 a | 1.0 a | 5.5 b | 5.5 a | 0.0 a |
| AxB/5 | xN | 95.6 a | 2.1 b | 3.2 a | 5.3 a | 1.7 b |
| SNC/6 | 1N | 97.5 a | 1.0 a | 2.8 b | 2.8 a | 0.0 a |
| AxB/6 | xN | 91.5 a | 1.4 b | 1.9 a | 2.5 a | 0.9 b |
| SNC (mean) | 95.4 - | 1.0 - | 5.5 - | 5.5 - | 0.0 - | |
| AxB (mean) | 96.5 - | 1.7 - | 2.9 - | 4.2 - | 1.5 - |
| Type of Culture | No of Growing Cultures [%] | Number of Shoots [pcs] | Length of the Longest Shoot [cm] | Total Length of Shoots [cm] | Callus Size [cm] |
|---|---|---|---|---|---|
| Old cultures after one harvest of shoots | 86.3 a | 1.3 a | 2.2 a | 2.7 a | 0.0 a |
| New culture | 100 a | 1.0 a | 5.5 b | 5.5 b | 0.0 a |
| Origin of Explant/ No of Subculture | Explant Type | No of Growing Cultures [%] | Number of Shoots [pcs] | Length of the Longest Shoot [cm] | Total Length of Shoots [cm] | Callus Size [cm] |
|---|---|---|---|---|---|---|
| from SNC/2 * | 1N ** | 93.8 a | 1.3 a | 2.7 b | 3.0 a | 2.0 b |
| from AxB/2 | xN | 100 a | 1.6 b | 2.0 a | 2.6 a | 1.6 a |
| from SNC/3 | 1N | 100 a | 1.2 a | 3.3 a | 3.7 a | 1.4 b |
| from AxB/3 | xN | 100 a | 1.7 b | 5.0 a | 7.0 b | 1.1 a |
| from SNC/5 | 1N | 100 a | 2.1 a | 1.7 a | 2.8 a | 1.9 b |
| from AxB/5 | xN | 95.6 a | 2.1 a | 3.2 b | 5.3 b | 1.7 a |
| from SNC/6 | 1N | 100 a | 1.6 a | 2.1 a | 2.9 a | 0.9 a |
| from AxB/6 | xN | 91.5 a | 1.4 a | 1.9 a | 2.5 a | 0.9 a |
| from SNC (mean) | 98.5 - | 1.6 - | 2.5 - | 3.1 - | 1.6 - | |
| from AxB (mean) | 96.8 - | 1.7 - | 3.0 - | 4.4 - | 1.3 - |
| Explant Type | No of Growing Cultures [%] | Number of Shoots [pcs] | Length of the Longest Shoot [cm] | No of Rooted Shoots [%] | Callus Size [cm] |
|---|---|---|---|---|---|
| 1N | 96.9 a | 1.0 a | 2.9 a | 100 a | 0.0 a |
| 2N | 100 a | 1.0 a | 2.5 a | 100 a | 0.0 a |
| Explant Type | No of Growing Cultures [%] | Number of Shoots [pcs] | Length of the Longest Shoot [cm] | Total Length of Shoots [cm] | Callus Size [cm] |
|---|---|---|---|---|---|
| xN | 95.8 a | 1.4 a | 1.7 a | 2.1 a | 0.9 a |
| ST | 91.3 a | 2.2 b | 3.4 b | 5.4 b | 0.8 a |
| Origin of Microshoots | No of Rooted Shoots [%] | No of Shoots [pcs] | Length of the Longest Shoot [cm] | Total Length of Shoots [cm] | Length of Leaf Blade [cm] | Width of Leaf Blade [cm] | Relative Chlorophyll Content [SPAD] |
|---|---|---|---|---|---|---|---|
| SNC | 100 a | 1.1 a | 6.0 a | 6.2 a | 3.5 a | 2.6 a | 23.3 a |
| AxB | 100 a | 1.3 a | 6.1 a | 6.8 a | 3.3 a | 2.7 a | 24.2 a |
| Method of Micropropagation | Axillary Branching (“AxB”) | Single-Node Culture (“SNC”) |
|---|---|---|
| Cost of media | higher | lower (reduced dose of sucrose and mineral nutrients) |
| Efficiency of micropropagation | ||
| Initiation of in vitro cultures (explants from young, nursery plants) | easy | easy |
| Initiation of in vitro cultures (explants from adult, field trees) | possible | possible although hard to get |
| Number of shoots | significantly higher | significantly lower |
| Length of shoots | significantly lower | significantly higher |
| Number of nodes on shoot | similar | similar |
| Estimated multiplication rate * | lower: 4 (2 × “ST”+ 2 × “xN” explants) | higher: 5 (1 × “ST” + 4 × “1N” explants) |
| Rooting and acclimation in vivo | similar | similar |
| Quality of cultures during multiplication | ||
| Growth of callus | too intensive | not observed |
| Physiological disorders (shoot-tip necrosis, vitrification, chlorosis, etc.) | sometimes observed | not observed |
| Development of adventitious shoots | rather not observed (overgrown callus complicates identification) | not observed |
| Risk of somaclonal variation | low | very low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litwińczuk, W.; Jacek, B. Micropropagation of Mountain Mulberry (Morus bombycis Koidz.) ‘Kenmochi’ on Cytokinin-Free Medium. Plants 2020, 9, 1533. https://doi.org/10.3390/plants9111533
Litwińczuk W, Jacek B. Micropropagation of Mountain Mulberry (Morus bombycis Koidz.) ‘Kenmochi’ on Cytokinin-Free Medium. Plants. 2020; 9(11):1533. https://doi.org/10.3390/plants9111533
Chicago/Turabian StyleLitwińczuk, Wojciech, and Beata Jacek. 2020. "Micropropagation of Mountain Mulberry (Morus bombycis Koidz.) ‘Kenmochi’ on Cytokinin-Free Medium" Plants 9, no. 11: 1533. https://doi.org/10.3390/plants9111533





