Advancing Timberline on Mt. Fuji between 1978 and 2018
Abstract
1. Introduction
2. Results
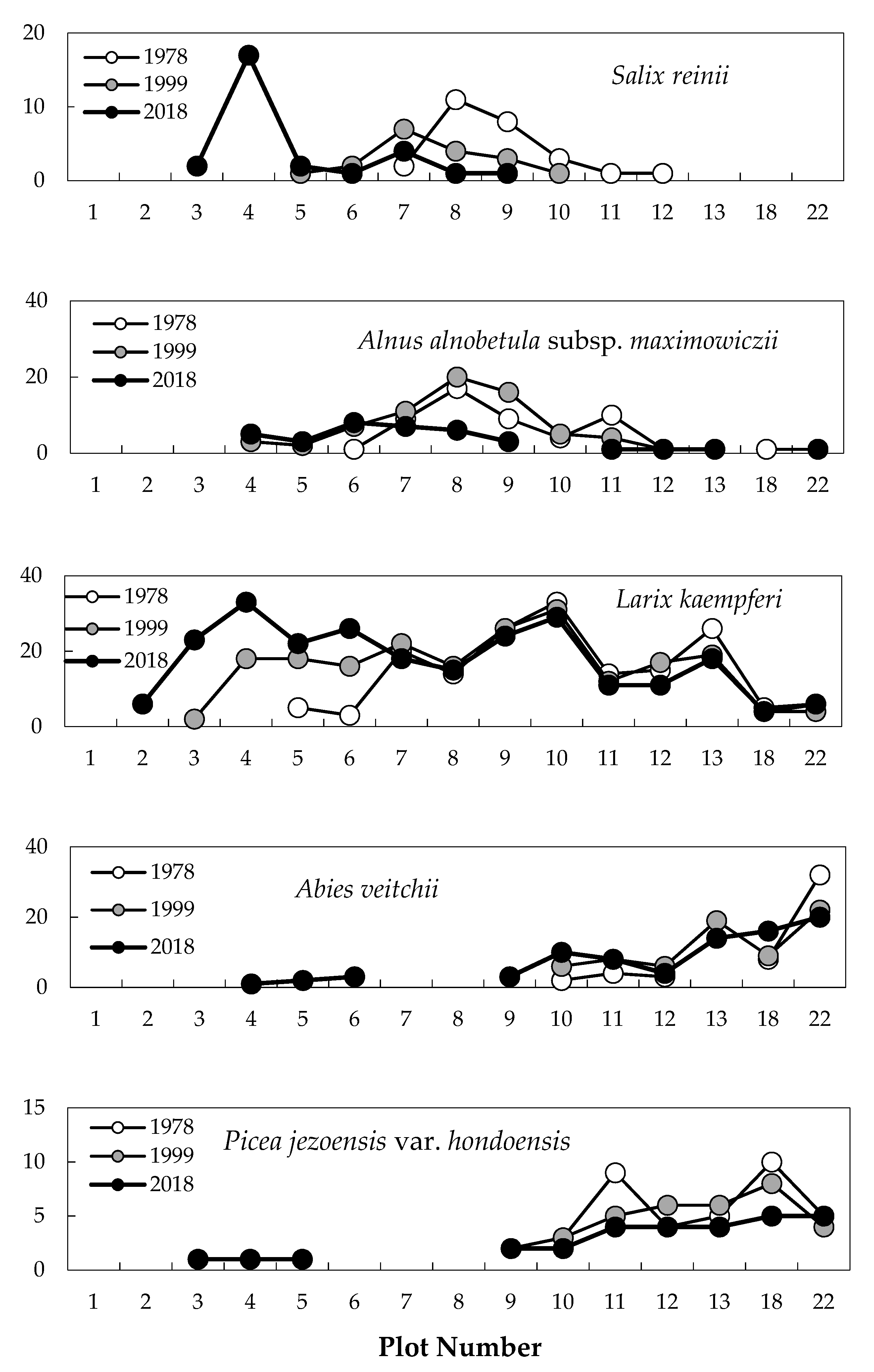
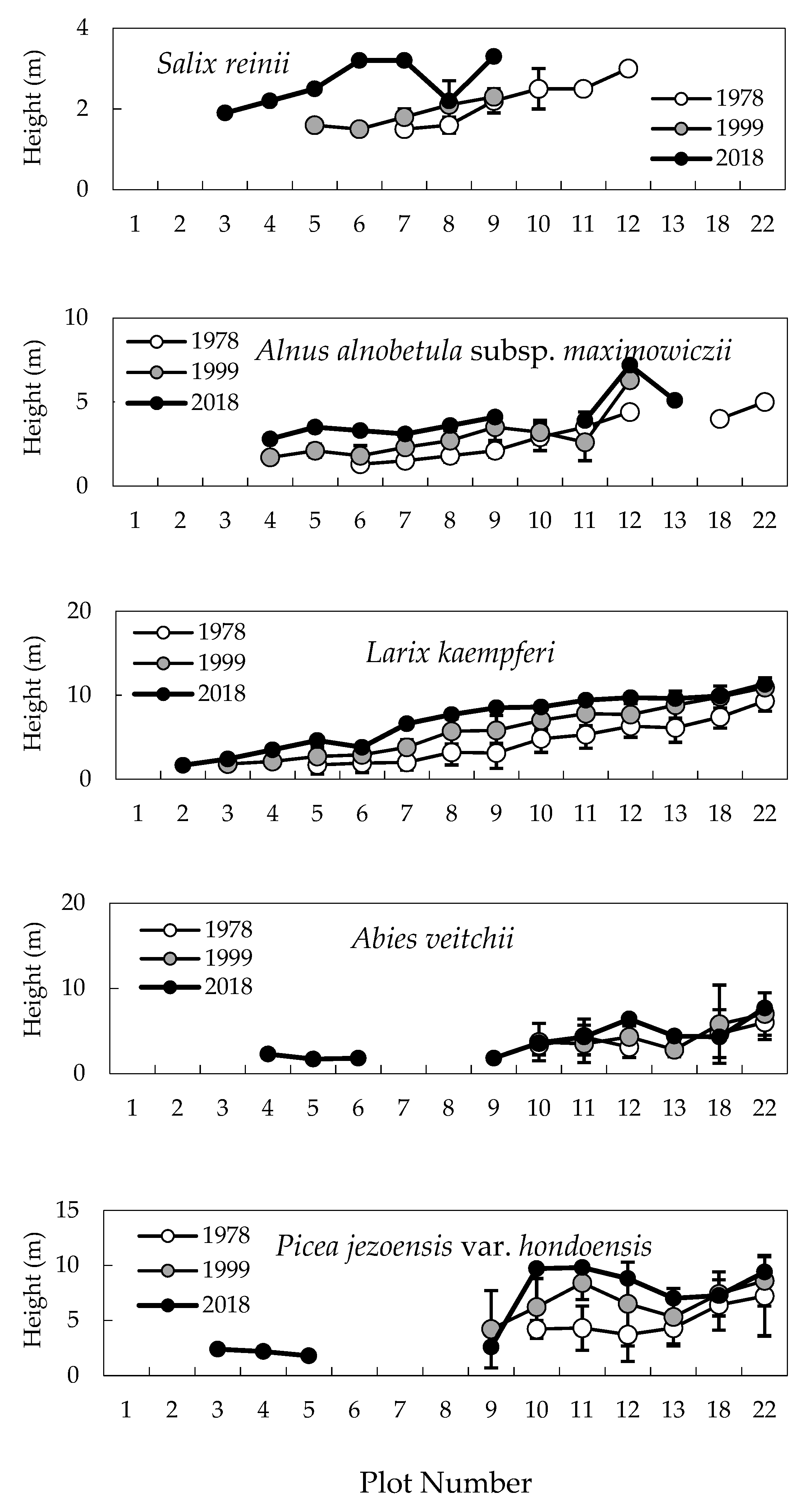
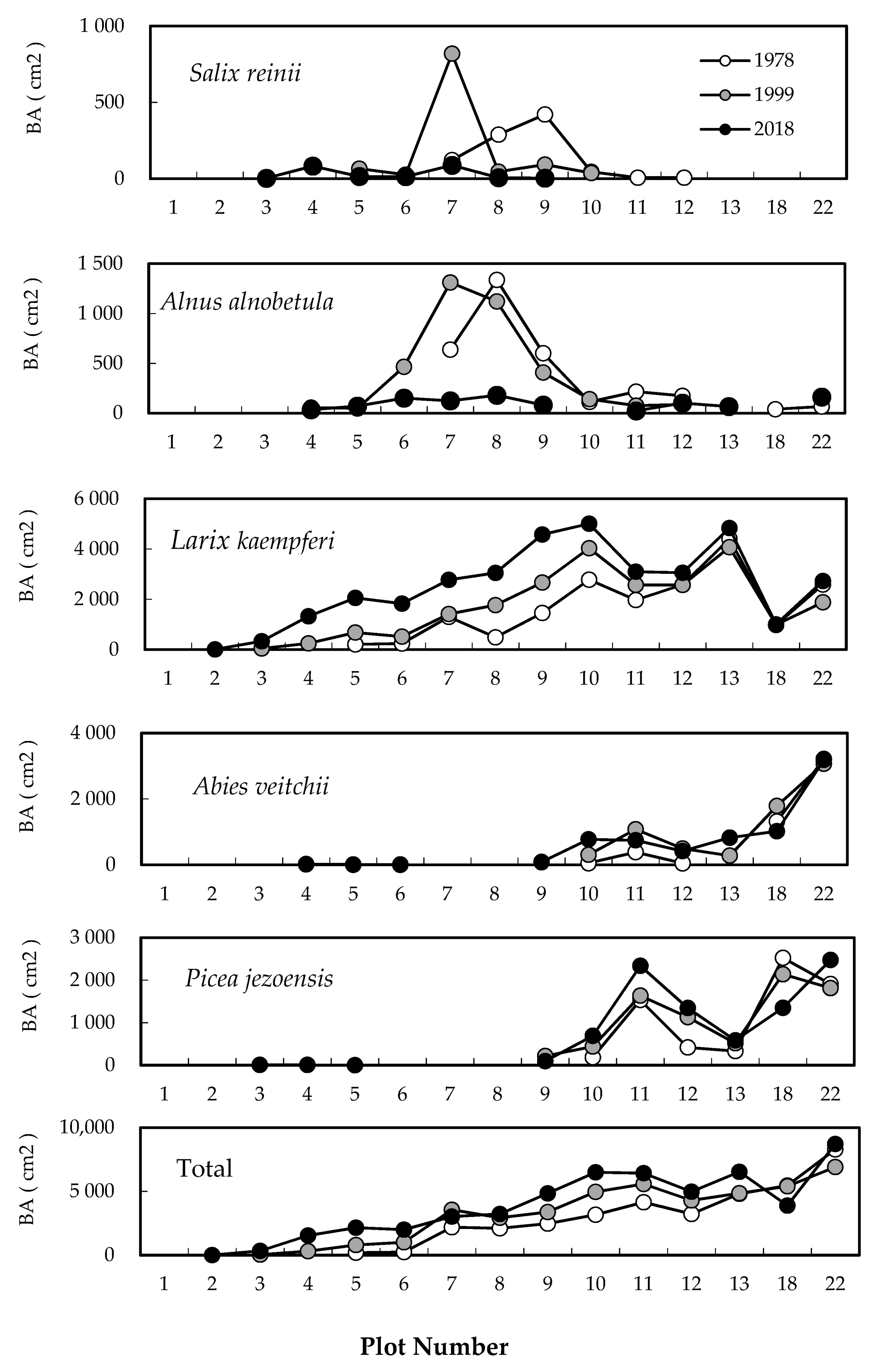
2.1. Change in Timberline Vegetation over Time
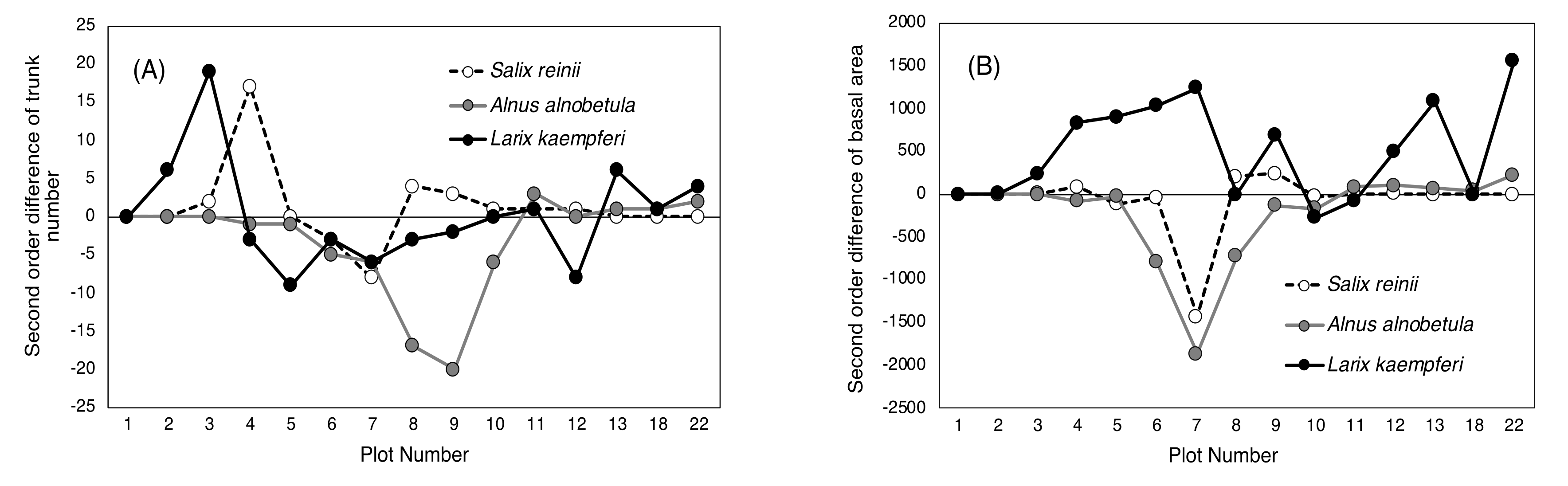
2.2. Difference in Changes between the Two 20-Year Periods
2.3. Establishment of Seedlings at the Upper Timberline
2.4. Degree of Vegetation Cover
3. Discussion
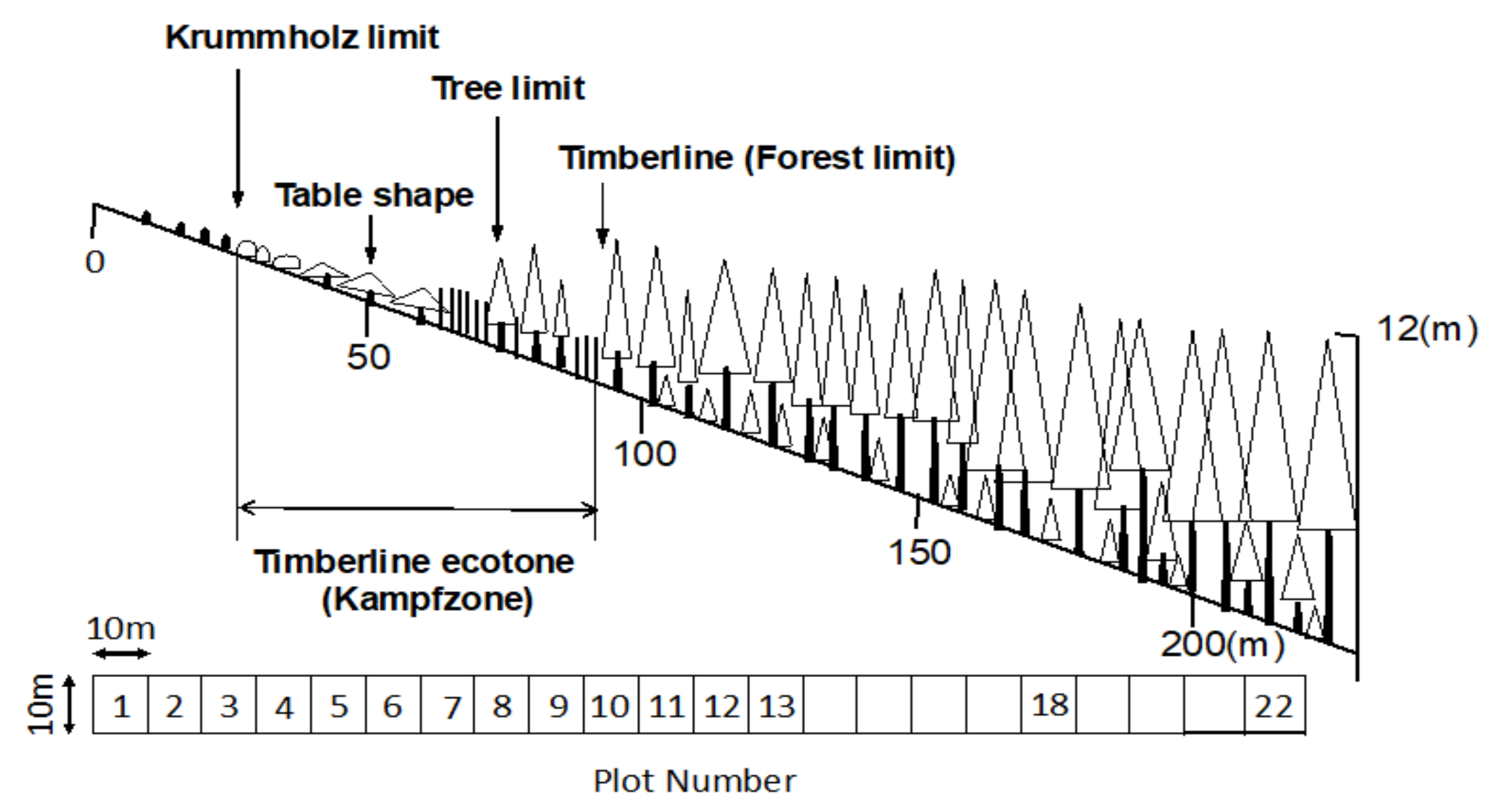
4. Study Site and Methods
4.1. Study Site
4.2. Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Evans, N.; Baierl, A.; Semenov, M.A.; Gladders, P.; Fitt, B.D.L. Range and severity of a plant disease increased by global warming. J. R. Soc. Interface 2008, 5, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Shakun, J.D.; Clark, P.U.; He, F.; Lifton, N.A.; Liu, Z.; Otto-Bliesner, B.L. Regional and global forcing of glacier retreat during the last deglaciation. Nat. Commun. 2015, 6, 8059. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, A.; Criado, M.G.; Myers-Smith, I.H.; Ravolainen, V.; Jónsdóttir, I.S.; Westergaard, K.B.; Lawler, J.P.; Aronsson, M.; Bennett, B.; Gardfjell, H.; et al. Status and trends in Arctic vegetation: Evidence from experimental warming and long-term monitoring. Ambio 2019, 49, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Rixen, C.; Wipf, S. Non-equilibrium in Alpine Plant Assemblages: Shifts in Europe’s Summit Floras. In High Mountain Conservation in a Changing World; Catalan, J., Ninot, J.M., Aniz, M.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 285–303. [Google Scholar]
- Gérard, M.; Vanderplanck, M.; Wood, T.; Michez, D. Global warming and plant–pollinator mismatches. Emerg. Top. Life Sci. 2020, 4, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Hoch, G.; Randin, C.F.; Lenz, A.; Kollas, C.; Körner, C. Tree recruitment of European tree species at their current upper elevational limits in the Swiss Alps. J. Biogeogr. 2012, 39, 1439–1449. [Google Scholar] [CrossRef]
- Mirtl, M.; Borer, E.T.; Djukic, I.; Forsius, M.; Haubold, H.; Hugo, W.; Jourdan, J.; Lindenmayer, D.; McDowell, W.H.; Muraoka, H.; et al. Genesis, goals and achievements of Long-Term Ecological Research at the global scale: A critical review of ILTER and future directions. Sci. Total Environ. 2018, 626, 1439–1462. [Google Scholar] [CrossRef]
- Biodiversity Center of Japan. Monitoring Sites 1000. Available online: http://www.biodic.go.jp (accessed on 20 September 2020).
- Tranquillini, W. Physiological Ecology of the Alpine Timber-Line—Tree Existence at High Altitude with Special Reference to the European Alps; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar] [CrossRef]
- Holtmeier, F.K. Mountain timberlines. Ecology, patchiness, and dynamics. Advances in Global Change Research 14; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Skre, O. Northern treelines as indicators of climate and land use changes—A literature review. Agrotechnology 2019, 9, 190. [Google Scholar] [CrossRef]
- Okitsu, S. Comparative studies on the Japanese alpine zone with special reference to the ecology of Pinus pumila thickets. Geogr. Rev. Jpn. 1984, 57, 791–802. [Google Scholar] [CrossRef]
- Okitsu, S. Consideration of vegetational zonation based on the establishment process of a Pinus pumila zone in the Hokkaido, northern Japan. Jap. J. Ecol. 1985, 35, 113–121, (In Japanese with English summary). [Google Scholar] [CrossRef]
- Payette, S.; Eronen, M.; Jasinski, P. The circumboreal tundra-taiga interface: Late Pleistocene and Holocene changes. Ambio 2002, 12, 15–22. [Google Scholar]
- Skre, O.; Baxter, R.; Crawford, R.M.M.; Challaghan, T.V.; Fedorkov, A. How will the tundra-taiga interface respond to climate change? Ambio 2002, 12, 37–46. [Google Scholar]
- Dalen, L.; Hofgaard, A. Differential Regional Treeline Dynamics in the Scandes Mountains. Arct. Antarct. Alp. Res. 2005, 37, 284–296. [Google Scholar] [CrossRef]
- Juntunen, V.; Neuvonen, S. Natural regeneration of Scots pine and Norway spruce close to the timberline in northern Finland. Silva Fenn. 2006, 40, 443–458. [Google Scholar] [CrossRef]
- Hofgaard, A.; Tømmervik, H.; Rees, G.; Hanssen, F. Latitudinal forest advance in northernmost Norway since the early 20th century. J. Biogeogr. 2012, 40, 938–949. [Google Scholar] [CrossRef]
- Bryn, A.; Potthoff, K. Elevational treeline and forest line dynamics in Norwegian mountain areas—A review. Landsc. Ecol. 2018, 33, 1225–1245. [Google Scholar] [CrossRef]
- Holtmeier, F.K. Geoecological aspects of timberlines in Northern and Central Europe. Arct. Antarct. Alp. Res. 1973, 5, 45–54. [Google Scholar]
- Rochefort, R.; Little, R.L.; Woodward, A.; Peterson, D.L. Changes in sub-alpine tree distribution in western North America: A review of climatic and other causal factors. Holocene 1994, 4, 89–100. [Google Scholar] [CrossRef]
- Treml, V.; Migoń, P. Controlling factors limiting timberline position and shifts in the Sudetes: A review. Geogr. Pol. 2015, 88, 55–70. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nashimoto, M.; Takeuchi, T.; Nakano, T. Distribution and ages of larch (Larix kaempferi) in different altitude on the west slope of Mt. Fuji. Mt. Fuji Res. 2012, 6, 55–60. (In Japanese) [Google Scholar]
- Maruta, E.; Masuyama, K. Elevation mechanism of timberline ecotone on the southern slope of Mt. Fuji. Mt. Fuji Res. 2009, 3, 1–12. [Google Scholar]
- Oka, S. The tree limit and its dynamics on the western and northwestern slopes of Mount Fuji, central Japan. Geogr. Rep. Tokyo Metrop. Univ. 1992, 27, 1–28. [Google Scholar]
- Masuzawa, T. Ecological studies on the timberline of Mt. Fuji I. Structure of plant community and soil development on the timberline. Bot. Mag. Tokyo. 1985, 98, 15–28. [Google Scholar] [CrossRef]
- Sakio, H.; Masuzawa, T. The advancing timberline on Mt. Fuji: Natural recovery or climate change? J. Plant Res. 2012, 125, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Yamamura, Y.; Tanaka, A.; Nakano, T.; Yasuda, T. Nurse-plant effects of a dwarf shrub on the establishment of tree seedlings in a volcanic desert on Mt. Fuji, central Japan. Arct. Antarct. Alp. Res. 2008, 40, 335–342. [Google Scholar] [CrossRef]
- Nabeta, K.; Yamamura, Y.; Nakano, T.; Yasuda, T. Effects of dwarf shrub Salix reinii on the establishment, survival and growth of tree seedlings Larix kaempferi in a volcanic desert on Mt. Fuji: Verification by monitoring for 14 years. Mt. Fuji Res. 2015, 9, 17–24. (In Japanese) [Google Scholar]
- Aradottir, Á.L.; Eysteinsson, T. Restoration of birch woodlands in Iceland. In Restoration of Boreal and Temperate Forests; Stanturf, J.A., Madsen, P., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 195–209. [Google Scholar]
- Chen, J.; Yang, Y.; Wang, S.; Sun, H.; Schöb, C. Shrub facilitation promotes selective tree establishment beyond the climatic treeline. Sci. Total Environ. 2020, 708, 134618. [Google Scholar] [CrossRef]
- Nara, K. Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol. 2006, 171, 187–198. [Google Scholar] [CrossRef]
- Sakio, H.; Masuzawa, T. Ecological studies on the timberline of Mt. Fuji II. Primary productivity of Alnus maximowiczii dwarf forest. Bot. Mag. Tokyo 1987, 100, 349–363. [Google Scholar] [CrossRef]
- Sakio, H.; Masuzawa, T. Ecological studies on the timber-line of Mt. Fuji III. Seasonal changes in nitrogen content in leaves of woody plants. Bot. Mag. Tokyo 1992, 105, 47–52. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Nakatsubo, T.; Ino, Y. Field measurements of nitrogen-fixing activity of intact saplings of Alnus maximowiczii in the subalpine zone of Mt Fuji. Ecol. Res. 1993, 8, 685–692. [Google Scholar] [CrossRef]
- Yura, H. Comparative ecophysiology of Larix kaempferi (Lamb.) Carr. and Abies veitchii Lindle. I. Seedling establishment on bare ground on Mt. Fuji. Ecol. Res. 1988, 3, 67–73. [Google Scholar] [CrossRef]
- Yura, H. Comparative ecophysiology of Larix kaempferi (Lamb.) Carr. and Abies veitchii Lindl. II. Mechanisms of higher drought resistance of seedlings of L. kaempferi as compared with A. veitchii. Ecol. Res. 1989, 4, 351–360. [Google Scholar] [CrossRef]
- Heikkinen, O.; Tuovinen, M.; Autio, J. What determines the timberline? Fennia 2002, 180, 67–74. [Google Scholar]
- Smith, W.K.; Germino, M.J.; Hancock, T.E.; Johnson, D.M. Another perspective on altitudinal limits of alpine timberlines. Tree Physiol. 2003, 23, 1101–1112. [Google Scholar] [CrossRef]
- Nakano, Y.; Sakio, H. The regeneration mechanisms of a Pterocarya rhoifolia population in a heavy snowfall region of Japan. Plant Ecol. 2018, 219, 1387–1398. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate effects on mountain plants. Nature 1994, 369, 448. [Google Scholar] [CrossRef]
- Kullman, L. 20th century climate warming and tree-limit rise in the southern Scandes of Sweden. Ambio 2001, 30, 72–80. [Google Scholar] [CrossRef]
- Sanz-Elorza, M.; Dana, E.D.; Gonzáalez, A.; Sobrino, E. Changes in the high-mountain vegetation of the Central Iberian Peninsula as a probable sign of global warming. Ann. Bot. 2003, 92, 273–280. [Google Scholar] [CrossRef]
- Parolo, G.; Rossi, G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic Appl. Ecol. 2008, 9, 100–107. [Google Scholar] [CrossRef]
- Nakazawa, T.; Aoki, S.; Fukabori, M.; Tanaka, M. The concentration of atmospheric carbon dioxide on the summit of Mt. Fuji (3776 m), Japan. J. Meteorol. Soc. Jpn. 1984, 62, 688–695. [Google Scholar] [CrossRef][Green Version]
- Sunaga, A.; Mukai, H.; Machida, T.; Nojiri, Y.; Conway, T.; Masarie, K.; Crotwell, A.; Dlugokenchy, E.J.; White, J.; Vaughn, B. Comparison of co-located air samples at Mauna Loa Observatory and CO2 observations at Mt. Fuji. In Proceedings of the Abstract of 39th NOAA Earth System Research Laboratory 2011 Global Monitoring Annual Conference, Boulder, CO, USA, 17 May 2011; NOAA: Boulder, CO, USA, 2011; p. 3. [Google Scholar]
- Nomura, S.; Mukai, H.; Terao, Y.; Machida, T.; Nojiri, Y. Six years of atmospheric CO2 observations at Mt. Fuji recorded with a battery-powered measurement system. Atmos. Meas. Tech. 2017, 10, 667–680. [Google Scholar] [CrossRef]
- Yazaki, K.; Ishida, S.; Kawagishi, T.; Fukatsu, E.; Maruyama, Y.; Kitao, M.; Tobita, H.; Koike, T.; Funada, R. Effects of elevated CO2 concentration on growth, annual ring structure and photosynthesis in Larix kaempferi seedlings. Tree Physiol. 2004, 24, 941–949. [Google Scholar] [CrossRef][Green Version]
- Menéndez, R.; Megías, A.G.; Hill, J.K.; Braschler, B.; Willis, S.G.; Collingham, Y.; Fox, R.; Roy, D.B.; Thomas, C.D. Species richness changes lag behind climate change. Proc. R. Soc. B. 2006, 273, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Bedford, F.E.; Whittaker, R.J.; Kerr, J.T. Systemic range shift lags among a pollinator species assemblage following rapid climate change. Botany 2012, 90, 587–597. [Google Scholar] [CrossRef]
- Oka, S. On the deformation of larches on Mt. Fuji and their causal factors. J. Geogr. 1980, 89, 97–112, (In Japanese with English summary). [Google Scholar] [CrossRef][Green Version]
- Ito, E. Climate of Mt. Fuji. Bull. Fac. Agric. Shizuoka Univ. 1964, 14, 117–187. (In Japanese) [Google Scholar]
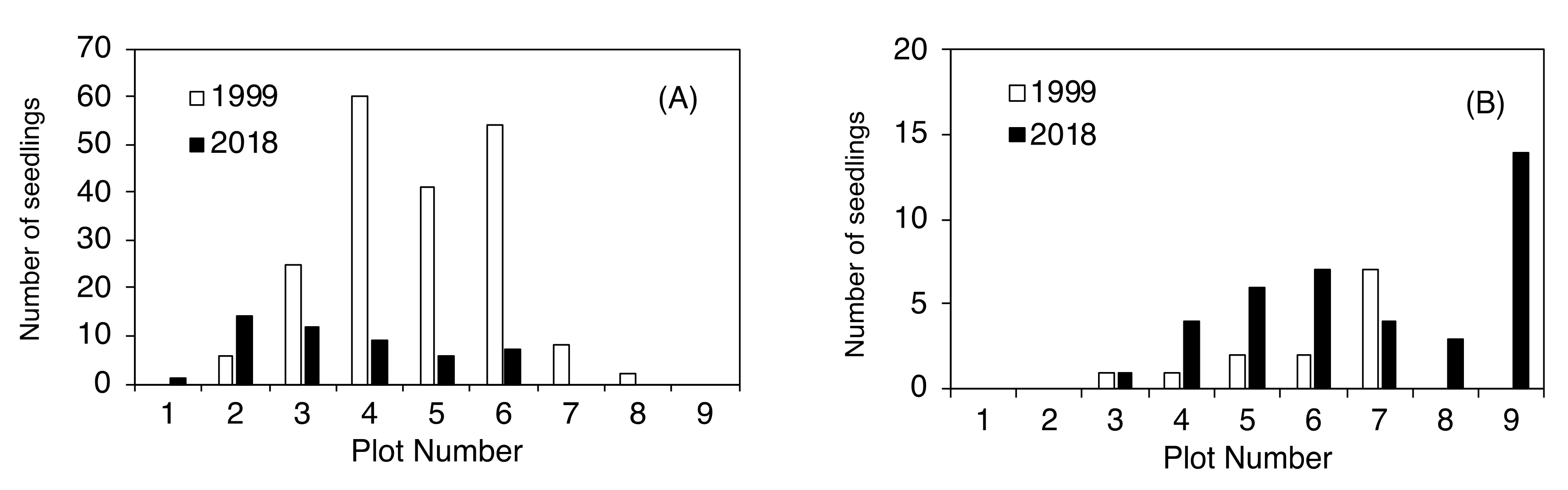
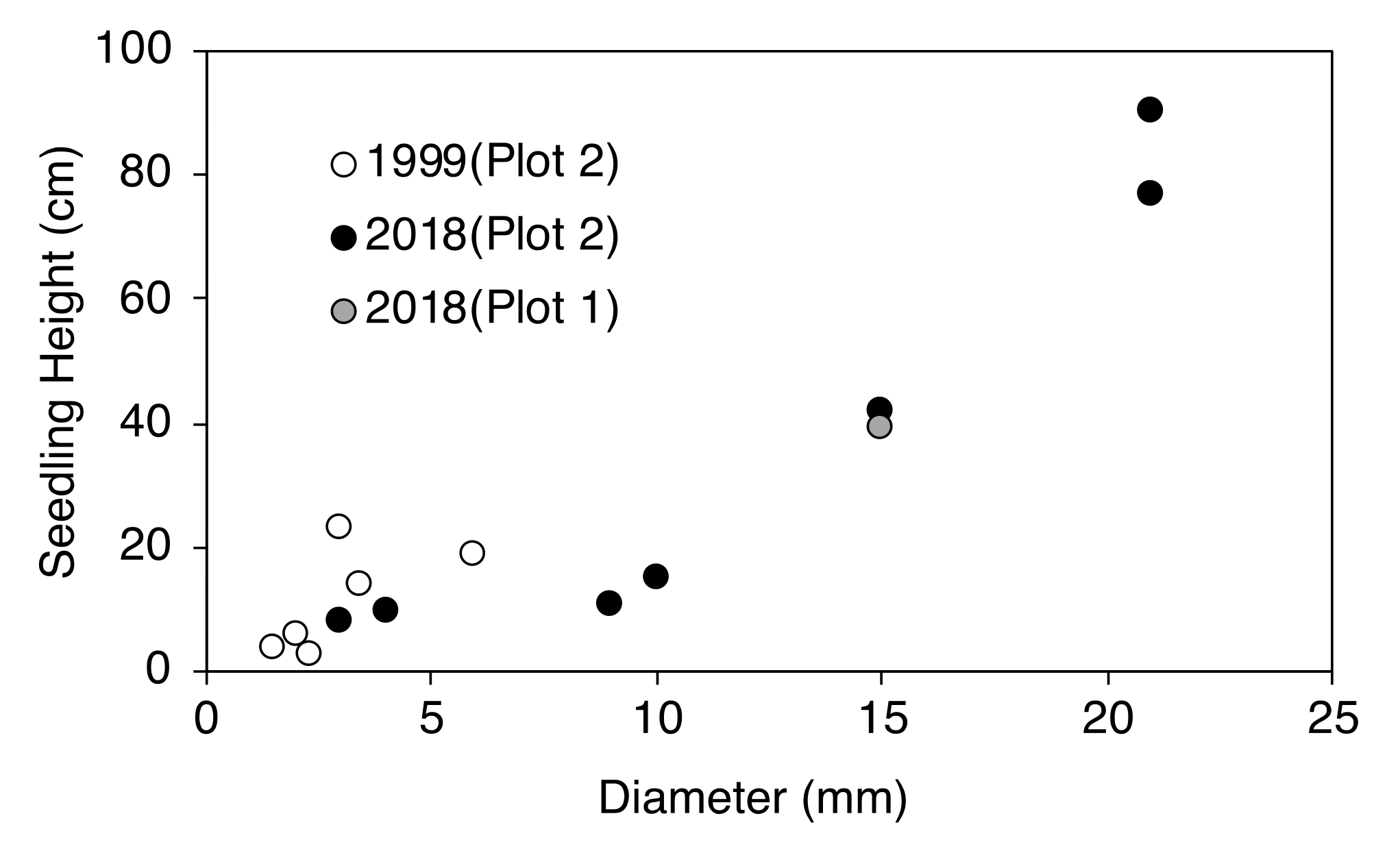
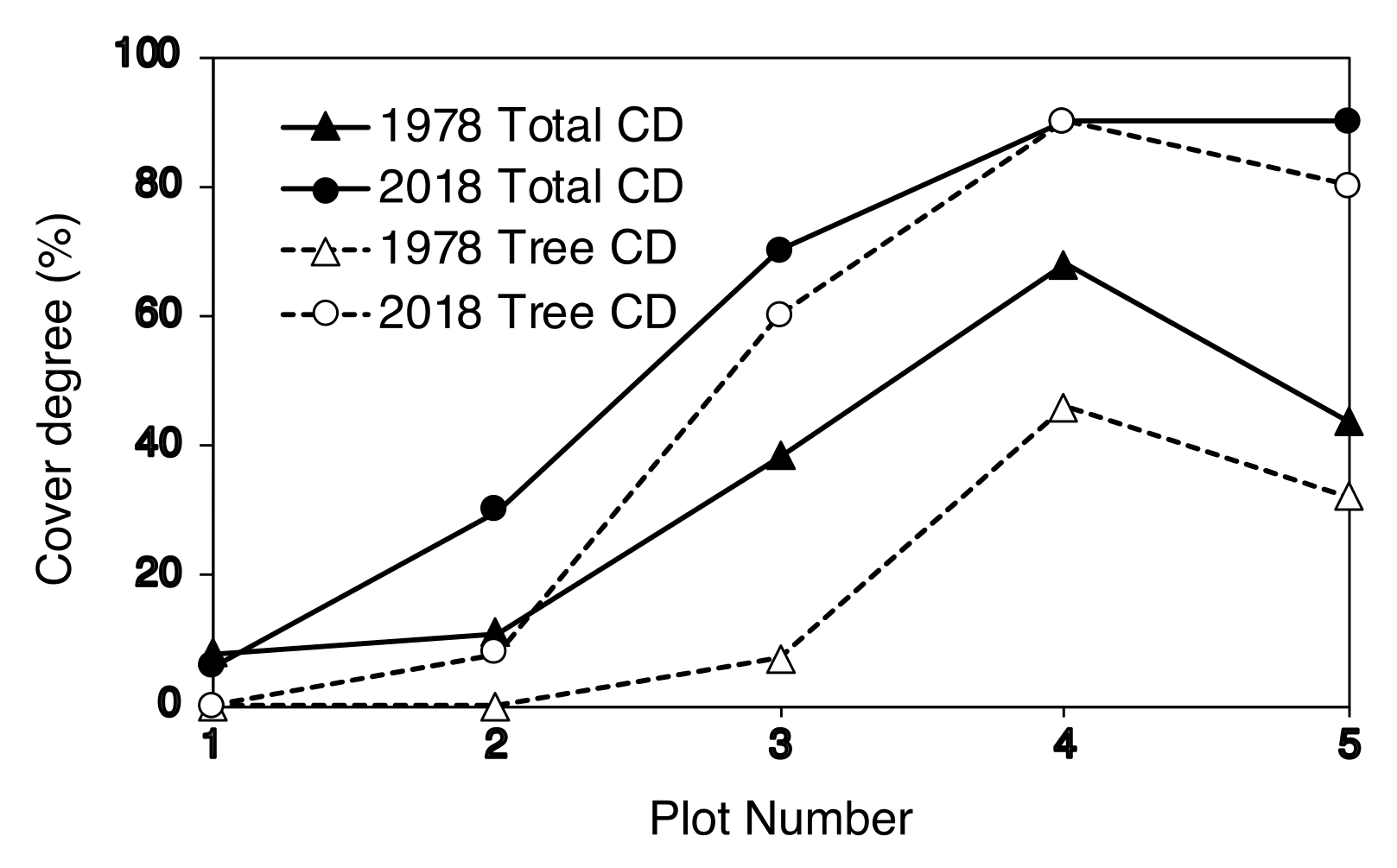

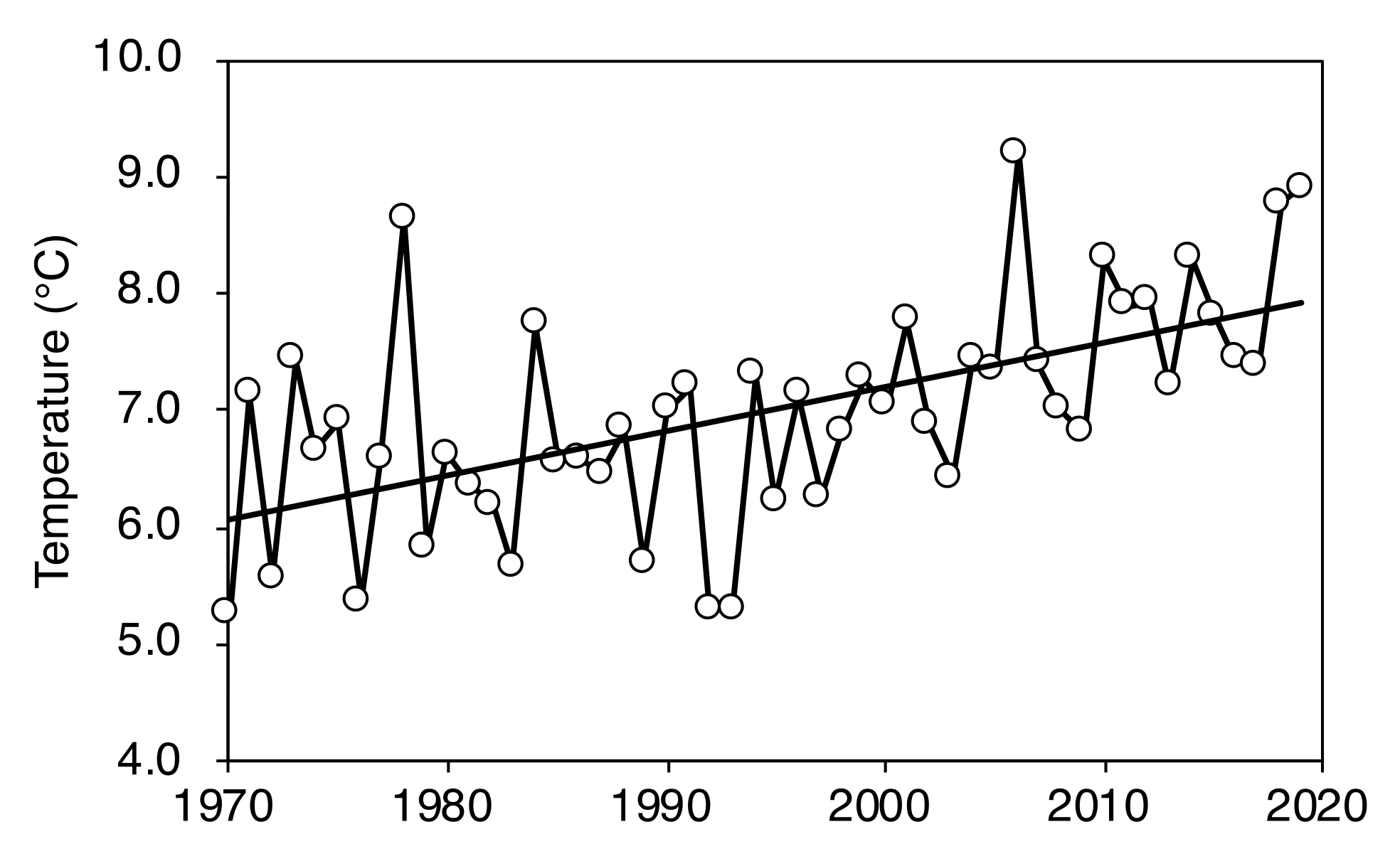

| Plot Number | No. of New Seedlings | Seedling Height (cm) | ||
|---|---|---|---|---|
| 1978–1999 | 1999–2018 | 1978–1999 | 1999–2018 | |
| 1 | 0 | 1 | - | 39 |
| 2 | 6 | 7 | 12 ± 8 | 36 ± 14 |
| 3 | 25 | 2 | 32 ± 38 | 18 |
| 4 | 60 | 1 | 52 ± 60 | 80 |
| 5 | 41 | 1 | 74 ± 79 | 11 |
| 6 | 54 | 0 | 77 ± 74 | - |
| 7 | 8 | 0 | 44 ± 23 | - |
| 8 | 2 | 0 | 11 ± 9 | - |
| 9 | 0 | 0 | - | - |
| Plot Number | No. of New Seedlings | Seedling Height (cm) | ||
|---|---|---|---|---|
| 1978–1999 | 1999–2018 | 1978–1999 | 1999–2018 | |
| 1 | 0 | 0 | - | - |
| 2 | 0 | 0 | - | - |
| 3 | 1 | 1 | 16 | 96 |
| 4 | 1 | 2 | 115 | 75 ± 45.3 |
| 5 | 2 | 4 | 29 ± 16 | 51.3 ± 36.7 |
| 6 | 2 | 1 | 23 ± 9.9 | 50 |
| 7 | 7 | 3 | 57.7 ± 76.3 | 43.3 ± 20.7 |
| 8 | 4 | 1 | 40 ± 23.6 | 78 |
| 9 | 0 | 0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakio, H.; Masuzawa, T. Advancing Timberline on Mt. Fuji between 1978 and 2018. Plants 2020, 9, 1537. https://doi.org/10.3390/plants9111537
Sakio H, Masuzawa T. Advancing Timberline on Mt. Fuji between 1978 and 2018. Plants. 2020; 9(11):1537. https://doi.org/10.3390/plants9111537
Chicago/Turabian StyleSakio, Hitoshi, and Takehiro Masuzawa. 2020. "Advancing Timberline on Mt. Fuji between 1978 and 2018" Plants 9, no. 11: 1537. https://doi.org/10.3390/plants9111537
APA StyleSakio, H., & Masuzawa, T. (2020). Advancing Timberline on Mt. Fuji between 1978 and 2018. Plants, 9(11), 1537. https://doi.org/10.3390/plants9111537





