Are Vulnerable Communities Thoroughly Informed on Mosquito Bio-Ecology and Burden?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Knowledge Attitude and Practices
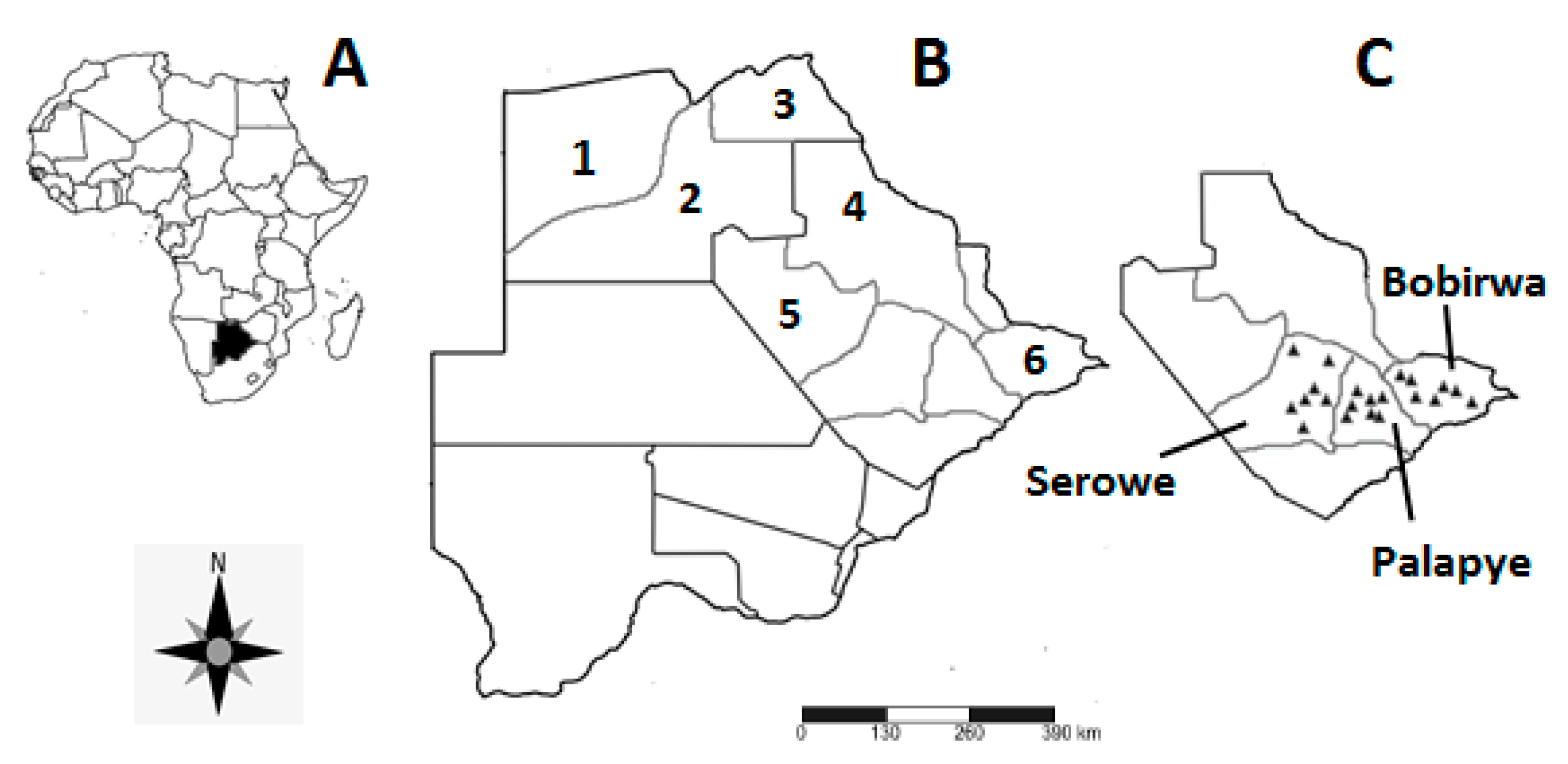
2.2. Study Area
2.3. Sampling Technique
2.4. Data Analysis
3. Results
3.1. Socio-Demographic Characteristics
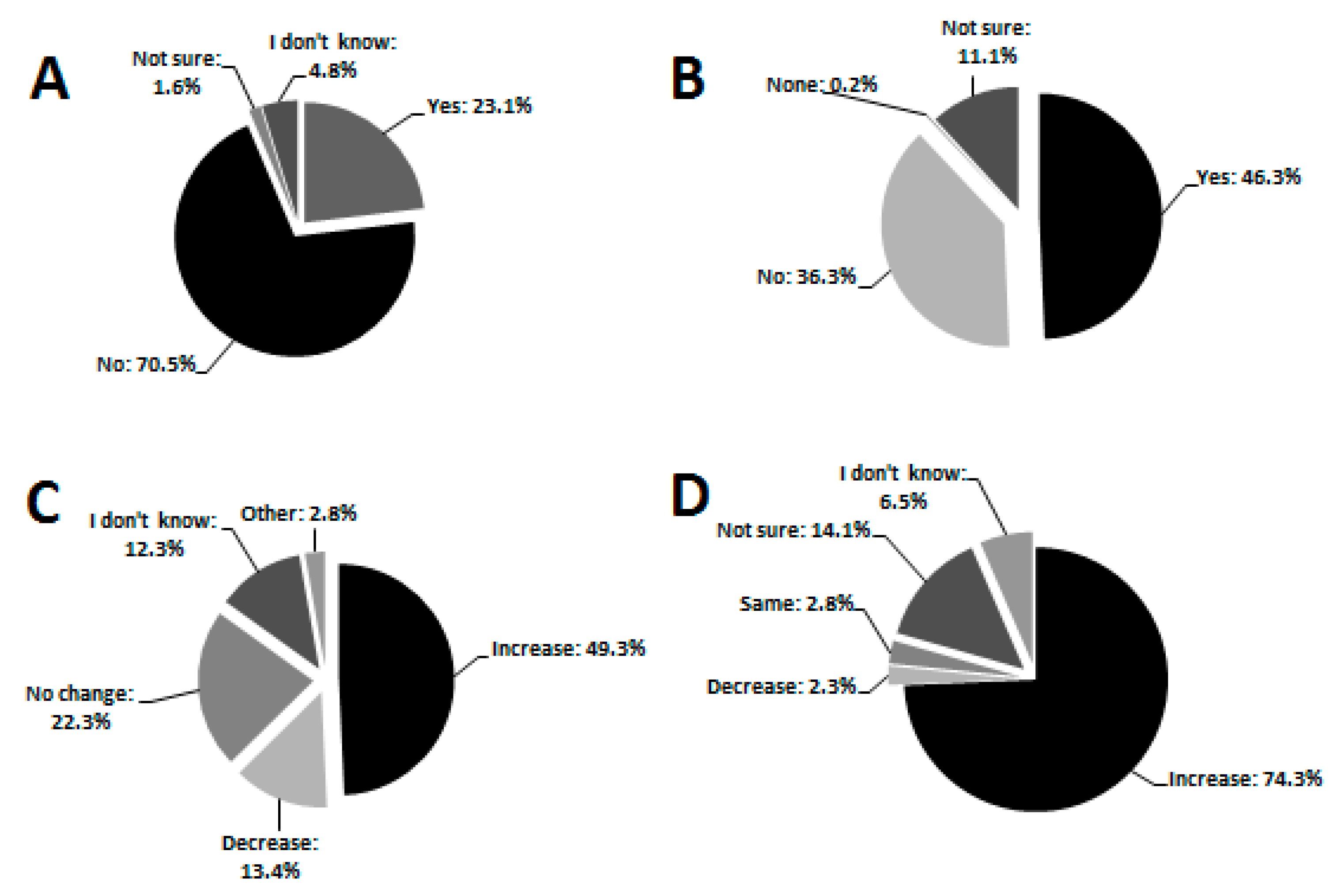
3.2. Knowledge
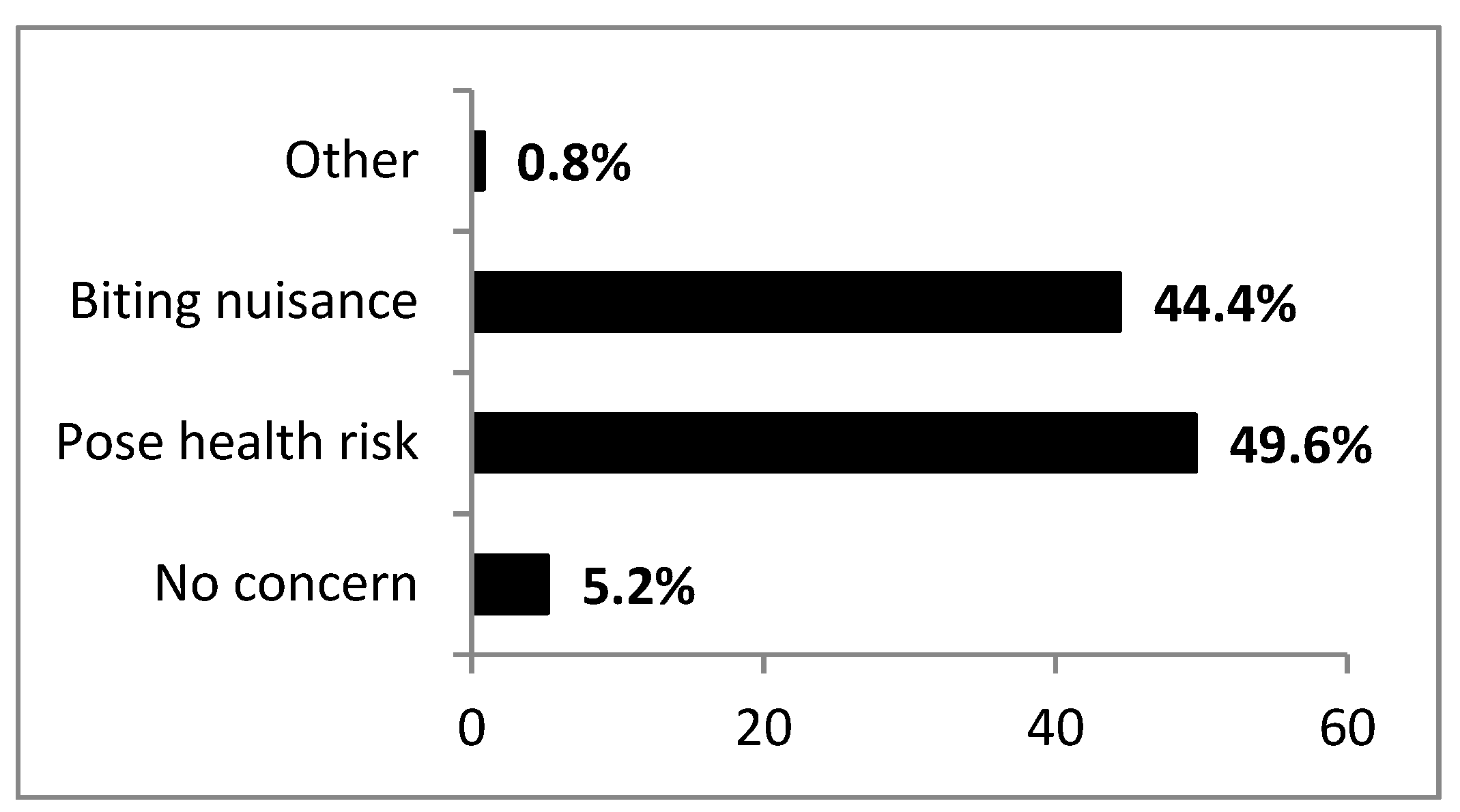
3.3. Attitude
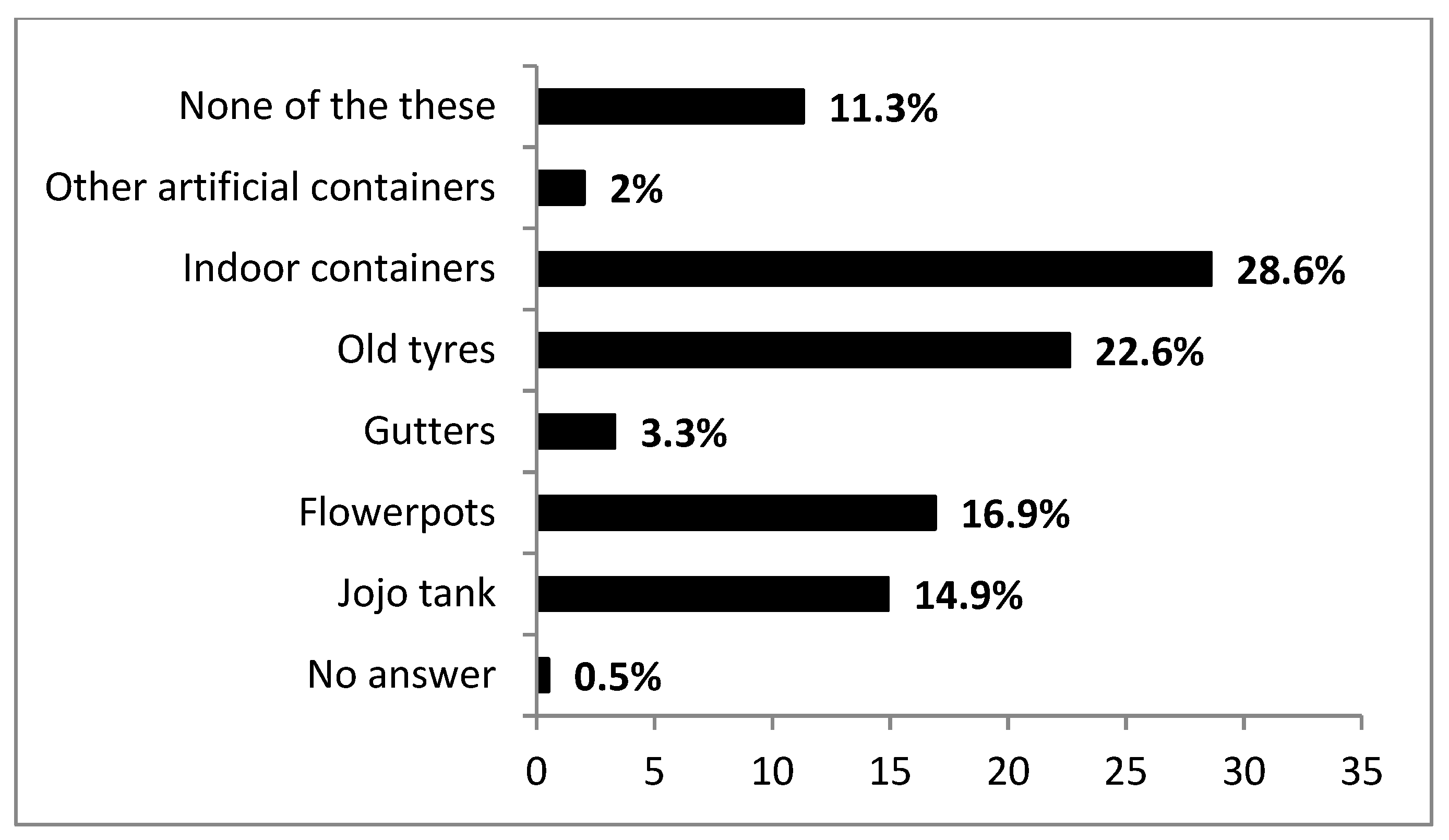
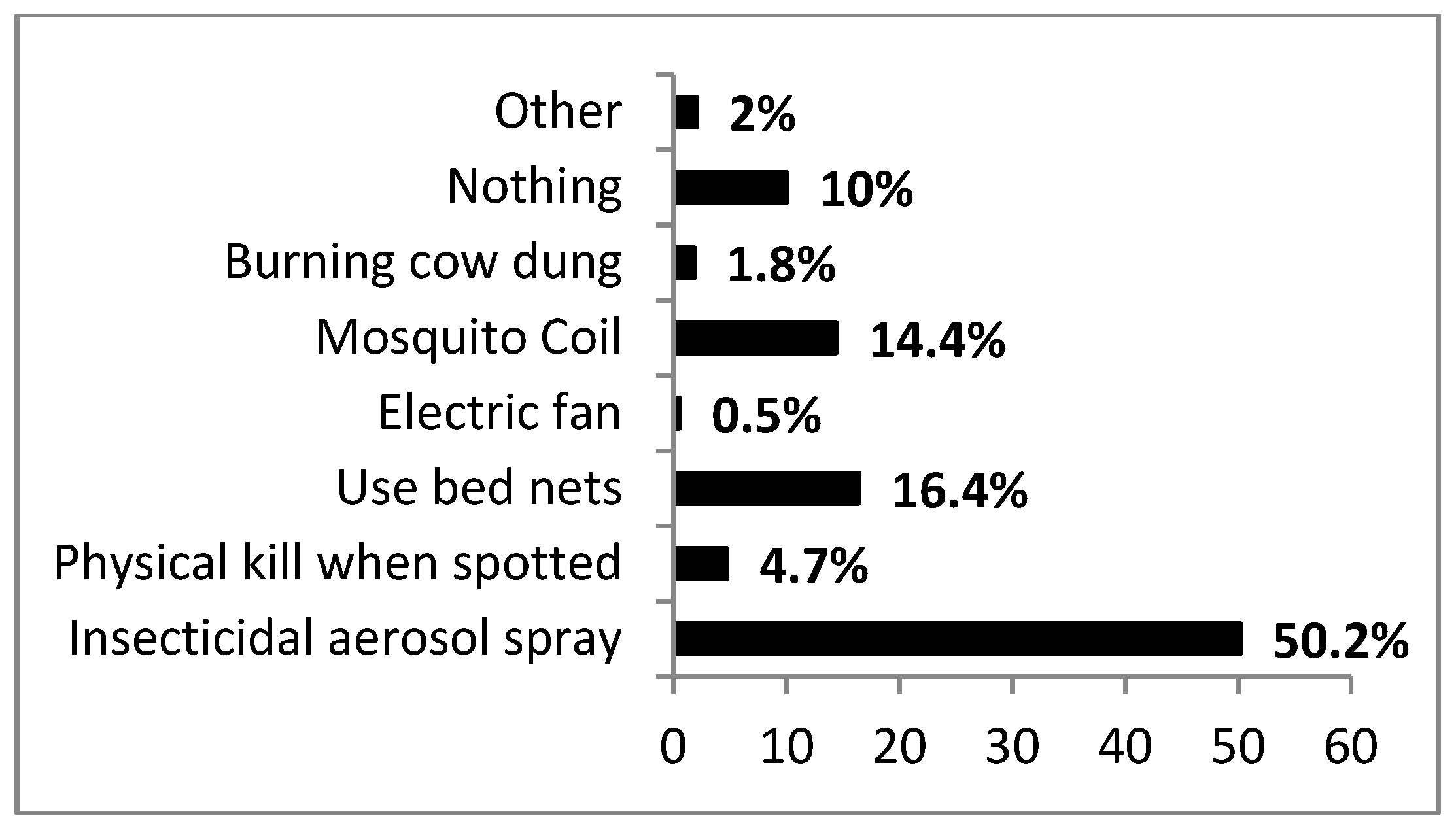
3.4. Practices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO (World Health Organization). Vector-Borne Diseases Factsheet; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- WHO (World Health Organization). A global Brief on Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, W.W.; Wali, T.; Kincaid, R.; Costero-Saint Denis, A. Arthropod vectors and disease transmission: Translational aspects. PLoS Negl. Trop. Dis. 2015, 9, e0004107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kweka, E.J.; Kimaro, E.E.; Kimaro, E.G.; Nagagi, Y.P.; Malele, I.I. Major Disease Vectors in Tanzania: Distribution, Control and Challenges. In Biological Control of Pest and Vector Insects; InTechOpen: London, UK, 2017; p. 257. [Google Scholar] [CrossRef] [Green Version]
- Tolle, M.A. Mosquito-borne diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2009, 39, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Mutheneni, S.R.; Morse, A.P.; Caminade, C.; Upadhyayula, S.M. Dengue burden in India: Recent trends and importance of climatic parameters. Emerg. Microbes Infect. 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brugman, V.; Hernández-Triana, L.; Medlock, J.; Fooks, A.; Carpenter, S.; Johnson, N. The role of Culex pipiens L. (Diptera: Culicidae) in virus transmission in Europe. Int. J. Environ. Res. Public Health 2018, 15, 389. [Google Scholar] [CrossRef] [Green Version]
- Paixão, E.S.; Teixeira, M.G.; Rodrigues, L.C. Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health 2018, 3, e000530. [Google Scholar] [CrossRef] [Green Version]
- Antonini, Y.; Lobato, D.N.C.; Norte, A.C.; Ramos, J.A.; Moreira, P.D.A.; Braga, E.M. Patterns of avian malaria in tropical and temperate environments: Testing the “The enemy release hypothesis”. Biota Neotrop. 2019, 19. [Google Scholar] [CrossRef]
- Coetzee, M.; Craig, M.; Le Sueur, D. Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol. Today 2000, 16, 74–77. [Google Scholar] [CrossRef]
- Braack, L.; de Almeida, A.P.G.; Cornel, A.J.; Swanepoel, R.; De Jager, C. Mosquito-borne arboviruses of African origin: Review of key viruses and vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef]
- Buxton, M.; Lebani, K.; Nyamukondiwa, C.; Wasserman, R.J. First record of Aedes (Stegomyia) aegypti (Linnaeus, 1762) (Diptera: Culicidae) in Botswana. BioInvasions Rec. 2019, 8, 551–557. [Google Scholar] [CrossRef]
- Eritja, R.; Palmer, J.R.; Roiz, D.; Sanpera-Calbet, I.; Bartumeus, F. Direct evidence of adult Aedes albopictus dispersal by car. Sci. Rep. 2017, 7, 14399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, R.S.; Hedeen, D.L.; Hedeen, M.W.; Hamer, G.L.; Mead, D.G.; Kitron, U.D. Avian species diversity and transmission of West Nile virus in Atlanta, Georgia. Parasites Vectors 2017, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, A.; Zheng, H.; Vrbova, L.; Drebot, M.A.; Iranpour, M.; Lindsay, L.R. Increased risk of endemic mosquito-borne diseases with climate change. CCDR 2019, 45, 4. [Google Scholar] [CrossRef] [PubMed]
- Asigau, S.; Parker, P.G. The influence of ecological factors on mosquito abundance and occurrence in Galápagos. J. Vector Ecol. 2018, 43, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Chirebvu, E.; Chimbari, M.J.; Ngwenya, B.N. Knowledge and practices on malaria in Tubu village, in a malaria-endemic area in northern Botswana: Implications for interventions. Malar. World J. 2013, 4, 1–9. [Google Scholar]
- Howes, R.E.; Mioramalala, S.A.; Ramiranirina, B.; Franchard, T.; Rakotorahalahy, A.J.; Bisanzio, D.; Gething, P.W.; Zimmerman, P.A.; Ratsimbasoa, A. Contemporary epidemiological overview of malaria in Madagascar: Operational utility of reported routine case data for malaria control planning. Malar. J. 2016, 15, 502. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Dhiman, S. Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect. Dis. Poverty. 2019, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Simon-Oke, I.A. Prevalence of Malaria Parasites among Pregnant Women and Children under Five years in Ekiti State, Southwest Nigeria. J. Biomed. Transl. Res. 2019, 5, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Chirebvu, E.; Chimbari, M.J.; Ngwenya, B.N. Assessment of risk factors associated with malaria transmission in Tubu village, northern Botswana. Malar. Res. Treat. 2014, 403069. [Google Scholar] [CrossRef]
- Motlaleng, M.; Edwards, J.; Namboze, J.; Butt, W.; Moakofhi, K.; Obopile, M.; Manzi, M.; Takarinda, K.C.; Zachariah, R.; Owiti, P.; et al. Driving towards malaria elimination in Botswana by 2018: Progress on case-based surveillance, 2013–2014. PHA 2018, 8, S24–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Menach, A.; Tatem, A.J.; Cohen, J.M.; Hay, S.I.; Randell, H.; Patil, A.P.; Smith, D.L. Travel risk, malaria importation and malaria transmission in Zanzibar. Sci. Rep. 2011, 1, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, B.; Zheng, J.; Qiu, H.; Yang, G.J.; Xia, S.; Zhou, X.N. Risk assessment of malaria transmission at the border area of China and Myanmar. Infect. Dis. Poverty. 2017, 6, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirebvu, E.; Chimbari, M.J. Characteristics of Anopheles arabiensis larval habitats in Tubu village, Botswana. J. Vector Ecol. 2015, 40, 129–138. [Google Scholar] [CrossRef]
- Pachka, H.; Annelise, T.; Alan, K.; Power, T.; Patrick, K.; Véronique, C.; Janusz, P.; Ferran, J. Rift Valley fever vector diversity and impact of meteorological and environmental factors on Culex pipiens dynamics in the Okavango Delta, Botswana. Parasites Vectors 2016, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Tawe, L.; Ramatlho, P.; Waniwa, K.; Muthoga, C.W.; Makate, N.; Ntebela, D.S.; Quaye, I.K.; Pombi, M.; Paganotti, G.M. Preliminary survey on Anopheles species distribution in Botswana shows the presence of Anopheles gambiae and Anopheles funestus complexes. Malar. J. 2017, 16, 106. [Google Scholar] [CrossRef]
- Meehl, G.A.; Tebaldi, C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef] [Green Version]
- Thuiller, W. Climate change and the ecologist. Nature 2007, 448, 550–552. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate change contribution to the emergence or re-emergence of parasitic diseases. Emerg. Infect. Dis. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef] [Green Version]
- Bango, Z.A.; Tawe, L.; Muthoga, C.W.; Paganotti, G.M. Past and current biological factors affecting malaria in the low transmission setting of Botswana: A review. Infect. Genet. Evol. 2020, 85, 104458. [Google Scholar] [CrossRef] [PubMed]
- Kgoroebutswe, T.K.; Ramatlho, P.; Reeder, S.; Makate, N.; Paganotti, G.M. Distribution of Anopheles mosquito species, their vectorial role and profiling of knock-down resistance mutations in Botswana. Parasitol. Res. 2020, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Makate, N.M. A Review of Insecticide Resistance Status in Botswana. Insect. Resist. 2016, 263. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Cornel, A.J.; Lee, Y.; Almeida, A.P.G.; Johnson, T.; Mouatcho, J.; Venter, M.; De Jager, C.; Braack, L. Mosquito community composition in South Africa and some neighboring countries. Parasites Vectors 2018, 11, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kengluecha, A.; Singhasivanon, P.; Tiensuwan, M.; Jones, J.W.; Sithiprasasna, R. Water quality and breeding habitats of anopheline mosquito in north-western Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 36, 46–53. [Google Scholar]
- Buxton, M.; Cuthbert, R.N.; Dalu, T.; Nyamukondiwa, C.; Wasserman, R.J. Cattle-induced eutrophication favours disease-vector mosquitoes. Sci. Total Environ. 2020, 715, 136952. [Google Scholar] [CrossRef]
- Simon, C.; Moakofhi, K.; Mosweunyane, T.; Jibril, H.B.; Nkomo, B.; Motlaleng, M.; Ntebela, D.S.; Chanda, E.; Haque, U. Malaria control in Botswana, 2008–2012: The path towards elimination. Malar. J. 2013, 12, 458. [Google Scholar] [CrossRef] [Green Version]
- Buxton, M.; Cuthbert, R.N.; Dalu, T.; Nyamukondiwa, C.; Wasserman, R.J. Complementary impacts of heterospecific predators facilitate improved biological control of mosquito larvae. Biol. Control 2020, 144, 104216. [Google Scholar] [CrossRef]
- Buxton, M.; Cuthbert, R.N.; Dalu, T.; Nyamukondiwa, C.; Wasserman, R.J. Predator density modifies mosquito regulation in increasingly complex environments. Pest Manag. Sci. 2020, 76, 2079–2084. [Google Scholar] [CrossRef]
- Quiroz-Martínez, H.; Rodríguez-Castro, A. Aquatic insects as predators of mosquito larvae. J. Am. Mosq. Control Assoc. 2007, 23, 110–118. [Google Scholar] [CrossRef]
- Shaalan, E.A.S.; Canyon, D.V. Aquatic insect predators and mosquito control. Trop. Biomed. 2009, 26, 223–261. [Google Scholar] [PubMed]
- Launiala, A. How much can a KAP survey tell us about people’s knowledge, attitudes and practices? Some observations from medical anthropology research on malaria in pregnancy in Malawi. Anthropol. Matters 2009, 11, 2009. [Google Scholar]
- Machekano, H.; Mvumi, B.M.; Nyamukondiwa, C. Plutella xylostella (L.): Pest status, control practices, perceptions and knowledge on existing and alternative management options in arid small-scale farming environments. Int. J. Pest Manag. 2020, 66, 48–64. [Google Scholar] [CrossRef]
- Chihanga, S.; Haque, U.; Chanda, E.; Mosweunyane, T.; Moakofhi, K.; Jibril, H.B.; Motlaleng, M.; Zhang, W.; Glass, G.E. Malaria elimination in Botswana, 2012–2014: Achievements and challenges. Parasites Vectors 2016, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Batisani, N.; Yarnal, B. Rainfall variability and trends in semi-arid Botswana: Implications for climate change adaptation policy. Appl. Geogr. 2010, 30, 483–489. [Google Scholar] [CrossRef]
- Hulsmans, A.; Vanschoenwinkel, B.; Pyke, C.; Riddoch, B.J.; Brendonck, L. Quantifying the hydroregime of a temporary pool habitat: A modelling approach for ephemeral rock pools in SE Botswana. Ecosystems 2008, 11, 89–100. [Google Scholar] [CrossRef]
- Bowling, A. Mode of questionnaire administration can have serious effects on data quality. J. Public Health 2005, 27, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Menjívar, C.; Agadjanian, V. Men’s migration and women’s lives: Views from rural Armenia and Guatemala. Soc. Sci. Q. 2007, 88, 1243–1262. [Google Scholar] [CrossRef]
- Yabiku, S.T.; Agadjanian, V.; Sevoyan, A. Husbands’ labour migration and wives’ autonomy, Mozambique 2000–2006. Popul. Stud. 2010, 64, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Gunn, J.K.; Ernst, K.C.; Center, K.E.; Bischof, K.; Nuñez, A.V.; Huynh, M.; Okello, A.; Hayden, M.H. Current strategies and successes in engaging women in vector control: A systematic review. BMJ Glob. Health 2018, 3, e000366. [Google Scholar] [CrossRef]
- Bonifay, T.; Douine, M.; Bonnefoy, C.; Hurpeau, B.; Nacher, M.; Djossou, F.; Epelboin, L. Poverty and arbovirus outbreaks: When chikungunya virus hits more precarious populations than dengue virus in French Guiana. Open Forum Infect. Dis. 2017, 4, ofx247. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, G.; Yadav, A.; Dudeja, P. Knowledge, awareness and practices regarding dengue among rural and slum communities in North Indian city, India. Int. J. Med. Sci. Public Health 2014, 3, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Nakagiri, A.; Niwagaba, C.B.; Nyenje, P.M.; Kulabako, R.N.; Tumuhairwe, J.B.; Kansiime, F. Are pit latrines in urban areas of Sub-Saharan Africa performing? A review of usage, filling, insects and odour nuisances. BMC Public Health 2015, 16, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emidi, B.; Kisinza, W.N.; Stanley, G.; Mosha, F. Seasonal variation of Culex quinquefasciatus densities emerged from Pit-Latrines in rural settings, Muheza, Tanzania. SM J. Public Health Epidemiol. 2017, 3, 1040. [Google Scholar]
- Nathan, M.B.; Toney, S.; Bramble, S.; Reid, V. Control of Culex quinquefasciatus in pit latrines, using shredded, waste polystyrene. Ann. Trop. Med. Parasit. 1996, 90, 207–212. [Google Scholar] [CrossRef]
- Chaggu, E.J. Sustainable Environmental Protection Using Modified Pit-latrines. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2004. [Google Scholar]
- Sivagnaname, N.; Amalraj, D.D.; Mariappan, T. Utility of expanded polystyrene (EPS) beads in the control of vector-borne diseases. Indian J. Med. Res. 2005, 122, 91. [Google Scholar]
- Castro, M.C.; Kanamori, S.; Kannady, K.; Mkude, S.; Killeen, G.F.; Fillinger, U. The importance of drains for the larval development of lymphatic filariasis and malaria vectors in Dar es Salaam, United Republic of Tanzania. PLoS Negl. Trop. Dis. 2010, 4, e693. [Google Scholar] [CrossRef]
- Bennett, K.L.; Martínez, C.G.; Almanza, A.; Rovira, J.R.; McMillan, W.O.; Enriquez, V.; Barraza, E.; Diaz, M.; Sanchez-Galan, J.E.; Whiteman, A.; et al. High infestation of invasive Aedes mosquitoes in used tires along the local transport network of Panama. Parasites Vectors 2019, 12, 264. [Google Scholar] [CrossRef] [Green Version]
- Medlock, J.M.; Vaux, A.G. Colonization of a newly constructed urban wetland by mosquitoes in England: Implications for nuisance and vector species. J. Vector Ecol. 2014, 39, 249–260. [Google Scholar] [CrossRef]
- Parker, C.; Garcia, F.; Menocal, O.; Jeer, D.; Alto, B. A Mosquito Workshop and Community Intervention: A Pilot Education Campaign to Identify Risk Factors Associated with Container Mosquitoes in San Pedro Sula, Honduras. Int. J. Environ. Res. Public Health 2019, 16, 2399. [Google Scholar] [CrossRef] [Green Version]
- Rueda, L.M. Pictorial Keys for the Identification of Mosquitoes (Diptera: Culicidae) Associated with Dengue Virus Transmission. Zootaxa 2004, 58, 519–524. [Google Scholar] [CrossRef]
- Tangena, J.A.A.; Thammavong, P.; Malaithong, N.; Inthavong, T.; Ouanesamon, P.; Brey, P.T.; Lindsay, S.W. Diversity of mosquitoes (Diptera: Culicidae) attracted to human subjects in rubber plantations, secondary forests, and villages in Luang Prabang province, Northern Lao PDR. J. Med. Entomol. 2017, 54, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Gandon, S. Evolutionary ecology of avian malaria: Past to present. Trends Parasitol. 2018, 34, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Chirebvu, E.; Chimbari, M.J.; Ngwenya, B.N.; Sartorius, B. Clinical malaria transmission trends and its association with climatic variables in Tubu Village, Botswana: A retrospective analysis. PLoS ONE 2016, 11, e0139843. [Google Scholar] [CrossRef] [PubMed]
- Karunamoorthi, K. Global malaria burden: Socialomics implications. J. Soc. 2012, 1, e108. [Google Scholar] [CrossRef]
- Snow, R.W. Sixty years trying to define the malaria burden in Africa: Have we made any progress? BMC Med. 2014, 12, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W. Changing transmission pattern of Plasmodium vivax malaria in the Republic of Korea: Relationship with climate change. Environ. Health Toxicol. 2011, 26, e2011001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjørnstad, O.N. The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk. R. Soc. Open Sci. 2017, 4, 160969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffel, T.R.; Hoverman, J.T.; Halstead, N.T.; Michel, P.J.; Rohr, J.R. Parasitism in a community context: Trait-mediated interactions with competition and predation. Ecology 2010, 91, 1900–1907. [Google Scholar] [CrossRef]
- Weis, J.S. Invasion and predation in aquatic ecosystems. Curr. Zool. 2011, 57, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Moi, M.L.; Takasaki, T.; Kotaki, A.; Tajima, S.; Lim, C.K.; Sakamoto, M.; Iwagoe, H.; Kobayashi, K.; Kurane, I. Importation of dengue virus type 3 to Japan from Tanzania and Côte d’Ivoire. Emerg. Infect. Dis. 2010, 16, 1770. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Fang, C.; Yan, J.; Yan, H.; Zhan, B.; Sun, Y.; Mao, H.; Cao, G.; Lv, L.; Zhang, Y. Chikungunya Fever Outbreak, Zhejiang Province, China, 2017. Emerg. Infect. Dis. 2019, 25, 1589. [Google Scholar] [CrossRef] [PubMed]
- Tatem, A.J.; Rogers, D.J.; Hay, S.I. Global transport networks and infectious disease spread. Adv. Parasit. 2006, 62, 293–343. [Google Scholar]
- Barnett, E.D.; Walker, P.F. Role of immigrants and migrants in emerging infectious diseases. Med. Clin. N. Am. 2008, 92, 1447–1458. [Google Scholar] [CrossRef]
- Shafie, A.; Roslan, M.A.; Ngui, R.; Lim, Y.A.L.; Sulaiman, W.Y.W. Mosquito biology and mosquito-borne disease awareness among island communities in Malaysia. J. Am. Mosq. Control Assoc. 2016, 32, 273–282. [Google Scholar] [CrossRef]
- Satapathy, S.; Taylor-Robinson, A.W. Bridging the Knowledge Gap in Transmission-Blocking Immunity to Malaria: Deciphering Molecular Mechanisms in Mosquitoes. Adv. Infect. Dis. 2016, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Finda, M.F.; Moshi, I.R.; Monroe, A.; Limwagu, A.J.; Nyoni, A.P.; Swai, J.K.; Ngowo, H.S.; Minja, E.G.; Toe, L.P.; Kaindoa, E.W.; et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE 2019, 14, e0217414. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Thomas, M.B. The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malar. J. 2011, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Pates, H.; Curtis, C. Mosquito behavior and vector control. Annu. Rev. Entomol. 2005, 50, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Rozendaal, J.A. Vector Control: Methods for Use by Individuals and Communities; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Breisch, N.L.; Golden, D.B.; Feldweg, A.M. Prevention of Arthropod and Insect Bites: Repellents and Other Measures. Up To Date, Waltham, MA. 2012. Available online: https://www.uptodate.com/contents/prevention-of-arthropod-and-insect-bites-repellents-and-other-measures (accessed on 18 November 2019).
- Lapshin, D.N.; Vorontsov, D.D. Low-Frequency Sounds Repel Male Mosquitoes Aedes diantaeus NDK (Diptera, Culicidae). Entomol. Rev. 2018, 98, 266–271. [Google Scholar] [CrossRef]
- Okumu, F.O.; Moore, S.J. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: A review of possible outcomes and an outline of suggestions for the future. Malar. J. 2011, 10, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muema, J.M.; Bargul, J.L.; Njeru, S.N.; Onyango, J.O.; Imbahale, S.S. Prospects for malaria control through manipulation of mosquito larval habitats and olfactory-mediated behavioural responses using plant-derived compounds. Parasites Vectors 2017, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Schrama, M.; Gorsich, E.E.; Hunting, E.R.; Barmentlo, S.H.; Beechler, B.; van Bodegom, P.M. Eutrophication and predator presence overrule the effects of temperature on mosquito survival and development. PLoS Negl. Trop. Dis. 2018, 12, e0006354. [Google Scholar] [CrossRef] [PubMed]
- Roux, O.; Robert, V. Larval predation in malaria vectors and its potential implication in malaria transmission: An overlooked ecosystem service? Parasites Vectors 2019, 12, 217. [Google Scholar] [CrossRef]
- Wagman, J.M.; Grieco, J.P.; Bautista, K.; Polanco, J.; Briceño, I.; King, R.; Achee, N.L. The field evaluation of a push-pull system to control malaria vectors in Northern Belize, Central America. Malar. J. 2015, 14, 184. [Google Scholar] [CrossRef] [Green Version]
- Schoepke, A.; Steffen, R.; Gratz, N. Effectiveness of personal protection measures against mosquito bites for malaria prophylaxis in travelers. J. Travel Med. 1998, 5, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Long, C.A.; Zavala, F. Malaria vaccines and human immune responses. Curr. Opin. Microbiol. 2016, 32, 96–102. [Google Scholar] [CrossRef] [Green Version]
| Variables | Category | Number of Respondents | Proportion (%) |
|---|---|---|---|
| Gender | Male | 174 | 28.5 |
| Female | 437 | 71.5 | |
| Marital Status | Single (never married) | 430 | 70.4 |
| Married | 122 | 20 | |
| Divorced | 9 | 1.5 | |
| Widowed | 50 | 8.2 | |
| Age (years) | 18–29 | 135 | 22.1 |
| 30–39 | 144 | 23.6 | |
| 40–49 | 110 | 18.0 | |
| 50–59 | 89 | 14.6 | |
| ≥60 | 133 | 21.8 | |
| Disability | Yes | 49 | 8.0 |
| No | 560 | 91.7 | |
| Prefer not to say | 2 | 0.3 | |
| Literacy | Literate | 542 | 88.7 |
| Illiterate | 65 | 10.6 | |
| Prefer not to say | 4 | 0.7 | |
| Education | None | 83 | 13.6 |
| Primary | 177 | 29.0 | |
| Junior Certificate | 180 | 29.5 | |
| Form 4–5 (Senior) | 90 | 14.7 | |
| Vocational | 50 | 8.2 | |
| Tertiary | 29 | 4.7 | |
| Prefer not to say | 1 | 0.2 | |
| Other | 1 | 0.2 | |
| Information access | Radio/TV | 311 | 50.9 |
| Health professionals | 207 | 33.9 | |
| Printed media | 11 | 1.8 | |
| Electronic sources | 2 | 0.3 | |
| Family/Friends | 29 | 4.7 | |
| Own experience | 25 | 4.1 | |
| Other | 26 | 4.3 | |
| Family size | 1–2 | 84 | 13.7 |
| 3–5 | 197 | 32.2 | |
| 6–10 | 229 | 37.5 | |
| >10 | 101 | 16.5 | |
| Pit latrine (toilet) | Yes | 516 | 84.5 |
| No | 95 | 15.5 | |
| Drainage system | Yes | 176 | 28.9 |
| No | 433 | 71.1 | |
| Stagnant water | Yes | 82 | 13.4 |
| No | 524 | 85.8 | |
| Not sure | 4 | 0.7 | |
| Don’t know | 1 | 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buxton, M.; Machekano, H.; Gotcha, N.; Nyamukondiwa, C.; Wasserman, R.J. Are Vulnerable Communities Thoroughly Informed on Mosquito Bio-Ecology and Burden? Int. J. Environ. Res. Public Health 2020, 17, 8196. https://doi.org/10.3390/ijerph17218196
Buxton M, Machekano H, Gotcha N, Nyamukondiwa C, Wasserman RJ. Are Vulnerable Communities Thoroughly Informed on Mosquito Bio-Ecology and Burden? International Journal of Environmental Research and Public Health. 2020; 17(21):8196. https://doi.org/10.3390/ijerph17218196
Chicago/Turabian StyleBuxton, Mmabaledi, Honest Machekano, Nonofo Gotcha, Casper Nyamukondiwa, and Ryan J. Wasserman. 2020. "Are Vulnerable Communities Thoroughly Informed on Mosquito Bio-Ecology and Burden?" International Journal of Environmental Research and Public Health 17, no. 21: 8196. https://doi.org/10.3390/ijerph17218196





