Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches
Abstract
1. Introduction
2. Pathophysiology
3. Treatment Strategies of NVAMD
3.1. Vascular Endothelial Growth Factor Inhibitors
3.1.1. Pegaptanib
3.1.2. Bevacizumab
3.1.3. Ranibizumab
3.1.4. Aflibercept
3.1.5. Brolucizumab
4. Real-World Outcomes
5. Limitations of Anti-VEGF Therapy
5.1. Non-Responders
5.2. Cost
6. NVAMD Management: Multi Target Approach
7. Designed Ankyrin Repeat Protein in NVAMD Treatment
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AEs | Adverse events |
| AMD | Age-related macular degeneration |
| Anti-VEGF | Vascular endothelial growth factor inhibitors |
| AREDS | Age-Related Eye Disease Study |
| ASRS | American Society of Retina Specialists |
| BCVA | Best corrected visual acuity |
| BLA | Biologics License Application |
| CANTREAT | Canadian Treat-and-Extend Analysis Trial with ranibizumab |
| CATT | Comparison of Age-Related Macular Degeneration Treatments Trials |
| CMT | Central macular thickness |
| CNV | Choroidal new vessels |
| DARPin | Designed ankyrin repeat protein |
| DME | Diabetic macular edema |
| EMAs | European Medicines Agency |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| FDA | Food and Drug Administration |
| GA | Geography atrophy |
| HIF-1 | Hypoxia-inducible factor-1 |
| IFSR | Intravitreal fluocinolone sustained released |
| IOI | Intraocular inflammation |
| ISIS | Intelligent Research in Sight |
| IVT-AFL | Intravitreal aflibercept |
| IVT-AFL-2W | Intravitreal aflibercept administered every 2 weeks |
| IVT-AFL-4W | Intravitreal aflibercept administered every 4 weeks |
| MAC | Membrane attack complex |
| MMP | Metalloproteinase |
| NVAMD | Neovascular age-related macular degeneration |
| OCT | optical coherence tomography |
| PDGF-B | Platelet-derived growth factor-B |
| PIGF | Placental growth factor |
| PMDA | Japanese Regulatory Agency |
| PRN | Pro re nata |
| Q4 | Every 4 weeks |
| Q8 | Every 8 weeks |
| Q12 | Every 12 weeks |
| RCT | Randomized clinical trial |
| RPE | Retinal pigment epithelium |
| RQ4 | Intravitreal ranibizumab administered every 4 weeks |
| SDF-1 | Stromal-derived factor-1 |
| TIMP | Tissue inhibitor of metalloproteinases |
| TREX | Treat-and-extend |
| VA | visual acuity |
| VEGF | Vascular endothelial growth factor |
References
- Cheung, L.K.; Eaton, A. Age-Related Macular Degeneration. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, A.; Mahdi, L.; Musat, O. Age-related macular degeneration. Rom. J. Ophthalmol. 2015, 59, 74–77. [Google Scholar] [PubMed]
- Handa, J.T.; Verzijl, N.; Matsunaga, H.; Aotaki-Keen, A.; Lutty, G.A.; Te Koppele, J.M.; Miyata, T.; Hjelmeland, L.M. Increase in the advanced glycation end productpentosidine in Bruch’s membrane with age. Investig. Ophthalmol. Vis. Sci. 1999, 40, 775–779. [Google Scholar]
- Wong, W.L.; Su, X.; Li, B.X.; Cheung, C.M.G.; Klein, B.E.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Vingerling, J.R.; Dielemans, I.; Hofman, A.; Grobbee, D.E.; Hijmering, M.; Kramer, C.F.; De Jong, P.T. The Prevalence of Age-related Maculopathy in the Rotterdam Study. Ophthalmology 1995, 102, 205–210. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, B.E.; Cruickshanks, K.J. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog. Retin. Eye Res. 1999, 18, 371–389. [Google Scholar] [CrossRef]
- Van Newkirk, M.R. The prevalence of age-related maculopathy the visual impairment project. Ophthalmology 2000, 107, 1593–1600. [Google Scholar] [CrossRef]
- Varma, R.; Fraser-Bell, S.; Tan, S.; Klein, B.E.; Azen, S.P. Prevalence of age-related macular degeneration in Latinos. Ophthalmology 2004, 111, 1288–1297. [Google Scholar] [CrossRef]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; Chen, S.-J.; Jonas, J.B.; Wang, J.J.; Mitchell, P.; Wong, T.Y. The Prevalence of Age-Related Macular Degeneration in Asians. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef]
- Korb, C.A.; Kottler, U.B.; Wolfram, C.; Hoehn, R.; Schulz, A.; Zwiener, I.; Wild, P.S.; Pfeiffer, N.; Mirshahi, A. Prevalence of age-related macular degeneration in a large European cohort: Results from the population-based Gutenberg Health Study. Graefe Arch. Clin. Exp. Ophthalmol. 2014, 252, 1403–1411. [Google Scholar] [CrossRef]
- Zapata, M.A.; Arcos, G.; Fonollosa, A.; Abraldes, M.; Oleñik, A.; Gutierrez, E.; García-Arumí, J. Telemedicine for a General Screening of Retinal Disease Using Nonmydriatic Fundus Cameras in Optometry Centers: Three-Year Results. Telemed. eHealth 2017, 23, 30–36. [Google Scholar] [CrossRef]
- García-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Linton, K.L. Prevalence of Age-related Maculopathy. Ophthalmology 2020, 127, S122–S132. [Google Scholar] [CrossRef]
- Al-Zamil, W.M.; A Yassin, S. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef]
- Buitendijk, G.H.; Rochtchina, E.; Myers, C.; van Duijn, C.M.; Lee, K.E.; Klein, B.E.; Meuer, S.M.; de Jong, P.T.; Holliday, E.G.; Tan, A.G.; et al. Prediction of Age-related Macular Degeneration in the General Population. Ophthalmology 2013, 120, 2644–2655. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.L.; DeAngelis, M.M. Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. 2016, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2019, 104, 1077–1084. [Google Scholar] [CrossRef]
- Singh, N.; Srinivasan, S.; Muralidharan, V.; Roy, R.; V, J.; Raman, R. Prevention of Age-Related Macular Degeneration. Asia Pacific J. Ophthalmol. 2017. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of Age-Related Macular Degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Drusen characterization with multimodal imaging. Retina 2010, 30, 1441–1454. [Google Scholar] [CrossRef]
- Ferris, F.L.; Wilkinson, C.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.G.; Sadda, S.R. Clinical Classification of Age-related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Green, W.R.; Enger, C. Age-related Macular Degeneration Histopathologic Studies. Ophthalmology 1993, 100, 1519–1535. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Michels, S.; Schmidt-Erfurth, U.; Rosenfeld, P.J. Promising new treatments for neovascular age-related macular degeneration. Expert Opin. Investig. Drugs 2006, 15, 779–793. [Google Scholar] [CrossRef]
- Bloch, S.B.; Larsen, M.; Munch, I.C. Incidence of Legal Blindness from Age-Related Macular Degeneration in Denmark: Year 2000 to 2010. Am. J. Ophthalmol. 2012, 153, 209–213. [Google Scholar] [CrossRef]
- Chong, V. Ranibizumab for the treatment of wet AMD: A summary of real-world studies. Eye 2015, 30, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Maguire, M.G.; Martin, D.F.; Ying, G.-S.; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.L.; Fine, S.L. Five-Year Outcomes with Anti–Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmology 2016, 123, 1751–1761. [Google Scholar] [CrossRef]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef]
- Kijlstra, A.; Berendschot, T. Age-Related Macular Degeneration: A Complementopathy? Ophthalmic Res. 2015, 54, 64–73. [Google Scholar] [CrossRef]
- Handa, J.T.; Rickman, C.B.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat. Commun. 2019, 10, 3347. [Google Scholar] [CrossRef]
- Smith, W.; Assink, J.; Klein, R.; Mitchell, P.; Klaver, C.C.; Klein, B.E.; Hofman, A.; Jensen, S.; Wang, J.J.; De Jong, P.T. Risk factors for age-related macular degeneration. Ophthalmology 2001, 108, 697–704. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Wong, M.S.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef]
- Okubo, A.; Rosa, R.H.; Bunce, C.V.; Alexander, R.A.; Fan, J.T.; Bird, A.C.; Luthert, P.J. The relationships of age changes in retinal pigment epithelium and Bruch’s membrane. Investig. Ophthalmol. Vis. Sci. 1999, 40, 443–449. [Google Scholar]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M.; et al. From The Cover: A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef]
- Edwards, A.O.; Ritter, R., 3rd; Abel, K.J.; Manning, A.; Panhuysen, C.; Farrer, L.A. Complement Factor H Polymorphism and Age-Related Macular Degeneration. Science 2005, 308, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.-Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Haines, J.L.; Hauser, M.A.; Schmidt, S.; Scott, W.K.; Olson, L.M.; Gallins, P.; Spencer, K.L.; Kwan, S.Y.; Noureddine, M.; Gilbert, J.R.; et al. Complement Factor H Variant Increases the Risk of Age-Related Macular Degeneration. Science 2005, 308, 419–421. [Google Scholar] [CrossRef]
- Ricci, F.; Zampatti, S.; D’Abbruzzi, F.; Missiroli, F.; Martone, C.; Lepre, T.; Pietrangeli, I.; Sinibaldi, C.; Peconi, C.; Novelli, G.; et al. Typing of ARMS2 and CFH in Age-Related Macular Degeneration. Arch. Ophthalmol. 2009, 127, 1368–1372. [Google Scholar] [CrossRef]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef]
- Bradt, B.M.; Kolb, W.P.; Cooper, N.R. Complement-dependent Proinflammatory Properties of the Alzheimer’s Disease β-Peptide. J. Exp. Med. 1998, 188, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Leitner, W.P.; Rivest, A.J.; Staples, M.K.; Radeke, M.J.; Anderson, D.H. The Alzheimer’s A-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 11830–11835. [Google Scholar] [CrossRef]
- Sohn, J.H.; Kaplan, H.J.; Suk, H.J.; Bora, P.S.; Bora, N.S. Chronic low level complementactivation within the eye is controlled by intraocular complement regulatoryproteins. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3492–3502. [Google Scholar]
- Thurman, J.M.; Renner, B.; Kunchithapautham, K.; Ferreira, V.P.; Pangburn, M.K.; Ablonczy, Z.; Tomlinson, S.; Holers, V.M.; Rohrer, B. Oxidative Stress Renders Retinal Pigment Epithelial Cells Susceptible to Complement-mediated Injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lauer, T.W.; Sick, A.; Hackett, S.F.; Campochiaro, P.A. Oxidative Stress Modulates Complement Factor H Expression in Retinal Pigmented Epithelial Cells by Acetylation of FOXO. J. Biol. Chem. 2007, 282, 22414–22425. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage–induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef]
- Moore, D.J.; Hussain, A.A.; Marshall, J. Age-related variation in the hydraulicconductivity of Bruch’s membrane. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1290–1297. [Google Scholar]
- Starita, C.; Hussain, A.A.; Pagliarini, S.; Marshall, J. Hydrodynamics of Ageing Bruch’s Membrane: Implications for Macular Disease. Exp. Eye Res. 1996, 62, 565–572. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Saint-Geniez, M.; Kurihara, T.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 2009, 106, 18751–18756. [Google Scholar] [CrossRef]
- Green, W.R.; McDonnell, P.J.; Yeo, J.H. Pathologic features of senile maculardegeneration. Ophthalmology 1985, 92, 615–627. [Google Scholar] [CrossRef]
- Sarks, J.P.; Sarks, S.H.; Killingsworth, M.C. Evolution of soft drusen in age-related macular degeneration. Eye 1994, 8, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Karwatowski, W.S.; Jeffries, T.E.; Duance, V.C.; Albon, J.; Bailey, A.J.; Easty, D.L. Preparation of Bruch’s membrane and analysis of the age-related changes in the structural collagens. Br. J. Ophthalmol. 1995, 79, 944–952. [Google Scholar] [CrossRef]
- Sarks, S.H.; Arnold, J.J.; Killingsworth, M.C.; Sarks, J.P. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: A clinicopathological study. Br. J. Ophthalmol. 1999, 83, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Presley, J.B.; Malek, G.; Medeiros, N.E.; Avery, D.V.; Kruth, H.S. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp. Eye Res. 2005, 81, 731–741. [Google Scholar] [CrossRef]
- Huang, J.-D.; Presley, J.B.; Chimento, M.F.; Curcio, C.A.; Johnson, M. Age-related changes in human macular Bruch’s membrane as seen by quick-freeze/deep-etch. Exp. Eye Res. 2007, 85, 202–218. [Google Scholar] [CrossRef]
- Pauleikhoff, D.; Harper, C.A.; Marshall, J.; Bird, A.C. Aging changes in Bruch’s membrane. A histochemical and morphologic study. Ophthalmology 1990, 97, 171–178. [Google Scholar] [CrossRef]
- Biesemeier, A.; Taubitz, T.; Julien, S.; Yoeruek, E.; Schraermeyer, U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol. Aging 2014, 35, 2562–2573. [Google Scholar] [CrossRef]
- Seddon, J.M.; McLeod, D.S.; Bhutto, I.A.; Villalonga, M.B.; Silver, R.E.; Wenick, A.S.; Edwards, M.M.; Lutty, G.A. Histopathological Insights into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 1272–1280. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Stefánsson, E.; Geirsdóttir, Á.; Sigurdsson, H. Metabolic physiology in age related macular degeneration. Prog. Retin. Eye Res. 2011, 30, 72–80. [Google Scholar] [CrossRef]
- Oshima, Y.; Oshima, S.; Nambu, H.; Kachi, S.; Hackett, S.F.; Melia, M.; Kaleko, M.; Connelly, S.; Esumi, N.; Zack, D.J.; et al. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J. Cell. Physiol. 2004, 201, 393–400. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.D.; Hackett, S.F.; Hirota, K.; Oshima, Y.; Cai, Z.; Berg-Dixon, S.; Rowan, A.; Yan, Z.; Campochiaro, P.A.; Semenza, G.L. Cell Type–Specific Regulation of Angiogenic Growth Factor Gene Expression and Induction of Angiogenesis in Nonischemic Tissue by a Constitutively Active Form of Hypoxia-Inducible Factor 1. Circ. Res. 2003, 93, 1074–1081. [Google Scholar] [CrossRef]
- Metzger, C.S.; Koutsimpelas, D.; Brieger, J. Transcriptional regulation of the VEGF gene in dependence of individual genomic variations. Cytokine 2015, 76, 519–526. [Google Scholar] [CrossRef]
- Blasiak, J.; Petrovski, G.; Veréb, Z.; Facskó, A.; Kaarniranta, K. Oxidative Stress, Hypoxia, and Autophagy in the Neovascular Processes of Age-Related Macular Degeneration. BioMed Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar]
- Roth, F.; Bindewald, A.; Holz, F.G. Keypathophysiologic pathways in age-related macular disease. Graefe Arch. Clin. Exp. Ophthalmol. 2004, 242, 710–716. [Google Scholar] [CrossRef]
- Kornzweig, A.L. Changes in the choriocapillaris associated with senile maculardegeneration. Ann. Ophthalmol. 1977, 9, 753–756. [Google Scholar]
- Killingsworth, M.C. Angiogenesis in early choroidal neovascularization secondary to age-related macular degeneration. Graefe Arch. Clin. Exp. Ophthalmol. 1995, 233, 313–323. [Google Scholar] [CrossRef]
- Sarks, J.P.; Sarks, S.H.; Killingsworth, M.C. Morphology of early choroidal neovascularisation in age-related macular degeneration: Correlation with activity. Eye 1997, 11, 515–522. [Google Scholar] [CrossRef]
- Das, A.; McGuire, P.G. Retinal and choroidal angiogenesis: Pathophysiology and strategies for inhibition. Prog. Retin. Eye Res. 2003, 22, 721–748. [Google Scholar] [CrossRef]
- Kijlstra, A.; La Heij, E.C.; Hendrikse, F. REVIEW ARTICLE, Immunological Factors in the Pathogenesis and Treatment of Age-Related Macular Degeneration. Ocul. Immunol. Inflamm. 2005, 13, 3–11. [Google Scholar] [CrossRef]
- Magnusson, K.P.; Duan, S.; Sigurdsson, H.; Petursson, H.; Yang, Z.; Zhao, Y.; Bernstein, P.S.; Ge, J.; Jonasson, F.; Stefansson, E.; et al. CFH Y402H Confers Similar Risk of Soft Drusen and Both Forms of Advanced AMD. PLoS Med. 2005, 3, e5. [Google Scholar] [CrossRef]
- Fagerness, J.A.; Maller, J.B.; Neale, B.M.; Reynolds, R.C.; Daly, M.J.; Seddon, J.M. Variation near complement factor I is associated with risk of advanced AMD. Eur. J. Hum. Genet. 2008, 17, 100–104. [Google Scholar] [CrossRef]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef]
- Skerka, C.; Chen, Q.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement factor H related proteins (CFHRs). Mol. Immunol. 2013, 56, 170–180. [Google Scholar] [CrossRef]
- Mattapallil, M.J.; Caspi, R.R. Compliments of Factor H: What’s in it for AMD? Immunity 2017, 46, 167–169. [Google Scholar] [CrossRef][Green Version]
- Nishiguchi, K.M.; Yasuma, T.R.; Tomida, D.; Nakamura, M.; Ishikawa, K.; Kikuchi, M.; Ohmi, Y.; Niwa, T.; Hamajima, N.; Furukawa, K.; et al. C9-R95X Polymorphism in Patients with Neovascular Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2012, 53, 508–512. [Google Scholar] [CrossRef]
- Natoli, R.; Fernando, N.; Jiao, H.; Racic, T.; Madigan, M.; Barnett, N.L.; Chu-Tan, J.A.; Valter, K.; Provis, J.M.; Rutar, M. Retinal Macrophages Synthesize C3 and Activate Complement in AMD and in Models of Focal Retinal Degeneration. Investig. Opthalmol. Vis. Sci. 2017, 58, 2977–2990. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, F.; Heid, I.M.; Weber, B.H.F.; International AMD Genomics Consortium (IAMDGC). Recombinant Haplotypes Narrow the ARMS2/HTRA1 Association Signal for Age-Related Macular Degeneration. Genetics 2017, 205, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, S.; Orlandi, P.; Figus, M.; Fioravanti, A.; Cascio, E.; Di Desidero, T.; Agosta, E.; Canu, B.; Sartini, M.S.; Danesi, R.; et al. The rs2071559 AAVEGFR-2Genotype Frequency Is Significantly Lower in Neovascular Age-Related Macular Degeneration Patients. Sci. World J. 2012, 2012, 420190. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A. Association between Vascular Endothelial Growth Factor Polymorphisms and Age-Related Macular Degeneration: An Updated Meta-Analysis. Dis. Markers 2016, 2016, 8486406. [Google Scholar] [CrossRef]
- Mammadzada, P.; Corredoira, P.M.; André, H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: A gene therapy perspective. Cell. Mol. Life Sci. 2020, 77, 819–833. [Google Scholar] [CrossRef]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-Related Macular Degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Blodi, B.A.; Shapiro, H.; Acharya, N.R. Angiographic and Optical Coherence Tomographic Results of the MARINA Study of Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology 2007, 114, 1868–1875. [Google Scholar] [CrossRef]
- Gragoudas, E.; Adamis, A.P.; Cunningham, E.T.; Feinsod, M.; Guyer, D.R. Pegaptanib for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef]
- Brown, D.M.; Michels, M.; Kaiser, P.K.; Heier, J.S.; Sy, J.P.; Ianchulev, T. Ranibizumab versus Verteporfin Photodynamic Therapy for Neovascular Age-Related Macular Degeneration: Two-Year Results of the ANCHOR Study. Ophthalmology 2009, 116, 57–65. [Google Scholar] [CrossRef]
- Regillo, C.D.; Brown, D.M.; Abraham, P.; Yue, H.; Ianchulev, T.; Schneider, S.; Shams, N. Randomized, Double-Masked, Sham-Controlled Trial of Ranibizumab for Neovascular Age-related Macular Degeneration: PIER Study Year 1. Am. J. Ophthalmol. 2008, 145, 239–248. [Google Scholar] [CrossRef]
- Abraham, P.; Yue, H.; Wilson, L. Randomized, Double-Masked, Sham-Controlled Trial of Ranibizumab for Neovascular Age-Related Macular Degeneration: PIER Study Year 2. Am. J. Ophthalmol. 2010, 150, 315–324. [Google Scholar] [CrossRef]
- Tufail, A.; Patel, P.J.; Egan, C.; Hykin, P.; Da Cruz, L.; Gregor, Z.; Dowler, J.; Majid, M.A.; Bailey, C.; Mohamed, Q.; et al. Bevacizumab for neovascular age related macular degeneration (ABC Trial): Multicentre randomised double masked study. BMJ 2010, 340, c2459. [Google Scholar] [CrossRef]
- Patel, P.J.; Chen, F.K.; Da Cruz, L.; Rubin, G.S.; Tufail, A. Contrast Sensitivity Outcomes in the ABC Trial: A Randomized Trial of Bevacizumab for Neovascular Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 3089–3093. [Google Scholar] [CrossRef][Green Version]
- Prager, F.; Michels, S.; Sacu, S.; Weigert, G.; Dunavölgyi, R.; Geitzenauer, W.; Schmidt-Erfurth, U. Intravitreal Bevacizumab (Avastin®) Monotherapy versus Photodynamic Therapy Plus Intravitreal Triamcinolone for Neovascular Age-Related Macular Degeneration: 12 Months Results of a Prospective, Randomized, Controlled Clinical Trial. Investigative Ophthalmology and Visual Science. Volume 49. ARVO E-Abstract. 2008. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2376251 (accessed on 23 October 2020).
- Weigert, G.; Michels, S.; Sacu, S.; Varga, A.; Prager, F.; Geitzenauer, W.; Schmidt-Erfurth, U. Intravitreal bevacizumab (Avastin) therapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age-related macular degeneration: 6-month results of a prospective, randomised, controlled clinical study. Br. J. Ophthalmol. 2008, 92, 356–360. [Google Scholar] [CrossRef][Green Version]
- Martin, D.F.; Maguire, M.G.; Ying, G.-S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.-S.; Jaffe, G.J.; Grunwald, J.E.; A Toth, C.; Redford, M.; Ferris, F.L. Ranibizumab and Bevacizumab for Treatment of Neovascular Age-related Macular Degeneration. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Harding, S.P.; Rogers, C.; Downes, S.M.; Lotery, A.J.; Wordsworth, S.; Reeves, B.C. Ranibizumab versus Bevacizumab to Treat Neovascular Age-related Macular Degeneration. Ophthalmoogy 2012, 119, 1399–1411. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Harding, S.P.; Rogers, C.; Downes, S.M.; Lotery, A.J.; Culliford, L.; Reeves, B.C. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013, 382, 1258–1267. [Google Scholar] [CrossRef]
- Biswas, P.; Sengupta, S.; Choudhary, R.; Home, S.; Paul, A.; Sinha, S. Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration. Indian J. Ophthalmol. 2011, 59, 191–196. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.M.; Dijkman, G.; Hooymans, J.M.; Verbraak, F.D.; Hoyng, C.B.; Dijkgraaf, M.G.; Peto, T.; Vingerling, J.R.; Schlingemann, R.O. Comparing the Effectiveness of Bevacizumab to Ranibizumab in Patients with Exudative Age-Related Macular Degeneration. The BRAMD Study. PLoS ONE 2016, 11, e0153052. [Google Scholar] [CrossRef]
- Kodjikian, L.; Souied, E.H.; Mimoun, G.; Mauget-FaŸsse, M.; Behar-Cohen, F.; Decullier, E.; Huot, L.; Aulagner, G. Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology 2013, 120, 2300–2309. [Google Scholar] [CrossRef]
- Berg, K.; Pedersen, T.R.; Sandvik, L.; Bragadóttir, R. Comparison of Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration According to LUCAS Treat-and-Extend Protocol. Ophthalmology 2015, 122, 146–152. [Google Scholar] [CrossRef]
- Berg, K.; Hadzalic, E.; Gjertsen, I.; Forsaa, V.; Berger, L.H.; Kinge, B.; Henschien, H.; Fossen, K.; Markovic, S.; Pedersen, T.R.; et al. Ranibizumab or Bevacizumab for Neovascular Age-Related Macular Degeneration According to the Lucentis Compared to Avastin Study Treat-and-Extend Protocol. Ophthalmology 2016, 123, 51–59. [Google Scholar] [CrossRef]
- Krebs, I.; Schmetterer, L.; Boltz, A.; Told, R.; Vécsei-Marlovits, V.; Egger, S.; Schönherr, U.; Haas, A.; Ansari-Shahrezaei, S.; Binder, S.; et al. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br. J. Ophthalmol. 2013, 97, 266–271. [Google Scholar] [CrossRef]
- Enseleit, F.; Michels, S.; Sudano, I.; Stahel, M.; Zweifel, S.; Schlager, O.; Becker, M.; Winnik, S.; Nägele, M.; Flammer, A.J.; et al. SAVE-AMD: Safety of VEGF Inhibitors in Age-Related Macular Degeneration. Ophthalmology 2017, 238, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Scholler, A.; Richter-Mueksch, S.; Weingessel, B.; Vécsei-Marlovits, P.-V. Differences of frequency in administration of ranibizumab and bevacizumab in patients with neovascular AMD. Wien. Klin. Wochenschr. 2014, 126, 355–359. [Google Scholar] [CrossRef]
- Subramanian, M.L.; Ness, S.; Abedi, G.; Ahmed, E.; Daly, M.K.; Feinberg, E.; Bhatia, S.; Patel, P.; Nguyen, M.; Houranieh, A. Bevacizumab vs. Ranibizumab for Age-Related Macular Degeneration: Early Results of a Prospective Double-Masked, Randomized Clinical Trial. Am. J. Ophthalmol. 2009, 148, 875–882. [Google Scholar] [CrossRef]
- Subramanian, M.L.; Abedi, G.; Ness, S.; Ahmed, E.; Fenberg, M.; Daly, M.K.; Houranieh, A.; Feinberg, E.B. Bevacizumab vs ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked randomised clinical trial. Eye 2010, 24, 1708–1715. [Google Scholar] [CrossRef]
- Busbee, B.G.; Ho, A.C.; Brown, D.M.; Heier, J.S.; Suñer, I.J.; Li, Z.; Rubio, R.G.; Lai, P. Twelve-Month Efficacy and Safety of 0.5 mg or 2.0 mg Ranibizumab in Patients with Subfoveal Neovascular Age-related Macular Degeneration. Ophthalmology 2013, 120, 1046–1056. [Google Scholar] [CrossRef]
- Ho, A.C.; Busbee, B.G.; Regillo, C.D.; Wieland, M.R.; Van Everen, S.A.; Li, Z.; Rubio, R.G.; Lai, P. Twenty-four-Month Efficacy and Safety of 0.5 mg or 2.0 mg Ranibizumab in Patients with Subfoveal Neovascular Age-Related Macular Degeneration. Ophthalmology 2014, 121, 2181–2192. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Croft, D.E.; Brown, D.M.; Wang, R.; Payne, J.F.; Clark, L.; Abdelfattah, N.S.; Sadda, S.R. Prospective Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration. Ophthalmology 2015, 122, 2514–2522. [Google Scholar] [CrossRef]
- Silva, R.; Berta, A.; Larsen, M.; MacFadden, W.; Feller, C.; Monés, J. Treat-and-Extend versus Monthly Regimen in Neovascular Age-Related Macular Degeneration. Ophthalmology 2018, 125, 57–65. [Google Scholar] [CrossRef]
- Kertes, P.J.; Galic, I.J.; Greve, M.; Williams, R.G.; Rampakakis, E.; Scarino, A.; Sheidow, T. Canadian Treat-and-Extend Analysis Trial with Ranibizumab in Patients with Neovascular Age-Related Macular Disease. Ophthalmology 2019, 126, 841–848. [Google Scholar] [CrossRef]
- Kertes, P.J.; Galic, I.J.; Greve, M.; Williams, G.; Baker, J.; Lahaie, M.; Sheidow, T. Efficacy of a Treat-and-Extend Regimen with Ranibizumab in Patients with Neovascular Age-Related Macular Disease. JAMA Ophthalmol. 2020, 138, 244. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal Aflibercept (VEGF Trap-Eye) in Wet Age-related Macular Degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Ohji, M.; Takahashi, K.; Okada, A.A.; Kobayashi, M.; Matsuda, Y.; Terano, Y. Efficacy and Safety of Intravitreal Aflibercept Treat-and-Extend Regimens in Exudative Age-Related Macular Degeneration: 52- and 96-Week Findings from ALTAIR. Adv. Ther. 2020, 37, 1173–1187. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Macugen®. Pegaptanib Sodium Injection Datasheet. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/macugen-epar-scientific-discussion_en.pdf (accessed on 23 October 2020).
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2019, 3, CD005139. [Google Scholar] [CrossRef]
- Iqbal, S.; Lenz, H.-J. Integration of novel agents in the treatment of colorectal cancer. Cancer Chemother. Pharmacol. 2004, 54, S32–S39. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.F.; Benson, A.B., 3rd. Bevacizumab in the treatment of colorectal cancer. Expert Opin. Biol. Ther. 2005, 5, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Moshfeghi, A.A.; Puliafito, C.A. Optical coherence tomographyfindings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg. Lasers Imaging 2005, 36, 331–335. [Google Scholar] [CrossRef]
- Avery, R.L.; Pieramici, D.J.; Rabena, M.D.; Castellarin, A.A.; Nasir, M.A.; Giust, M.J. Intravitreal Bevacizumab (Avastin) for Neovascular Age-Related Macular Degeneration. Ophthalmology 2006, 113, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.; Thomas, S.M.; Lillie, E.; Lee, T.; Hamid, J.; Richter, T.; Janoudi, G.; Agarwal, A.; Sharpe, J.P.; Scott, A.; et al. Anti-vascular endothelial growth factor treatment for retinal conditions: A systematic review and meta-analysis. BMJ Open 2019, 9, e022031. [Google Scholar] [CrossRef]
- Ferrara, N.; Damico, L.; Shams, N.; Lowman, H.; Kim, R. Development of ranibizumab, an anti–vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006, 26, 859–870. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.M.; Eldem, B.; Guymer, R.; Korobelnik, J.-F.; Schlingemann, R.O.; Axer-Siegel, R.; Wiedemann, P.; Simader, C.; Gekkieva, M.; Weichselberger, A. Efficacy and Safety of Monthly versus Quarterly Ranibizumab Treatment in Neovascular Age-related Macular Degeneration: The EXCITE Study. Ophthalmology 2011, 118, 831–839. [Google Scholar] [CrossRef]
- Mantel, I.; Deli, A.; Iglesias, K.; Ambresin, A. Prospective study evaluating the predictability of need for retreatment with intravitreal ranibizumab for age-related macular degeneration. Graefe Arch. Clin. Exp. Ophthalmol. 2012, 251, 697–704. [Google Scholar] [CrossRef]
- Mantel, I.; Niderprim, S.-A.; Gianniou, C.; Deli, A.; Ambresin, A. Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br. J. Ophthalmol. 2014, 98, 1192–1196. [Google Scholar] [CrossRef]
- Gianniou, C.; Dirani, A.; Ferrini, W.; Marchionno, L.; Decugis, D.; Deli, A.; Ambresin, A.; Mantel, I. Two-year outcome of an observe-and-plan regimen for neovascular age-related macular degeneration: How to alleviate the clinical burden with maintained functional results. Eye 2014, 29, 342–349. [Google Scholar] [CrossRef]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Le, K.; et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 2014, 98, 1636–1641. [Google Scholar] [CrossRef]
- Economides, A.N.; Carpenter, L.R.; Rudge, J.S.; Wong, V.; Koehler-Stec, E.M.; Hartnett, C.; Pyles, E.A.; Xu, X.; Daly, T.J.; Young, M.R.; et al. Cytokine traps: Multi-component, high-affinity blockers of cytokine action. Nat. Med. 2002, 9, 47–52. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Kaiser, P.K.; Korobelnik, J.-F.; Brown, D.M.; Chong, V.; Nguyen, Q.D.; Ho, A.C.; Ogura, Y.; Simader, C.; Jaffe, G.J.; et al. Intravitreal Aflibercept Injection for Neovascular Age-related Macular Degeneration. Ophthalmology 2014, 121, 193–201. [Google Scholar] [CrossRef]
- Gillies, M.C.; Hunyor, A.P.; Arnold, J.J.; Guymer, R.H.; Wolf, S.; Ng, P.; Pecheur, F.L.; McAllister, I.L. Effect of Ranibizumab and Aflibercept on Best-Corrected Visual Acuity in Treat-and-Extend for Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 372–379. [Google Scholar] [CrossRef]
- Markham, A. Brolucizumab: First Approval. Drugs 2019, 79, 1997–2000. [Google Scholar] [CrossRef]
- Dugel, P.U.; Jaffe, G.J.; Sallstig, P.; Warburton, J.; Weichselberger, A.; Wieland, M.; Singerman, L. Brolucizumab versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial. Ophthalmology 2017, 124, 1296–1304. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef]
- Sanson-Fisher, R.W.; Bonevski, B.; Green, L.W.; D’Este, C. Limitations of the Randomized Controlled Trial in Evaluating Population-Based Health Interventions. Am. J. Prev. Med. 2007, 33, 155–161. [Google Scholar] [CrossRef]
- Mehta, H.; Tufail, A.; Daien, V.; Lee, A.Y.; Nguyen, V.; Ozturk, M.; Barthelmes, D.; Gillies, M.C. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog. Retin. Eye Res. 2018, 65, 127–146. [Google Scholar] [CrossRef]
- Rao, P.; Lum, F.; Wood, K.; Salman, C.; Burugapalli, B.; Hall, R.; Singh, S.; Parke, D.W.; Williams, G.A. Real-World Vision in Age-Related Macular Degeneration Patients Treated with Single Anti–VEGF Drug Type for 1 Year in the IRIS Registry. Ophthalmology 2018, 125, 522–528. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K. Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON. Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Bhisitkul, R.B.; Desai, S.J.; Boyer, D.S.; Sadda, S.R.; Zhang, K. Fellow Eye Comparisons for 7-Year Outcomes in Ranibizumab-Treated AMD Subjects from ANCHOR, MARINA, and HORIZON (SEVEN-UP Study). Ophthalmology 2016, 123, 1269–1277. [Google Scholar] [CrossRef]
- Holz, F.G.; Tadayoni, R.; Beatty, S.; Berger, A.; Cereda, M.G.; Cortez, R.; Hoyng, C.B.; Hykin, P.; Staurenghi, G.; Heldner, S.; et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br. J. Ophthalmol. 2014, 99, 220–226. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Lasave, A.F.; Wu, L.; Acón, D.; Berrocal, M.H.; Diaz-Llopis, M.; Gallego-Pinazo, R.; Serrano, M.; Alezzandrini, A.A.; Rojas, S.; et al. Intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration: 5-Year Results of the Pan-American Collaborative Retina Study Group. Retina 2016, 36, 859–867. [Google Scholar] [CrossRef]
- Ozkaya, A.; Alkin, Z.; Togac, M.; Ahmet, S.; Perente, I.; Taskapili, M. Five-year Outcomes of Ranibizumab in Neovascular Age-related Macular Degeneration: Real Life Clinical Experience. Korean J. Ophthalmol. 2017, 31, 424–430. [Google Scholar] [CrossRef]
- Holz, F.G.; Figueroa, M.S.; Bandello, F.; Yang, Y.; Ohji, M.; Dai, H.; Wykrota, H.; Sharma, S.; Dunger-Baldauf, C.; Lacey, S.; et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: Results From luminous, a Global Real-World Study. Retina 2019, 40, 1673–1685. [Google Scholar] [CrossRef]
- Kim, L.N.; Mehta, H.; Barthelmes, D.; Nguyen, V.; Gillies, M.C. Metaanalysis of real-world outcomes of intravitreal Ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 2016, 36, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
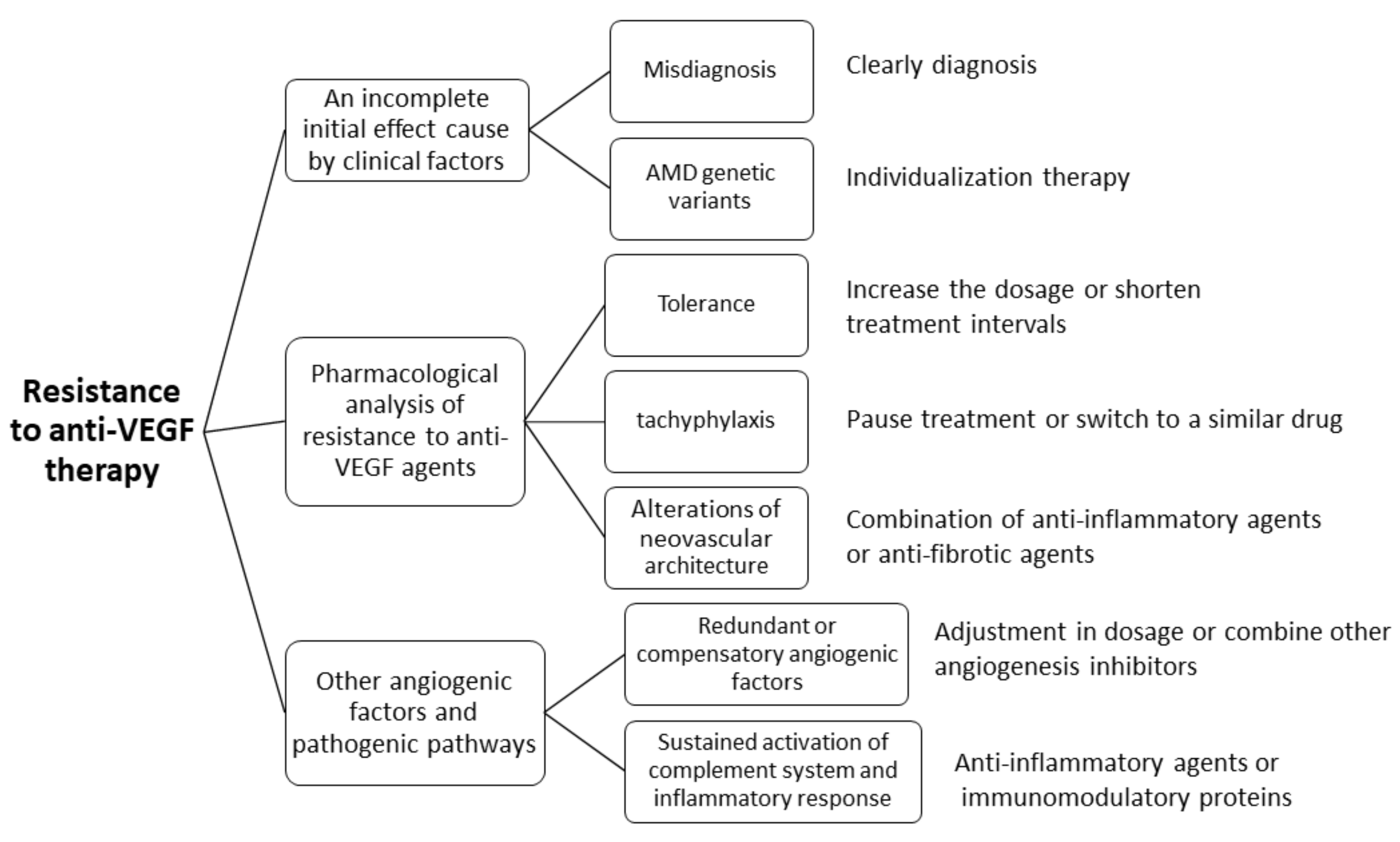
- Broadhead, G.K.; Hong, T.; Chang, A. Treating the untreatable patient: Current options for the management of treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2014, 92, 713–723. [Google Scholar] [CrossRef]
- Sun, X.; Yang, S.; Zhao, J. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Dev. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef]
- Otsuji, T.; Nagai, Y.; Sho, K.; Tsumura, A.; Koike, N.; Tsuda, M.; Nishimura, T.; Takahashi, K. Initial non-responders to ranibizumab in the treatment of age-related macular degeneration (AMD). Clin. Ophthalmol. 2013, 7, 1487–1490. [Google Scholar] [CrossRef]
- Zuber-Laskawiec, K.; Kubicka-Trzaska, A.; Karska-Basta, I.; Pociej-Marciak, W.; Romanowska-Dixon, B. Non-responsiveness and tachyphylaxis to anti-vascularendothelial growth factor treatment in naive patients with exudative age-relatedmacular degeneration. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Ferris, F.L.; Maguire, M.G.; Glassman, A.R.; Ying, G.-S.; Martin, D.F. Evaluating Effects of Switching Anti–Vascular Endothelial Growth Factor Drugs for Age-Related Macular Degeneration and Diabetic Macular Edema. JAMA Ophthalmol. 2017, 135, 145–149. [Google Scholar] [CrossRef]
- Zarbin, M.; Tsuboi, M.; Hill, L.; Stoilov, I. Simulating an Anti–Vascular Endothelial Growth Factor Switch in Neovascular Age-Related Macular Degeneration. Ophthalmology 2019, 126, 849–855. [Google Scholar] [CrossRef]
- Spooner, K.; Hong, T.; Wijeyakumar, W.; A Chang, A. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: A systematic review with meta-analysis. Clin. Ophthalmol. 2017, 11, 161–177. [Google Scholar] [CrossRef] [PubMed]
- L’uso dei Farmaci in Italia-Rapporto os Med. Available online: https://www.aifa.gov.it/web/guest/-/rapporto-osmed-20-1 (accessed on 23 October 2020).
- Ciulla, T.A.; Hussain, R.M.; Pollack, J.S.; Williams, D.F. Visual Acuity Outcomes and Anti–Vascular Endothelial Growth Factor Therapy Intensity in Neovascular Age-Related Macular Degeneration Patients. Ophthalmol. Retin. 2020, 4, 19–30. [Google Scholar] [CrossRef]
- Carrasco, J.; Pietsch, G.-A.; Nicolas, M.-P.; Koerber, C.; Bennison, C.; Yoon, J. Real-World Effectiveness and Real-World Cost-Effectiveness of Intravitreal Aflibercept and Intravitreal Ranibizumab in Neovascular Age-Related Macular Degeneration: Systematic Review and Meta-Analysis of Real-World Studies. Adv. Ther. 2020, 37, 300–315. [Google Scholar] [CrossRef]
- Van Asten, F.; Michels, C.T.J.; Hoyng, C.B.; Van Der Wilt, G.J.; Klevering, B.J.; Rovers, M.M.; Grutters, J.P.C. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration—A cost-effectiveness analysis from a societal perspective. PLoS ONE 2018, 13, e0197670. [Google Scholar] [CrossRef]
- Ophthotech. Ophthotech Announces Results from Third Phase 3 Trial of Fovista_ in Wet Age-Related Macular Degeneration. Press Release. Available online: https://investors.ivericbio.com/news-releases/news-release-details/ophthotech-announces-results-third-phase-3-trial-fovistar-wet (accessed on 28 September 2020).
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration. JAMA Ophthalmol. 2018, 136, 666–677. [Google Scholar] [CrossRef]
- Ohr Pharmaceuticals. Ohr Pharmaceutical Announces Efficacy Results from the MAKO Study in Wet-AMD. Press Release. Available online: https://www.globenewswire.com/news-release/2018/01/05/1284092/0/en/Ohr-Pharmaceutical-Announces-Efficacy-Results-from-the-MAKO-Study-in-Wet-AMD.html (accessed on 28 September 2020).
- Papadopoulos, K.P.; Kelley, R.K.; Tolcher, A.W.; Razak, A.R.A.; Van Loon, K.; Patnaik, A.; Bedard, P.L.; Alfaro, A.A.; Beeram, M.; Adriaens, L.; et al. A Phase I First-in-Human Study of Nesvacumab (REGN910), a Fully Human Anti–Angiopoietin-2 (Ang2) Monoclonal Antibody, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 22, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Regeneron Pharmaceuticals. Regeneron Provides Update on EYLEA® (aflibercept) Injection and Nesvacumab (Ang2 Antibody) Combination Program. Press Release. Available online: https://investor.regeneron.com/news-releases/news-release-details/regeneron-provides-update-eylear-aflibercept-injection-and/ (accessed on 28 September 2020).
- Regula, J.T.; Von Leithner, P.L.; Foxton, R.; Barathi, V.A.; Cheung, C.M.G.; Tun, S.B.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific Cross MA b optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. [Google Scholar] [CrossRef]
- Sahni, J.; Patel, S.S.; Dugel, P.U.; Khanani, A.M.; Jhaveri, C.D.; Wykoff, C.C.; Hershberger, V.S.; Pauly-Evers, M.; Sadikhov, S.; Szczesny, P.; et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema. Ophthalmology 2019, 126, 1155–1170. [Google Scholar] [CrossRef]
- Patel, S.S.; Sahni, J.; Sadikhov, S.; Pauly-Evers, M.; Szczesny, P.; Weikert, R. Anti-VEGF/anti–angiopoietin-2 bispecific antibody faricimab (RG7716) in neovascular AMD. In Proceedings of the Retina Society 43rd Annual Scientific Meeting, San Francisco, CA, USA, 12–15 September 2018. [Google Scholar]
- Khanani, A.M. Simultaneous inhibition of ang-2 and VEGF with faricimab in neovascular AMD: STAIRWAY phase 2 results. In Proceedings of the American Academy of Ophthalmology Retina Subspecialty Day, Chicago, IL, USA, 26–27 October 2018. [Google Scholar]
- Opthea. Opthea Meets Primary Endpoint in Phase 2b Study of OPT-302 in Wet AMD. Press Release. Available online: https://www.opthea.com/wp-content/uploads/2019/08/Opthea-Limited-Opthea-Results-of-Wet-AMD-Clinical-Trial-10001623-070819_V2.pdf (accessed on 28 September 2020).
- Dugel, P.U.; Boyer, D.S.; Antoszyk, A.N.; Steinle, N.C.; Varenhorst, M.P.; Pearlman, J.A.; Gillies, M.C.; Finger, R.P.; Baldwin, M.E.; Leitch, I.M. Phase 1 Study of OPT-302 Inhibition of Vascular Endothelial Growth Factors C and D for Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2020, 4, 250–263. [Google Scholar] [CrossRef]
- Forrer, P.; Stumpp, M.T.; Binz, H.K.; Plückthun, A. A novel strategy to design binding molecules harnessing the modular nature of repeat proteins. FEBS Lett. 2003, 539, 2–6. [Google Scholar] [CrossRef]
- Li, J.; Mahajan, A.A.; Tsai, M.-D. Ankyrin Repeat: A Unique Motif Mediating Protein−Protein Interactions. Biochemistry 2006, 45, 15168–15178. [Google Scholar] [CrossRef] [PubMed]
- Binz, H.; Stumpp, M.T.; Forrer, P.; Amstutz, P.; Plückthun, A. Designing Repeat Proteins: Well-expressed, Soluble and Stable Proteins from Combinatorial Libraries of Consensus Ankyrin Repeat Proteins. J. Mol. Biol. 2003, 332, 489–503. [Google Scholar] [CrossRef]
- Stumpp, M.T.; Binz, H.K.; Amstutz, P. DARPins: A new generation of protein therapeutics. Drug Discov. Today 2008, 13, 695–701. [Google Scholar] [CrossRef]
- Hammill, J.A.; VanSeggelen, H.; Helsen, C.W.; Denisova, G.F.; Evelegh, C.; Tantalo, D.G.M.; Bassett, J.D.; Bramson, J.L. Designed ankyrin repeat proteins are effective targeting elements for chimeric antigen receptors. J. Immunother. Cancer 2015, 3, 55. [Google Scholar] [CrossRef]
- Plückthun, A. Designed Ankyrin Repeat Proteins (DARPins): Binding Proteins for Research, Diagnostics, and Therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef]
- Theurillat, J.-P.; Dreier, B.; Nagy-Davidescu, G.; Seifert, B.; Behnke, S.; Zürrer-Härdi, U.; Ingold, F.; Plückthun, A.; Moch, H. Designed ankyrin repeat proteins: A novel tool for testing epidermal growth factor receptor 2 expression in breast cancer. Mod. Pathol. 2010, 23, 1289–1297. [Google Scholar] [CrossRef]
- Martin-Killias, P.; Stefan, N.; Rothschild, S.; Zangemeister-Wittke, U.; Plückthun, A. A Novel Fusion Toxin Derived from an EpCAM-Specific Designed Ankyrin Repeat Protein Has Potent Antitumor Activity. Clin. Cancer Res. 2010, 17, 100–110. [Google Scholar] [CrossRef]
- Tamaskovic, R.; Simon, M.; Stefan, N.; Schwill, M.; Plückthun, A. Designed Ankyrin Repeat Proteins (DARPins). Methods Enzymol. 2012, 503, 101–134. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Stefan, N.; Borsig, L.; Plückthun, A.; Zangemeister-Wittke, U. Increasing the Antitumor Effect of an EpCAM-Targeting Fusion Toxin by Facile Click PEGylation. Mol. Cancer Ther. 2013, 13, 375–385. [Google Scholar] [CrossRef]
- Binz, H.K.; Bakker, T.R.; Phillips, D.J.; Cornelius, A.; Zitt, C.; Göttler, T.; Sigrist, G.; Fiedler, U.; Ekawardhani, S.; Dolado, I.; et al. Design and characterization of MP0250, a tri-specific anti-HGF/anti-VEGF DARPin® drug candidate. MAbs 2017, 9, 1262–1269. [Google Scholar] [CrossRef]
- Caputi, A.P.; Navarra, P. Beyond antibodies: Ankyrins and DARPins. From basic research to drug approval. Curr. Opin. Pharmacol. 2020, 51, 93–101. [Google Scholar] [CrossRef]
- Syed, B.A.; Evans, J.B.; Bielory, L. Wet AMD market. Nat. Rev. Drug Discov. 2012, 11, 827. [Google Scholar] [CrossRef]
- Stahl, A.; Stumpp, M.T.; Schlegel, A.; Ekawardhani, S.; Lehrling, C.; Martin, G.; Gulotti-Georgieva, M.; Villemagne, D.; Forrer, P.; Agostini, H.T.; et al. Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis 2013, 16, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.A.; Mason, M.; Christie, L.-A.; Hansen, C.; Hernandez, L.M.; Burke, J.; Luhrs, K.A.; Hohman, T.C. Functional Characterization of Abicipar-Pegol, an Anti-VEGF DARPin Therapeutic That Potently Inhibits Angiogenesis and Vascular Permeability. Investig. Opthalmol. Vis. Sci. 2018, 59, 5836–5846. [Google Scholar] [CrossRef]
- Souied, E.; Devin, F.; Mauget-Faÿsse, M.; Kolář, P.; Wolf-Schnurrbusch, U.; Framme, C.; Gaucher, D.; Querques, G.; Stumpp, M.T.; Wolf, S. Treatment of Exudative Age-Related Macular Degeneration with a Designed Ankyrin Repeat Protein that Binds Vascular Endothelial Growth Factor: A Phase I/II Study. Am. J. Ophthalmol. 2014, 158, 724–732. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Channa, R.; Berger, B.B.; Heier, J.S.; Brown, D.M.; Fiedler, U.; Hepp, J.; Stumpp, M.T. Treatment of Diabetic Macular Edema With a Designed Ankyrin Repeat Protein That Binds Vascular Endothelial Growth Factor: A Phase I/II Study. Am. J. Ophthalmol. 2013, 155, 697–704. [Google Scholar] [CrossRef]
- Callanan, D.G.; Kunimoto, D.; Maturi, R.K.; Patel, S.S.; Staurenghi, G.; Wolf, S.; Cheetham, J.K.; Hohman, T.C.; Kim, K.; López, F.J.; et al. Double-Masked, Randomized, Phase 2 Evaluation of Abicipar Pegol (an Anti-VEGF DARPin Therapeutic) in Neovascular Age-Related Macular Degeneration. J. Ocul. Pharmacol. Ther. 2018, 34, 700–709. [Google Scholar] [CrossRef]
- Kunimoto, D.; Ohji, M.; Maturi, R.K.; Sekiryu, T.; Wang, Y.; Pan, G.; Li, X.-Y.; Schneider, S. For the BAMBOO and CYPRESS Study Groups Evaluation of Abicipar Pegol (an Anti-VEGF DARPin Therapeutic) in Patients With Neovascular Age-Related Macular Degeneration: Studies in Japan and the United States. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, e10–e22. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, D.; Yoon, Y.H.; Wykoff, C.C.; Chang, A.; Khurana, R.N.; Maturi, R.K.; Agostini, H.; Souied, E.; Chow, D.R.; Lotery, A.J.; et al. Efficacy and Safety of Abicipar in Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1331–1344. [Google Scholar] [CrossRef]
- Khurana, R.N. Abicipar for Neovascular Age-Related Macular Degeneration: Two-Year Results from CEDAR and SEQUOIA Phase 3. In Proceedings of the American Academy of Ophthalmology Annual Meeting, San Francisco, CA, USA, 11–15 October 2019; Available online: https://www.healio.com/ophthalmology/retina-vitreous/news/online/%7B41a2229d-ec29-4d17-8c30-8084a36601fa%7D/phase-3-data-presented-for-cedar-sequoia-studies (accessed on 23 October 2020).
- Molecular Partners. Allergan and Molecular Partners Announce Topline Safety Results from MAPLE Study of Abicipar Pegol. Press Release. Available online: https://www.molecularpartners.com/allergan-and-molecular-partners-announce-topline-safety-results-from-maple-study-of-abicipar-pegol/ (accessed on 23 October 2020).



| Study Treatment Period | Study Groups | |||
|---|---|---|---|---|
| Pegaptanib vs. Control | ||||
| Gragoudas et al. [92] 2 years; re−randomized at end of first year | Group I | Group II | Group III | Group IV |
| 0.3 mg pegaptanib every 6 weeks | 1.0 mg pegaptanib every 6 weeks | 3.0 mg pegaptanib every 6 weeks | Sham every 6 weeks | |
| Ranibizumab vs. Control | ||||
| ANCHOR [90,93] 2 years | Group I | Group II | Group III | |
| 0.3 mg ranibizumab monthly plus sham verteporfin PDT | 0.5 mg ranibizumab monthly plus sham verteporfin PDT | Sham intravitreal injection plus verteporfin PDT | ||
| MARINA [89,91] 2 years | Group I | Group II | Group III | |
| 0.3 mg ranibizumab monthly | 0.5 mg ranibizumab monthly | Sham intravitreal injection monthly | - | |
| PIER [94,95] 2 years | Group I | Group II | Group III | |
| 0.3 mg ranibizumab monthly for 3 months, then every 3 months | 0.5 mg ranibizumab monthly for 3 months, then every 3 months | Sham intravitreal injection monthly for 3 months, then every 3 months | - | |
| Bevacizumab vs. Control | ||||
| ABC [96,97] 1 year | Group I | Group II | ||
| 1.25 mg bevacizumab given first 3 injections every 6 weeks, then as needed | Standard therapy (0.3 mg pegaptanib every 6 weeks, verteporfin PDT, or sham injection) | - | - | |
| Sacu [98,99] 1 year | Group I | Group II | ||
| 1.0 mg bevacizumab monthly for 3 months, then as needed | Verteporfin PDT plus same day 4 mg triamcinolone acetonide | - | - | |
| Bevacizumab vs. Ranibizumab | ||||
| CATT [28,100,101] 2 years; re-randomized at end of first year | Group I | Group II | Group III | Group IV |
| 1.25 mg bevacizumab monthly for 1 year; at 1 year, re-randomization to ranibizumab monthly or variable dosing | 0.5 mg ranibizumab monthly for 1 year; at 1 year, re-randomization to ranibizumab monthly or variable dosing | 1.25 mg bevacizumab as needed after first injection for 2 years | 0.5 mg ranibizumab as needed after first injection for 2 years | |
| IVAN [102,103] 2 years; ongoing | Group I | Group II | Group III | Group IV |
| 1.25 mg bevacizumab monthly for 2 years | 0.5 mg ranibizumab monthly for 2 years | 1.25 mg bevacizumab monthly for 3 months, then as needed in 3-month cycles | 0.5 mg ranibizumab monthly for 3 months, then as needed in 3-month cycles | |
| Biswas et al. [104] 18 months | Group I | Group II | ||
| 1.25 mg bevacizumab monthly for 3 months, then as needed | 0.5 mg ranibizumab monthly for 3 months, then as needed | - | - | |
| BRAMD [105] 1 year | Group I | Group II | ||
| 1.25 mg bevacizumab monthly for 1 year | 0.5 mg ranibizumab monthly for 1 year | - | - | |
| GEFAL [106] 1 year | Group I | Group II | ||
| 1.25 mg bevacizumab; maximum of 1 injection per month | 0.5 mg ranibizumab; maximum of 1 injection per month | - | - | |
| LUCAS [107,108] 1 year | Group I | Group II | ||
| 1.25 mg bevacizumab; treat and extend protocol | 0.5 mg ranibizumab; treat and extend protocol | - | - | |
| Group I | Group II | |||
| MANTA [109] 1 year | 1.25 mg bevacizumab monthly for 3 months, then as needed | 0.5 mg ranibizumab monthly for 3 months, then as needed | - | - |
| SAVE-AMD [110] 1 year | Group I | Group II | ||
| 1.25 mg bevacizumab at day 1 and at week 4, then as needed | 0.5 mg ranibizumab at day 1 and at week 4, then as needed | - | - | |
| Scholler et al. [111] 1 year | Group I | Group II | ||
| 1.25 mg bevacizumab for 3 months, at 30-day intervals, then as needed | 0.5 mg ranibizumab for 3 months, at 30-day intervals, then as needed | - | - | |
| Subramanian et al. [112,113] 1 year | Group I | Group II | ||
| 0.05 mL bevacizumab monthly for 3 months, then as needed | 0.05 mL ranibizumab monthly for 3 months, then as needed | - | - | |
| Study | Ref | Duration (w) | Regimen | N | BCVA (ETDRS Letters) | CMT (µm) | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | |||||
| ABC | [96] | 54 | Beva 1.25 mg | 65 | 50 (43–61) * | +7.0 | 328 (271–376) * | −93.5 (−144.5–−26) |
| Control | 66 | 53 (47–60) * | –9.4 | 330 (256–359) * | −55 (−150–7) | |||
| CATT | [100] | 52 | Beva 1.25 mg (monthly) | 286 | 60.2 (13.1) | 8.0 (1.0) ** | 463 (196) | −164 (181) |
| Beva 1.25 (PRN) | 300 | 60.4 (13.4) | 5.9 (1.0) ** | 461 (175) | −152 (178) | |||
| Rani 0.5 mg (monthly) | 301 | 60.1 (14.3) | 8.5 (0.8) ** | 458 (184) | −196 (176) | |||
| Rani 0.5 mg (PRN) | 298 | 61.5 (13.2) | 6.8 (0.8) ** | 458 (193) | −168 (186) | |||
| CATT | [101] | 104 | Beva 1.25 mg (M/M) | 135 | 60.2 (13.6) | 7.8 (15.5) | 462 (205) | −180 (196) |
| Beva 1.25 (PRN/PRN) | 270 | 60.6 (13.0) | 5.0 (17.9) | 459 (173) | −153 (189) | |||
| Beva 1.25 mg (M/PRN) | 131 | 60.4 (12.4) | NA | 471 (185) | NA | |||
| Rani 0.5 mg (M/M) | 146 | 59.9 (14.2) | 8.8 (15.9) | 460 (190) | −190 (172) | |||
| Rani 0.5 mg (PRN/PRN) | 287 | 61.6 (13.1) | 6.7 (14.6) | 462 (195) | −166 (190) | |||
| Rani 0.5 mg (MM/PRN) | 138 | 60.9 (14.3) | NA | 462 (184) | NA | |||
| CATT | [28] | 5 years | Beva 1.25 mg | 319 | 60.2 (24.1) | −2.1 (22.3) | 460 | 278 † |
| Rani 0.5 mg | 328 | 57.7 (42.1) | −4.5 (22.3) | 466 | 289 † | |||
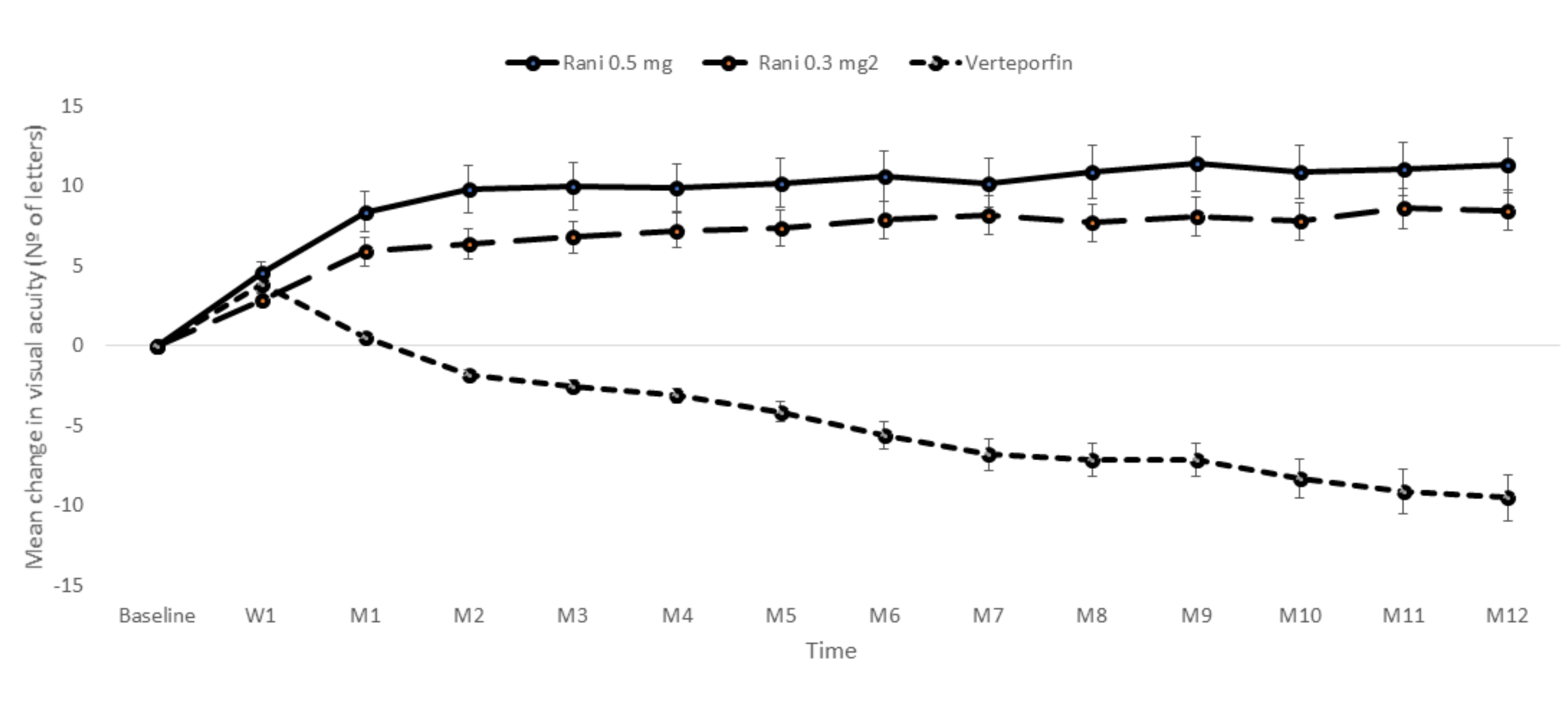
| ANCHOR | [90] | 52 | Rani 0.3 mg | 140 | 47.0 (13.1) | 8.5 (14.6) | 1.89 (1.44) ‡ | 0.36 (1.06) |
| Rani 0.5 mg | 140 | 47.1 (13.2) | 11.3 (14.6) | 1.79 (1.54) ‡ | 0.28 (1.29) | |||
| Verteporfin | 143 | 45.5 (13.1) | −9.5 (16.4) | 1.88 (1.40) ‡ | 2.56 (3.09) | |||
| ANCHOR | [93] | 104 | Rani 0.3 mg | 140 | 47.0 (13.1) | 8.1 (16.2) | 1.89 (1.44) ‡ | 0.52 (1.34) |
| Rani 0.5 mg | 140 | 47.1 (13.2) | 10.7 (16.5) | 1.79 (1.54) ‡ | 0.39 (1.34) | |||
| Verteporfin | 143 | 45.5 (13.1) | −9.8 (17.6) | 1.88 (1.40) ‡ | 2.89 (3.33) | |||
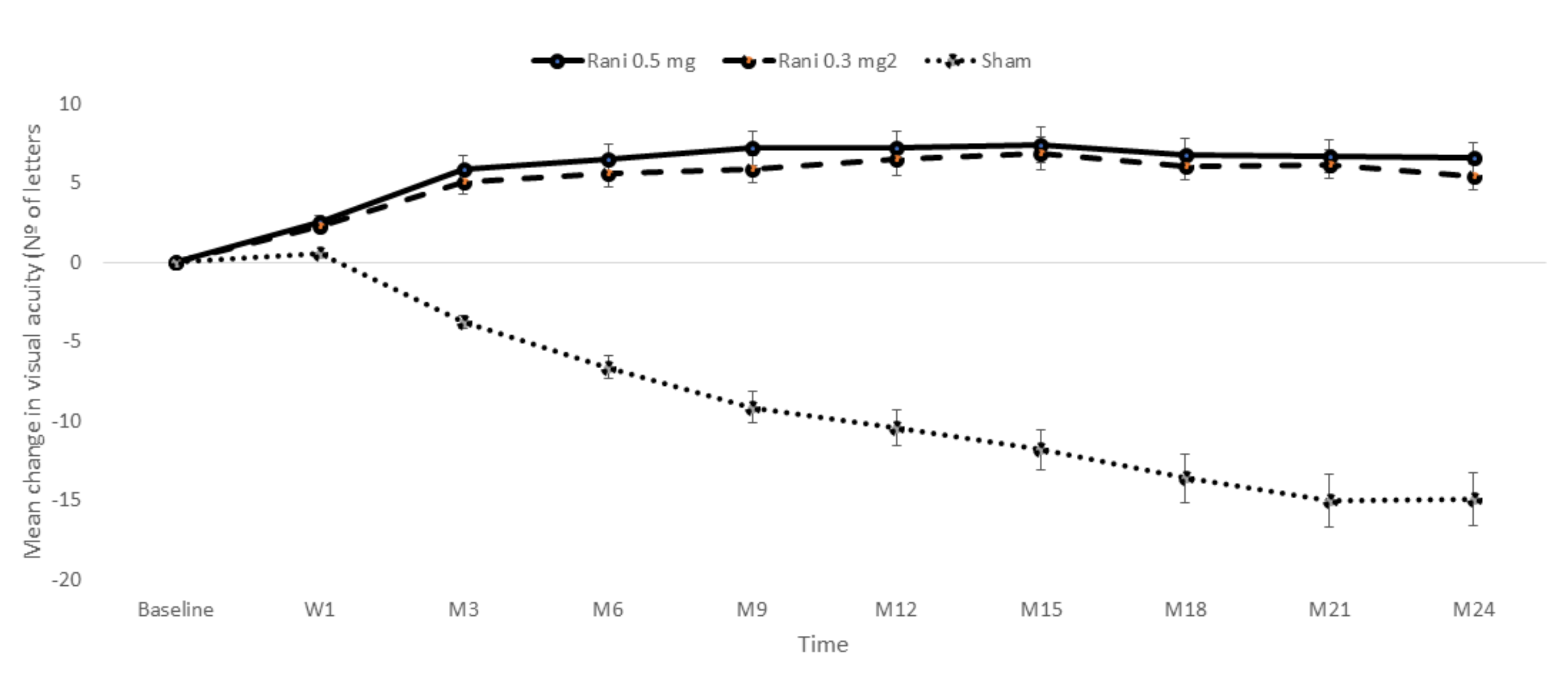
| MARINA | [89] | 52 | Rani 0.3 mg | 238 | 53.1 (12.9) | 6.5 | 4.3 (2.5) | −0.27 (2.07) |
| Rani 0.5 mg | 240 | 53.7 812.8) | 7.2 | 4.5 (2.6) | −0.01 (1.98) | |||
| Sham | 238 | 53.6 (14.1) | −10.4 | 4.4 (2.5) | 1.91 (2.81) | |||
| MARINA | [91] | 104 | Rani 0.3 mg | 238 | 53.1 (12.9) | 5.4 | 4.3 (2.5) | −0.32 (2.41) |
| Rani 0.5 mg | 240 | 53.7 812.8) | 6.6 | 4.5 (2.6) | −0.00 (2.04) | |||
| Sham | 238 | 53.6 (14.1) | −14.9 | 4.4 (2.5) | 2.58 (2.81) | |||
| HARBOR | [114] | 52 | Rani 0.5 monthly | 275 | 52.4 (13.3) | 10.1 | 348.3 (146.3) | –172.0 |
| Rani 0.5 PRN | 275 | 54.5 (11.7) | 8.2 | 347.8 (143.8) | −161.2 | |||
| Rani 2.0 mg monthly | 274 | 53.5 (13.1) | 9.2 | 332.9 (138.7) | −163.3 | |||
| Rani 2.0 mg PRN | 273 | 53.5 (13.2) | 8.6 | 347.9 (142.9) | −172.4 | |||
| HARBOR | [115] | 104 | Rani 0.5 monthly | 275 | 52.4 (13.3) | 9.1 | 348.3 (146.3) | −182.5 |
| Rani 0.5 PRN | 275 | 54.5 (11.7) | 7.9 | 347.8 (143.8) | −172.0 | |||
| Rani 2.0 mg monthly | 274 | 53.5 (13.1) | 8.0 | 332.9 (138.7) | −171.8 | |||
| Rani 2.0 mg PRN | 273 | 53.5 (13.2) | 7.6 | 347.9 (142.9) | −181.0 | |||
| TREX-AMD | [116] | 52 | Rani 0.5 monthly | 20 | 60.3 (2.4) ** | 9.2 | 533 (45) ** | −173 |
| Rani 0.5 PRN | 40 | 59.9 (2.4) ** | 10.5 | 489 (28) ** | −246 | |||
| TREND | [117] | 104 | Rani 0.5 monthly | 327 | 60.6 (13.9) | 7.9 | 497.7 (187.2) | −173.3 |
| Rani 0.5 PRN | 323 | 59.5 (13.2) | 6.6 | 504.0 (189.9) | −169.2 | |||
| CANTREAT | [118] | 52 | Rani 0.5 monthly | 293 | 59.5 | 6.0 (11.9) | 374.2 (111.9) | NA |
| Rani 0.5 PRN | 287 | 58.9 | 8.4 (11.9) | 382.5 (113.2) | NA | |||
| CANTREAT | [119] | 104 | Rani 0.5 monthly | 293 | 59.5 | 6.0 (12.6) | 374.2 (111.9) | NA |
| Rani 0.5 PRN | 287 | 58.9 | 6.8 (14.1) | 382.5 (113.2) | NA | |||
| VIEW 1 | [120] | 52 | Rani 0.5 mg Q4 | 304 | 54.0 (13.4) | 8.1 (15.3) | 315.3 (108.3) | −116.8 (109.0) |
| Afli 0.5 mg Q4 | 301 | 55.6 (13.1) | 6.9 (13.4) | 313.2 (106.0) | −115.6 (104.1) | |||
| Afli 2.0 mg Q4 | 304 | 55.2 (13.2) | 10.9 (13.8) | 313.6 (103.4) | −116.5 (98.4) | |||
| Afli 2.0 mg Q8 | 301 | 55.7 (12.8) | 7.9 (15.0) | 324.4 (111.2) | −128.5 (108.5) | |||
| VIEW 2 | [120] | 52 | Rani 0.5 mg Q4 | 291 | 53.8 (13.5) | 9.4 (13.5) | 325.9 (110.9) | −138.5 (122.2) |
| Afli 0.5 mg Q4 | 296 | 51.6 (14.2) | 9.7 (14.1) | 326.5 (116.5) | −129.8 (114.8) | |||
| Afli 2.0 mg Q4 | 309 | 52.8 (13.9) | 7.6 (12.6) | 334.6 (119.8) | −156.8 (122.8) | |||
| Afli 2.0 mg Q8 | 306 | 51.6 (13.9) | 8.9 (14.4) | 342.6 (124.0) | −149.2 (119.7) | |||
| ALTAIR | [121] | 52 | IVT-AFL-2W | 123 | 54.8 (13.1) | 9.0 | 386.2 (159.2) | −134.4 |
| IVT-AFL-4W | 123 | 55.3 (12.0) | 8.4 | 370.3 (120.0) | −126.1 | |||
| ALTAIR | [121] | 96 | IVT-AFL-2W | 123 | 54.8 (13.1) | 7.6 | 386.2 (159.2) | −130.5 |
| IVT-AFL-4W | 123 | 55.3 (12.0) | 6.1 | 370.3 (120.0) | −125.3 | |||
| HAWK | [122] | 48 | Broluzizumab 3 mg | 358 | 61.0 (13.6) | 6.1 (0.7) ** | 466.6 (167.4) | −167.4 (6.9) ** |
| Broluzizumab 6 mg | 360 | 60.8 (13.7) | 6.6 (0.7) ** | 463.1 (166.2) | −172.8 (6.7) ** | |||
| Aflibercept 2 mg | 360 | 60.0 (13.9) | 6.8 (0.7) ** | 457.9 (146.4) | −143.7 (6.7) ** | |||
| HARRIER | [122] | 48 | Broluzizumab 6 mg | 370 | 61.5 (12.6) | 6.9 (0.6) ** | 473.6 (171.4) | −193.8 (6.8) ** |
| Aflibercept 2 mg | 369 | 60.8 (12.9) | 7.6 (0.6) ** | 465.3 (151.2) | −143.9 (6.8) ** | |||
| Ocular Adverse Event | 0.3 Mg Pegaptanib N = 295 | 1.0 Mg Pegaptanib N = 301 | 3.0 Mg Pegaptanib N = 296 | All Doses Pegaptanib N = 892 | Control N = 298 | RR (95% CI) All Doses vs. Control |
|---|---|---|---|---|---|---|
| Any eye disorder | 9 (3.1%) | 4 (1.3%) | 10 (3.4%) | 23 (2.6%) | 2 (0.7%) | 3.84 (0.91 to 16.20) |
| Endophthalmitis | 6 (2.0%) | 3 (1.0%) | 3 (1.0%) | 12 (1.3%) | 0 | 8.37 (0.50 to 140.95) |
| Retinal detachment | 1 (0.3%) | 2 (0.7%) | 2 (0.7%) | 5 (0.6%) | 0 | 3.68 (0.20 to 66.41) |
| Traumatic cataract | 1 (0.3%) | 2 (0.7%) | 2 (0.7%) | 5 (0.6 1%) | 0 | 3.68 (0.20 to 66.41] |
| Retinal hemorrhage | 1 (0.3%) | 0 | 1 (0.3%) | 2 (0.2%) | 0 | 1.67 (0.08 to 34.77) |
| Vitreous hemorrhage | 0 | 0 | 1 (0.3%) | 1 (0.1%) | 0 | 1.00 (0.04 to 24.59) |
| Uveitis | 0 | 0 | 1 (0.3%) | 1 (0.1%) | 0 | 1.00 (0.04 to 24.59) |
| Elevated intraocular pressure | 1 (0.3%) | 0 | 0 | 1 (0.1%) | 0 | 1.00 (0.04 to 24.59) |
| Papilledema | 0 | 0 | 0 | 0 | 1 (0.3%) | 0.11 (0.00 to 2.73) |
| Serious Ocular Adverse Event a | Studies Reporting Adverse Events 1 | Bevacizumab | Ranibizumab | RR (95% CI) Bevacizumab vs. Ranibizumab | ||
|---|---|---|---|---|---|---|
| Number with Event | Total Participants | Number with Event | Total Participants | |||
| Endophthalmitis a | CATT [100]; GEFAL [106]; LUCAS [107] | 5 (0.5%) | 1052 | 3 (0.3%) | 1059 | 1.68 (0.40 to 7.00) |
| Retinal detachment | CATT [100]; GEFAL [106] | 3 (0.4%) | 832 | 0 | 838 | 7.05 (0.36 to 136.28) |
| Retinal pigment epithelial tear | CATT [100]; IVAN [102]; LUCAS [107] | 4 (0.4%) | 1102 | 3 (0.3%) | 1134 | 1.37 (0.31 to 6.12) |
| Traumatic cataract | CATT [100]; GEFAL [106]; LUCAS [107] | 1 (0.09%) | 1128 | 2 (0.2%) | 1152 | 0.51 (0.05 to 5.62) |
| Severe uveitis | CATT [100]; IVAN [102] | 4 (0.5%) | 882 | 1 (0.1%) | 913 | 4.14 (0.46 to 36.97) |
| Serious Ocular Adverse Event a | Studies Reporting Adverse Events 2 | Bevacizumab | Ranibizumab | RR (95% CI) Bevacizumab vs. Ranibizumab | ||
| Number With Event | Total Participants | Number With Event | Total Participants | |||
| Endophthalmitis | CATT [101] | 7 | 586 | 4 | 599 | 1.79 (0.53 to 6.08) |
| Traumatic cataract | IVAN [103] | 1 (0.3%) | 296 | 1 (0.3) | 314 | 1.06 (0.07 to 16.88) |
| Severe uveitis | IVAN [103] | 1 (0.3%) | 296 | 0 | 314 | 3.18 (0.13 to 77.80) |
| Retinal detachment | IVAN [103] | 0 | 296 | 1 (0.3) | 314 | 0.35 (0.01 to 8.64) |
| Retinal pigment epithelial tear | IVAN [103] | 1 (0.3%) | 296 | 3 (1%) | 314 | 0.35 (0.04 to 3.38) |
| Ocular Adverse Event a | 0.3 Mg Ranibizumab N = 196 | 0.5 Mg Ranibizumab N = 201 | All Doses Ranibizumab N = 397 | Control N = 206 | RR [95% CI] All Doses vs. Control |
|---|---|---|---|---|---|
| Endophthalmitis | 0 | 2 (1%) | 2 (0.5%) | 0 | 2.60 (0.13 to 53.92) |
| Retinal detachment | 1 (0.5%) | 0 | 1 (0.3%) | 1 (0.5%) | 0.52 (0.03 to 8.25) |
| Traumatic cataract | 18 (9.2%) | 22 (10.9%) | 40 (10%) | 14 (6.8%) | 1.48 (0.83 to 2.66) |
| Retinal hemorrhage | 2 (1%) | 0 | 2 (0.5%) | 2 (1%) | 0.52 (0.07 to 3.66) |
| Vitreous hemorrhage | 1 (0.5%) | 0 | 1 (0.3%) | 0 | 1.56 (0.06 to 38.13) |
| Uveitis | 0 | 1 (0.5%) | 1 (0.3%) | 0 | 1.56 (0.06 to 38.13) |
| Elevated intraocular pressure (≥30 mmHg increase) | 13 (6.6%) | 17 (8.5%) | 30 (7.6%) | 7 (3.4%) | 2.22 (0.99 to 4.98) |
| Ocular inflammation (trace to 4+) | 21 (10.7%) | 26 (12.9%) | 47 (11.8%) | 9 (4.4%) | 2.71 (1.36 to 5.42) |
| Ocular adverse event b | 0.3 mg ranibizumab n = 434 | 0.5 mg ranibizumab n = 440 | All doses ranibizumab n = 874 | Control n = 441 | RR [95% CI] All doses vs. control |
| Endophthalmitis | 2 (0.5%) | 6 (1.4%) | 8 (0.9%) | 0 | 8.59 (0.50 to 148.44) |
| Retinal detachment | 2 (0.5%) | 0 | 2 (0.2%) | 2 (0.5%) | 0.50 (0.07 to 3.57) |
| Traumatic cataract | 65 (15%) | 76 (17.3%) | 141 (16.1%) | 57 (12.9%) | 1.25 (0.94 to 1.66) |
| Retinal hemorrhage | 1 (0.2%) | 0 | 1 (<0.1%) | 1 (0.2%) | 0.50 (0.03 to 8.05) |
| Vitreous hemorrhage | 3 (0.7%) | 1 (0.2%) | 4 (<0.5%) | 2 (0.5%) | 1.01 (0.19 to 5.49) |
| Uveitis | 3 (0.7%) | 4 (0.9%) | 7 (0.8%) | 0 | 7.58 (0.43 to 132.36) |
| Elevated intraocular pressure (≥30 mmHg increase) c | 45 (15.2%) | 61 (20.3%) | 106 (17.8%) | 11 (3,7%) | 4.81 (2.63 to 8.81) |
| Ocular inflammation (1+ to 4+) | 32 (7%) | 30 (6.8%) | 62 (7.1%) | 8 (1.8%) | 3.91 [1.89 to 8.09] |
| VIEW 1 | VIEW 2 | |||
|---|---|---|---|---|
| Rani | AFL | Rani | AFL | |
| N = 304 | N = 303 | N = 291 | N = 307 | |
| n (%) | n (%) | n (%) | n (%) | |
| AE | ||||
| Patients with ≥1 TEAEs, N (%) | 290 (95.4) | 289 (95.4) | 250 (85.9) | 277 (90.2) |
| Any ocular TEAE | 263 (86.5) | 257 (84.8) | 210 (72.2) | 220 (71.7 |
| Study eye | 246 (80.9) | 238 (78.5) | 187 (64.3) | 198 (64.5) |
| Fellow eye | 150 (49.3) | 143 (47.2) | 124 (42.6) | 123 (40.1) |
| SAE | ||||
| Patients with ≥1 serious TEAEs, N (%) | 68 (22.4) | 56 (18.5) | 35 (12.0) | 48 (15.6) |
| Most common ocular SAEs | ||||
| Endophthalmitis | 3 (1.0) | 0 | NR | NR |
| Reduced VA | 2 (0.7) | 0 | 1 (0.3) | 5 (1.6) |
| Retinal hemorrhage | 2 (0.7) | 2 (0.7) | 1 (0.3) | 1 (0.3) |
| Most common injection-related ocular SAEs | ||||
| Endophthalmitis | 3 (1.0) | 0 | NR | NR |
| WDAE | ||||
| WDAEs, N (%) (discontinuation from study) | 4 (1.3) | 4 (1.3) | 2 (0.7) | 9 (2.9) |
| Most common reasons | ||||
| Retinal hemorrhage | 1 (0.3) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Endophthalmitis | 1 (0.3) | 0 | NR | NR |
| Deaths | ||||
| Deaths, N (%) | 5 (1.6) | 8 (2.6) | 2 (0.7) | 2 (0.6) |
| Notable Harms | ||||
| Retinal detachment | NR | NR | 15 (5.2) | 12 (3.9) |
| ATE | 5 (1.6) | 12 (4.0) | 0 | 0 |
| Adverse Event, n (%) | HAWK | HARRIER | |||
|---|---|---|---|---|---|
| Brolucizumab 3 mg (N = 358) | Brolucizumab 6 mg (N = 360) | Aflibercept 2 mg (N = 360) | Brolucizumab 6 mg (N = 370) | Aflibercept 2 mg (N = 369) | |
| Patients with ≥1 event | 175 (48.9) | 179 (49.7) | 170 (47.2) | 122 (33.0) | 119 (32.2) |
| Conjunctival hemorrhage | 30 (8.4) | 23 (6.4) | 20 (5.6) | 7 (1.9) | 12 (3.3) |
| Visual acuity reduced | 23 (6.4) | 19 (5.3) | 24 (6.7) | 20 (5.4) | 20 (5.4) |
| Vitreous floaters | 24 (6.7) | 18 (5.0) | 11 (3.1) | 11 (3.0) | 3 (0.8) |
| Eye pain | 21 (5.9) | 16 (4.4) | 15 (4.2) | 10 (2.7) | 12 (3.3) |
| Dry eye | 11 (3.1) | 14 (3.9) | 15 (4.2) | 8 (2.2) | 6 (1.6) |
| Retinal hemorrhage | 10 (2.8) | 13 (3.6) | 16 (4.4) | 5 (1.4) | 2 (0.5) |
| Retinal pigment epithelial tear | 5 (1.4) | 12 (3.3) | 4 (1.1) | 6 (1.6) | 4 (1.1) |
| Vitreous detachment | 16 (4.5) | 10 (2.8) | 13 (3.6) | 7 (1.9) | 5 (1.4) |
| Eye irritation | 8 (2.2) | 10 (2.8) | 8 (2.2) | 3 (0.8) | 1 (0.3) |
| Intraocular pressure increased | 11 (3.1) | 9 (2.5) | 8 (2.2) | 12 (3.2) | 9 (2.4) |
| Posterior capsule opacification | 5 (1.4) | 9 (2.5) | 7 (1.9) | 5 (1.4) | 1 (0.3) |
| Uveitis | 5 (1.4) | 8 (2.2) | 1 (0.3) | 3 (0.8) | 0 |
| Blepharitis | 4 (1.1) | 8 (2.2) | 7 (1.9) | 8 (2.2) | 3 (0.8) |
| Iritis | 1 (0.3) | 8 (2.2) | 0 | 0 | 1 (0.3) |
| Cataract | 10 (2.8) | 7 (1.9) | 8 (2.2) | 4 (1.1) | 12 (3.3) |
| Visual field defect | 7 (2.0) | 7 (1.9) | 3 (0.8) | 1 (0.3) | 0 |
| Conjunctivitis | 2 (0.6) | 7 (1.9) | 3 (0.8) | 10 (2.7) | 3 (0.8) |
| Vision blurred | 11 (3.1) | 6 (1.7) | 5 (1.4) | 1 (0.3) | 2 (0.5) |
| Visual impairment | 10 (2.8) | 6 (1.7) | 10 (2.8) | 0 | 2 (0.5) |
| Punctate keratitis | 5 (1.4) | 6 (1.7) | 8 (2.2) | 1 (0.3) | 3 (0.8) |
| Corneal abrasion | 5 (1.4) | 5 (1.4) | 8 (2.2) | 0 | 1 (0.3) |
| Lenticular opacities | 6 (1.7) | 0 | 3 (0.8) | 8 (2.2) | 7 (1.9) |
| Bevacizumab * | Ranibizumab | Aflibercept | |
|---|---|---|---|
| Cost, € | |||
| Mean 95% CI | 27,087 (22,818 to 31,789) | 33,137 (28,883 to 37,926) | 31,119 (26,979 to 35,766) |
| Differences in cost, € | |||
| Mean | N.A. | 6,050 | 4,032 |
| Mean effectiveness, QALY | |||
| Mean 95% CI | 0.69 (0.66 to 0.73) | 0.69 (0.66 to 0.73) | 0.71 (0.67 to 0.74) |
| ICER, Δ€/ΔQALY (€) | N.A. | Dominated † | 278,099 |
| Study | Ref | Duration (w) | Regimen | N | BCVA (ETDRS Letters) | CRT (µm) | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | |||||
| Souied et al. | [191] | 16 | Abicipar 0.04 mg | 9 | N.A. | N.A. | 352 (107.8) * | 7 |
| Abicipar 0.15 mg | 7 | −12 | ||||||
| Abicipar 0.4 mg | 6 | −62 | ||||||
| Abicipar 1.0 mg | 6 | −95 | ||||||
| Abicipar 2.0 mg | 4 | −111 | ||||||
| Abicipar 3.6 mg | 0 | N.A. | ||||||
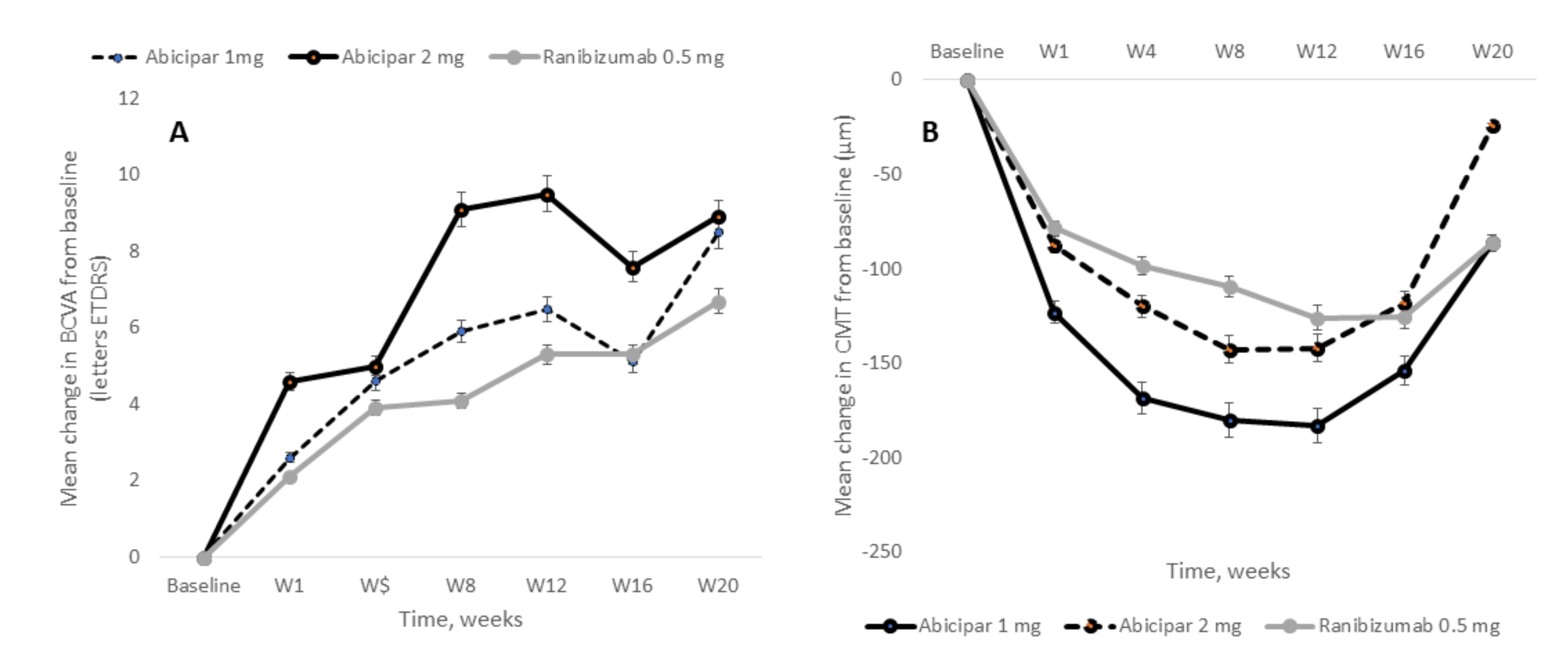
| REACH | [193] | 20 | Abicipar 1 mg | 25 | 58 (13) | 8.5 (8.1) | 526 (165) | −86.2 (124.4) |
| Abicipar 2 mg | 23 | 59 (14) | 8.9 (5.5) | 466 (126) | −24.3 (54.1) | |||
| Ranibizumab 0.5 mg | 16 | 60 (16) | 6.7 (7.7) | 463 (95) | −86.1 (113.4) | |||
| BAMBOO | [194] | 20 | Abicipar 1 mg | 10 | 54.3 (24−72) † | 7.8 (2.7) ‡ | 475.1 (296−840) † | −187.3 (46.1) |
| Abicipar 2 mg | 10 | 58.5 (27−75) † | 8.9 (2.9) ‡ | 438.7 (288−591) † | −196.5 (39.3) | |||
| Ranibizumab 0.5 mg | 5 | 55.8 (47−70) † | 17.4 (3.6) ‡ | 470.0 (363−538) † | −230.4 (26.5) | |||
| CYPRESS | [194] | 20 | Abicipar 1 mg | 10 | 55.2 (33−70)† | 4.4 (2.8) ‡ | 443.8 (285−643) † | −106.5 (40.6) |
| Abicipar 2 mg | 10 | 59.0 (42−74) † | 10.1 (3.3) ‡ | 383.8 (278−792) † | −112.8 (53.7) | |||
| Ranibizumab 0.5 mg | 5 | 57.6 (30−72) † | 15.2 (3.0) ‡ | 348.8 (313−395) † | −124.4 (22.1) | |||
| CEDAR | [195] | 52 | Abicipar Q8 | 265 | 56.8 (12.8) ** | 22.6% ⁋ | 382.5 (130.7) ** | −134.6 (4.8) |
| Abicipar Q12 | 262 | 56.5 (12.7) ** | 19.2% ⁋ | 378.3 (121.4) ** | −141.0 (4.8) | |||
| Ranibizumab Q4 | 290 | 56.8 812.4) ** | 27.2% ⁋ | 380.3 (125.5) ** | −143.2 (4.7) | |||
| SEQUOIA | [195] | 52 | Abicipar Q8 | 267 | 56.8 (12.8) ** | 28.2% ⁋ | 382.5 (130.7) ** | −141.8 (4.2) |
| Abicipar Q12 | 265 | 56.5 (12.7) ** | 24.2% ⁋ | 378.3 (121.4) ** | −139.4 (4.2) | |||
| Ranibizumab Q4 | 299 | 56.8 812.4) ** | 26.7% ⁋ | 380.3 (125.5) ** | −145.3 (4.1) | |||
| Khurana RN 1 | [196] | 104 | Abicipar Q8 | 630 | 56.8 (12.8) ** | 7.8 | 382.5 (130.7) ** | N.A. |
| Abicipar Q12 | 628 | 56.5 (12.7) ** | 6.1 | 378.3 (121.4) ** | ||||
| Ranibizumab Q4 | 630 | 56.8 812.4) ** | 8.5 | 380.3 (125.5) ** | ||||
| Study | Ref | TRAE | Treatment Regime | ||
|---|---|---|---|---|---|
| REACH | [193] | Abicipar 1 mg (n = 25) | Abicipar 2 mg (n = 23) | Ranibizumab 0.5 mg (n = 16) | |
| Overall incidence a | 11 (44.0) | 7 (30.4) | 5 (31.3) | ||
| Vitreous floaters | 3 (12.0) | 1 (4.3) | 1 (6.3) | ||
| IOI * | 3 (12.0) | 2 (8.7) | 0 (0.0) | ||
| Vitreous detachment | 2 (8.0) | 2 (8.7) | 0 | ||
| Retinal hemorrhage | 3 (12.0) | 0 | 2 (12.5) | ||
| Eye pain | 1 (4.0) | 2 (8.7) | 1 (6.3) | ||
| Conjunctival hemorrhage | 2 (8.0) | 0 | 0 | ||
| Macular scar | 0 | 0 | 2 (12.5) | ||
| BAMBOO and CYPRESS | [194] | TRAE | Abicipar 1 mg (n = 20) | Abicipar 2 mg (n = 20) | Ranibizumab 0.5 mg (n = 10) |
| Conjunctival hemorrhage | 4 (20.0) | 0 (0.0) | 1 (10) | ||
| Dry eye | 2 (10.0) | 1 (5.0) | 0 (0.0) | ||
| Cataract | 1 (5.0) | 0 (0.0) | 1 (10.0) | ||
| Eye pain | 0 (0.0) | 1 (5.0) | 1 (10.0) | ||
| FBS | 0 (0.0) | 1 (5.0) | 1 (10.0) | ||
| Increased IOP | 0 (0.0) | 0 (0.0) | 2 (20.0) | ||
| Iritis | 1 (5.0) | 1 (5.0) | 0 (0.0) | ||
| Edema peripheral | 0 (0.0) | 1 (5.09 | 1 (10.0) | ||
| Pneumonia | 0 (0.0) | 1 (5.0) | 1 (10.0) | ||
| Vitreous floaters | 1 (5.0) | 1 (5.0) | 0 (0.0) | ||
| Vitreous opacities | 1 (5.0) | 1 (5.0) | 0 (0.0) | ||
| Vitritis | 1 (5.0) | 1 (5.0) | 0 (0.0) | ||
| CEDAR and SEQUOIA | [195] | TRAE | Abicipar Q8 (n = 625) | Abicipar Q12 (n = 626) | Ranibizumab Q4 (n = 625) |
| Any | 203 (32.5) | 233 (37.2) | 152 (24.3) | ||
| Study drug related | 105 (16.8) | 128 (20.4) | 28 (4.5) | ||
| Study procedure related | 142 (22.7) | 168 (26.8) | 143 (22.9) | ||
| Serious | 43 (6.9) | 42 (6.7) | 2 (0.3) | ||
| IOI | 99 (15.8) | 96 (15.6) | 1 (0.2) ** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, F.; Bandello, F.; Navarra, P.; Staurenghi, G.; Stumpp, M.; Zarbin, M. Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches. Int. J. Mol. Sci. 2020, 21, 8242. https://doi.org/10.3390/ijms21218242
Ricci F, Bandello F, Navarra P, Staurenghi G, Stumpp M, Zarbin M. Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches. International Journal of Molecular Sciences. 2020; 21(21):8242. https://doi.org/10.3390/ijms21218242
Chicago/Turabian StyleRicci, Federico, Francesco Bandello, Pierluigi Navarra, Giovanni Staurenghi, Michael Stumpp, and Marco Zarbin. 2020. "Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches" International Journal of Molecular Sciences 21, no. 21: 8242. https://doi.org/10.3390/ijms21218242
APA StyleRicci, F., Bandello, F., Navarra, P., Staurenghi, G., Stumpp, M., & Zarbin, M. (2020). Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches. International Journal of Molecular Sciences, 21(21), 8242. https://doi.org/10.3390/ijms21218242






