A Review of the Important Role of CYP2D6 in Pharmacogenomics
Abstract
1. Introduction
1.1. Background to Cytochrome P450 Enzymes
1.2. Background to Pharmacogenomics
1.3. Background to CYP2D6
2. An Overview of CYP2D6 Variation
2.1. Pseudogenes
2.2. Copy Number Variation
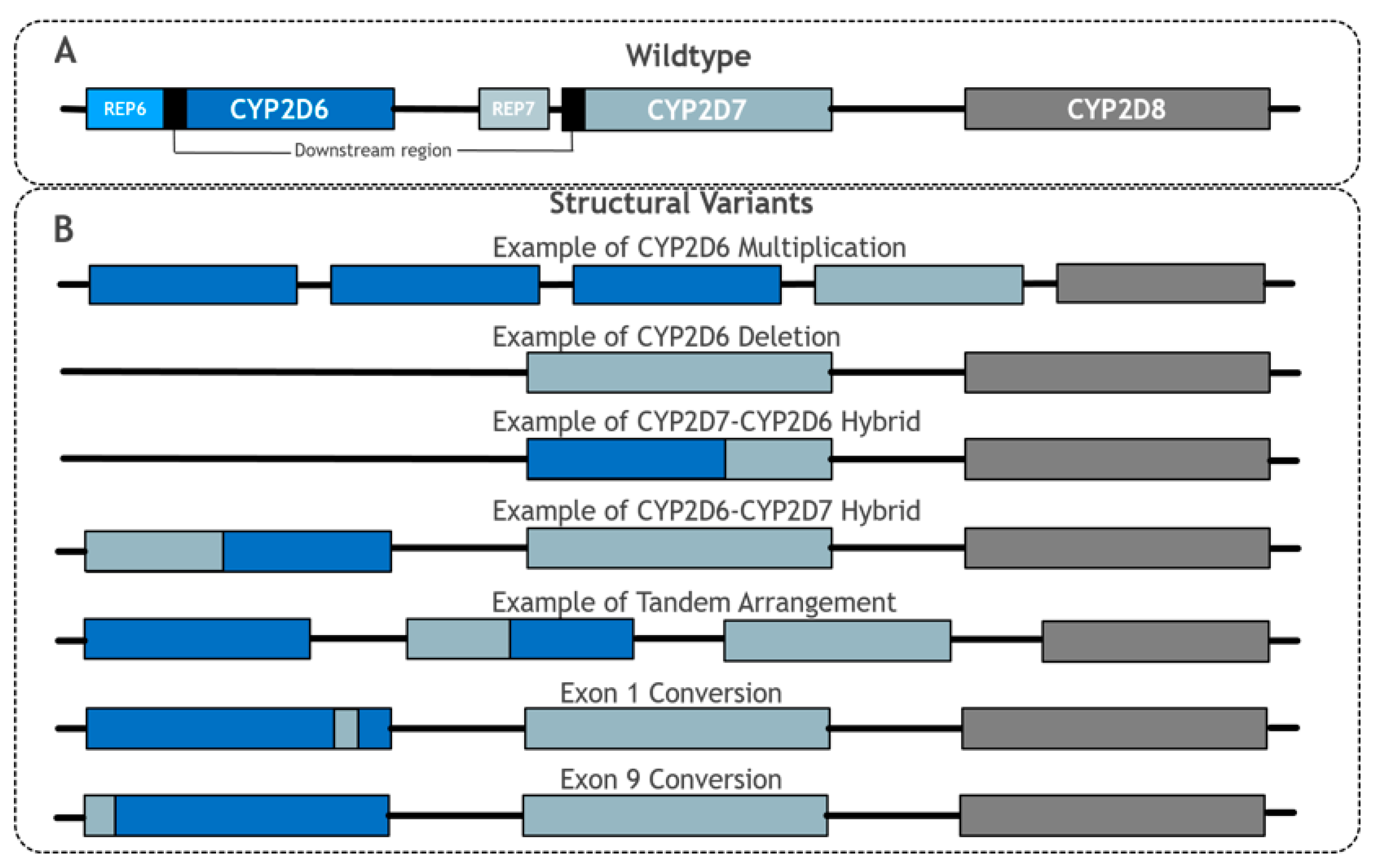
2.3. Hybridisations, Tandem Arrangements and Conversions
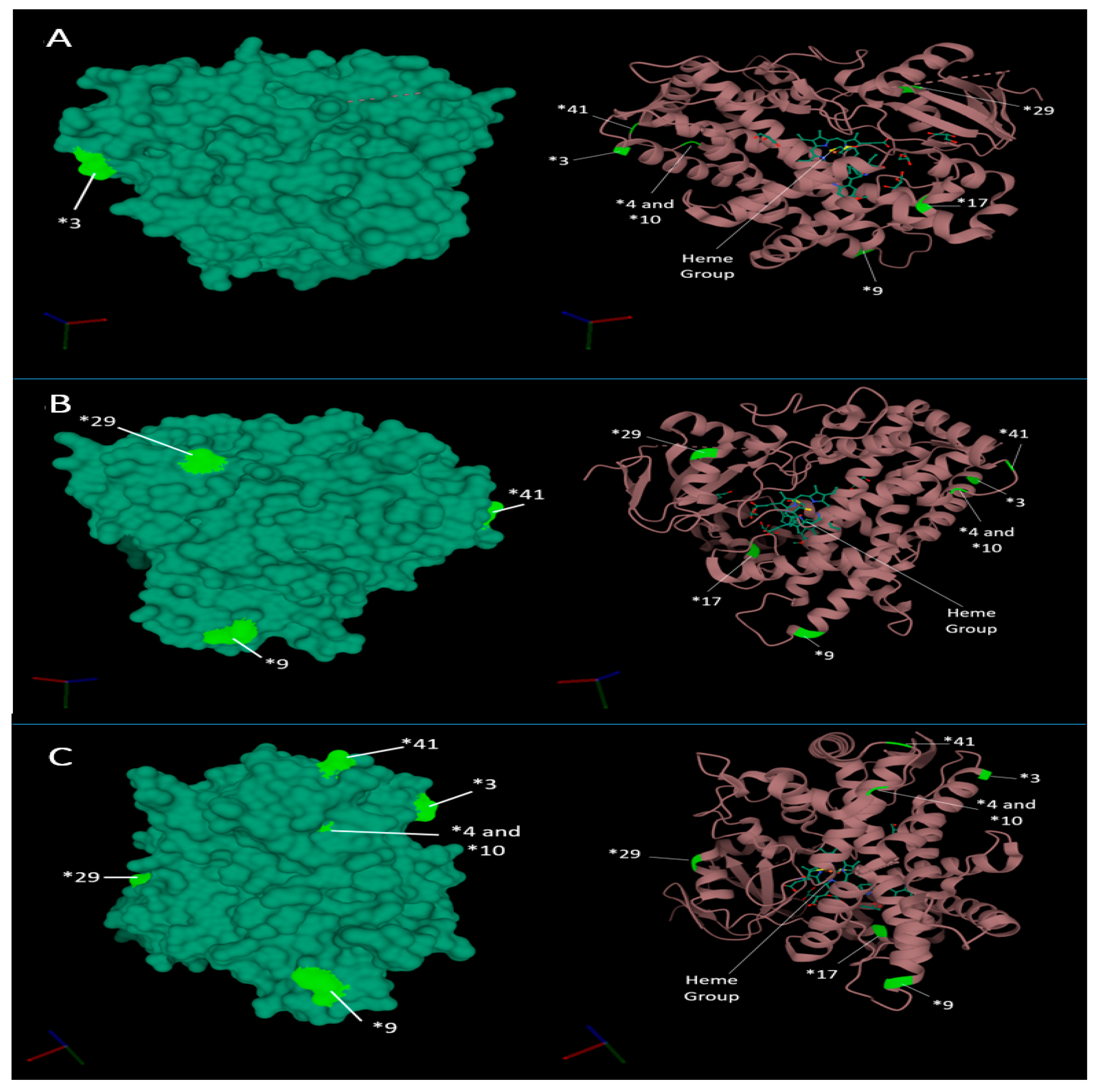
2.4. Structural Impact of Star Alleles
3. CYP2D6 Metaboliser Status
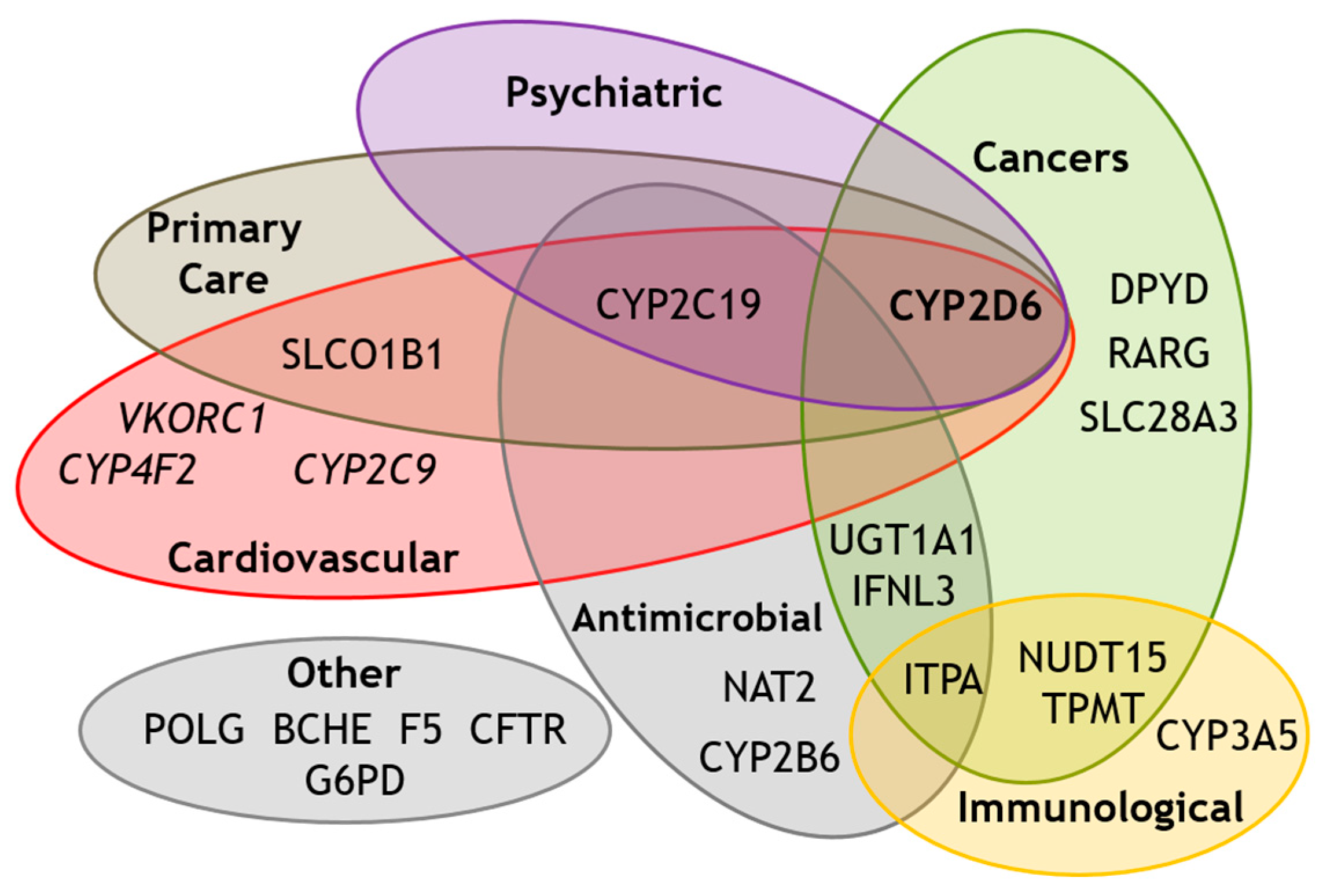
4. CYP2D6 Star Allele Frequencies
5. Detection and Interpretation of CYP2D6 Genotype
6. Factors Impacting CYP2D6 Function beyond CYP2D6 Genetics
7. CYP2D6 Clinical Impact
Clinical Guidelines and Pharmacogenomics Implementation
8. CYP2D6-Drug Case Studies
8.1. Codeine
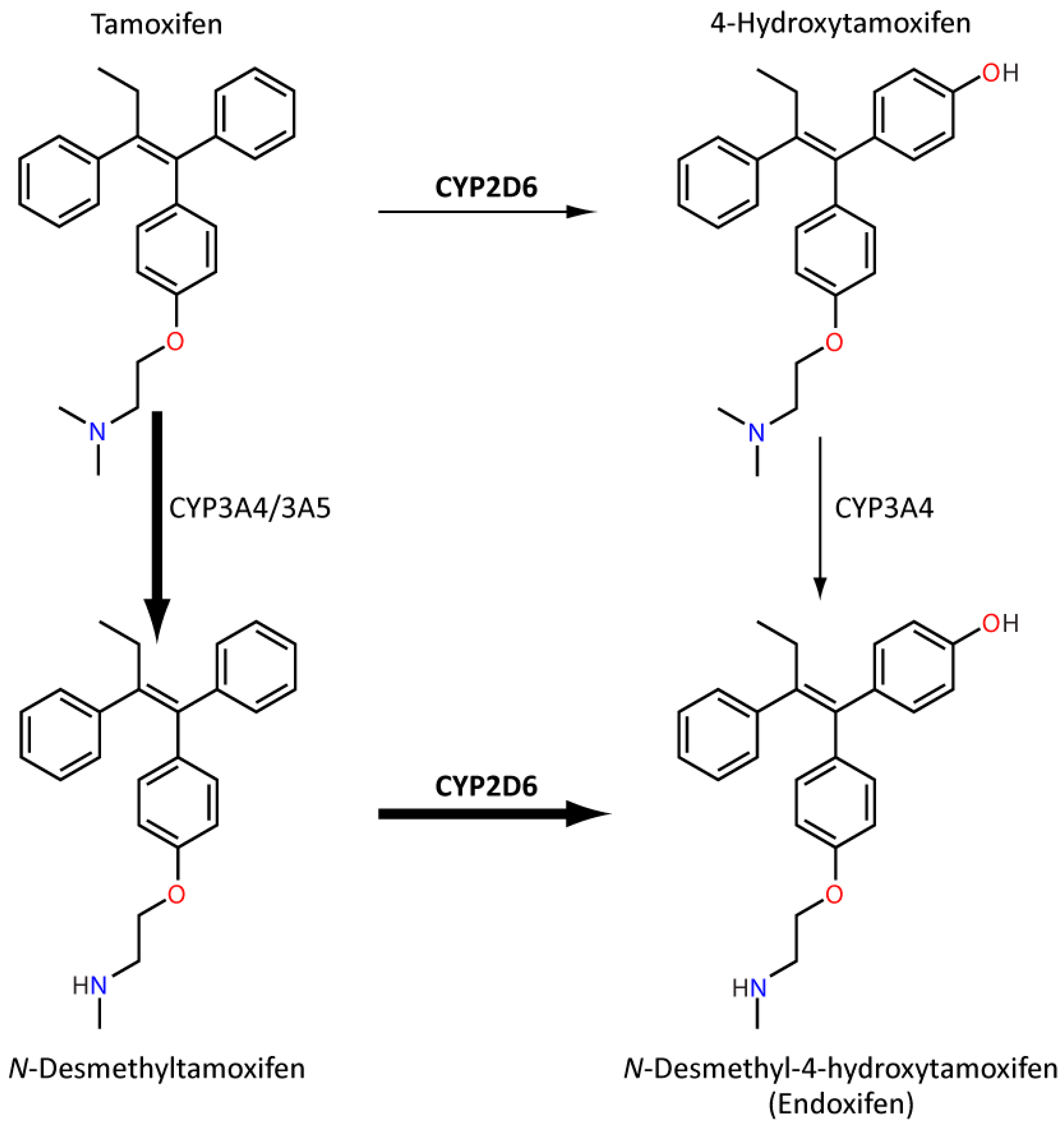
8.2. Tamoxifen
9. Novel Approaches to CYP2D6 Phenotyping
10. Veterinary Pharmacogenomics and CYP2D6 Orthologues
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Munro, A.W.; Lindsay, J.G. Bacterial cytochromes P-450. Mol. Microbiol. 1996, 20, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, A.; Satta, Y. Substrate-dependent evolution of cytochrome P450: Rapid turnover of the detoxification-type and conservation of the biosynthesis-type. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, reviews3003.1. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Šrejber, M.; Navrátilová, V.; Paloncýová, M.; Bazgier, V.; Berka, K.; Anzenbacher, P.; Otyepka, M. Membrane-attached mammalian cytochromes P450: An overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J. Inorg. Biochem. 2018, 183, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Otyepka, M.; Skopalík, J.; Anzenbacherová, E.; Anzenbacher, P. What common structural features and variations of mammalian P450s are known to date? Biochim. Biophys. Acta-Gen. Subj. 2007, 1770, 376–389. [Google Scholar] [CrossRef]
- Hasemann, C.A.; Kurumbail, R.G.; Boddupalli, S.S.; Peterson, J.A.; Deisenhofer, J. Structure and function of cytochromes P450: A comparative analysis of three crystal structures. Structure 1995, 3, 41–62. [Google Scholar] [CrossRef]
- Porter, T.D. New insights into the role of cytochrome P450 reductase (POR) in microsomal redox biology. Acta Pharm. Sin. B 2012, 2, 102–106. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.F.; Li, Z.H.; Liu, J.Y.; Liu, T.T.; Wang, P.; Fang, Y.; Zhou, J.; Cui, M.Z.; Gao, N.; Tian, X.; et al. Correlation of cytochrome P450 oxidoreductase expression with the expression of 10 isoforms of cytochrome P450 in human liver. Drug Metab. Dispos. 2016, 44, 1193–1200. [Google Scholar] [CrossRef]
- Gopisankar, M.G. CYP2D6 pharmacogenomics. Egypt. J. Med. Hum. Genet. 2017, 18, 309–313. [Google Scholar] [CrossRef]
- Spear, B.; Heath-Chiozzi, M.; Huff, J. Clinical application of pharmacogenetics. Trends Mol. Med. 2001, 7, 201–204. [Google Scholar] [CrossRef]
- Pirmohamed, M.; James, S.; Meakin, S.; Green, C.; Scott, A.K.; Walley, T.J.; Farrar, K.; Park, B.K.; Breckenridge, A.M. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. Br. Med. J. 2004, 329, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Bouvy, J.C.; De Bruin, M.L.; Koopmanschap, M.A. Epidemiology of Adverse Drug Reactions in Europe: A Review of Recent Observational Studies. Drug Saf. 2015, 38, 437–453. [Google Scholar] [CrossRef]
- Davies, E.C.; Green, C.F.; Taylor, S.; Williamson, P.R.; Mottram, D.R. Adverse Drug Reactions in Hospital In-Patients: A Prospective Analysis of 3695 Patient-Episodes. PLoS ONE 2009, 4, e4439. [Google Scholar] [CrossRef]
- Rodrigues, M.C.S.; De Oliveira, C. Drug-drug interactions and adverse drug reactions in polypharmacy among older adults: An integrative review. Rev. Lat. Am. Enferm. 2016, 24, 1–17. [Google Scholar] [CrossRef]
- Ferner, R.E.; McGettigan, P. Adverse drug reactions. BMJ 2018, 363. [Google Scholar] [CrossRef]
- NICE Costing Statement: Medicines Optimisation: Implementing the NICE Guideline on Medicines Optimisation (NG5). Available online: https://www.nice.org.uk/guidance/ng5/resources/costing-statement-6916717 (accessed on 12 September 2020).
- Nebert, D.W.; Zhang, G.; Vesell, E.S. From human genetics and genomics to pharmacogenetics and pharmacogenomics: Past lessons, future directions. Drug Metab. Rev. 2008, 40, 187–224. [Google Scholar] [CrossRef]
- Van der Wouden, C.H.; Cambon-Thomsen, A.; Cecchin, E.; Cheung, K.C.; Dávila-Fajardo, C.L.; Deneer, V.H.; Dolžan, V.; Ingelman-Sundberg, M.; Jönsson, S.; Karlsson, M.O.; et al. Implementing Pharmacogenomics in Europe: Design and Implementation Strategy of the Ubiquitous Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 2017, 101, 341–358. [Google Scholar] [CrossRef]
- Daly, A.K. Pharmacogenetics: A general review on progress to date. Br. Med. Bull. 2017, 124, 65–79. [Google Scholar] [CrossRef]
- Turner, R.M.; Pirmohamed, M. Cardiovascular pharmacogenomics: Expectations and practical benefits. Clin. Pharmacol. Ther. 2014, 95, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Table of Pharmacogenomic Biomarkers in Drug Labeling|FDA. Available online: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 28 August 2020).
- PharmGKB Clinical Guideline Annotations. Available online: https://www.pharmgkb.org/guidelineAnnotations (accessed on 15 September 2020).
- Dutch Pharmacogenetic Working Group. DPWG Recommendations; Dutch Pharmacogenetic Working Group: Den Haag, The Netherlands, 2020. [Google Scholar]
- The Pharmacogenetics Implementation Consortium Guidelines—CPIC. Available online: https://cpicpgx.org/guidelines/ (accessed on 13 October 2020).
- Canadian Pharmacogenomics Network for Drug Safety Pharmacogenomics—Canadian Pharmacogenomics Network for Drug Safety. Available online: http://cpnds.ubc.ca/faqs/pharmacogenomics (accessed on 13 October 2020).
- Pharmacogénétiqu, the R.N. de the Réseau National de Pharmacogénétiqu Recommendations. Available online: http://www.pharmacogenetics.fr/7.html (accessed on 13 October 2020).
- Mahgoub, A.; Dring, L.G.; Idle, J.R.; Lancaster, R.; Smith, R.L. Polymorphic Hydroxylation of Debrisoquin in Man. Lancet 1977, 310, 584–586. [Google Scholar] [CrossRef]
- Eichelbaum, M.; Ingelman-Sundberg, M.; Evans, W.E. Pharmacogenetics and individualized drug therapy. Annu. Rev. Med. 2005, 57, 119–156. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, F.; Dauchy, S.; Diry, M.; Sazdovitch, V.; Cloarec, O.; Mellottée, L.; Bièche, I.; Ingelman-Sundberg, M.; Flinois, J.P.; De Waziers, I.; et al. Xenobiotic-metabolizing enzymes and transporters in the normal human brain: Regional and cellular mapping as a basis for putative roles in cerebral function. Drug Metab. Dispos. 2009, 37, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Mantione, K.J.; Cadet, P.; Zhu, W.; Kream, R.M.; Sheehan, M.; Fricchione, G.L.; Goumon, Y.; Esch, T.; Stefano, G.B. Endogenous morphine signaling via nitric oxide regulates the expression of CYP2D6 and COMT: Autocrine/paracrine feedback inhibition. Addict. Biol. 2008, 13, 118–123. [Google Scholar] [CrossRef]
- Funae, Y.; Kishimoto, W.; Cho, T.; Niwa, T.; Hiroi, T. CYP2D in the Brain. Drug Metab. Pharmacokinet. 2003, 18, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Cao, J. Finite-time synchronization of coupled neural networks via discontinuous controllers. Cogn. Neurodyn. 2011, 5, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Dong, G.; Yue, J. The endogenous substrates of brain CYP2D. Eur. J. Pharmacol. 2014, 724, 211–218. [Google Scholar] [CrossRef]
- Williams, I.S.; Gatchie, L.; Bharate, S.B.; Chaudhuri, B. Biotransformation, using recombinant CYP450-expressing baker’s yeast cells, identifies a novel cyp2d6.10 a122v variant which is a superior Metabolizer of codeine to morphine than the wild-type enzyme. ACS Omega 2018, 3, 8903–8912. [Google Scholar] [CrossRef]
- Yang, Y.; Botton, M.R.; Scott, E.R.; Scott, S.A. Sequencing the CYP2D6 gene: From variant allele discovery to clinical pharmacogenetic testing. Pharmacogenomics 2017, 18, 673–685. [Google Scholar] [CrossRef]
- Jarvis, J.P.; Peter, A.P.; Shaman, J.A. Consequences of CYP2D6 copy-number variation for pharmacogenomics in psychiatry. Front. Psychiatry 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Gaedigk, A. Complexities of CYP2D6 gene analysis and interpretation. Int. Rev. Psychiatry 2013, 25, 534–553. [Google Scholar] [CrossRef]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Muller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Beoris, M.; Wilson, J.A.; Garces, J.A.; Lukowiak, A.A. CYP2D6 copy number distribution in the US population. Pharm. Genom. 2016, 26, 96–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fleeman, N.; Dundar, Y.; Dickson, R.; Jorgensen, A.; Pushpakom, S.; McLeod, C.; Pirmohamed, M.; Walley, T. Cytochrome P450 testing for prescribing antipsychotics in adults with schizophrenia: Systematic review and meta-analyses. Pharm. J. 2011, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- PharmGKB CYP2D6—Drug Label Annotations. Available online: https://www.pharmgkb.org/gene/PA128/labelAnnotation (accessed on 15 September 2020).
- Pan, X.; Ning, M.; Jeong, H. Transcriptional regulation of CYP2D6 expression. Drug Metab. Dispos. 2017, 45, 42–48. [Google Scholar] [CrossRef]
- Chan, W.; Li, M.S.; Sundaram, S.K.; Tomlinson, B.; Cheung, P.Y.; Tzang, C.H. CYP2D6 allele frequencies, copy number variants, and tandems in the population of Hong Kong. J. Clin. Lab. Anal. 2019, 33. [Google Scholar] [CrossRef]
- Pharmacogene Variation Consortium PharmVar CYP2D6 Alleles. Available online: https://www.pharmvar.org/gene/CYP2D6 (accessed on 12 October 2020).
- Nofziger, C.; Turner, A.J.; Sangkuhl, K.; Whirl-Carrillo, M.; Agúndez, J.A.G.; Black, J.L.; Dunnenberger, H.M.; Ruano, G.; Kennedy, M.A.; Phillips, M.S.; et al. PharmVar GeneFocus: CYP2D6. Clin. Pharmacol. Ther. 2020, 107, 154–170. [Google Scholar] [CrossRef]
- PharmVar Structural Variation CYP2D6. Available online: https://www.pharmvar.org/gene-support/Variation_CYP2D6.pdf (accessed on 9 September 2020).
- Ramírez, B.; Niño-Orrego, M.J.; Cárdenas, D.; Ariza, K.E.; Quintero, K.; Contreras Bravo, N.C.; Tamayo-Agudelo, C.; González, M.A.; Laissue, P.; Fonseca Mendoza, D.J. Copy number variation profiling in pharmacogenetics CYP-450 and GST genes in Colombian population. BMC Med. Genom. 2019, 12, 110. [Google Scholar] [CrossRef]
- Gaedigk, A.; Fuhr, U.; Johnson, C.; Bérard, L.A.; Bradford, D.; Leeder, J.S. CYP2D7-2D6 hybrid tandems: Identification of novel CYP2D6 duplication arrangements and implications for phenotype prediction. Pharmacogenomics 2010, 11, 43–53. [Google Scholar] [CrossRef]
- Petrović, J.; Pešić, V.; Lauschke, V.M. Frequencies of clinically important CYP2C19 and CYP2D6 alleles are graded across Europe. Eur. J. Hum. Genet. 2020, 28, 88–94. [Google Scholar] [CrossRef]
- Del Tredici, A.L.; Malhotra, A.; Dedek, M.; Espin, F.; Roach, D.; Zhu, G.; Voland, J.; Moreno, T.A. Frequency of CYP2D6 alleles including structural variants in the United States. Front. Pharmacol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Daly, A.K.; Idle, J.R.; Fairbrother, K.S.; Andreassen, O.A.; London, S.J.; Steen, V.M. Characterization and PCR-based detection of two different hybrid CYP2D7P/CYP2D6 alleles associated with the poor metabolizer phenotype. Pharmacogenetics 1996, 6, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kramer, W.E.; Walker, D.L.; O’Kane, D.J.; Mrazek, D.A.; Fisher, P.K.; Dukek, B.A.; Bruflat, J.K.; Black, J.L. CYP2D6: Novel genomic structures and alleles. Pharm. Genom. 2009, 19, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Black, J.L.; Walker, D.L.; O’Kane, D.J.; Harmandayan, M. Frequency of undetected CYP2D6 hybrid genes in clinical samples: Impact on phenotype prediction. Drug Metab. Dispos. 2012, 40, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gaedigk, A.; Bradford, L.D.A.; Alander, S.W.; Leeder, J.S. CYP2D6*36 gene arrangements within the CYP2D6 locus: Association of CYP2D6*36 with poor metabolizer status. Drug Metab. Dispos. 2006, 34, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kiyotani, K.; Shimizu, M.; Kumai, T.; Kamataki, T.; Kobayashi, S.; Yamazaki, H. Limited effects of frequent CYP2D6*36-*10 tandem duplication allele on in vivo dextromethorphan metabolism in a Japanese population. Eur. J. Clin. Pharmacol. 2010, 66, 1065–1068. [Google Scholar] [CrossRef]
- Wang, A.; Savas, U.; Hsu, M.H.; Stout, C.D.; Johnson, E.F. Crystal structure of human cytochrome P450 2D6 with prinomastat bound. J. Biol. Chem. 2012, 287, 10834–10843. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.P.; Zhang, X.Z.; Huang, S.Q.; Bartlam, M.; Zhou, S.F. New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme. Drug Metab. Rev. 2009, 41, 573–643. [Google Scholar] [CrossRef]
- Sehnal, D.; Rose, A.; Koca, J.; Burley, S.; Velankar, S. Mol*: Towards a common library and tools for web molecular graphics. In Proceedings of the Workshop on Molecular Graphics and Visual Analysis of Molecular Data, Brno, Czech Republic, 4 June 2018; pp. 29–33. [Google Scholar]
- Caudle, K.E.; Sangkuhl, K.; Whirl-Carrillo, M.; Swen, J.J.; Haidar, C.E.; Klein, T.E.; Gammal, R.S.; Relling, M.V.; Scott, S.A.; Hertz, D.L.; et al. Standardizing CYP2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 2020, 13, 116–124. [Google Scholar] [CrossRef]
- Gaedigk, A.; Sangkuhl, K.; Whirl-Carrillo, M.; Klein, T.; Steven Leeder, J. Prediction of CYP2D6 phenotype from genotype across world populations. Genet. Med. 2017, 19, 69–76. [Google Scholar] [CrossRef]
- Parveen, F.; Faridi, R.M.; Das, V.; Tripathi, G.; Agrawal, S. Genetic association of phase I and phase II detoxification genes with recurrent miscarriages among North Indian women. Mol. Hum. Reprod. 2010, 16, 207–214. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Ingelman-Sundberg, M.; Lauschke, V.M. Worldwide Distribution of Cytochrome P450 Alleles: A Meta-analysis of Population-scale Sequencing Projects. Clin. Pharmacol. Ther. 2017, 102, 688–700. [Google Scholar] [CrossRef]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; M€ Uller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2D6 and CYP2C19 Genotypes and Dosing of Tricyclic Antidepressants: 2016 Update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bousman, C.A.; Jaksa, P.; Pantelis, C. Systematic evaluation of commercial pharmacogenetic testing in psychiatry: A focus on CYP2D6 and CYP2C19 allele coverage and results reporting. Pharm. Genom. 2017, 27, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Dorado, P.; López-Torres, E.; Peñas-Lledó, E.M.; Martínez-Antón, J.; Llerena, A. Neurological toxicity after phenytoin infusion in a pediatric patient with epilepsy: Influence of CYP2C9, CYP2C19 and ABCB1 genetic polymorphisms. Pharm. J. 2013, 13, 359–361. [Google Scholar] [CrossRef]
- Puaprasert, K.; Chu, C.; Saralamba, N.; Day, N.P.J.; Nosten, F.; White, N.J.; Dondorp, A.M.; Imwong, M. Real time PCR detection of common CYP2D6 genetic variants and its application in a Karen population. Malar. J. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Fouad, A.F.; Rôças, I.N. Pyrosequencing as a tool for better understanding of human microbiomes. J. Oral Microbiol. 2012, 4, 10743. [Google Scholar] [CrossRef]
- Scantamburlo, G.; Tziolia, K.; Zopf, M.; Bernardinelli, E.; Soyal, S.M.; Civello, D.A.; Vanoni, S.; Dossena, S.; Patsch, W.; Patrinos, G.P.; et al. Allele Drop Out Conferred by a Frequent CYP2D6 Genetic Variation for Commonly Used CYP2D6*3 Genotyping Assays. Cell. Physiol. Biochem. 2017, 43, 2297–2309. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Flockhart, D.A.; Hosono, N.; Kubo, M.; Nakamura, Y.; Skaar, T.C. Differential quantification of CYP2D6 gene copy number by four different quantitative real-time PCR assays. Pharm. Genom. 2010, 20, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Kamenski, G.; Ayazseven, S.; Berndt, A.; Fink, W.; Kamenski, L.; Zehetmayer, S.; Pühringer, H. Clinical Relevance of CYP2D6 Polymorphisms in Patients of an Austrian Medical Practice: A Family Practice-Based Observational Study. Drugs Real World Outcomes 2020, 7, 63–73. [Google Scholar] [CrossRef]
- Heller, T.; Kirchheiner, J.; Armstrong, V.W.; Luthe, H.; Tzvetkov, M.; Brockm??ller, J.; Oellerich, M. AmpliChip CYP450 GeneChip: A new gene chip that allows rapid and accurate CYP2D6 genotyping. Ther. Drug Monit. 2006, 28, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Bank, P.C.D.; Swen, J.J.; Guchelaar, H.J.; Van Der Straaten, T. GenoChip CYP2D6 macroarray as a method to genotype for CYP2D6 variants: Results of a validation study in a Caucasian population. Pharmacogenomics 2015, 16, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Wheeler, M.M.; Patterson, K.; McGee, S.; Dalton, R.; Woodahl, E.L.; Gaedigk, A.; Thummel, K.E.; Nickerson, D.A. Stargazer: A software tool for calling star alleles from next-generation sequencing data using CYP2D6 as a model. Genet. Med. 2019, 21, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Twist, G.P.; Gaedigk, A.; Miller, N.A.; Farrow, E.G.; Willig, L.K.; Dinwiddie, D.L.; Petrikin, J.E.; Soden, S.E.; Herd, S.; Gibson, M.; et al. Constellation: A tool for rapid, automated phenotype assignment of a highly polymorphic pharmacogene, CYP2D6, from whole-genome sequences. NPJ Genom. Med. 2016, 1. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Shen, F.; Gonzaludo, N.; Malhotra, A.; Rogert, C.; Taft, R.J.; Bentley, D.R.; Eberle, M.A.; Eberle, M. Accurate CYP2D6 genotyping using whole genome sequencing data. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ratain, M.J.; Nakamura, Y.; Cox, N.J. CYP2D6 genotype and tamoxifen activity: Understanding interstudy variability in methodological quality. Clin. Pharmacol. Ther. 2013, 94, 185–187. [Google Scholar] [CrossRef]
- Hosein, A.N.; Song, S.; McCart Reed, A.E.; Jayanthan, J.; Reid, L.E.; Kutasovic, J.R.; Cummings, M.C.; Waddell, N.; Lakhani, S.R.; Chenevix-Trench, G.; et al. Evaluating the repair of DNA derived from formalin-fixed paraffin-embedded tissues prior to genomic profiling by SNP-CGH analysis. Lab. Investig. 2013, 93, 701–710. [Google Scholar] [CrossRef]
- Gaedigk, A.; Dinh, J.C.; Jeong, H.; Prasad, B.; Leeder, J.S. Ten years’ experience with the CYP2D6 activity score: A perspective on future investigations to improve clinical predictions for precision therapeutics. J. Pers. Med. 2018, 8, 15. [Google Scholar] [CrossRef]
- Westergaard, N.; Nielsen, R.S.; Jørgensen, S.; Vermehren, C. Drug use in Denmark for drugs having pharmacogenomics (PGx) based dosing guidelines from CPIC or DPWG for CYP2D6 and CYP2C19 drug–gene pairs: Perspectives for introducing PGx test to polypharmacy patients. J. Pers. Med. 2020, 10, 3. [Google Scholar] [CrossRef]
- Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers|FDA. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers (accessed on 29 July 2020).
- Yasuda, K.; Ikushiro, S.; Kamakura, M.; Ohta, M.; Sakaki, T. Metabolism of sesamin by cytochrome P450 in human liver microsomes. Drug Metab. Dispos. 2010, 38, 2117–2123. [Google Scholar] [CrossRef]
- Sasaki, T.; Sato, Y.; Kumagai, T.; Yoshinari, K.; Nagata, K. Effect of health foods on cytochrome P450-mediated drug metabolism. J. Pharm. Health Care Sci. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Tay-Sontheimer, J.; Shireman, L.M.; Beyer, R.P.; Senn, T.; Witten, D.; Pearce, R.E.; Gaedigk, A.; Gana Fomban, C.L.; Lutz, J.D.; Isoherranen, N.; et al. Detection of an endogenous urinary biomarker associated with CYP2D6 activity using global metabolomics. Pharmacogenomics 2014, 15, 1947–1962. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Smith, R.L. Addressing phenoconversion: The Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015, 79, 222–240. [Google Scholar] [CrossRef]
- Tolson, A.H.; Wang, H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv. Drug Deliv. Rev. 2010, 62, 1238–1249. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Molony, C.; Chudin, E.; Hao, K.; Zhu, J.; Gaedigk, A.; Suver, C.; Zhong, H.; Leeder, J.S.; et al. Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Res. 2010, 20, 1020–1036. [Google Scholar] [CrossRef]
- Jiang, F.; Yeo, C.W.; Lee, S.S.; Oh, M.K.; Ghim, J.L.; Shon, J.H.; Kim, H.S.; Kim, E.Y.; Kim, D.H.; Shin, J.G. Effect of HNF4α genetic polymorphism G60D on the pharmacokinetics of CYP2D6 substrate tolterodine in healthy Korean individuals. Pharm. Genom. 2013, 23, 175–179. [Google Scholar] [CrossRef]
- Koh, K.H.; Pan, X.; Shen, H.W.; Arnold, S.L.M.; Yu, A.M.; Gonzalez, F.J.; Isoherranen, N.; Jeong, H. Altered expression of small Heterodimer Partner Governs Cytochrome P450 (CYP) 2D6 induction during Pregnancy in CYP2D6-humanized mice. J. Biol. Chem. 2014, 289, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.H.; Pan, X.; Zhang, W.; McLachlan, A.; Urrutia, R.; Jeong, H. Krüppel-like factor 9 promotes hepatic cytochrome P450 2D6 expression during pregnancy in CYP2D6-humanized mice. Mol. Pharmacol. 2014, 86, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Bank, P.C.D.; Caudle, K.E.; Swen, J.J.; Gammal, R.S.; Whirl-Carrillo, M.; Klein, T.E.; Relling, M.V.; Guchelaar, H.J. Comparison of the Guidelines of the Clinical Pharmacogenetics Implementation Consortium and the Dutch Pharmacogenetics Working Group. Clin. Pharmacol. Ther. 2018, 103, 599–618. [Google Scholar] [CrossRef]
- Caudle, K.E.; Thorn, C.F.; Klein, T.E.; Swen, J.J.; McLeod, H.L.; Diasio, R.B.; Schwab, M. Clinical pharmacogenetics implementation consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin. Pharmacol. Ther. 2013, 94, 640–645. [Google Scholar] [CrossRef]
- Egan, A. Highlights of Prescribing Information—CERDELGATM (Eliglustat) Capsules. Available online: www.fda.gov/medwatch (accessed on 10 October 2020).
- Bank, P.C.D.; Swen, J.J.; Guchelaar, H.J. Estimated nationwide impact of implementing a preemptive pharmacogenetic panel approach to guide drug prescribing in primary care in The Netherlands. BMC Med. 2019, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mignat, C.; Wille, U.; Ziegler, A. Affinity profiles of morphine, codeine, dihydrocodeine and their glucuronides at opioid receptor subtypes. Life Sci. 1995, 56, 793–799. [Google Scholar] [CrossRef]
- Thorn, C.F.; Klein, T.E.; Altman, R.B. Codeine and Morphine Pathway. Available online: http://journals.lww.com/01213011-200907000-00009 (accessed on 24 October 2020).
- Dean, L. Codeine Therapy and CYP2D6 Genotype. In Medical Genetics Summaries; National Center for Biotechnology Information: Bethesda, MD, USA, 2012; pp. 1–9. [Google Scholar]
- Boyle, K.L.; Rosenbaum, C.D. Oxycodone overdose in the pediatric population: Case files of the University of Massachusetts Medical Toxicology Fellowship. J. Med. Toxicol. 2014, 10, 280–285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Racoosin, J.A.; Roberson, D.W.; Pacanowski, M.A.; Nielsen, D.R. New evidence about an old drug—Risk with codeine after adenotonsillectomy. N. Engl. J. Med. 2013, 368, 2155–2157. [Google Scholar] [CrossRef] [PubMed]
- Use of Codeine and Tramadol Products in Breastfeeding Women—Questions and Answers|FDA. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/use-codeine-and-tramadol-products-breastfeeding-women-questions-and-answers (accessed on 15 September 2020).
- Goetz, M.P.; Sangkuhl, K.; Guchelaar, H.-J.; Schwab, M.; Province, M.; Whirl-Carrillo, M.; Symmans, W.F.; Mcleod, H.L.; Ratain, M.J.; Zembutsu, H.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and Tamoxifen Therapy. Clin. Pharmacol. Ther. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Dueñas, E.; Ochoa Aranda, E.; Blancas Lopez-Barajas, I.; Ferrer Magdalena, T.; Bandrés Moya, F.; Chicharro García, L.M.; Gómez Capilla, J.A.; Zafra Ceres, M.; de Haro, T.; Romero Llorens, R.; et al. Adjusting the dose of tamoxifen in patients with early breast cancer and CYP2D6 poor metabolizer phenotype. Breast 2014, 23, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Lee, H.J.; Lee, K.S.; Lee, E.S.; Jang, I.J.; Ro, J. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J. Clin. Oncol. 2007, 25, 3837–3845. [Google Scholar] [CrossRef]
- Rae, J.M.; Drury, S.; Hayes, D.F.; Stearns, V.; Thibert, J.N.; Haynes, B.P.; Salter, J.; Sestak, I.; Cuzick, J.; Dowsett, M. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J. Natl. Cancer Inst. 2012, 104, 452–460. [Google Scholar] [CrossRef]
- Regan, M.M.; Leyland-Jones, B.; Bouzyk, M.; Pagani, O.; Tang, W.; Kammler, R.; Dell’Orto, P.; Biasi, M.O.; Thürlimann, B.; Lyng, M.B.; et al. CYP2D6 Genotype and Tamoxifen Response in Postmenopausal Women with Endocrine-Responsive Breast Cancer: The Breast International Group 1-98 Trial. J. Natl. Cancer 2008, 37, 1–9. [Google Scholar] [CrossRef]
- Chan, C.W.H.; Law, B.M.H.; So, W.K.W.; Chow, K.M.; Waye, M.M.Y. Pharmacogenomics of breast cancer: Highlighting CYP2D6 and tamoxifen. J. Cancer Res. Clin. Oncol. 2020, 146, 1395–1404. [Google Scholar] [CrossRef]
- Wei, X.; Sun, H.; Zhuang, J.; Weng, X.; Zheng, B.; Lin, Q.; Zhang, G.; Cai, J. Cost-effectiveness analysis of CYP2D6*10 pharmacogenetic testing to guide the adjuvant endocrine therapy for postmenopausal women with estrogen receptor positive early breast cancer in China. Clin. Drug Investig. 2020, 40, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Kamal, A.; Ames, M.M. Tamoxifen pharmacogenomics: The role of CYP2D6 as a predictor of drug response. Clin. Pharmacol. Ther. 2008, 83, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.J.; Thorn, C.F.; Desta, Z.; Flockhart, D.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Tamoxifen pathway, pharmacokinetics. Pharm. Genom. 2013, 23, 643–647. [Google Scholar] [CrossRef]
- Hicks, J.K.; Swen, J.J.; Thorn, C.F.; Sangkuhl, K.; Kharasch, E.D.; Ellingrod, V.L.; Skaar, T.C.; Müller, D.J.; Gaedigk, A.; Stingl, J.C. Clinical pharmacogenetics implementation consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 2013, 93, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Suiter, C.C.; Moriyama, T.; Matreyek, K.A.; Yang, W.; Scaletti, E.R.; Nishii, R.; Yang, W.; Hoshitsuki, K.; Singh, M.; Trehan, A.; et al. Massive parallel variant characterization identifies NUDT15 alleles associated with thiopurine toxicity. bioRxiv 2020, 1–42. [Google Scholar] [CrossRef]
- Mcinnes, G.; Dalton, R.; Sangkuhl, K.; Whirl-Carrillo, M.; Lee, S.-B.; Tsao, P.S.; Gaedigk, A.; Altman, R.B.; Woodahl, E.L. Transfer learning enables prediction of CYP2D6 haplotype function. bioRxiv 2020, 1–30. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Ingelman-Sundberg, M. Emerging strategies to bridge the gap between pharmacogenomic research and its clinical implementation. NPJ Genom. Med. 2020, 5, 1–7. [Google Scholar] [CrossRef]
- Liau, Y.; Maggo, S.; Miller, A.L.; Pearson, J.F.; Kennedy, M.A.; Cree, S.L. Nanopore sequencing of the pharmacogene CYP2D6 allows simultaneous haplotyping and detection of duplications. Pharmacogenomics 2019, 20, 1033–1047. [Google Scholar] [CrossRef]
- Napolitano, G.; Stingl, J.C.; Schmid, M.; Viviani, R. Predicting CYP2D6 phenotype from resting brain perfusion images by gradient boosting. Psychiatry Res. Neuroimaging 2017, 259, 16–24. [Google Scholar] [CrossRef]
- McInnes, G.; Dalton, R.; Sangkuhl, K.; Whirl-Carrillo, M.; Lee, S.; Altman, R.B.; Woodahl, E.L. Hubble2D6: A deep learning approach for predicting drug metabolic activity. bioRxiv 2019, 684357. [Google Scholar] [CrossRef]
- Van Der Lee, M.; Allard, W.G.; Vossen, R.H.A.M.; Baak-Pablo, R.F.; Menafra, R.; Deiman, B.A.L.M.; Deenen, M.J.; Neven, P.; Johansson, I.; Gastaldello, S.; et al. A unifying model to predict variable drug response for personalised medicine. bioRxiv 2020, 1–20. [Google Scholar] [CrossRef]
- Campion, D.P.; Dowell, F.J. Translating pharmacogenetics and pharmacogenomics to the clinic: Progress in human and veterinary medicine. Front. Vet. Sci. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Mealey, K.L. Adverse drug reactions in herding-breed dogs: The role of P-glycoprotein. Compend. Contin. Educ. Pract. Vet. 2006, 28, 23–33. [Google Scholar]
- Fleischer, S.; Sharkey, M.; Mealey, K.; Ostrander, E.A.; Martinez, M. Pharmacogenetic and metabolic differences between dog breeds: Their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS J. 2008, 10, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Paulson, S.K.; Engel, L.; Reitz, B.; Bolten, S.; Burton, E.G.; Maziasz, T.J.; Yan, B.; Schoenhard, G.L. Evidence for polymorphism in the canine metabolism of the cyclooxygenase 2 inhibitor, celecoxib. Drug Metab. Dispos. 1999, 27, 1133–1142. [Google Scholar]
- Corado, C.R.; McKemie, D.S.; Young, A.; Knych, H.K. Evidence for polymorphism in the cytochrome P450 2D50 gene in horses. J. Vet. Pharmacol. Ther. 2016, 39, 245–254. [Google Scholar] [CrossRef]
- Court, M.H. Canine Cytochrome P-450 Pharmacogenetics. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1027–1038. [Google Scholar]

| Drug | Drug Class | Guideline Committee Recommendations | PharmGKB Evidence Level | ||
|---|---|---|---|---|---|
| CPIC | DPWG | Other | |||
| Amiodarone | Antiarrhythmic | - | No Recommendation | - | - |
| Amitriptyline | Antidepressant | CD or AD | CD or AD | - | 1A |
| Aripiprazole | Antipsychotic | - | CD | - | 3 |
| Atenolol | β blocker | - | No Recommendation | - | - |
| Atomoxetine | ADHD treatment | CD | CD or AD | - | 1A |
| Bisoprolol | β blocker | - | No Recommendation | - | - |
| Brexpiprazole | Antipsychotic | - | CD | - | - |
| Carvedilol | β blocker | - | No Recommendation | - | 3 |
| Citalopram | Antidepressant | - | No Recommendation | - | 3 |
| Clomipramine | Antidepressant | CD or AD | CD or AD | - | 1A |
| Clonidine | Antihypertensive | - | No Recommendation | - | - |
| Clozapine | Antipsychotic | - | No Recommendation | - | - |
| Codeine | Analgesic | AD | CD or AD | CPNDs: AD | 1A |
| Desipramine | Antidepressant | CD or AD | - | - | 1A |
| Disopyramide | Antiarrhythmic | - | No Recommendation | - | - |
| Doxepin | Antidepressant | CD or AD | CD or AD | - | 1A |
| Duloxetine | Antidepressant | - | No Recommendation | - | - |
| Eliglustat | Gaucher’s disease | - | CD with CM or AD | - | - |
| Escitalopram | Antidepressant | - | No Recommendation | - | 3 |
| Flecainide | Antiarrhythmic | - | CD | - | 2A |
| Fluoxetine | Antidepressant | - | No Recommendation | - | 3 |
| Flupenthixol | Antipsychotic | - | No Recommendation | - | - |
| Fluphenazine | Antipsychotic | - | No Recommendation | - | - |
| Fluvoxamine | Antidepressant | CD or AD | No Recommendation | - | 1A |
| Gefitinib | Cancer treatment | - | No Recommendation | - | 3 |
| Haloperidol | Antipsychotic | - | CD or AD | - | 3 |
| Imipramine | Antidepressant | CD or AD | CD | - | 1A |
| Methylphenidate | ADHD treatment | - | No Recommendation | - | 4 |
| Metoprolol | β blocker | - | CD or AD | - | 2A |
| Mirtazapine | Antidepressant | - | No Recommendation | - | 3 |
| Nortriptyline | Antidepressant | CD or AD | CD or AD | - | 1A |
| Olanzapine | Antipsychotic | - | No Recommendation | - | 3 |
| Ondansetron | Antiemetic | AD | - | - | 1A |
| Oxycodone | Analgesic | - | No Recommendation | - | 2A |
| Paroxetine | Antidepressant | CD or AD | AD | - | 1A |
| Pimozide | Antipsychotic | - | CD | - | 4 |
| Propafenone | Antiarrhythmic | - | CD or AD | - | 2A |
| Quetiapine | Antipsychotic | - | No Recommendation | - | 4 |
| Quinidine | Antiarrhythmic | - | No Recommendation | - | - |
| Risperidone | Antipsychotic | - | CD or AD | - | 1B |
| Sertraline | Antidepressant | - | No Recommendation | - | 3 |
| Sotalol | Antiarrhythmic | - | No Recommendation | - | - |
| Tamoxifen | Cancer treatment | CD or AD | CD or AD | CPNDS: AD, RNPGx: No Recommendation | 1A |
| Tramadol | Analgesic | - | CD or AD | - | 1B |
| Trimipramine | Antidepressant | CD or AD | - | - | 1A |
| Tropisetron | Antiemetic | AD | - | - | 1A |
| Venlafaxine | Antidepressant | - | CD or AD | - | 2A |
| Zuclopenthixol | Antipsychotic | - | CD or AD | - | 3 |
| CYP2D6 Allele | Frequency | Predicted Function | |||
|---|---|---|---|---|---|
| AFR | EUR | EAS | SAS | ||
| *xN | 0.07 | 0.03 | 0.02 | 0.02 | Allele Specific |
| *1xN | 0.03 | 0.01 | 0.01 | 0.01 | Increased Metaboliser |
| *2 | 0.27 | 0.34 | 0.14 | 0.30 | Normal Metaboliser |
| *3 | 0.00 | 0.04 | 0.00 | 0.00 | Decreased Metaboliser |
| *4 | 0.12 | 0.15 | 0.00 | 0.09 | None Functional |
| *4xN | 0.02 | 0.00 | 0.00 | 0.00 | None Functional |
| *5 | 0.04 | 0.03 | 0.07 | 0.03 | None Functional |
| *6 | 0.00 | 0.02 | 0.00 | 0.00 | None Functional |
| *9 | 0.00 | 0.02 | 0.00 | 0.01 | Decreased Metaboliser |
| *10/114(A) | 0.03 | 0.00 | 0.59 | 0.20 | Decreased Metaboliser |
| *17 | 0.20 | 0.00 | 0.00 | 0.00 | Decreased Metaboliser |
| *29 | 0.09 | 0.00 | 0.00 | 0.00 | Decreased Metaboliser |
| *33 | 0.00 | 0.01 | 0.00 | 0.00 | Normal Metaboliser |
| *35 | 0.00 | 0.06 | 0.00 | 0.00 | Normal Metaboliser |
| *36 | 0.00 | 0.00 | 0.01 | 0.01 | Decreased Metaboliser |
| *41 | 0.03 | 0.03 | 0.03 | 0.08 | Decreased Metaboliser |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A Review of the Important Role of CYP2D6 in Pharmacogenomics. Genes 2020, 11, 1295. https://doi.org/10.3390/genes11111295
Taylor C, Crosby I, Yip V, Maguire P, Pirmohamed M, Turner RM. A Review of the Important Role of CYP2D6 in Pharmacogenomics. Genes. 2020; 11(11):1295. https://doi.org/10.3390/genes11111295
Chicago/Turabian StyleTaylor, Christopher, Ian Crosby, Vincent Yip, Peter Maguire, Munir Pirmohamed, and Richard M. Turner. 2020. "A Review of the Important Role of CYP2D6 in Pharmacogenomics" Genes 11, no. 11: 1295. https://doi.org/10.3390/genes11111295
APA StyleTaylor, C., Crosby, I., Yip, V., Maguire, P., Pirmohamed, M., & Turner, R. M. (2020). A Review of the Important Role of CYP2D6 in Pharmacogenomics. Genes, 11(11), 1295. https://doi.org/10.3390/genes11111295





