Application of Metal-Organic Frameworks and Covalent Organic Frameworks as (Photo)Active Material in Hybrid Photovoltaic Technologies
Abstract
:1. Introduction
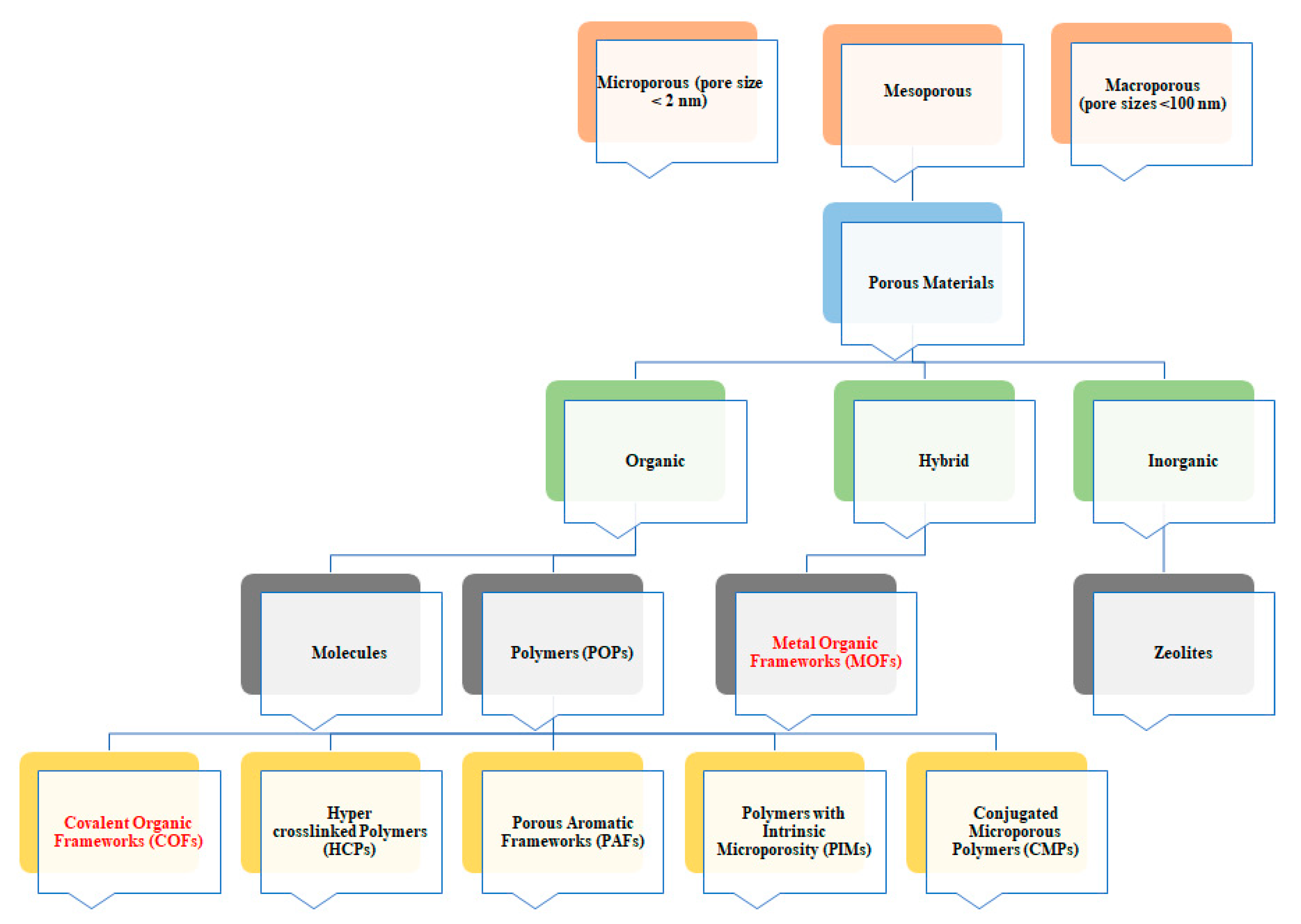
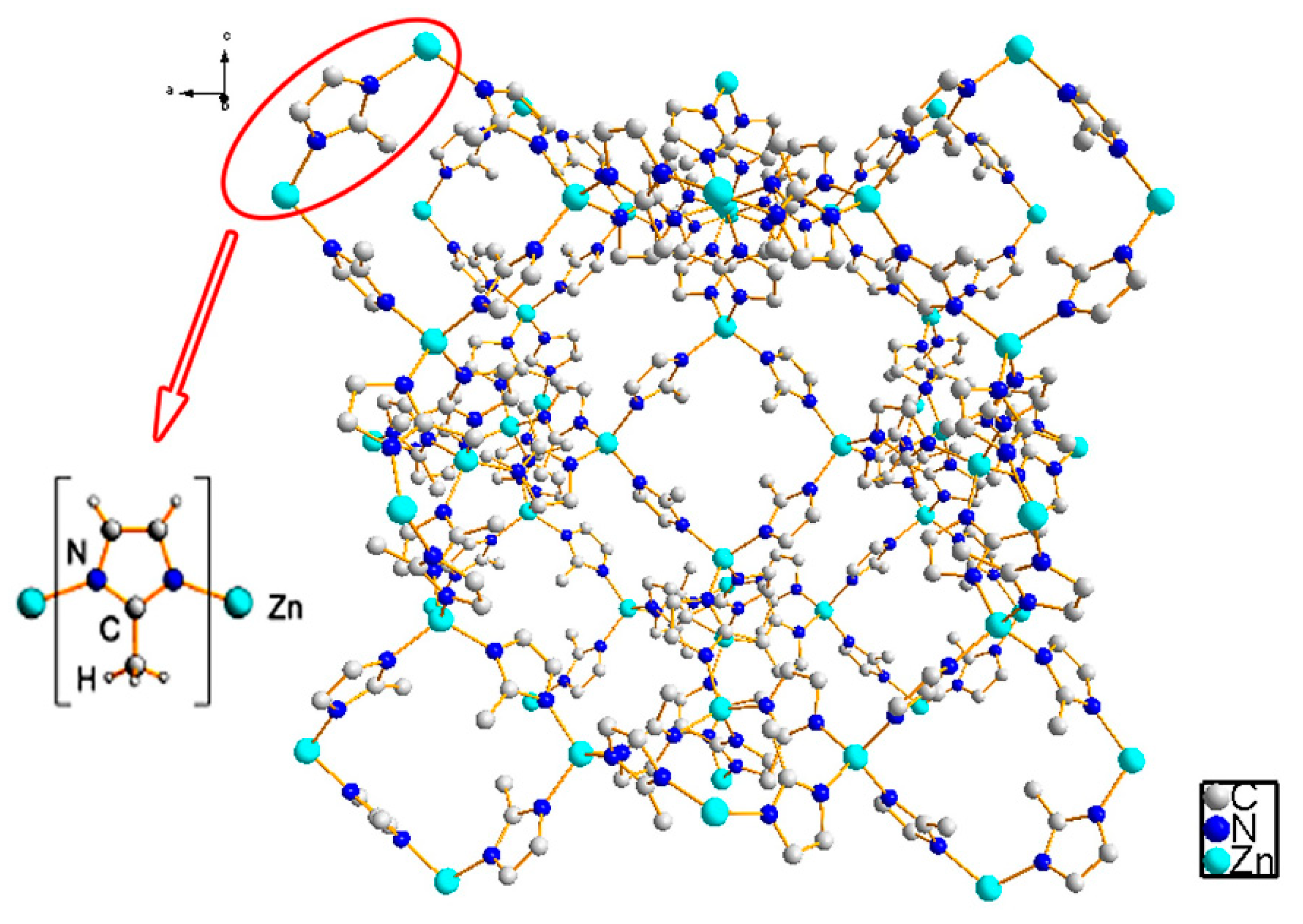
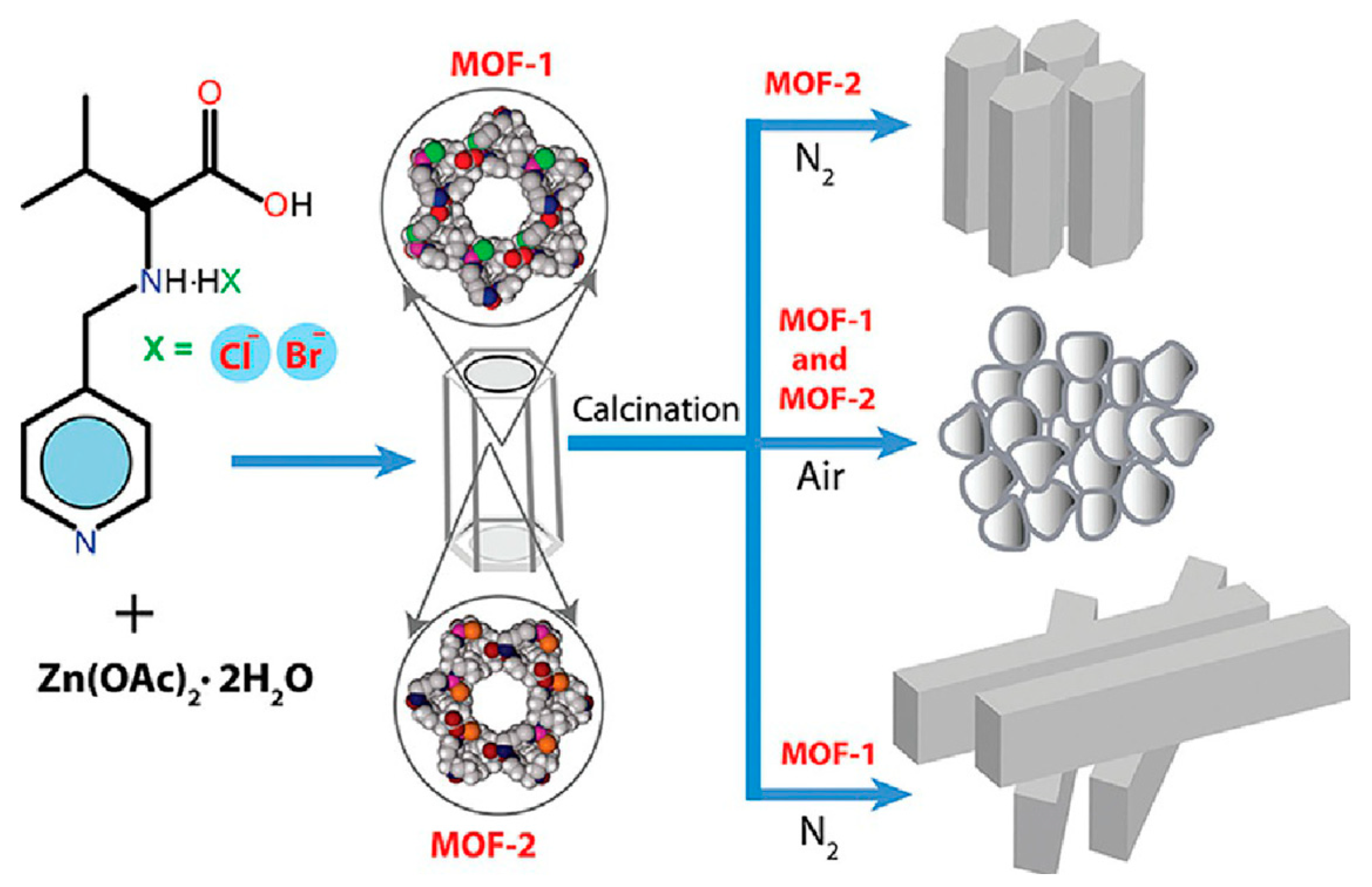
1.1. Metal-Organic Frameworks (MOFs)
1.2. Covalent Organic Frameworks (COFs)
2. Dye-Sensitized Solar Cells (DSSCs)
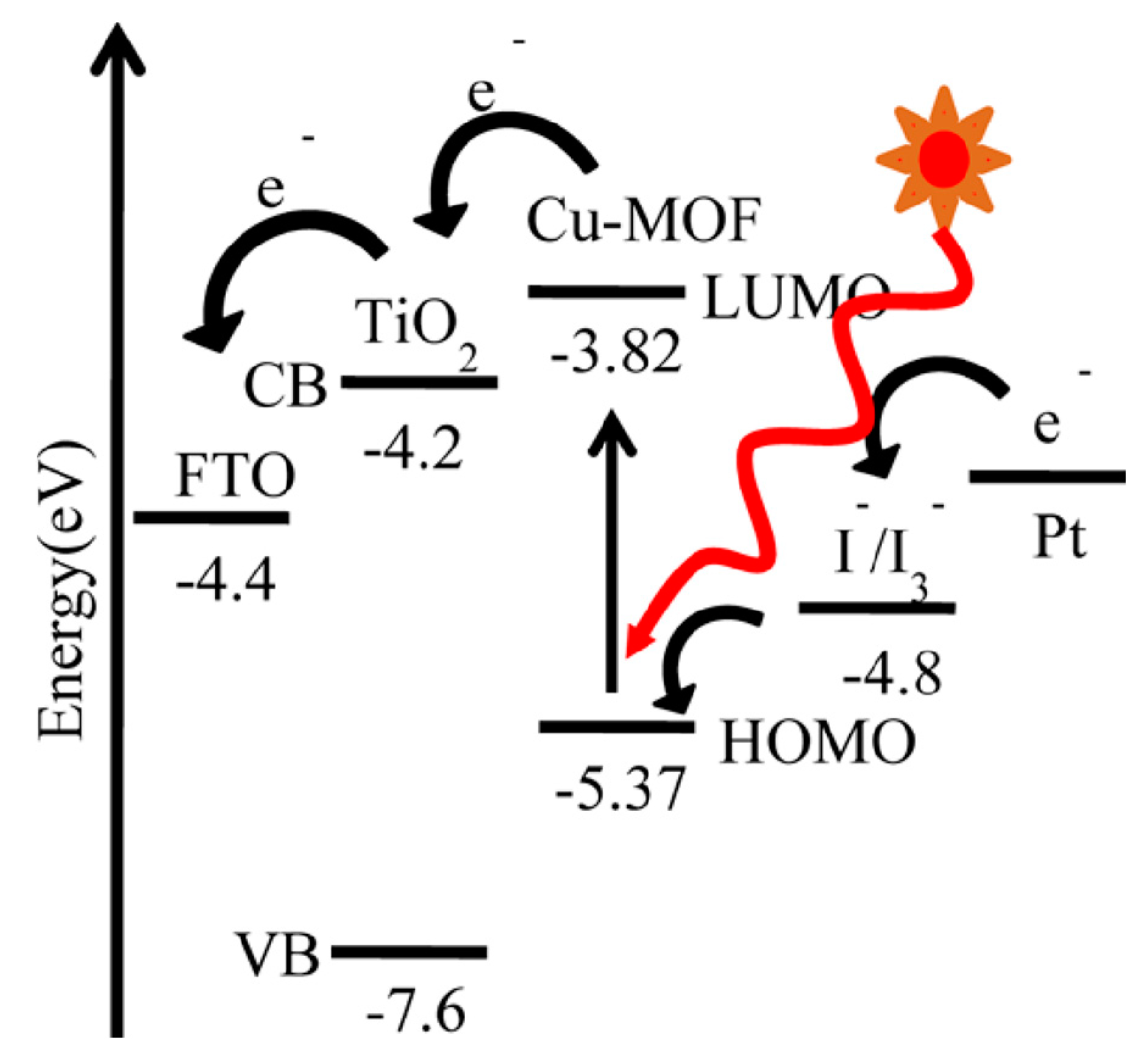
2.1. MOFs in DSSCs
2.1.1. MOF as Photoelectrode
2.1.2. MOFs as Sensitizers
2.1.3. Counter-Electrode CE
2.2. Covalent Organic Frameworks (COFs) in DSSCs
COFs as Photoactive Materials
3. Perovskite Solar Cells (PSCs)
3.1. MOFs in PSCs
3.2. COFs in PSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | 3-Dimensional |
| ACN | Acetonitrile |
| BDBA | 1,4-Benzenediboronic acid |
| BDBA | 1,4-Benzenediboronic acid |
| CE | Counter-electrode |
| CNR | Carbon nanorode |
| COF | Covalent organic framework |
| DAPV | Diaminopropyl viologen |
| DEF | Diethylformamide |
| DSSC | Dye-sensitized solar cells |
| DTP-ANDI | Naphtaline diimide |
| DTP-APyrDI | Pyrromellitic diimide |
| EIS | Electrochemical impedance spectroscopy |
| ETL | Electron transporting layer |
| FF | Fill factor |
| FP-TRMC | Flash photolysis time resolved microwave method |
| FTO | Fluorine-doped Tin Oxide |
| H4DOBDC | 2,5-Dihydroxyterephthalic acid |
| HHTP | 2,3,6,7,10,11-Hexahydroxytriphenylene |
| HHTP | 2,3,6,7,10,11-Hexahydroxytriphenylene |
| HOMO | Highest occupied molecular orbital |
| HTL | Hole transporting layer |
| HTM | Hole transport materials |
| ITO | Indium tin oxide |
| JSC | Short circuit photocurrent |
| LED | Light emitting electrochemical diodes |
| LEEC | Light emitting electrochemical cells |
| LDH | Layered double hydroxide |
| LUMO | Lowest unoccupied molecular orbital |
| MOF | Metal-organic framework |
| MPN | 3-Methoxypropionitrile |
| MW | Microwave |
| MWCNT | Multi-walled carbon nanotube |
| NH2-bdc | 2-Aminoterephthalic acid |
| NiPc-BTDA | Nickel phthalocyanine-3,3′,4,4′-benzophenonetetracarboxylic acid dianhydride |
| NIR | Near infrared |
| NMP | N-Methyl-2-pyrrolidone |
| NP | Nanoparticle |
| NT | Nanotube |
| OPV | Organic photovoltaic |
| PBBA | 1,4-Phenylenebis(boronic acid) |
| PCBM | Phenyl-C61-butyric acid methyl ester |
| PCE | Power conversion efficiency |
| PCP | Porous coordination polymer |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEGDGE | Poly(ethylene glycol) di-glycidyl ether |
| PET | Polyethylene Terephthalate |
| PMMA | Poly(methyl methacrylate |
| PPF | Pillared porphyrin framework |
| PPy | Polypyrrole |
| PSC | Perovskite solar cell |
| PSK | Perovskite |
| PT | tert-Butylpyrenetetraone |
| PVs | Photovoltaics |
| QD | Quantum dot |
| QSDSSC | Quasi-solid dye-sensitized solar cells |
| RT | Room temperature |
| RGO | Reduced graphene oxide |
| SC | Solar cell |
| SLG | Single-layer graphene |
| SP | Spirobifluorene |
| SURMOF | Surface-anchored metal-organic framework |
| TBP | tert-Butylpyridine |
| TCO | Transparent conductive oxide |
| TPHA | Triphenylene hexamine |
| TTBA | Thieno-[3,2-b]-thiophene-2,5-diyldiboronic acid |
| UV | Ultraviolet |
| VOC | Open-circuit voltage |
| ZIF | Zeolitic imidazolate framework |
| ZnPc[OH]8 | 2,3,9,1016,17,23,24-(Octahydroxyphthalocyanito) zinc |
| Zn-TCPP | Zinc(II) meso-tetrakis(4-carboxyphenyl)porphyrin |
| η | Power conversion efficiency |
References
- Grätzel, M. Photovoltaic and photoelectrochemical conversion of solar energy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Meillaud, F.; Boccard, M.; Bugnon, G.; Despeisse, M.; Hänni, S.; Haug, F.-J.; Persoz, J.; Schüttauf, J.-W.; Stuckelberger, M.; Ballif, C. Recent advances and remaining challenges in thin-film silicon photovoltaic technology. Mater. Today 2015, 18, 378–384. [Google Scholar] [CrossRef]
- Mehmood, H.; Tauqeer, T.; Hussain, S. Recent progress in silicon-based solid-state solar cells. Int. J. Electron. 2018, 105, 1568–1582. [Google Scholar] [CrossRef]
- Chapin, D.M.; Fuller, C.S.; Pearson, G.L. A new silicon p-n junction photocell for converting solar radiation into electrical power. J. Appl. Phys. 1954, 25, 676–677. [Google Scholar] [CrossRef]
- Alta, F.; Asu, E.S. National Renewable Energy Labs (NREL) efficiency chart. 2019. 2020. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200925.pdf (accessed on 30 August 2020).
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Phot. 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Wu, T.-C.; Long, Y.-S.; Hsu, S.-T.; Wang, E.-Y. Efficiency Rating of Various PV Technologies under Different Indoor Lighting Conditions. Energy Procedia 2017, 130, 66–71. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Jose, R.; Brown, T.M.; Fabregat-Santiago, F.; Bisquert, J. A perspective on the production of dye-sensitized solar modules. Energy Environ. Sci. 2014, 7, 3952–3981. [Google Scholar] [CrossRef]
- McConnell, I.; Li, G.; Brudvig, G.W. Energy Conversion in Natural and Artificial Photosynthesis. Chem. Biol. 2010, 17, 434–447. [Google Scholar] [CrossRef] [Green Version]
- Schulze, T.F.; Schmidt, T.W. Photochemical upconversion: Present status and prospects for its application to solar energy conversion. Energy Environ. Sci. 2015, 8, 103–125. [Google Scholar] [CrossRef] [Green Version]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.S.; Dangi, T.; Tak, P.; Sharma, S.; Ameta, R. Organic photovoltaic cells. Sol. Energy Convers. Storage Photochem. Modes 2015, 10, 55–84. [Google Scholar] [CrossRef]
- Segura, J.L.; Martín, N.; Guldi, D.M. Materials for organic solar cells: The C60/π-conjugated oligomer approach. Chem. Soc. Rev. 2005, 34, 31–47. [Google Scholar] [CrossRef]
- Wong, W.Y.; Ho, C.L. Organometallic Photovoltaics: A New and Versatile Approach for Harvesting Solar Energy Using Conjugated Polymetallaynes. Acc. Chem. Res. 2010, 43, 1246–1259. [Google Scholar] [CrossRef]
- Cavallo, C.; Di Pascasio, F.; Latini, A.; Bonomo, M.; Dini, D. Nanostructured Semiconductor Materials for Dye-Sensitized Solar Cells. J. Nanomater. 2017, 2017, 5323164. [Google Scholar] [CrossRef]
- Luceño-Sánchez, J.; Díez-Pascual, A.; Peña Capilla, R. Materials for Photovoltaics: State of Art and Recent Developments. Int. J. Mol. Sci. 2019, 20, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.D.D.; Sick, T.; Bein, T. Photoactive and Conducting Covalent Organic Frameworks. Adv. Energy Mater. 2017, 7, 1700387. [Google Scholar] [CrossRef]
- Wu, S.; Li, Z.; Li, M.Q.; Diao, Y.; Lin, F.; Liu, T.; Zhang, J.; Tieu, P.; Gao, W.; Qi, F.; et al. 2D metal–organic framework for stable perovskite solar cells with minimized lead leakage. Nat. Nanotechnol. 2020, in press. [Google Scholar] [CrossRef]
- Chueh, C.C.; Chen, C.I.; Su, Y.A.; Konnerth, H.; Gu, Y.J.; Kung, C.W.; Wu, K.C.W. Harnessing MOF materials in photovoltaic devices: Recent advances, challenges, and perspectives. J. Mater. Chem. A 2019, 7, 17079–17095. [Google Scholar] [CrossRef]
- Wu, X.-P.; Choudhuri, I.; Truhlar, D.G. Computational Studies of Photocatalysis with Metal–Organic Frameworks. Energy Environ. Mater. 2019, 2, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Nasalevich, M.A.; Hendon, C.H.; Santaclara, J.G.; Svane, K.; Van Der Linden, B.; Veber, S.L.; Fedin, M.V.; Houtepen, A.J.; Van Der Veen, M.A.; Kapteijn, F.; et al. Electronic origins of photocatalytic activity in d0 metal organic frameworks. Sci. Rep. 2016, 6, 23676. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best practices for the synthesis, activation, and characterization of metal−organic frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. New synthetic strategies toward covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 2852–2868. [Google Scholar] [CrossRef]
- Auras, F.; Ascherl, L.; Hakimioun, A.H.; Margraf, J.T.; Hanusch, F.C.; Reuter, S.; Bessinger, D.; Döblinger, M.; Hettstedt, C.; Karaghiosoff, K.; et al. Synchronized Offset Stacking: A Concept for Growing Large-Domain and Highly Crystalline 2D Covalent Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 16703–16710. [Google Scholar] [CrossRef]
- Ascherl, L.; Sick, T.; Margraf, J.T.; Lapidus, S.H.; Calik, M.; Hettstedt, C.; Karaghiosoff, K.; Döblinger, M.; Clark, T.; Chapman, K.W.; et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 2016, 8, 310–316. [Google Scholar] [CrossRef]
- Alahakoon, S.B.; Thompson, C.M.; Occhialini, G.; Smaldone, R.A. Design Principles for Covalent Organic Frameworks in Energy Storage Applications. ChemSusChem 2017, 10, 2116–2129. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Y. Narrowing the Band Gap: The Key to High-Performance Organic Photovoltaics. Acc. Chem. Res. 2020, 53, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Ye, P.; Lu, J.; Wu, X.; Chen, Y.; Zhu, T.; Peng, A.; Huang, H. Tellurophene-based metal-organic framework nanosheets for high-performance organic solar cells. J. Power Sources 2018, 401, 13–19. [Google Scholar] [CrossRef]
- Zhang, W.; Li, W.; He, X.; Zhao, L.; Chen, H.; Zhang, L.; Tian, P.; Xin, Z.; Fang, W.; Zhang, F. Dendritic Fe-based polyoxometalates @ metal–organic framework (MOFs) combined with ZnO as a novel photoanode in solar cells. J. Mater. Sci. Mater. Electron. 2018, 29, 1623–1629. [Google Scholar] [CrossRef]
- Sasitharan, K.; Bossanyi, D.G.D.G.; Vaenas, N.; Parnell, A.J.A.J.; Clark, J.; Iraqi, A.; Lidzey, D.G.D.G.; Foster, J.A.J.A. Metal-organic framework nanosheets for enhanced performance of organic photovoltaic cells. J. Mater. Chem. A 2020, 8, 6067–6075. [Google Scholar] [CrossRef]
- Bildirir, H.; Gregoriou, V.G.; Avgeropoulos, A.; Scherf, U.; Chochos, C.L. Porous organic polymers as emerging new materials for organic photovoltaic applications: Current status and future challenges. Mater. Horizons 2017, 4, 546–556. [Google Scholar] [CrossRef]
- Su, C.Y.; Dong, Y. Bin Metal-organic frameworks (MOFs). J. Solid State Chem. 2015, 223, 1. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Biondi, C.; Bonamico, M.; Torelli, L.; Vaciago, A. On the structure and water content of copper(II) tricyanomethanide. Chem. Commun. 1965, 191–192. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and Construction of a New Class of Scaffolding-like Materials Comprising Infinite Polymeric Frameworks of 3D-Linked Molecular Rods. A Reappraisal of the Zn(CN)2 and Cd(CN)2 Structures and the Synthesis and Structure of the Diamond-Related Framework. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.B.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.R.; Habas, S.; Yang, P. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Li, A.; Wiggin, S.B.; Tao, A.; Maloney, A.G.P.; Wood, P.A.; Ward, S.C.; Fairen-Jimenez, D. Development of a Cambridge Structural Database Subset: A Collection of Metal-Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Washizu, S.; Ogura, K.; Kwon, Y.J. Preparation, Clathration Ability, and Catalysis of a Two-Dimensional Square Network Material Composed of Cadmium(II) and 4, 4ʹ-Bipyridine. J. Am. Chem. Soc. 1994, 116, 1151–1152. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [Green Version]
- Services, R.; Issue, L.; Issues, P.; Service, E.; Links, R.; Blog, C.W.; Newsvine, S.; Products, R. Instant insight: Nothing but surface. Chem. Soc. Rev. 2009, 38, 12–14. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Atzori, C.; Civalleri, B.; Barbero, N.; Barolo, C.; Bonino, F. Design and Characterization of MOFs (Metal–Organic Frameworks) for Innovative Applications. In Hybrid Organic-Inorganic Interfaces: Towards Advanced Functional Materials; Delville, M., Taubert, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; ISBN 9783527342556. [Google Scholar]
- Nasalevich, M.A.; Goesten, M.G.; Savenije, T.J.; Kapteijn, F.; Gascon, J. Enhancing optical absorption of metal-organic frameworks for improved visible light photocatalysis. Chem. Commun. 2013, 49, 10575–10577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, B.J.; Nguyen, S.T.; Hupp, J.T.; Farha, O.K.; Lee, C.Y.; Sarjeant, A.A. Light-Harvesting Metal–Organic Frameworks (MOFs): Efficient Strut-to-Strut Energy Transfer in Bodipy and Porphyrin-Based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef]
- Zhang, X.; Ballem, M.A.; Hu, Z.J.; Bergman, P.; Uvdal, K. Nanoscale light-harvesting metal-organic frameworks. Angew. Chemie Int. Ed. 2011, 50, 5729–5733. [Google Scholar] [CrossRef]
- Yan, D.; Tang, Y.; Lin, H.; Wang, D. Tunable two-color luminescence and host-guest energy transfer of fluorescent chromophores encapsulated in metal-organic frameworks. Sci. Rep. 2014, 4, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Kent, C.A.; Mehl, B.P.; Ma, L.; Papanikolas, J.M.; Meyer, T.J.; Lin, W. Energy transfer dynamics in metal-organic frameworks. J. Am. Chem. Soc. 2010, 132, 12767–12769. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, structure, and metalation of two new highly porous zirconium metal-organic frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, O.; Derkus, B. Triazine-based 2D covalent organic frameworks improve the electrochemical performance of enzymatic biosensors. J. Mater. Sci. 2020, 55, 3034–3044. [Google Scholar] [CrossRef]
- Bonomo, M. Synthesis and characterization of NiO nanostructures: A review. J. Nanoparticle Res. 2018, 20, 222. [Google Scholar] [CrossRef]
- Mollamahaleh, Y.B.; Hosseini, D.; Mazaheri, M.; Sadrnezhaad, S.K. Surfactant-Free Production of Ni-Based Nanostructures. Mater. Sci. Appl. 2011, 02, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Demazeau, G. Solvothermal reactions: An original route for the To cite this version: HAL Id: Hal-00269253. J. Mater. Sci. 2008, 7, 2104–2114. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Xia, L.; Liu, X. Covalent organic frameworks (COFs): Perspectives of industrialization. CrystEngComm 2018, 20, 1613–1634. [Google Scholar] [CrossRef]
- Campbell, N.L.; Clowes, R.; Ritchie, L.K.; Cooper, A.I. Rapid Microwave Synthesis and Purification of Porous Covalent Organic Frameworks. Chem. Mater. 2009, 21, 204–206. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjee, R. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef]
- Yang, C.X.; Liu, C.; Cao, Y.M.; Yan, X.P. Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem. Commun. 2015, 51, 12254–12257. [Google Scholar] [CrossRef]
- Bella, F.; Gerbaldi, C.; Barolo, C.; Grätzel, M. Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3431–3473. [Google Scholar] [CrossRef] [Green Version]
- Barbero, N.; Sauvage, F. Low-cost electricity production from sunlight: Third-generation photovoltaics and the dye-sensitized solar cell. In Materials for Sustainable Energy Applications: Conversion, Storage, Transmission, and Consumption; Munoz-Rojas, D., Moya, X., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2016; ISBN 9789814411820. [Google Scholar]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–739. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, M.; Li, J.Y.; Pootrakulchote, N.; Alibabaei, L.; Ngoc-Le, C.H.; Decoppet, J.D.; Tsai, J.H.; Grätzel, C.; Wu, C.G.; et al. Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells. ACS Nano 2009, 3, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, T.; Ezure, T.; Okada, K.; Matsui, H.; Goto, K.; Tanabe, N. FTO/ITO double-layered transparent conductive oxide for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2004, 164, 199–202. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.; Dini, D.; Decker, F. Electrochemical and photoelectrochemical properties of nickel oxide (NiO) with nanostructured morphology for photoconversion applications. Front. Chem. 2018, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Q. Ruthenium Sensitizers and Their Applications in Dye-Sensitized Solar Cells. Int. J. Photoenergy 2012, 2012, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Bella, F.; Bongiovanni, R. Photoinduced polymerization: An innovative, powerful and environmentally friendly technique for the preparation of polymer electrolytes for dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2013, 16, 1–21. [Google Scholar] [CrossRef]
- Thomas, S.; Deepak, T.G.; Anjusree, G.S.; Arun, T.A.; Nair, S.V.; Nair, A.S. A review on counter electrode materials in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 4474–4490. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
- Hardin, B.E.; Snaith, H.J.; McGehee, M.D. The renaissance of dye-sensitized solar cells. Nat. Photonics 2012, 6, 162–169. [Google Scholar] [CrossRef]
- Lee, D.Y.; Shinde, D.V.; Yoon, S.J.; Cho, K.N.; Lee, W.; Shrestha, N.K.; Han, S.H. Cu-based metal-organic frameworks for photovoltaic application. J. Phys. Chem. C 2014, 118, 16328–16334. [Google Scholar] [CrossRef]
- Maza, W.A.; Haring, A.J.; Ahrenholtz, S.R.; Epley, C.C.; Lin, S.Y.; Morris, A.J. Ruthenium(II)-polypyridyl zirconium(IV) metal-organic frameworks as a new class of sensitized solar cells. Chem. Sci. 2016, 7, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Kim, E.K.; Shin, C.Y.; Shinde, D.V.; Lee, W.; Shrestha, N.K.; Lee, J.K.; Han, S.H. Layer-by-layer deposition and photovoltaic property of Ru-based metal-organic frameworks. RSC Adv. 2014, 4, 12037–12042. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Liu, J.; Fujimori, Y.; Higashino, T.; Imahori, H.; Jiang, X.; Zhao, J.; Sakurai, T.; Hattori, Y.; et al. A new class of epitaxial porphyrin metal-organic framework thin films with extremely high photocarrier generation efficiency: Promising materials for all-solid-state solar cells. J. Mater. Chem. A 2016, 4, 12739–12747. [Google Scholar] [CrossRef]
- Bella, F.; Bongiovanni, R.; Kumar, R.S.; Kulandainathan, M.A.; Stephan, A.M. Light cured networks containing metal organic frameworks as efficient and durable polymer electrolytes for dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 9033–9036. [Google Scholar] [CrossRef]
- Lopez, H.A.; Dhakshinamoorthy, A.; Ferrer, B.; Atienzar, P.; Alvaro, M.; Garcia, H. Photochemical response of commercial MOFs: Al2(BDC)3 and its use as active material in photovoltaic devices. J. Phys. Chem. C 2011, 115, 22200–22206. [Google Scholar] [CrossRef]
- Kundu, T.; Sahoo, S.C.; Banerjee, R. Solid-state thermolysis of anion induced metal-organic frameworks to ZnO microparticles with predefined morphologies: Facile synthesis and solar cell studies. Cryst. Growth Des. 2012, 12, 2572–2578. [Google Scholar] [CrossRef]
- Li, Y.; Pang, A.; Wang, C.; Wei, M. Metal-organic frameworks: Promising materials for improving the open circuit voltage of dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 17259–17264. [Google Scholar] [CrossRef]
- Park, J.T.; Moon, J.; Choi, G.H.; Lim, S.M.; Kim, J.H. Facile graft copolymer template synthesis of mesoporous polymeric metal-organic frameworks to produce mesoporous TiO2: Promising platforms for photovoltaic and photocatalytic applications. J. Ind. Eng. Chem. 2020, 84, 384–392. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Zhao, K.; Hao, M.; Zhang, Z.; Li, L.; Zhang, Y.; Zhang, W. A facile hydrothemal synthesis of MoS2@Co3S4 composites based on metal organic framework compounds as a high-efficiency liquid-state solar cell counter electrode. J. Alloys Compd. 2020, 831, 154910. [Google Scholar] [CrossRef]
- Wu, J.; Pan, W.; Lin, Y.Y.; Zhu, J.; Jiang, Q.S.Q.-S.; Zhao, Y.Y.; Ji, R.; Zhang, Y. Metal–organic framework-derived cobalt diselenide as an efficient electrocatalyst for dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2020, 31, 12309–12316. [Google Scholar] [CrossRef]
- Padmanathan, S.; Prakasam, A. Incorporation of Carbon Dots on the ZnO Nanosheets as Metal–Organic Framework Photoanodes for High Efficient Dye Sensitized Solar Cell Applications. J. Clust. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Gao, J.; Miao, J.; Li, P.Z.; Teng, W.Y.; Yang, L.; Zhao, Y.; Liu, B.; Zhang, Q. A p-type Ti(iv)-based metal-organic framework with visible-light photo-response. Chem. Commun. 2014, 50, 3786–3788. [Google Scholar] [CrossRef]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’Arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the optical response of the titanium-MIL-125 metal-organic framework through ligand functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Wang, S.; Ding, Z.; Wang, S.; Zhou, Y.; Huang, L.; Wang, X. Amine-functionalized zirconium metal-organic framework as efficient visible-light photocatalyst for aerobic organic transformations. Chem. Commun. 2012, 48, 11656–11658. [Google Scholar] [CrossRef]
- Sarwar, S.; Lee, M.S.; Park, S.; Dao, T.T.; Ullah, A.; Hong, S.; Han, C.H. Transformation of a liquid electrolyte to a gel inside dye sensitized solar cells for better stability and performance. Thin Solid Films 2020, 704, 138024. [Google Scholar] [CrossRef]
- Oszajca, M.; Podborska, A.; Szaciłowski, K. Unconventional molecular scale logic devices. In Handbook of Organic Materials for Optical and (Opto)electronic Devices; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780857098764. [Google Scholar]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical applications of TiO2 nanostructures: Recent advances. Int. J. Nanomedicine 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupuy, L.; Haller, S.; Rousset, J.; Donsanti, F.; Guillemoles, J.F.; Lincot, D.; Decker, F. Impedance measurements of nanoporosity in electrodeposited ZnO films for DSSC. Electrochem. commun. 2010, 12, 697–699. [Google Scholar] [CrossRef]
- Minoura, H.; Yoshida, T. Electrodeposition of ZnO/dye hybrid thin films for dye-sensitized solar cells. Electrochemistry 2008, 76, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Turković, A.; Orel, Z.C. Dye-sensitized solar cell with CeO2 and mixed CeO2/SnO2 photoanodes. Sol. Energy Mater. Sol. Cells 1997, 45, 275–281. [Google Scholar] [CrossRef]
- Zheng, H.; Tachibana, Y.; Kalantar-Zadeh, K. Dye-sensitized solar cells based on WO3. Langmuir 2010, 26, 19148–19152. [Google Scholar] [CrossRef]
- Burnside, S.; Moser, J.-E.; Brooks, K.; Grätzel, M.; Cahen, D. Nanocrystalline mesoporous strontium titanate as photoelectrode material for photosensitized solar devices: Increasing photovoltage through flatband potential engineering. J. Phys. Chem. B 1999, 103, 9328–9332. [Google Scholar] [CrossRef] [Green Version]
- Sayama, K.; Sugihara, H.; Arakawa, H. Photoelectrochemical properties of a porous Nb2O5 electrode sensitized by a ruthenium dye. Chem. Mater. 1998, 10, 3825–3832. [Google Scholar] [CrossRef]
- Silva, C.G.; Corma, A.; García, H. Metal-organic frameworks as semiconductors. J. Mater. Chem. 2010, 20, 3141–3156. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Llabrés I Xamena, F.X.; Garcia, H. Semiconductor behavior of a metal-organic framework (MOF). Chem. A Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, W.; Zhou, H.; Li, J.; Xu, D. Hydrothermal synthesis of TiO2(B) nanowires with ultrahigh surface area and their fast charging and discharging properties in Li-ion batteries. Chem. Commun. 2011, 47, 3439–3441. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.L.; Liu, B.; Lan, Y.Q.; Kuratani, K.; Akita, T.; Shioyama, H.; Zong, F.; Xu, Q. From metal-organic framework to nanoporous carbon: Toward a very high surface area and hydrogen uptake. J. Am. Chem. Soc. 2011, 133, 11854–11857. [Google Scholar] [CrossRef]
- Snurr, R.Q.; Wilmer, C.E.; Farha, O.K.; Jeong, N.C.; Yazaydın, A.Ö.; Nguyen, S.T.; Hupp, J.T.; Hauser, B.G.; Sarjeant, A.A.; Eryazici, I. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Dou, J.; Li, Y.; Chen, C.; Sun, X. Metal-Organic Frameworks at Interfaces in Dye-Sensitized Solar Cells. ChemSusChem 2014, 7, 2469–2472. [Google Scholar] [CrossRef]
- Joly, F.; Devaux, P.; Loiseau, T.; Arab, M.; Morel, B.; Volkringer, C. Optimization of the synthesis of UiO-66(Zr) in ionic liquids. Microporous Mesoporous Mater. 2019, 288, 109564. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Z.; Wang, W.; Fu, L. Metal organic frameworks derived high-performance photoanodes for DSSCs. J. Alloys Compd. 2020, 825, 154089. [Google Scholar] [CrossRef]
- Chi, W.S.; Roh, D.K.; Lee, C.S.; Kim, J.H. A shape- and morphology-controlled metal organic framework template for high-efficiency solid-state dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 21599–21608. [Google Scholar] [CrossRef]
- Moad, G.; Jones, R.G.; Alemán, J.V.; Kratochvíl, P.; Stepto, R.F.T.; Hess, M.; Chadwick, A.V.; Penczek, S.; Mita, I.; Horie, K.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Tao, L.; Huo, Z.; Ding, Y.; Li, Y.; Dai, S.; Wang, L.; Zhu, J.; Pan, X.; Zhang, B.; Yao, J.; et al. High-efficiency and stable quasi-solid-state dye-sensitized solar cell based on low molecular mass organogelator electrolyte. J. Mater. Chem. A 2015, 3, 2344–2352. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, P.; Cong, S.; Zhao, J.; Zou, G. Carbon nanotubes embedding organic ionic plastic crystals electrolytes for high performance solid-state dye-sensitized solar cells. Carbon N. Y. 2015, 92, 262–270. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Kumar, P.R.; Mothi, E.M.; Karuppasamy, K.; Kim, H.S.; Maiyalagan, T.; Shajan, X.S. Highly interconnected porous TiO2-Ni-MOF composite aerogel photoanodes for high power conversion efficiency in quasi-solid dye-sensitized solar cells. Appl. Surf. Sci. 2019, 496, 143646. [Google Scholar] [CrossRef]
- Alwin, S.; Ramasubbu, V.; Sahaya Shajan, X. TiO2 aerogel–metal organic framework nanocomposite: A new class of photoanode material for dye-sensitized solar cell applications. Bull. Mater. Sci. 2018, 41, 27. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Che, Z.; Sun, X.; Dou, J.; Wei, M. Metal-organic framework derived hierarchical ZnO parallelepipeds as an efficient scattering layer in dye-sensitized solar cells. Chem. Commun. 2014, 50, 9769–9772. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Shin, C.Y.; Yoon, S.J.; Lee, H.Y.; Lee, W.; Shrestha, N.K.; Lee, J.K.; Han, S.H. Enhanced photovoltaic performance of Cu-based metal-organic frameworks sensitized solar cell by addition of carbon nanotubes. Sci. Rep. 2014, 4, 3930. [Google Scholar] [CrossRef]
- Gordillo, M.A.; Panda, D.K.; Saha, S. Efficient MOF-Sensitized Solar Cells Featuring Solvothermally Grown [100]-Oriented Pillared Porphyrin Framework-11 Films on ZnO/FTO Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 3196–3206. [Google Scholar] [CrossRef]
- Khajavian, R.; Ghani, K. Cu-based metal-organic framework thin films: A morphological and photovoltaic study. J. Solid State Chem. 2018, 262, 94–99. [Google Scholar] [CrossRef]
- Carella, A.; Borbone, F.; Centore, R. Research progress on photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundaraprabhu, R.; Satish Kumar, T.; Prabavathy, N.; Senthilarasu, S.; Prasanna, S. Status and outlook of sensitizers/dyes used in dye sensitized solar cells (DSSC): A review. Int. J. Energy Res. 2016, 40, 1303–1320. [Google Scholar] [CrossRef]
- Saccone, D.; Galliano, S.; Barbero, N.; Quagliotto, P.; Viscardi, G.; Barolo, C. Polymethine Dyes in Hybrid Photovoltaics: Structure-Properties Relationships. European J. Org. Chem. 2016, 2016, 2244–2259. [Google Scholar] [CrossRef]
- Park, J.; Viscardi, G.; Barolo, C.; Barbero, N. Near-infrared sensitization in dye-sensitized solar cells. Int. J. Chem. 2013, 67, 129–135. [Google Scholar] [CrossRef]
- Saygili, Y.; Stojanovic, M.; Flores-Díaz, N.; Zakeeruddin, S.M.; Vlachopoulos, N.; Grätzel, M.; Hagfeldt, A. Metal coordination complexes as redox mediators in regenerative dye-sensitized solar cells. Inorganics 2019, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Jennings, J.R.; Parameswaran, M.; Wang, Q. An organic redox mediator for dye-sensitized solar cells with near unity quantum efficiency. Energy Environ. Sci. 2011, 4, 564–571. [Google Scholar] [CrossRef]
- Hagberg, D.P.; Edvinsson, T.; Marinado, T.; Boschloo, G.; Hagfeldt, A.; Sun, L. A novel organic chromophore for dye-sensitized nanostructured solar cells. Chem. Commun. 2006, 2245–2247. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Fischer, M.K.R.; Büuerle, P. Metal-Free organic dyes for dye-Sensitized solar cells: From structure: Property relationships to design rules. Angew. Chemie Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Bonomo, M.; Barbero, N.; Naponiello, G.; Giordano, M.; Dini, D.; Barolo, C. Sodium Hydroxide Pretreatment as an Effective Approach to Reduce the Dye / Holes Recombination Reaction in P-Type DSCs. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, W.; Liu, J.; Howard, I.; Kilibarda, G.; Schlabach, S.; Coupry, D.; Addicoat, M.; Yoneda, S.; Tsutsui, Y.; et al. Photoinduced Charge-Carrier Generation in Epitaxial MOF Thin Films: High Efficiency as a Result of an Indirect Electronic Band Gap? Angew. Chemie Int. Ed. 2015, 54, 7441–7445. [Google Scholar] [CrossRef] [PubMed]
- Spoerke, E.D.; Small, L.J.; Foster, M.E.; Wheeler, J.; Ullman, A.M.; Stavila, V.; Rodriguez, M.; Allendorf, M.D. MOF-Sensitized Solar Cells Enabled by a Pillared Porphyrin Framework. J. Phys. Chem. C 2017, 121, 4816–4824. [Google Scholar] [CrossRef]
- Joyce, J.T.; Laffir, F.R.; Silien, C. Layer-by-layer growth and photocurrent generation in metal-organic coordination films. J. Phys. Chem. C 2013, 117, 12502–12509. [Google Scholar] [CrossRef]
- Dou, J.; Li, Y.; Xie, F.; Ding, X.; Wei, M. Metal-Organic Framework Derived Hierarchical Porous Anatase TiO2 as a Photoanode for Dye-Sensitized Solar Cell. Cryst. Growth Des. 2016, 16, 121–125. [Google Scholar] [CrossRef]
- Tang, R.; Xie, Z.; Zhou, S.; Zhang, Y.; Yuan, Z.; Zhang, L.; Yin, L. Cu2ZnSnS4 Nanoparticle Sensitized Metal-Organic Framework Derived Mesoporous TiO2 as Photoanodes for High-Performance Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 22201–22212. [Google Scholar] [CrossRef]
- Du, X.; Fan, R.; Wang, X.; Yu, G.; Qiang, L.; Wang, P.; Gao, S.; Yang, Y. Cooperative Crystallization of Chiral Heterometallic Indium(III)-Potassium(I) Metal-Organic Frameworks as Photosensitizers in Luminescence Sensors and Dye-Sensitized Solar Cells. Cryst. Growth Des. 2016, 16, 1737–1745. [Google Scholar] [CrossRef]
- Meier, S.B.; Tordera, D.; Pertegás, A.; Roldán-Carmona, C.; Ortí, E.; Bolink, H.J. Light-emitting electrochemical cells: Recent progress and future prospects. Mater. Today 2014, 17, 217–223. [Google Scholar] [CrossRef]
- Chen, H.-W.; Lee, J.-H.; Lin, B.-Y.; Chen, S.; Wu, S.-T. Liquid crystal display and organic light-emitting diode display: Present status and future perspectives. Light Sci. Appl. 2018, 7, 17168. [Google Scholar] [CrossRef] [PubMed]
- Babu, H.V.; Bai, M.G.M.; Rajeswara Rao, M. Functional π-Conjugated Two-Dimensional Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2019, 11, 11029–11060. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.Y.; Lee, D.Y.; Shin, C.Y.; Bui, H.T.; Shrestha, N.K.; Giebeler, L.; Noh, Y.Y.; Han, S.H. Novel Solid-State Solar Cell Based on Hole-Conducting MOF-Sensitizer Demonstrating Power Conversion Efficiency of 2.1%. ACS Appl. Mater. Interfaces 2017, 9, 12930–12935. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe-TiO2 architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Nozik, A.J. Quantum dot solar cells. Phys. E Low-Dimens. Syst Nanostruct. 2002, 14, 115–120. [Google Scholar] [CrossRef]
- Brus, L. Size, dimensionality, and strong electron correlation in Nanoscience. Acc. Chem. Res. 2014, 47, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Quantum dot solar cells. The next big thing in photovoltaics. J. Phys. Chem. Lett. 2013, 4, 908–918. [Google Scholar] [CrossRef]
- Farha, O.K.; Wiederrecht, G.P.; Hupp, J.T.; Son, H.-J.; Jin, S. Energy Transfer from Quantum Dots to Metal–Organic Frameworks for Enhanced Light Harvesting. J. Am. Chem. Soc. 2013, 135, 955–958. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, A.L.; Kim, K.H.; Deep, A. A novel CdTe/Eu-MOF photoanode for application in quantum dot-sensitized solar cell to improve power conversion efficiency. J. Ind. Eng. Chem. 2017, 53, 77–81. [Google Scholar] [CrossRef]
- Kaur, R.; Rana, A.; Singh, R.K.; Chhabra, V.A.; Kim, K.H.; Deep, A. Efficient photocatalytic and photovoltaic applications with nanocomposites between CdTe QDs and an NTU-9 MOF. RSC Adv. 2017, 7, 29015–29024. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Nattestad, A.; Yu, H.; Bai, Y.; Wang, L.; Dou, S.X.; Kim, J.H. Highly connected hierarchical textured TiO2 spheres as photoanodes for dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 8902–8909. [Google Scholar] [CrossRef]
- Lin, J.; Heo, Y.U.; Nattestad, A.; Sun, Z.; Wang, L.; Kim, J.H.; Dou, S.X. 3D hierarchical rutile TiO2 and metal-free organic sensitizer producing dye-sensitized solar cells 8.6% conversion efficiency. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Hu, Y.H. Graphene as a counter electrode material for dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 8182–8188. [Google Scholar] [CrossRef]
- Ahmad, I.; McCarthy, J.E.; Bari, M.; Gun’ko, Y.K. Carbon nanomaterial based counter electrodes for dye sensitized solar cells. Sol. Energy 2014, 102, 152–161. [Google Scholar] [CrossRef]
- Park, B.W.; Pazoki, M.; Aitola, K.; Jeong, S.; Johansson, E.M.J.; Hagfeldt, A.; Boschloo, G. Understanding interfacial charge transfer between metallic PEDOT counter electrodes and a cobalt redox shuttle in dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 2074–2079. [Google Scholar] [CrossRef]
- Wang, M.; Anghel, A.M.; Marsan, B.; Ha, N.L.C.; Pootrakulchote, N.; Zakeeruddin, S.M.; Grätzel, M. CoS supersedes Pt as efficient electrocatalyst for triiodide reduction in dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 15976–15977. [Google Scholar] [CrossRef] [PubMed]
- Congiu, M.; Albano, L.G.S.; Decker, F.; Graeff, C.F.O. Single precursor route to efficient cobalt sulphide counter electrodes for dye sensitized solar cells. Electrochim. Acta 2015, 151, 517–524. [Google Scholar] [CrossRef]
- Congiu, M.; Lanuti, A.; di Carlo, A.; Graeff, C.F.O. A novel and large area suitable water-based ink for the deposition of cobalt sulfide films for solar energy conversion with iodine-free electrolytes. Sol. Energy 2015, 122, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Bonomo, M.; Congiu, M.; De Marco, M.L.; Dowling, D.P.; Di Carlo, A.; Graeff, C.F.O.; Dini, D. Limits on the use of cobalt sulfide as anode of p-type dye-sensitized solar cells. J. Phys. D. Appl. Phys. 2017, 50, 215501. [Google Scholar] [CrossRef]
- Hsu, S.H.; Li, C.T.; Chien, H.T.; Salunkhe, R.R.; Suzuki, N.; Yamauchi, Y.; Ho, K.C.; Wu, K.C.W. Platinum-free counter electrode comprised of metal-organic-framework (MOF)-derived cobalt sulfide nanoparticles for efficient dye-sensitized solar cells (DSSCs). Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhu, J.; Guo, W.; An, L.; Xia, D.; Zou, R. Well-defined carbon polyhedrons prepared from nano metal-organic frameworks for oxygen reduction. J. Mater. Chem. A 2014, 2, 11606–11613. [Google Scholar] [CrossRef]
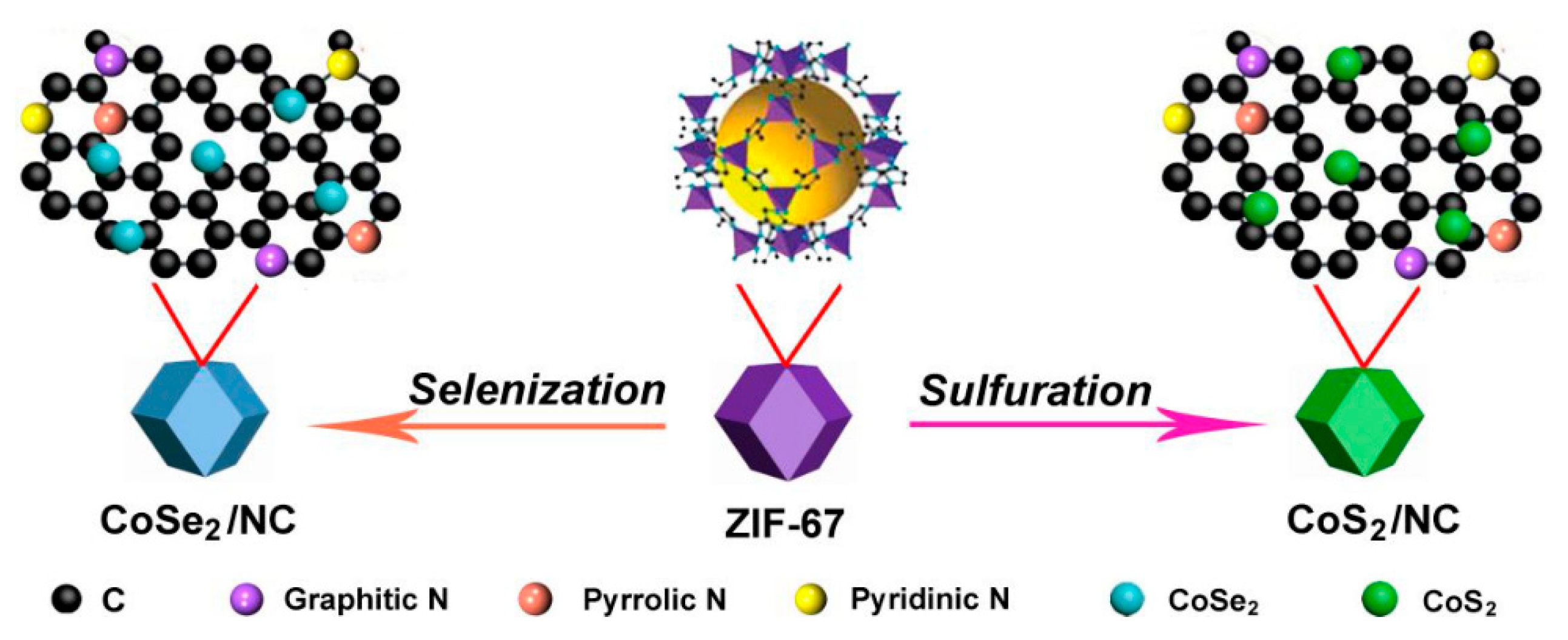
- Wang, X.; Xie, Y.; Bateer, B.; Pan, K.; Zhang, X.; Wu, J.; Fu, H. CoSe 2 /N-Doped Carbon Hybrid Derived from ZIF-67 as High-Efficiency Counter Electrode for Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 2784–2791. [Google Scholar] [CrossRef]
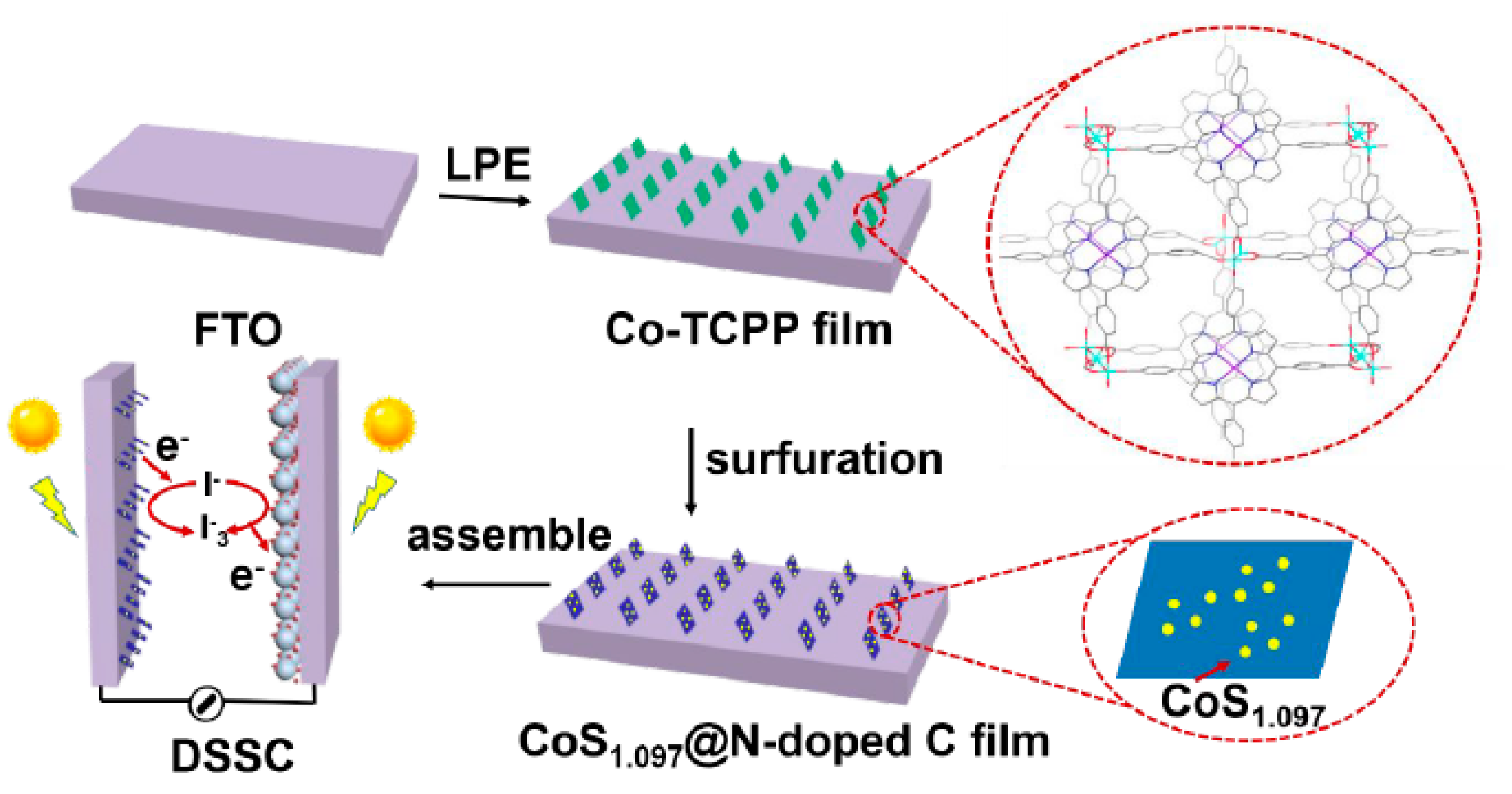
- Ou, J.; Xiang, J.; Liu, J.; Sun, L. Surface-Supported Metal-Organic Framework Thin-Film-Derived Transparent CoS 1.097 @N-Doped Carbon Film as an Efficient Counter Electrode for Bifacial Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 14862–14870. [Google Scholar] [CrossRef]
- Tian, Y.B.; Wang, Y.Y.; Chen, S.M.; Gu, Z.G.; Zhang, J. Epitaxial Growth of Highly Transparent Metal-Porphyrin Framework Thin Films for Efficient Bifacial Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 1078–1083. [Google Scholar] [CrossRef]
- Cui, X.; Xie, Z.; Wang, Y. Novel CoS2embedded carbon nanocages by direct sulfurizing metal-organic frameworks for dye-sensitized solar cells. Nanoscale 2016, 8, 11984–11992. [Google Scholar] [CrossRef]
- Lin, Y.; Song, H.; Rao, H.; Du, Z.; Pan, Z.; Zhong, X. MOF-Derived Co,N Codoped Carbon/Ti Mesh Counter Electrode for High-Efficiency Quantum Dot Sensitized Solar Cells. J. Phys. Chem. Lett. 2019, 10, 4974–4979. [Google Scholar] [CrossRef]
- Zhao, L.; Du, J.; Wang, W.; Zhong, X.; Du, Z.; Wang, Y.; Cao, X.-M.; Zhao, L.; Li, Y. Metal–organic framework derived Co,N-bidoped carbons as superior electrode catalysts for quantum dot sensitized solar cells. J. Mater. Chem. A 2017, 6, 2129–2138. [Google Scholar] [CrossRef]
- Kang, J.S.; Kang, J.; Chung, D.Y.; Son, Y.J.; Kim, S.; Kim, S.; Kim, J.; Jeong, J.; Lee, M.J.; Shin, H.; et al. Tailoring the porosity of MOF-derived N-doped carbon electrocatalysts for highly efficient solar energy conversion. J. Mater. Chem. A 2018, 6, 20170–20183. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, M.; Zhao, L.; Wang, W.; Xue, W.; Li, Y. Cu x S nanoparticle@carbon nanorod composites prepared from metal-organic frameworks as efficient electrode catalysts for quantum dot sensitized solar cells. J. Mater. Chem. A 2019, 7, 2210–2218. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, H.; Shi, D.; Jiao, Q.; Zhao, Y.; Feng, C.; Bai, X.; Wang, H.; Wu, Q. Rational design of metal organic framework derived hierarchical structural nitrogen doped porous carbon coated CoSe/nitrogen doped carbon nanotubes composites as a robust Pt-free electrocatalyst for dye-sensitized solar cells. J. Power Sources 2019, 422, 122–130. [Google Scholar] [CrossRef]
- Vittal, R.; Chen, T.-Y.; Ho, K.-C.; Li, C.-T.; Kung, C.-W.; Huang, Y.-J. Metal-organic framework/sulfonated polythiophene on carbon cloth as a flexible counter electrode for dye-sensitized solar cells. Nano Energy 2016, 32, 19–27. [Google Scholar] [CrossRef]
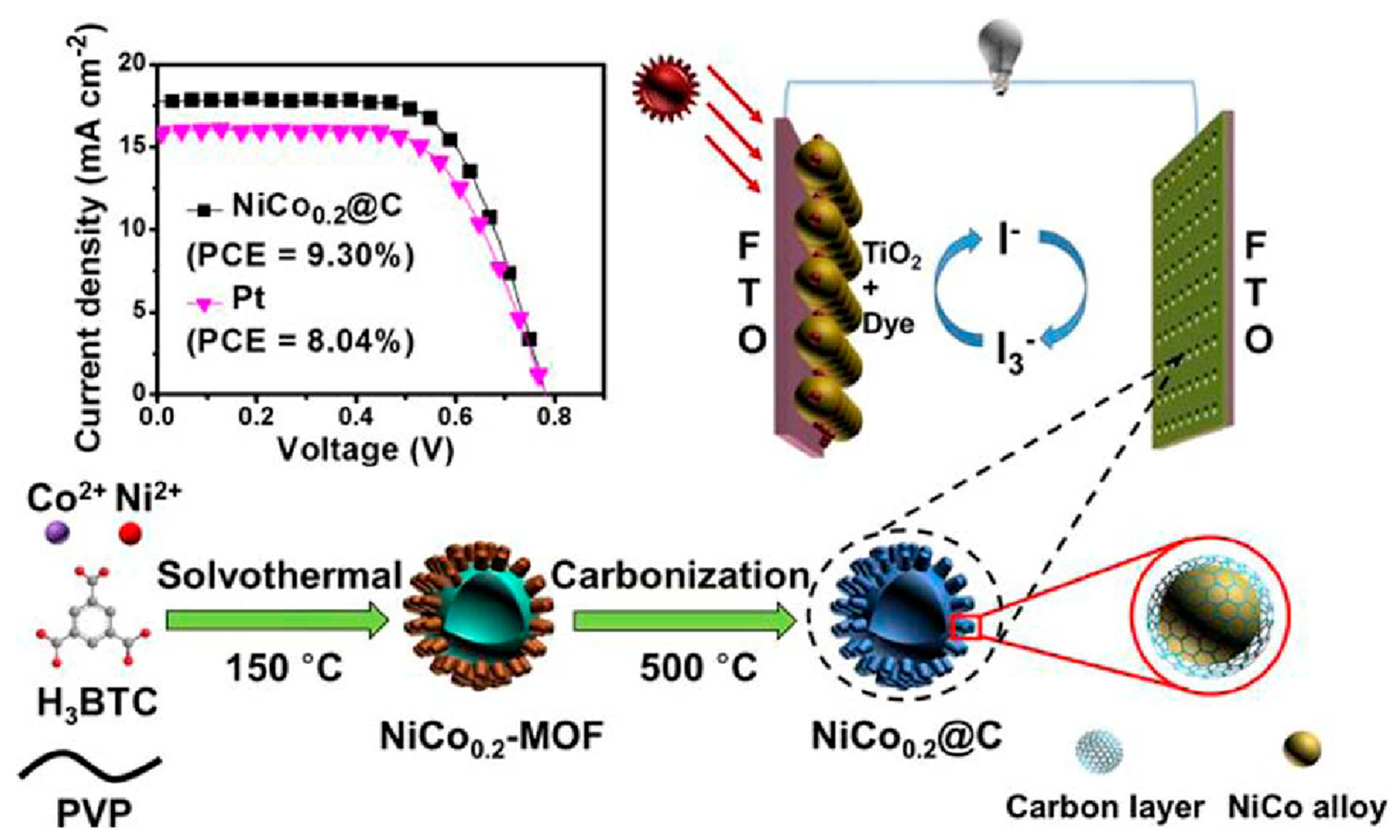
- Jiang, X.; Li, H.; Li, S.; Huang, S.; Zhu, C.; Hou, L. Metal-organic framework-derived Ni–Co alloy@carbon microspheres as high-performance counter electrode catalysts for dye-sensitized solar cells. Chem. Eng. J. 2018, 334, 419–431. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Z.Y.; Jiang, H.L.; Yu, S.H. Nanowire-directed templating synthesis of metal-organic framework nanofibers and their derived porous doped carbon nanofibers for enhanced electrocatalysis. J. Am. Chem. Soc. 2014, 136, 14385–14388. [Google Scholar] [CrossRef]
- Wei, Q.; Xiong, F.; Tan, S.; Huang, L.; Lan, E.H.; Dunn, B.; Mai, L. Porous One-Dimensional Nanomaterials: Design, Fabrication and Applications in Electrochemical Energy Storage. Adv. Mater. 2017, 29, 1602300. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, B.K.; Lee, W.J. Electrospun activated carbon nanofibers with hollow core/highly mesoporous shell structure as counter electrodes for dye-sensitized solar cells. J. Power Sources 2013, 239, 122–127. [Google Scholar] [CrossRef]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A photoconductive covalent organic framework: Self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chemie Int. Ed. 2009, 48, 5439–5442. [Google Scholar] [CrossRef]
- Maitarad, P.; Feng, X.; Guo, J.; Seki, S.; Guo, J.; Nagase, S.; Saeki, A.; Ding, X.; Jiang, D.; Honsho, Y. Synthesis of Metallophthalocyanine Covalent Organic Frameworks That Exhibit High Carrier Mobility and Photoconductivity. Angew. Chemie Int. Ed. 2010, 50, 1289–1293. [Google Scholar] [CrossRef]
- Saeki, A.; Seki, S.; Feng, X.; Nagase, S.; Ding, X.; Honsho, Y.; Jiang, D.; Guo, J.; Chen, L.; Saengsawang, O.; et al. An n -Channel Two-Dimensional Covalent Organic Framework. J. Am. Chem. Soc. 2011, 133, 14510–14513. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, X.; Liu, L.; Saeki, A.; Nagai, A.; Honsho, Y.; Jiang, D.; Irle, S.; Seki, S. High-Rate Charge-Carrier Transport in Porphyrin Covalent Organic Frameworks: Switching from Hole to Electron to Ambipolar Conduction. Angew. Chemie Int. Ed. 2012, 51, 2618–2622. [Google Scholar] [CrossRef]
- Colson, J.W.; Woll, A.R.; Mukherjee, A.; Levendorf, M.P.; Spitler, E.L.; Shields, V.B.; Spencer, M.G.; Park, J.; Dichtel, W.R. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 2011, 332, 228–231. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Jin, S.; Chen, L.; Kaji, T.; Honsho, Y.; Addicoat, M.A.; Kim, J.; Saeki, A.; Ihee, H.; et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 2013, 4, 2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogru, M.; Handloser, M.; Auras, F.; Kunz, T.; Medina, D.; Hartschuh, A.; Knochel, P.; Bein, T. A photoconductive thienothiophene-based covalent organic framework showing charge transfer towards included fullerene. Angew. Chemie Int. Ed. 2013, 52, 2920–2924. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.T.; Berard, P.G.; Clancy, P. A kinetic monte carlo study of fullerene adsorption within a Pc-PBBA covalent organic framework and implications for electron transport. J. Chem. Theory Comput. 2015, 11, 1172–1180. [Google Scholar] [CrossRef]
- Chen, L.; Furukawa, K.; Gao, J.; Nagai, A.; Nakamura, T.; Dong, Y.; Jiang, D. Photoelectric covalent organic frameworks: Converting open lattices into ordered donor-acceptor heterojunctions. J. Am. Chem. Soc. 2014, 136, 9806–9809. [Google Scholar] [CrossRef]
- Medina, D.D.; Werner, V.; Auras, F.; Tautz, R.; Dogru, M.; Schuster, J.; Linke, S.; Döblinger, M.; Feldmann, J.; Knochel, P.; et al. Oriented thin films of a benzodithiophene covalent organic framework. ACS Nano 2014, 8, 4042–4052. [Google Scholar] [CrossRef] [PubMed]
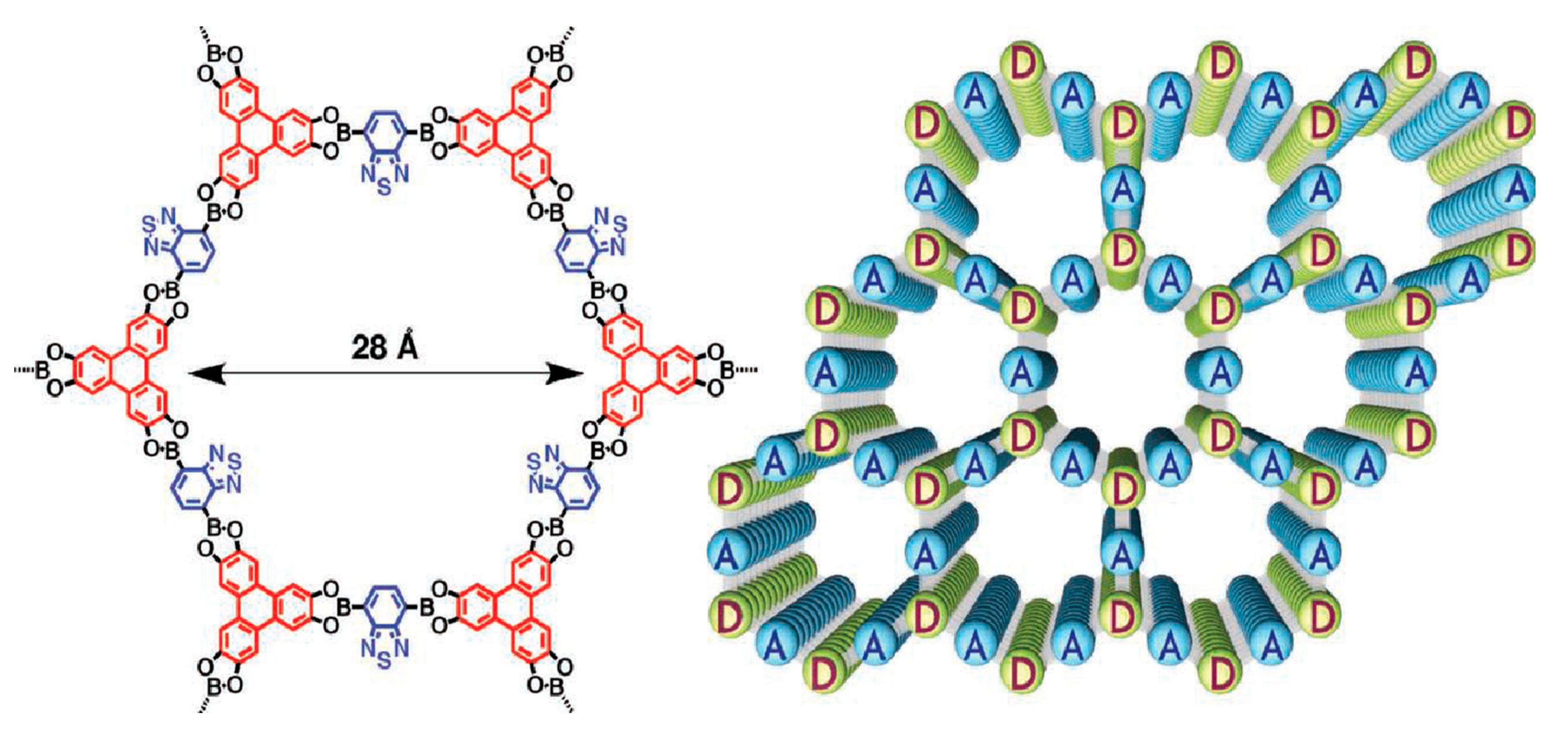
- Feng, X.; Chen, L.; Honsho, Y.; Saengsawang, O.; Liu, L.; Wang, L.; Saeki, A.; Irle, S.; Seki, S.; Dong, Y.; et al. An ambipolar conducting covalent organic framework with self-sorted and periodic electron donor-acceptor ordering. Adv. Mater. 2012, 24, 3026–3031. [Google Scholar] [CrossRef]
- Jin, S.; Ding, X.; Feng, X.; Supur, M.; Furukawa, K.; Takahashi, S.; Addicoat, M.; El-Khouly, M.E.; Nakamura, T.; Irle, S.; et al. Charge dynamics in a donor-acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chemie Int. Ed. 2013, 52, 2017–2021. [Google Scholar] [CrossRef]
- Jin, S.; Supur, M.; Addicoat, M.; Furukawa, K.; Chen, L.; Nakamura, T.; Fukuzumi, S.; Irle, S.; Jiang, D. Creation of Superheterojunction Polymers via Direct Polycondensation: Segregated and Bicontinuous Donor-Acceptor π-Columnar Arrays in Covalent Organic Frameworks for Long-Lived Charge Separation. J. Am. Chem. Soc. 2015, 137, 7817–7827. [Google Scholar] [CrossRef]
- Jin, S.; Furukawa, K.; Addicoat, M.; Chen, L.; Takahashi, S.; Irle, S.; Nakamura, T.; Jiang, D. Large pore donor—acceptor covalent organic frameworks. Chem. Sci. 2013, 4, 4505–4511. [Google Scholar] [CrossRef]
- Calik, M.; Auras, F.; Salonen, L.M.; Bader, K.; Grill, I.; Handloser, M.; Medina, D.D.; Dogru, M.; Löbermann, F.; Trauner, D.; et al. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 2014, 136, 17802–17807. [Google Scholar] [CrossRef]
- You, Y.-M.; Wang, Z.; Niu, X.; Liao, W.-Q.; Zhao, D.; Gao, S.; Ye, H.-Y.; Liu, J.-M.; Fu, D.-W.; Li, J.; et al. An organic-inorganic perovskite ferroelectric with large piezoelectric response. Science 2017, 357, 306–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Shrestha, N.; Wang, C.; Podraza, N.J.; Xiao, Y.; Grice, C.R.; Zhao, D.; Yu, Y.; Cimaroli, A.J.; Liao, W.; et al. Fabrication of Efficient Low-Bandgap Perovskite Solar Cells by Combining Formamidinium Tin Iodide with Methylammonium Lead Iodide. J. Am. Chem. Soc. 2016, 138, 12360–12363. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, D.; Yu, Y.; Grice, C.R.; Wang, C.; Cimaroli, A.J.; Schulz, P.; Meng, W.; Zhu, K.; Xiong, R.G.; et al. Lead-Free Inverted Planar Formamidinium Tin Triiodide Perovskite Solar Cells Achieving Power Conversion Efficiencies up to 6.22%. Adv. Mater. 2016, 28, 9333–9340. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.Q.; Zhang, Y.; Hu, C.L.; Mao, J.G.; Ye, H.Y.; Li, P.F.; Huang, S.D.; Xiong, R.G. A lead-halide perovskite molecular ferroelectric semiconductor. Nat. Commun. 2015, 6, 7338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinova, N.; Valero, S.; Delgado, J.L. Organic and perovskite solar cells: Working principles, materials and interfaces. J. Colloid Interface Sci. 2017, 488, 373–389. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Almora, O.; Jiménez-Tejada, J.A.; Lopez-Varo, P.; García-Rosell, M.; Ravishankar, S. Device Physics of Hybrid Perovskite Solar cells: Theory and Experiment. Adv. Energy Mater. 2018, 8, 1702772. [Google Scholar] [CrossRef]
- Chen, B.; Yang, M.; Priya, S.; Zhu, K. Origin of J-V Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 905–917. [Google Scholar] [CrossRef]
- De Wolf, S.; Holovsky, J.; Moon, S.J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.J.; Yum, J.H.; Ballif, C. Organometallic halide perovskites: Sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef]
- Im, J.H.; Lee, C.R.; Lee, J.W.; Park, S.W.; Park, N.G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [Green Version]
- Ball, J.M.; Lee, M.M.; Hey, A.; Snaith, H.J. Low-temperature processed meso-superstructured to thin-film perovskite solar cells. Energy Environ. Sci. 2013, 6, 1739–1743. [Google Scholar] [CrossRef]
- Kim, H.S.; Im, S.H.; Park, N.G. Organolead halide perovskite: New horizons in solar cell research. J. Phys. Chem. C 2014, 118, 5615–5625. [Google Scholar] [CrossRef]
- You, Y.; Tong, X.; Wang, W.; Sun, J.; Yu, P.; Ji, H.; Niu, X.; Wang, Z.M. Eco-Friendly Colloidal Quantum Dot-Based Luminescent Solar Concentrators. Adv. Sci. 2019, 6, 1801967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Bai, Y.; Yu, Z.; Li, T.; Zheng, X.; Dong, Q.; Shen, L.; Boccard, M.; Gruverman, A.; Holman, Z.; et al. Efficient Semitransparent Perovskite Solar Cells for 23.0%-Efficiency Perovskite/Silicon Four-Terminal Tandem Cells. Adv. Energy Mater. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef]
- Mariotti, N.; Bonomo, M.; Barolo, C. Emerging photovoltaic technologies and eco-design—Criticisms and potential improvements. In Criticisms and Potential Improvements, Reliability and Ecological Aspects of Photovoltaic Modules; Gok, A., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Yang, G.; Tao, H.; Qin, P.; Ke, W.; Fang, G. Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 3970–3990. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Lee, Y.H.; Bolink, H.J.; Graetzel, M.; Malinkiewicz, O.; Espallargas, G.M.; Yella, A. Perovskite solar cells employing organic charge-transport layers. Nat. Photonics 2013, 8, 128–132. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Zaake-Hertling, H.; Hey-Hawkins, E.; Agafonov, A.V.; Seisenbaeva, G.A.; Kessler, V.G.; Vinogradov, V.V. The first depleted heterojunction TiO2-MOF-based solar cell. Chem. Commun. 2014, 50, 10210–10213. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Bark, C.W. Synthesis of Cobalt-Doped TiO2 Based on Metal-Organic Frameworks as an Effective Electron Transport Material in Perovskite Solar Cells. ACS Omega 2020, 5, 2280–2286. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Humphry-Baker, R.; Nazeeruddin, M.K.; Burschka, J.; Gao, P.; Pellet, N.; Grätzel, M.; Moon, S.-J. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Im, J.H.; Jang, I.H.; Pellet, N.; Grätzel, M.; Park, N.G. Growth of CH3 NH3 PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, R.; Bi, D.; Park, B.W.; Oscarsson, J.; Gorgoi, M.; Siegbahn, H.; Odelius, M.; Johansson, E.M.J.; Rensmo, H. Electronic structure of TiO2/CH3NH 3PbI3 perovskite solar cell interfaces. J. Phys. Chem. Lett. 2014, 5, 648–653. [Google Scholar] [CrossRef]
- Boopathi, K.M.; Huang, T.-Y.; Chen, H.-W.; Kung, C.-W.; Ho, K.-C.; Kao, S.-Y.; Lu, H.-C.; Chu, C.-W.; Lee, M.-H.; Chang, T.-H. Planar Heterojunction Perovskite Solar Cells Incorporating Metal-Organic Framework Nanocrystals. Adv. Mater. 2015, 27, 7229–7235. [Google Scholar] [CrossRef]
- Kung, C.W.; Chang, T.H.; Chou, L.Y.; Hupp, J.T.; Farha, O.K.; Ho, K.C. Post metalation of solvothermally grown electroactive porphyrin metal-organic framework thin films. Chem. Commun. 2015, 51, 2414–2417. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Pang, A.; Li, Y.; Dou, J.; Wei, M. Metal-organic frameworks at interfaces of hybrid perovskite solar cells for enhanced photovoltaic properties. Chem. Commun. 2018, 54, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Li, B.; Fu, L.; Li, Q.; Yin, L.-W. MOF-derived ZnO as electron transport layer for improving light harvesting and electron extraction efficiency in perovskite solar cells. Electrochim. Acta 2020, 330, 135280. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, Z.G.; Fu, W.Q.; Wang, F.; Zhang, J. A Confined Fabrication of Perovskite Quantum Dots in Oriented MOF Thin Film. ACS Appl. Mater. Interfaces 2016, 8, 28737–28742. [Google Scholar] [CrossRef]
- Li, M.; Xia, D.; Yang, Y.; Du, X.; Dong, G.; Jiang, A.; Fan, R. Doping of [In2(phen)3Cl6]·CH3CN·2H2O Indium-Based Metal–Organic Framework into Hole Transport Layer for Enhancing Perovskite Solar Cell Efficiencies. Adv. Energy Mater. 2018, 8, 1702052. [Google Scholar] [CrossRef]
- Choi, K.M.; Jeong, H.M.; Park, J.H.; Zhang, Y.B.; Kang, J.K.; Yaghi, O.M. Supercapacitors of nanocrystalline metal-organic frameworks. ACS Nano 2014, 8, 7451–7457. [Google Scholar] [CrossRef] [PubMed]
- Ryu, U.J.; Jee, S.; Park, J.S.; Han, I.K.; Lee, J.H.; Park, M.; Choi, K.M. Nanocrystalline Titanium Metal-Organic Frameworks for Highly Efficient and Flexible Perovskite Solar Cells. ACS Nano 2018, 12, 4968–4975. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Chen, C.-I.; Liao, Y.-T.; Wu, K.C.W.; Chueh, C.-C. Enhancing Efficiency and Stability of Photovoltaic Cells by Using Perovskite/Zr-MOF Heterojunction Including Bilayer and Hybrid Structures. Adv. Sci. 2019, 6, 1801715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, J.; He, J.; Li, B.; Zhang, Y.; Hu, J.; Wang, H.; Zhang, D.; Liu, Q. Porous Anatase TiO 2 Nanocrystal Derived from the Metal–Organic Framework as Electron Transport Material for Carbon-Based Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 6180–6187. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Jiang, A.; Xia, D.; Du, X.; Dong, Y.; Wang, P.; Fan, R.; Yang, Y. Metal organic framework doped Spiro-OMeTAD with increased conductivity for improving perovskite solar cell performance. Sol. Energy 2019, 188, 380–385. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, L.; Fan, R.; Wang, A.; Ye, H.; Tian, C.; Hao, S.; Yang, Y. Metal–Organic Framework-Derived N-Rich Porous Carbon as an Auxiliary Additive of Hole Transport Layers for Highly Efficient and Long-Term Stable Perovskite Solar Cells. Sol. RRL 2020, 4, 1900380. [Google Scholar] [CrossRef]
- Seo, S.; Jeong, S.; Bae, C.; Park, N.-G.; Shin, H. Perovskite Solar Cells with Inorganic Electron- and Hole-Transport Layers Exhibiting Long-Term (≈500 h) Stability at 85 °C under Continuous 1 Sun Illumination in Ambient Air. Adv. Mater. 2018, 30, 1801010. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.-B.T.B.; Guo, T.F.T.-F.; Chang, W.H.W.-H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; Liu, Y.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, L.; Fan, R.; Ye, H.; Tian, C.; Hao, S.; Yang, Y. Toward high-efficiency and thermally-stable perovskite solar cells: A novel metal-organic framework with active pyridyl sites replacing 4-tert-butylpyridine. J. Power Sources 2020, 473, 228556. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, X.; Wu, R.; Shi, C.; Xue, R.; Zou, J.; Xu, C.; Zhao, J.; Zeng, W. Oriented haloing metal-organic framework providing high efficiency and high moisture-resistance for perovskite solar cells. J. Power Sources 2019, 433, 226699. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Yang, Y.; Qiu, L.; Xia, D.; Lin, K.; Wang, J.; Fan, X.; Fan, R. Self-Assembly of Hybrid Oxidant POM@Cu-BTC for Enhanced Efficiency and Long-Term Stability of Perovskite Solar Cells. Angew. Chemie Int. Ed. 2019, 58, 17610–17615. [Google Scholar] [CrossRef] [PubMed]
- Sardashti, M.K.; Zendehdel, M.; Nia, N.Y.; Karimian, D.; Sheikhi, M. High Efficiency MAPbI3 Perovskite Solar Cell Using a Pure Thin Film of Polyoxometalate as Scaffold Layer. ChemSusChem 2017, 10, 3773–3779. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Yang, Y.; Qiu, L.; Dong, G.; Xia, D.; Liu, X.; Li, M.; Fan, R. Polyoxometalate-Based Inorganic-Organic Hybrid [Cu(phen)2]2[(α-Mo8O26)]: A New Additive to Spiro-OMeTAD for Efficient and Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 4224–4233. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Liu, H.; Duan, C.; Pan, Q.; Zhu, J.; Hu, F.; Ma, X.; Jiu, T.; Li, Z.; et al. Highly Conjugated Three-Dimensional Covalent Organic Frameworks Based on Spirobifluorene for Perovskite Solar Cell Enhancement. J. Am. Chem. Soc. 2018, 140, 10016–10024. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Lee, C.C.; EL-Mahdy, A.F.M.; Lüder, J.; Yu, M.H.; Li, Z.; Zhu, Z.; Chueh, C.C.; Kuo, S.W. Exploitation of two-dimensional conjugated covalent organic frameworks based on tetraphenylethylene with bicarbazole and pyrene units and applications in perovskite solar cells. J. Mater. Chem. A 2020, 8, 11448–11459. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Xu, T.; Xie, Z.; Liu, J.; Yu, X.; Ma, S.; Qin, T.; Chen, L. De Novo Design and Facile Synthesis of 2D Covalent Organic Frameworks: A Two-in-One Strategy. J. Am. Chem. Soc. 2019, 141, 13822–13828. [Google Scholar] [CrossRef] [PubMed]






| Special Role | MOFs | η (%) | Ref. | |
|---|---|---|---|---|
| Metals | Organic Linkers | |||
| Interfacial Layer | Zn | ZIF-8 | 5.34 | [93] |
| Zn |  | 2.34 | [124] | |
| Scattering Layer | Zn | MOF-5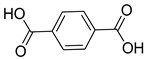 | 3.67 | [125] |
| Ti | MIL-125(Ti) | 7.1 | [126] | |
| Zn |  | 0.15 | [92] | |
| Cu | 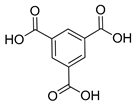 | 0.008 | [86] | |
| Cu | 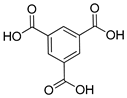 | 0.27 | [126] | |
| Cu | 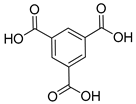 | 1.22 | [88] | |
| Ru | 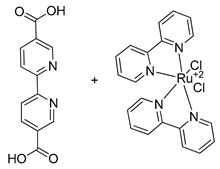 | 0.125 | [87] | |
| Zn |  | 0.86 | [127] | |
| Cu |  | 0.1 | [128] | |
| MOFs | η (%) | Ref. | |
|---|---|---|---|
| Metals | Organic Linker(s) | ||
| In/K |  | 8.07 | [143] |
| Zn | 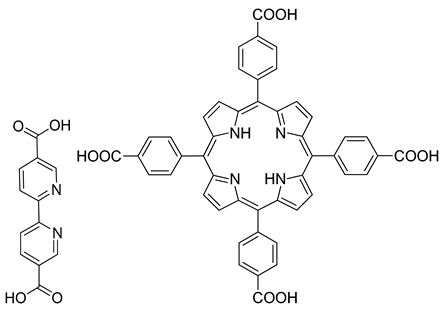 | 0.0023 | [139] |
| Co | Co-DAPV MOF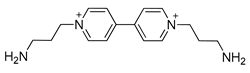 | 2.1 | [147] |
| Eu | Eu-MOF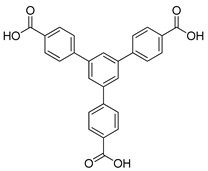 | 2.2 | [153] |
| Ti | titanium-based MOF ‘NTU-9,’ | 3.20 | [154] |
| Zn | 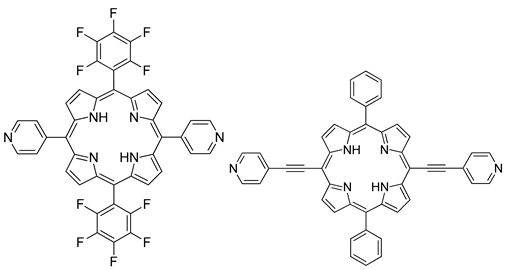 | n.a. | [152] |
| MOFs | η (%) | Ref. | |
|---|---|---|---|
| Metals | Organic Linkers | ||
| Co | PISA-1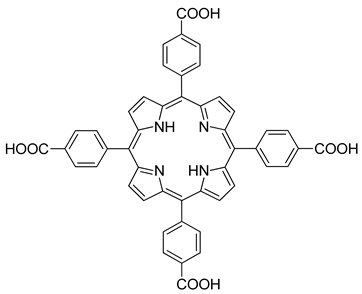 | 9.11 | [167] |
| Co | ZIF-67, | 8.2 | [169] |
| Co Zn | ZIF-67, ZIF-8 | 13.50 9.12 | [170] [171] |
| Zn | Carbonaceous-ZIF-8 | 9.03 | [172] |
| Cu | HKUST-1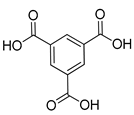 | 9.50 | [173] |
| Co | ZIF-67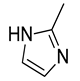 | 7.58 | [174] |
| Zr Zn | MOF-525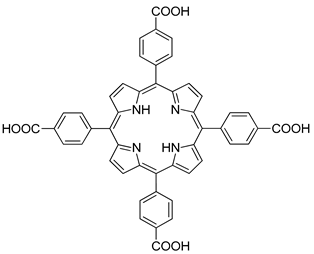 | 9.75 5.48 | [175] [168] |
| Ni/Co | NiCo0.2/MOF | 9.30 | [176] |
| Role | COFs | η (%) | Ref. | |
|---|---|---|---|---|
| Name | Building Blocks | |||
| Photoactive Material | PPy-COF (Hexagonal) |  | n.a. | [180] |
| Photoactive Material | Metallophthalocyanine-COF (Tetragonal) | 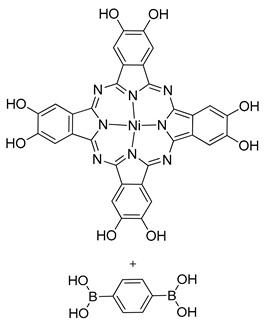 | n.a. | [181] |
| Photoactive Material | NiPc-PBBA COF (Tetragonal) | 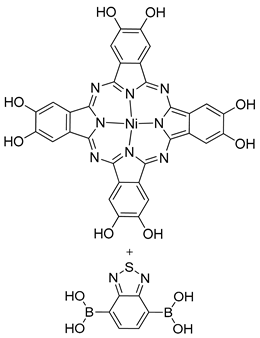 | n.a. | [182] |
| Photoactive Material | MP-COF (M = H2, Zn, Cu) | 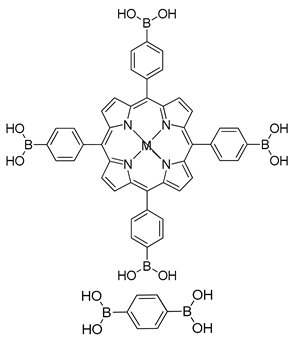 | n.a. | [183] |
| Photoactive Material | TP-COF, NiPBBA COF | 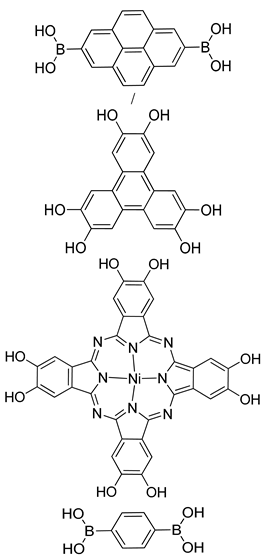 | n.a. | [184] |
| Photoactive Material | CS-COF/C60 (Hexagonal) | 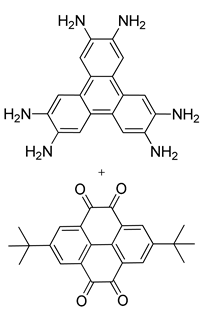 | n.a. | [185] |
| Photoactive Material | TT-COF:PCBM (Hexagonal) | 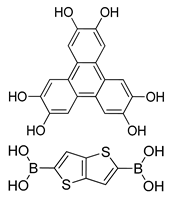 | 0.05 | [186] |
| Photoactive Material | [C60]y-ZnPc-COFs | 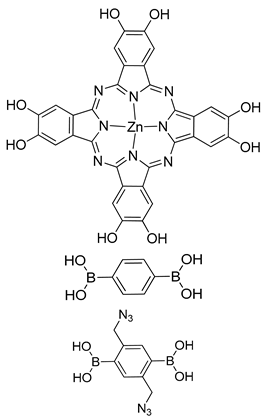 | n.a. | [188] |
| Photoactive Material | BDT-COF:[60]PCBM | 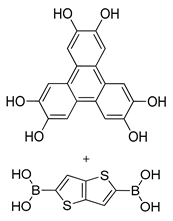 | n.a. | [189] |
| Photoactive Material | D-A COF (Hexagonal). | 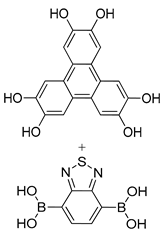 | n.a. | [190] |
| Photoactive Material | DZnPc-ANDI-COF | 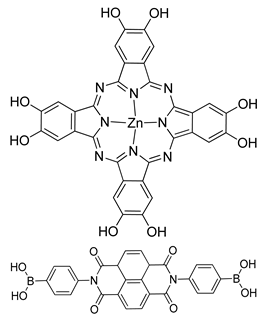 | n.a. | [191] |
| DZnPc-ANDI-COF DTP-APyrDI-COF | 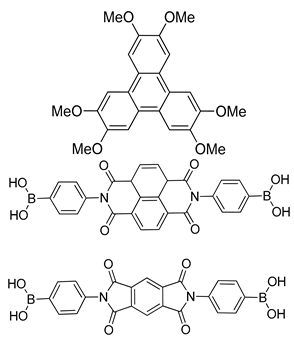 | n.a. | [193] | |
| Photo electrode | triphenylene−porphyrin COF (Hexagonal), | 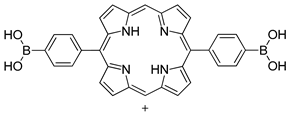 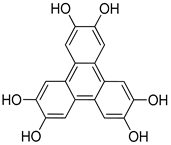 | n.a. | [194] |
| Role | MOFs | η (%) | Ref. | |
|---|---|---|---|---|
| Metals | Organic Linker(s) | |||
| Photoactive Material | Ti | MIL-125(Ti)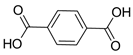 | 6.4 | [213] |
| Photoactive Material | Zr | MOF-525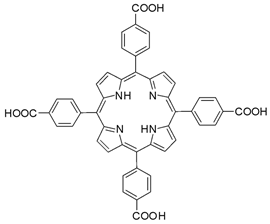 | 12 | [220] |
| Photoactive Material | Zn | ZIF-8 | 16.99 | [221] |
| Photoactive Material | Cu | HKUST-1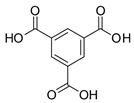 | n.a. | [223] |
| Hole Transport Material (HTM) | In | 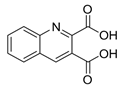 | 15,8 | [225] |
| Electron Transport Layer (ETL) | Ti | MIL-125(Ti)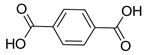 | 18.9 | [226] |
| Electron Transport Layer (ETL) | Zr | UiO-66, MOF-808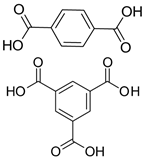 | 17 | [228] |
| Hole Transport Material (HTM) | In | 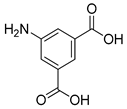 | 18.51 | [207] |
| Hole Transport Material (HTM) | In | 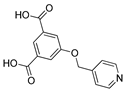 | 19.47 | [208] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, O.; Bonomo, M.; Barbero, N.; Atzori, C.; Civalleri, B.; Bonino, F.; Viscardi, G.; Barolo, C. Application of Metal-Organic Frameworks and Covalent Organic Frameworks as (Photo)Active Material in Hybrid Photovoltaic Technologies. Energies 2020, 13, 5602. https://doi.org/10.3390/en13215602
Yildirim O, Bonomo M, Barbero N, Atzori C, Civalleri B, Bonino F, Viscardi G, Barolo C. Application of Metal-Organic Frameworks and Covalent Organic Frameworks as (Photo)Active Material in Hybrid Photovoltaic Technologies. Energies. 2020; 13(21):5602. https://doi.org/10.3390/en13215602
Chicago/Turabian StyleYildirim, Onur, Matteo Bonomo, Nadia Barbero, Cesare Atzori, Bartolomeo Civalleri, Francesca Bonino, Guido Viscardi, and Claudia Barolo. 2020. "Application of Metal-Organic Frameworks and Covalent Organic Frameworks as (Photo)Active Material in Hybrid Photovoltaic Technologies" Energies 13, no. 21: 5602. https://doi.org/10.3390/en13215602






