A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease
Abstract
1. Introduction
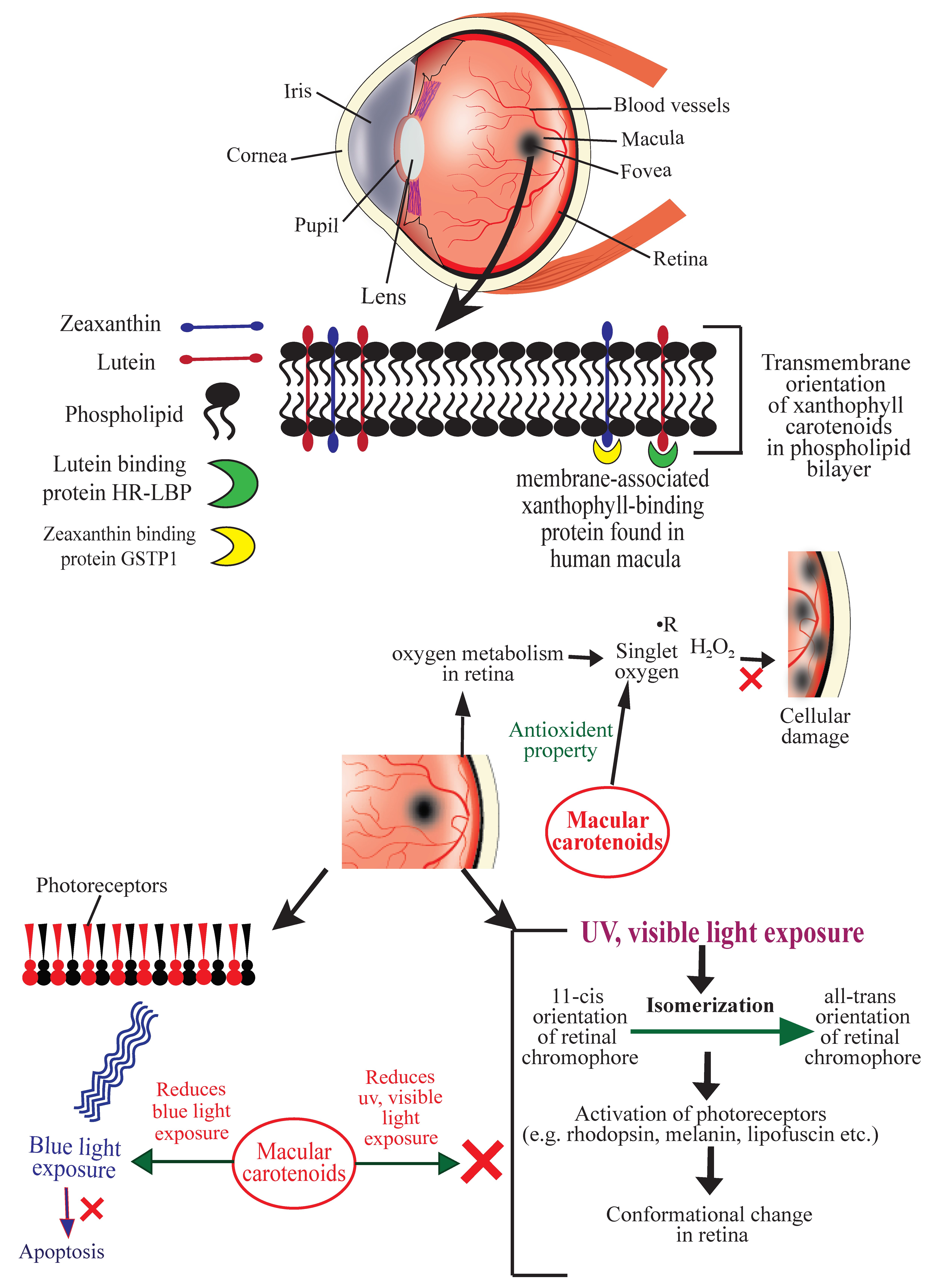
2. Age-Related Macular Degeneration (AMD)
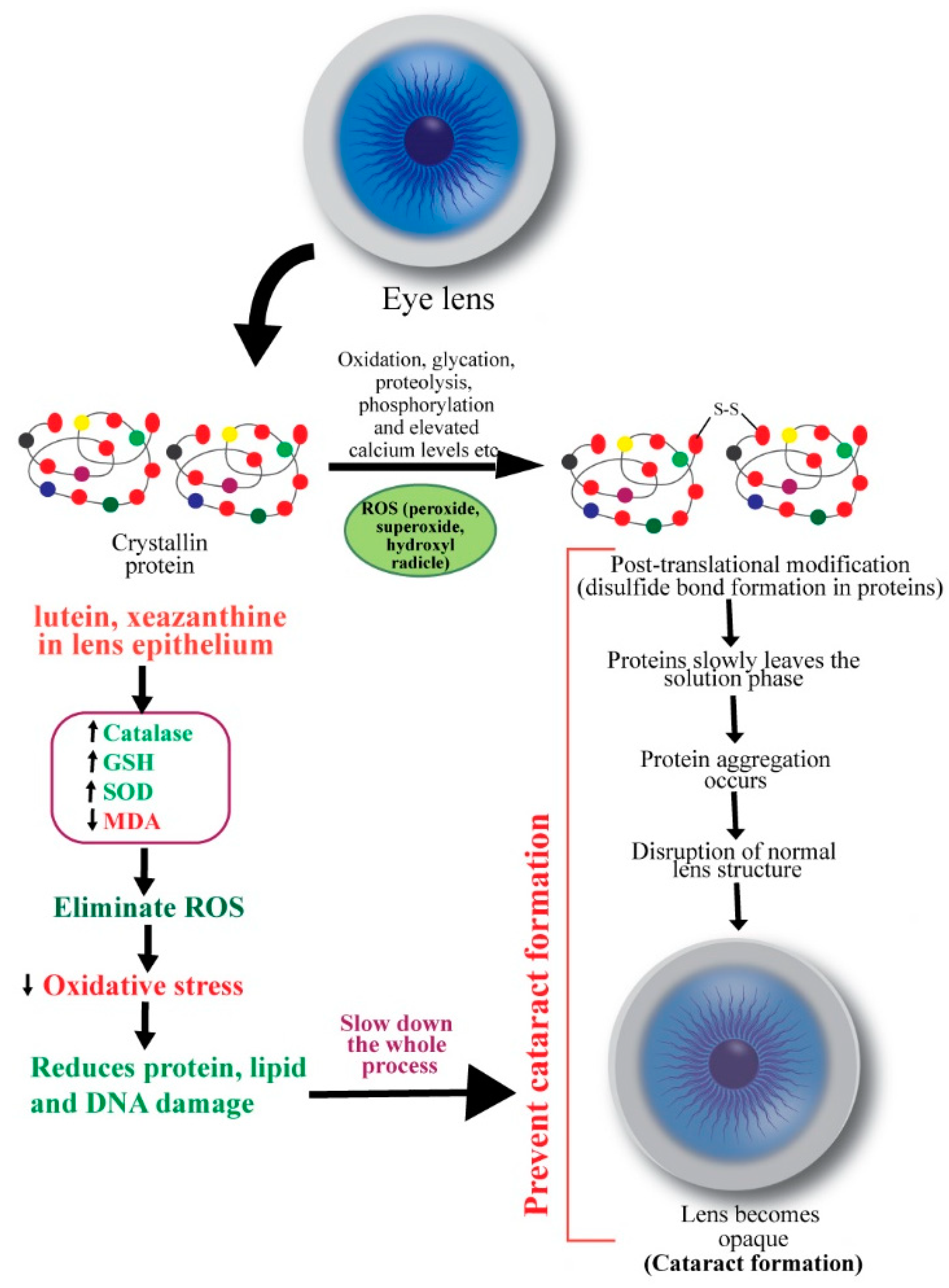
3. Cataracts
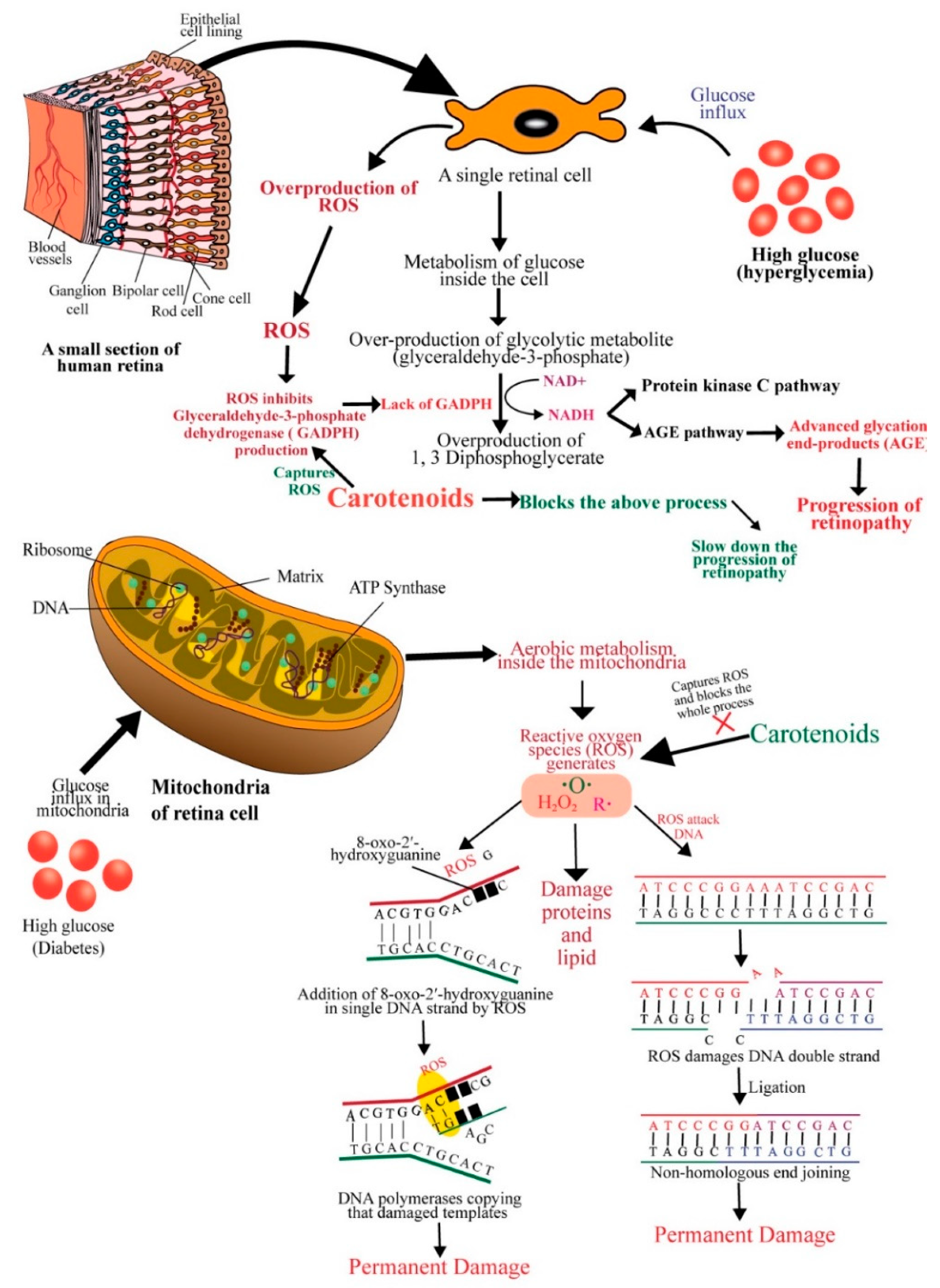
4. Diabetic Retinopathy
5. Safety of Carotenoids
6. Clinical Trials
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and ophthalmic diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.; Das, A.; Jonas, J.; Keeffe, J.; Kempen, J. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, 1221–1234. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M.; Thandampallayam, M.; Putt, D.; Gierhart, D.L. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. (Lond.) 2014, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Javitt, J.; Wang, F.; West, S. Blindness Due to Cataract: Epidemiology and Prevention. Annu. Rev. Public Health 1996, 17, 159–177. [Google Scholar] [CrossRef]
- Subczynski, W.; Wisniewska, A.; Widomska, J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch. Biochem. Biophys. 2010, 504, 61–66. [Google Scholar] [CrossRef]
- Snodderly, D.M.; Auran, J.D.; Delori, F.C. The macular pigment. II. Spatial distribution in primate retinas. Investig. Ophthalmol. Vis. Sci. 1984, 25, 674–685. [Google Scholar]
- Hammond, B.R., Jr.; Wooten, B.R.; Snodderly, D.M. Individual variations in the spatial profile of human macular pigment. J. Opt. Soc. Am. B 1997, 14, 1187–1196. [Google Scholar] [CrossRef]
- Manayi, A.; Abdollahi, M.; Raman, T.; Nabavi, S.; Habtemariam, S.; Daglia, M.; Nabavi, S.M. Lutein and cataract: From bench to bedside. Crit. Rev. Biotechnol. 2015, 36, 829–839. [Google Scholar] [CrossRef]
- Fraser, P.; Bramley, P. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharm. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Namitha, K.K.; Negi, P.S. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Hatade, K.; Asai, A.; Kimura, F.; Sookwong, P.; Tsuduki, T.; Arai, H.; Miyazawa, T. Development of a high-performance liquid chromatography-based assay for carotenoids in human red blood cells: Application to clinical studies. Anal. Biochem. 2008, 381, 129–134. [Google Scholar] [CrossRef]
- Priyadarshani, A. Insights of hypercarotenaemia: A brief review. Clin. Nutr. Espen. 2018, 23, 19–24. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2016, 174, 1290–1324. [Google Scholar] [CrossRef]
- Harikumar, K.; Nimita, C.; Preethi, K.; Kuttan, R.; Shankaranarayana, M.; Deshpande, J. Toxicity Profile of Lutein and Lutein Ester Isolated from Marigold Flowers (Tagetes erecta). Int. J. Toxicol. 2008, 27, 1–9. [Google Scholar] [CrossRef]
- Krinsky, N.; Landrum, J.; Bone, R. Biologicmechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Calvo, C.M.; Conrady, C.D.; Bernstein, P.S. What do we know about the macular pigment in AMD: The past, the present, and the future. Eye 2018, 32, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: A review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Crocker, E.; Eilers, M.; Hornak, V.; Hirshfeld, A.; Ziliox, M.; Syrett, N.; Reeves, P.J.; Khorana, H.G.; Sheves, M.; et al. Location of the Retinal Chromophore in the Activated State of Rhodopsin. J. Biol. Chem. 2009, 284, 10190–10201. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.; Wald, G.J. Cis-Trans Isomers of Vitamin a and Retinene in the Rhodopsin System. Gen. Physiol. 1952, 36, 269–315. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Larson, A.; Frederick, J.; Southwick, K.; Thulin, C.; Bernstein, P. Identification and Characterization of a Pi Isoform of GlutathioneS-Transferase (GSTP1) as a Zeaxanthin-binding Protein in the Macula of the Human Eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef]
- Bhosale, P.; Li, B.; Sharifzadeh, M.; Gellermann, W.; Frederick, J.; Tsuchida, K.; Bernstein, P.S. Purification and Partial Characterization of a Lutein-Binding Protein from Human Retina. Biochemistry 2009, 48, 4798–4807. [Google Scholar] [CrossRef]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. 1991, 11, 41–47. [Google Scholar] [CrossRef]
- Oliveros, E.; Braun, A.M.; Aminian-Saghafi, T.; Sliwka, H. Quenching of singlet oxygen (1Dg) by carotenoid derivatives: Kinetic analysis by near infra-red luminescence. New J. Chem. 1994, 18, 535–539. [Google Scholar]
- Krinsky, N.I.; Deneke, S.M. Interaction of oxygen and oxy-radicals with carotenoids. J. Natl. Cancer Inst. 1982, 69, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta 1997, 1336, 575–586. [Google Scholar] [CrossRef]
- Landrum, J.; Bone, R.; Joa, H.; Kilburn, M.; Moore, L.; Sprague, K. A One Year Study of the Macular Pigment: The Effect of 140 Days of a Lutein Supplement. Exp. Eye Res. 1997, 65, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Spector, A. Oxidative stress-induced cataract: Mechanisms of action. FASEB 1995, 9, 1173–1182. [Google Scholar] [CrossRef]
- Meyer, C.H.; Sekundo, W. Nutritional Supplementation to Prevent Cataract Formation. Dev. Ophthalmol. 2005, 38, 103–119. [Google Scholar]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y. Cataract in diabetes mellitus. World J. Diabetes 2019, 10, 140–153. [Google Scholar] [CrossRef]
- Brown, G.C.; Brown, M.M.; Busbee, B.G. Cost-utility analysis of cataract surgery in the United States for the year 2018. J. Cataract. Refract. Surg. 2019, 45, 927–938. [Google Scholar] [CrossRef]
- Cumming, R.G.; Mitchell, P. Alcohol, smoking, and cataracts: The Blue Mountains Eye Study. Arch. Ophthalmol. 1997, 115, 1296–1303. [Google Scholar] [CrossRef]
- Masters, P.M.; Bada, J.L.; Zigler, J.S. Aspartic acid racemisation in the human lens during ageing and in cataract formation. Nature 1977, 268, 71–73. [Google Scholar] [CrossRef]
- Garner, W.H.; Spector, A. Racemization in human lens: Evidence of rapid insolubilization of specific polypeptides in cataract formation. Proc. Natl. Acad. Sci. USA 1978, 75, 3618–3620. [Google Scholar] [CrossRef]
- Gupta, S.; Selvan, V.; Agrawal, S.; Saxena, R. Advances in pharmacological strategies for the prevention of cataract development. Indian J. Ophthalmol. 2009, 57, 175–183. [Google Scholar] [CrossRef]
- Micelli-Ferrari, T.; Vendemiale, G.; Grattagliano, I.; Boscia, F.; Arnese, L.; Altomare, E.; Cardia, L. Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. J. Ophthalmol. 1996, 80, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Gupta, V.; Christopher, A.F.; Mali, M.A.; Bansal, P. Nutraceuticals in prevention of cataract—An evidence based approach. Saudi J. Ophthalmol. 2017, 31, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J.; Augusteyn, R.C. Oxidative Changes in Human Lens Proteins during Senile Nuclear Cataract Formation. Biochim. Biophys. Acta 1977, 492, 43–52. [Google Scholar] [CrossRef]
- Truscott, R.J.W. Age-related nuclear cataract: A lens transport problem. Ophthalmic Res. 2000, 32, 185–194. [Google Scholar] [CrossRef]
- Garner, M.H.; Spector, A. Sulfur oxidation in selected human cortical cataracts and nuclear cataracts. Exp. Eye Res. 1980, 31, 361–369. [Google Scholar] [CrossRef]
- Bhuyan, K.C.; Bhuyan, D.K. Lipid peroxidation in cataract of the human. Life Sci. 1986, 38, 1463–1471. [Google Scholar] [CrossRef]
- Kellogg III, E.W.; Fridovich, I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J. Biol. Chem. 1975, 250, 8812–8817. [Google Scholar]
- Babizhayev, M.A. Failure to withstand oxidative stress induced by phospholipid hydroperoxides as a possible cause of the lens opacities in systemic diseases and ageing. Biochim. Biophys. Acta 1996, 1315, 87–99. [Google Scholar] [CrossRef]
- Christen, W.G. Antioxidants and eye disease. Am. J. Med. 1994, 97, 7–14. [Google Scholar] [CrossRef]
- Yeum, K.J.; Taylor, A.; Tang, G.; Russell, R.M. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2756–2761. [Google Scholar]
- Yeum, K.J.; Shang, F.; Schalch, W.; Russell, R.M.; Taylor, A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr. Eye Res. 1999, 19, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, O.; Yeum, K.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2 induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar]
- Chang, D.; Zhang, X.; Rong, S.; Sha, Q.; Liu, P.; Han, T.; Pan, H. Serum antioxidative enzymes levels and oxidative stress products in age-related cataract patients. Oxid. Med. Cell. Longev. 2013, 2013, 587826. [Google Scholar] [CrossRef]
- Kaur, J.; Kukreja, S.; Kaur, A.; Malhotra, N.; Kaur, R. The oxidative stress in cataract patients. J. Clin. Diagn. Res. 2012, 6, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Girach, A.; Manner, D.; Porta, M. Diabetic microvascular complications: Can patients at risk be identified? A review. Int. J. Clin. Pract. 2006, 60, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Moss, S.E. Relation of Glycemic Control to Diabetic Microvascular Complications in Diabetes Mellitus. Ann. Intern. Med. 1996, 124, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Jeong, J.S.; Kim, M.K.; Kwon, H.S.; Baek, K.H.; Ko, S.H.; Ahn, Y.B. Discordance in risk factors for the progression of diabetic retinopathy and diabetic nephropathy in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2019, 10, 745–752. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharm. 2017, 91, 31–42. [Google Scholar] [CrossRef]
- Julia, M.S.; Ghulam, M.; Qing, Z.; Renu, A.K. Diabetic Retinopathy, Superoxide Damage and Antioxidants. Curr. Pharm. Biotechnol. 2011, 12, 352–361. [Google Scholar] [CrossRef]
- Madsen-Bouterse, S.A.; Kowluru, R.A. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev. Endocr. Metab. Disord. 2008, 9, 315–327. [Google Scholar] [CrossRef]
- Kim, D.; Lee, D.; Trackman, P.C.; Roy, S. Effects of High Glucose–Induced Lysyl Oxidase Propeptide on Retinal Endothelial Cell Survival: Implications for Diabetic Retinopathy. J. Pathol. 2019, 189, 1945–1952. [Google Scholar] [CrossRef]
- Trudeau, K.; Molina, A.; Guo, W.; Roy, S. High Glucose Disrupts Mitochondrial Morphology in Retinal Endothelial Cells. J. Pathol. 2010, 177, 447–455. [Google Scholar] [CrossRef]
- Kim, D.; Roy, S. Effects of Diabetes on Mitochondrial Morphology and Its Implications in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 10. [Google Scholar] [CrossRef]
- Kowluru, R. Diabetic Retinopathy: Mitochondrial Dysfunction and Retinal Capillary Cell Death. Antioxid. Redox. Signal. 2005, 7, 1581. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E. DNA Damage: Air-breaks? Curr. Biol. 2002, 12, 262–264. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–603. [Google Scholar] [CrossRef] [PubMed]
- Karanjawala, Z.; Murphy, N.; Hinton, D.; Hsieh, C.; Lieber, M. Oxygen Metabolism Causes Chromosome Breaks and Is Associated with the Neuronal Apoptosis Observed in DNA Double-Strand Break Repair Mutants. Curr. Biol. 2002, 12, 397–402. [Google Scholar] [CrossRef]
- Santos, J.; Tewari, S.; Kowluru, R.A. Compensatory mechanism protects retinal mitochondria from initial insult in diabetic retinopathy. Free Radic. Biol. Med. 2012, 53, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Madsen-Bouterse, S.; Zhong, Q.; Mohammad, G.; Ho, Y.; Kowluru, R. Oxidative damage of mitochondrial DNA in diabetes and its protection by manganese superoxide dismutase. Free Radic. Res. 2010, 44, 313–321. [Google Scholar] [CrossRef]
- Aso, Y.; Inukai, T.; Tayama, K.; Takemura, Y. Serum concentrations of advanced glycation endproducts are associated with the development of atherosclerosis as well as diabetic microangiopathy in patients with type 2 diabetes. Acta Diabetol. 2000, 37, 87–92. [Google Scholar] [CrossRef]
- Khangholi, S.; Majid, F.; Berwary, N.; Ahmad, F.; Aziz, R. The Mechanisms of Inhibition of Advanced Glycation End Products Formation through Polyphenols in Hyperglycemic Condition. Planta Medica 2015, 82, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Genuth, S.; Sun, W.; Cleary, P.; Gao, X.; Sell, D.R.; Lachin, J. Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes 2015, 64, 266–278. [Google Scholar] [CrossRef]
- Ames, B.; Shigenaga, M.; Hagen, T. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.; Fernandez, M. Potential of Dietary Non-Provitamin A Carotenoids in the Prevention and Treatment of Diabetic Microvascular Complications. Adv. Nutr. 2016, 7, 14–24. [Google Scholar] [CrossRef]
- Scanlon, G.; Loughman, J.; Farrell, D.; McCartney, D. A review of the putative causal mechanisms associated with lower macular pigment in diabetes mellitus. Nutr. Res. Rev. 2019, 32, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef]
- Wang, W.; Tam, K.C.; Ng, T.C.; Goit, R.K.; Chan, K.L.S.; Lo, A.C.Y. Long-term lutein administration attenuates retinal inflammation and functional deficits in early diabetic retinopathy using the Ins2Akita/+ mice. BMJ Open Diabetes Res. Care 2020, 8, e001519. [Google Scholar] [CrossRef] [PubMed]
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Li, C.; Miao, X.; Li, F.; Wang, S.; Liu, Q.; Wang, Y.; Sun, J. Oxidative stress-related mechanisms and antioxidant therapy in diabetic retinopathy. Oxid. Med. Cell. Longev. 2017, 2017, 9702820. [Google Scholar] [CrossRef]
- Keegan, G.; Pardhan, S.; Chichger, H. Lutein and zeaxanthin attenuates VEGF-induced neovascularisation in human retinal microvascular endothelial cells through a Nox4-dependent pathway. Exp. Eye Res. 2020, 197, 108104. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Han, S.G.; Lee, C.H.; Seo, H.G. Lutein suppresses hyperglycemia-induced premature senescence of retinal pigment epithelial cells by up-regulating SIRT1. J. Food Biochem. 2018, 42, e12495. [Google Scholar] [CrossRef]
- Ravikrishnan, R.; Rusia, S.; Ilamurugan, G.; Salunkhe, U.; Deshpande, J.; Shankaranarayanan, J.; Shankaranarayana, M.L.; Soni, M.G. Safety assessment of lutein and zeaxanthin (Lutemax™ 2020): Subchronic toxicity and mutagenicity studies. Food Chem. Toxicol. 2011, 49, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.P.; Ravikumar, C.; Eswarappa, R.; Krishnappa, H.; Rao, K.S.; Quiroga, J.T.; Raviyadava. Sub-chronic (90 day) oral toxicity study in rats with lutein diacetate. Toxicol. Int. 2009, 16, 55–62. [Google Scholar]
- Nidhi, B.; Baskaran, V. Acute and Subacute Toxicity Assessment of Lutein in Lutein-Deficient Mice. J. Food Sci. 2013, 78, 1636–1642. [Google Scholar] [CrossRef]
- Ranganathan, A.; Hindupur, R.; Vallikannan, B. Biocompatible lutein-polymer-lipid nanocapsules: Acute and subacute toxicity and bioavailability in mice. Mater. Sci. Eng. C 2016, 69, 1318–1327. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Shao, B.; Sun, X.; Ho, C.; Li, S. Safety evaluation of meso-zeaxanthin. Food Control 2013, 32, 678–686. [Google Scholar] [CrossRef]
- Ravi, K.B.; Reddy, K.R.R.; Shankaranarayanan, J.; Deshpande, J.V.; Juturu, V.; Soni, M.G. Safety Evaluation of Zeaxanthin Concentrate (Omnixan™): Acute, Subchronic Toxicity and Mutagenicity Studies. Food Chem. Toxicol. 2014, 72, 30–39. [Google Scholar] [CrossRef]
- Thurnham, D.; Howard, A. Studies on Meso-Zeaxanthin for Potential Toxicity and Mutagenicity. Food Chem. Toxicol. 2013, 59, 455–463. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.; Elman, M.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.; Fish, G.; et al. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration. JAMA 2013, 309, 2005. [Google Scholar] [CrossRef]
- Akuffo, K.; Nolan, J.; Howard, A.; Moran, R.; Stack, J.; Klein, R. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye 2015, 29, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Chakravarthy, U.; Nolan, J.; Muldrew, K.; Woodside, J.; Denny, F.; Stevenson, M.R. Secondary Outcomes in a Clinical Trial of Carotenoids with Coantioxidants versus Placebo in Early Age-related Macular Degeneration. Ophthalmology 2013, 120, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Berrow, E.; Bartlett, H.; Eperjesi, F.; Gibson, J. The effects of a lutein-based supplement on objective and subjective measures of retinal and visual function in eyes with age-related maculopathy—A randomised controlled trial. Br. J. Nutr. 2012, 109, 2008–2014. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T. Dose-dependent response of serum lutein and macular pigment optical density to supplementation with lutein esters. Arch. Biochem. Biophys. 2010, 504, 50–55. [Google Scholar] [CrossRef]
- Dawczynski, J.; Jentsch, S.; Schweitzer, D.; Hammer, M.; Lang, G.; Strobel, J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: The LUTEGA study. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 2711–2723. [Google Scholar] [CrossRef]
- Fujimura, S.; Ueda, K.; Nomura, Y.; Yanagi, Y. Preliminary analysis of the relationship between serum lutein and zeaxanthin levels and macular pigment optical density. Clin. Ophthalmol. 2016, 10, 2149–2155. [Google Scholar] [CrossRef]
- Hammond, B.; Fletcher, L.; Roos, F.; Wittwer, J.; Schalch, W. A Double-Blind, Placebo-Controlled Study on the Effects of Lutein and Zeaxanthin on Photostress Recovery, Glare Disability, and Chromatic Contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef]
- Huang, Y.; Dou, H.; Huang, F.; Xu, X.; Zou, Z.; Lu, X. Changes following supplementation with lutein and zeaxanthin in retinal function in eyes with early age-related macular degeneration: A randomised, double-blind, placebo-controlled trial. Br. J. Ophthalmol. 2014, 99, 371–375. [Google Scholar] [CrossRef]
- Stringham, J.M.; Stringham, N.T. Serum and retinal responses to three different doses of macular carotenoids over 12 weeks of supplementation. Exp. Eye Res. 2016, 151, 1–8. [Google Scholar] [CrossRef]
- Christen, W.G.; Liu, S.; Glynn, R.J.; Gaziano, J.M.; Buring, J.E. Dietary Carotenoids, Vitamins C and E, and Risk of Cataract in Women. Arch. Ophthalmol. 2008, 126, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Lyle, B.J.; Mares-Perlman, J.A.; Klein, B.E.K.; Klein, R.; Palta, M.; Bowen, P.E.; Greger, J.L. Serum carotenoids and tocopherols and incidence of age-related nuclear cataract. Am. J. Clin. Nutr. 1999, 69, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Lyle, B.J.; Mares-Perlman, J.A.; Klein, B.E.; Klein, R.; Greger, J.L. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am. J. Epidemiol. 1999, 149, 801–809. [Google Scholar] [CrossRef]
- Brown, L.; Rimm, E.; Seddon, J.; Giovannucci, E.; Chasan-Taber, L.; Spiegelman, D.; Willett, W.C.; Hankinson, S.E. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am. J. Clin. Nutr. 1999, 70, 517–524. [Google Scholar] [CrossRef]
- Chasan-Taber, L.; Willett, W.; Seddon, J.; Stampfer, M.; Rosner, B.; Colditz, G.; Speizer, F.E.; Hankinson, S.E. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am. J. Clin. Nutr. 1999, 70, 509–516. [Google Scholar] [CrossRef]
- Glaser, T.; Doss, L.; Shih, G.; Nigam, D.; Sperduto, R.; Ferris, F.; Agron, E.; Clemons, T.E.; Chew, E.Y. The Association of Dietary Lutein plus Zeaxanthin and B Vitamins with Cataracts in the Age-Related Eye Disease Study. Ophthalmology 2015, 122, 1471–1479. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch. Ophthalmol. 2001, 119, 1439–1452. [Google Scholar] [CrossRef]
- Papudesu, C.; Clemons, T.E.; Agrón, E.; Chew, E.Y. Age-Related Eye Disease Study 2 Research Group, Association of Mortality with Ocular Diseases and Visual Impairment in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 13. Ophthalmology 2018, 125, 512–521. [Google Scholar] [CrossRef]
- Christen, W.G.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M.; Sperduto, R.D.; Buring, J.E.; Hennekens, C.H. A Randomized Trial of Beta Carotene and Age-Related Cataract in US Physicians. Arch. Ophthalmol. 2003, 121, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.; Glynn, R.; Sperduto, R.; Chew, E.; Buring, J. Age-related cataract in a randomized trial of beta-carotene in women. Ophthalmic Epidemiol. 2004, 11, 401–412. [Google Scholar] [CrossRef]
- Chylack, L.T.; Brown, N.P.; Bron, A.; Hurst, M.; Köpcke, W.; Thien, U.; Schalch, W. The Roche European American Cataract Trial (REACT): A randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. 2002, 9, 49–80. [Google Scholar] [CrossRef]
- Teikari, J.M.; Virtamo, J.; Rautalahti, M.; Palmgren, J.; Liesto, K.; Heinonen, O.P. Long-term supplementation with alpha-tocopherol and beta-carotene and age-related cataract. Acta Ophthalmol. Scand. 1997, 75, 634–640. [Google Scholar] [CrossRef]
- Azar, G.; Maftouhi, M.Q.; Masella, J.J.; Mauget-Faÿsse, M. Macular pigment density variation after supplementation of lutein and zeaxanthin using the Visucam ® 200 pigment module: Impact of age-related macular degeneration and lens status. J. Fr. Ophtalmol. 2017, 40, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla, B.; Granado, F.; Blanco, I.; Vaquero, M. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: A 2-y double-blind, placebo-controlled pilot study. Nutrition 2003, 19, 21–24. [Google Scholar] [CrossRef]
- Korobelnik, J.; Rougier, M.; Delyfer, M.; Bron, A.; Merle, B.M.J.; Savel, H.; Chene, G.; Delcourt, C.; Creuzot-Garcher, C. Effect of Dietary Supplementation With Lutein, Zeaxanthin, and ω-3 on Macular Pigment: A Randomized Clinical Trial. JAMA Ophthalmol. 2017, 135, 1259–1266. [Google Scholar] [CrossRef]
- Bahrami, H.; Melia, M.; Dagnelie, G. Lutein supplementation in retinitis pigmentosa: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial [NCT00029289]. BMC Ophthalmol. 2006, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Hathcock, J.N. Risk assessment for the carotenoids lutein and lycopene. Regul. Toxicol. Pharm. 2006, 45, 289–298. [Google Scholar] [CrossRef]
- Celentano, J.C.; Burke, J.D.; Hammond, B.R., Jr. In Vivo Assessment of Retinal Carotenoids: Macular Pigment Detection Techniques and Their Impact on Monitoring Pigment Status. J. Nutr. 2002, 132, 535–539. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015, 133, 1415. [Google Scholar] [CrossRef]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V. Lutein and Zeaxanthin—Food Sources, Bioavailability and Dietary Variety in Age-Related Macular Degeneration Protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef]
- Green-Gomez, M.; Prado-Cabrero, A.; Moran, R.; Power, T.; Gómez-Mascaraque, L.G.; Stack, J.; Nolan, J.M. The Impact of Formulation on Lutein, Zeaxanthin, and meso-Zeaxanthin Bioavailability: A Randomised Double-Blind Placebo-Controlled Study. Antioxidants 2020, 9, 767. [Google Scholar] [CrossRef] [PubMed]
| Reference | Type and Number of Animal Used | Duration of Therapy | Name and Dose of Carotenoids Administered | Parameters Observed | Findings and Observation |
|---|---|---|---|---|---|
| [84] | 120 wistar strain rats (60 males and 60 females) | 90 days | Lutein/zeaxanthin at the concentrate of 0, 4, 40, and 400 mg/kg body weight/day. It was given as extract of marigold flowers (Tagetes erecta L) containing minimum 80% carotenoids of which 67% is lutein and 13.5% is zeaxanthin isomers. | Body weights, urine parameters (volume, specific gravity, color, clarity, pH, RBC, WBC, bilirubin, ketone bodies, proteins, glucose and nitrite), hematological analysis, serum parameter analysis (glucose, urea, creatinine, cholesterol, triglycerides, AST, ALT, ALP, bilirubin, sodium, potassium, chloride, total protein, albumin, globulin and A/G ratio), and gene mutation | No adverse effect was observed. NOAEL: 400 mg/kg/day HED: 64.8 mg/kg/day |
| [85] | 20 rats (10 male, 10 female) | 90 days | Lutein diacetate 2.1, 22.5, and 210 mg/kg body weight/day | Body weights, net body weight gains, feed intake, neurological observations, hematology and clinical chemistry | No adverse effect was observed. It is relatively safe in rats up to this dose. NOAEL: 210 mg/kg/day HED: 34.02 mg/kg/day |
| [20] | 70 wistar rats (35 males and 35 females) for short-term toxicity study and 70 more rats for subchronic toxicity study | Short-term toxicity study: 4 weeks, subchronic toxicity study: 13 weeks | Lutein and its ester was administered orally at doses of 4, 40, and 400 mg/kg body weight/day | Body weight, food consumption pattern, organ weight, other adverse side reactions, alteration in hepatic and renal function, change in the hematological parameters and in lipid profile | No adverse effect was observed. No mortality was produced by lutein and lutein ester up to a concentration of 4 g/kg. Lutein and its ester form was found nontoxic. NOAEL: 400 mg/kg/day HED: 64.8 mg/kg/day |
| [86] | 80 weanling male albino mice (50 for acute toxicity study, 30 for subacute toxicity study) | Acute toxicity study: 14 days, subacute toxicity study: 4 weeks | Lutein 0.57, 100, 1000, and 10,000 mg/kg body weight for acute toxicity study; 0, 100, and 1000 mg/kg body weight/day for subacute toxicity study | Clinical observation, ophthalmic examinations, body, organ weights, hematological, histopathological, and other clinical chemistry parameters | LD50 exceeded 10,000 mg/kg body weight. No toxicity was observed up to this dose. NOAEL: 1000 mg/kg/day HED: 81 mg/kg/day |
| [87] | 90 female swiss albino mice (50 for acute toxicity study, 40 for subacute toxicity study) | Acute toxicity study: 2 weeks, subacute toxicity study: 4 weeks | Lutein-poly-(lactic-co-glycolic acid) (PLGA)-phospholipid (PL) nano-capsules 0.1, 1, 10, and 100 mg/kg body weight for acute toxicity study; 1 and 10 mg/kg body weight/day for subacute toxicity study | Mortality, ophthalmic examinations, body and organ weights, hematology, histopathology and other blood and tissue clinical chemistry parameters | No toxicity or adverse effect was observed at a dose of 10 mg/kg body weight. NOAEL: 10 mg/kg/day HED: 0.81 mg/kg/day |
| [88] | 100 SD rats | Subchronic toxicity: 90 days, acute toxicity: 2 weeks | Maximum tolerable dose was more than 10.0 g/kg body weight (acute oral toxicity tests), Highest dosage of 300 mg/kg/day meso-zeaxanthin | Acute toxicity, genetic toxicity (Ames test, mice bone marrow erythrocyte micronucleus and mice sperm abnormality) | No acute toxicity and no genotoxicity was observed. NOAEL: 300 mg/kg/day HED: 48.6 mg/kg/day |
| [89] | 120 wistar rats (60 males and 60 females) | 90 days | Zeaxanthin concentrate at doses of 0, 4, 40, and 400 mg/kg body weight/day for sub-chronic toxicity study; additional 100, 200, 400, and 1000 mg/kg body weight/day; 4 rats were given zeaxanthin 2000 mg/kg bw/day for acute toxicity study | Body weight, feed consumption; ophthalmoscopic examination, clinical pathology, neurological examination, clinical chemistry, hematology, urine analysis, necropsy, organ weight, histopathology, mutagenic activity | No mortality, toxicity, and mutagenicity was observed. NOAEL: 400 mg/kg/day HED: 64.8 mg/kg/day |
| [90] | 50 han wistar rats | 13 weeks followed by a 4-week recovery period | Meso-zeaxanthin 2, 20, and 200 mg/kg/day. | Potential toxicity and genotoxicity | No adverse effect was observed up to 200 mg/kg/day. NOAEL: 200 mg/kg/day HED: 32.4 mg/kg/day |
| Reference | Trial ID | Study Type | Study Duration | Characteristics of Participants | Intervention Group(s)/Comparison Group(s)/Assessment Criteria | Result |
|---|---|---|---|---|---|---|
| [91] | NCT00345176 | Multicenter, randomized, double-masked, placebo-controlled phase 3 study | 7 years | Patients N = 4203 Age range: 50–85 years Age (mean ± SD): 73.1 ± 7.7 Sex: 56.8% Female | Primary randomization: G 1: AREDS formulation G 2: AREDS, L 10 mg, Z 2 mg G 3: AREDS, DHA 350 mg, EPA 650 mg G 4: AREDS, L 10 mg, Z 2 mg, DHA 350 mg, EPA 650 mg | No statistically significant reduction in progression to advanced AMD was observed (p = 0.12 for L + Z). |
| Secondary randomization: G 1: AREDS supplement G 2: AREDS supplement with no beta carotene G 3: AREDS with low dose zinc (25 mg) G 4: AREDS supplement with no beta carotene and with low-dose zinc | ||||||
| [92] | ISRCTN60816411 | Single-blind, randomized controlled clinical trial | 3 years | Patients N = 67 (52 completed Age (mean ± SD): 66 ± 8 Sex: 65.4% Female | G 1: 20 mg L, 0.86 mg Z G 2: 10 mg MZ, 10 mg L, 2 mg Z G 3: 17 mg MZ, 3 mg L, 2 mg Z | No statistically significant change in AMD grade between intervention groups (p = 0.29, Fisher’s exact test). |
| [93] | ISRCTN 94557601 | Randomized double-masked placebo-controlled clinical trial | Each participant was followed up for up to 3 years | Patients N = 433 Age range: 50–95 years Age (mean ± SD): 75.9 ± 7.7 Sex: 57.3% Female | G1: A tablet was taken twice daily to deliver a daily dose of 12 mg L, 0.6 mg Z, 15 mg d-α-tocopherol, 150 mg ascorbic acid, 20 mg zinc oxide, and 0.4 mg copper gluconate G2: Placebo (made up of cellulose, lactose, and magnesium stearate) | 4.8 letters better BCVA was seen in active group than placebo group, which was statistically significant (p = 0.04). Visual acuity increased by 1.4 letters with 1-log-unit increase in serum lutein. Morphologic severity progressed slowly. |
| [94] | ISRCTN 17842302 | Randomized clinical trial on participants with AMD | 60 weeks | Patients N = 14 Age range: 56–83 years Age (mean ± SD): 67.3 ± 8.5 | G1: Ascorbic acid 150 mg, cupric oxide 400 µg, DL-α-tocopherol 15 mg, zinc oxide 20 mg, lutein 12 mg, Z 0.6 mg, EPA 240 mg, DHA 840 mg G2: No supplement | A nonsignificant latency and reduced amplitudes of multifocal electroretinography (mfERG) was seen on supplement withdrawal (ring 3 N2 latency, p = 0.041 and ring 4 P1 latency, p = 0.016). |
| [95] | - | Double-blind and placebo-controlled clinical trial | 140 days | Patients N = 100 Age range: 18–64 years Sex: 48% Female | G1: Placebo G2: 5 mg L G3: 10 mg L G4: 20 mg L (younger group) G5: 20 mg L (older group) | A linear, dose-dependent increase in macular pigment optical density (MPOD) was observed (p < 0.0001). |
| [96] | NCT00763659 | A prospective, randomized double-blind, placebo controlled clinical trial on patients with non-exudative AMD | 12 months | Patients N = 172 Age (mean ± SD): 70 ± 10 Sex: 54.7% Female | G1: 10 mg L, 1 mg Z, 255 mg concentrated fish oil (100 mg DHA, 30 mg EPA), 60 mg vitamin C, 20 mg vitamin E, 10 mg zinc, 0.25 mg copper G2: 20 mg L, 2 mg Z, 500 mg concentrated fish oil (200 mg DHA, 60 mg EPA) and 120 mg vitamin C, 40 mg vitamin E, 20 mg zinc, 0.5 mg copper G3: Placebo | Statistically significant increase in MPOD was seen in both G1 and G2 (p < 0.001). Supplementation with L and Z improved and stabilized BCVA in AMD patients. |
| [97] | - | Clinical trial on patients with unilateral wet AMD and patients with unilateral cCSC | 6 months | Patients N = 20 Age: >56 years Age (mean ± SD): 66 ± 4 Sex: 30% Female | Sante Lutax 20 plus DHA (20 mg L, 1 mg Z, and 200 mg DHA) | MPOD (p = 0.032) and contrast sensitivity (p < 0.05) improved. L, Z, DHA dietary supplement had beneficial effects. |
| [98] | NCT00909090 | Randomized, double-blind, placebo-controlled clinical trial | 400 days | Patients N = 115 (109 completed) Age (mean ± SD): 23.2 ± 4 Sex: 59.6% Female | G1: 10 mg L, 2 mg Z G2: Placebo | MPOD (p < 0.0001), chromatic contrast (p < 0.0001) and photostress recovery time (p = 0.002) improved significantly. |
| [99] | NCT10528605 | Randomized, double-blind, placebo controlled trial on patients with early AMD | 2 years | Patients N = 112 Age: >50 years Age (mean ± SD): 69.1 ± 7.3 Sex: 57.4% Female | G1: Placebo G2: 10 mg L G3: 20 mg L G4: L 10 mg, Z 10 mg | MPOD, retinal sensitivity, N1P1 response densities in ring 1 and ring 2 increased significantly in G2, G3, G4 (all p < 0.05) |
| [100] | ISRCTN54990825 | A double-blind, placebo-controlled | 12 weeks | Healthy subjects N = 32 Age range: 18–25 years | G1: Placebo G2: 6.18 mg L, 0.73 mg Z, 0.53 mg MZ (total 7.44 mg) G3: 10.86 mg L, 1.33 mg Z, 0.94 mg MZ (total 13.13 mg) G4: 22.33 mg L, 2.70 mg Z, 2 mg MZ (total 27.03 mg) | Serum level increased linearly with increased dose. Group 3 showed the highest ratio of MPOD change, which was statistically significant (p = 0.021). |
| [101] | - | Randomized, double masked, placebo-controlled trial | 10 years | Patients and healthy subjects N = 39,876 Age range: ≥45 years Age (mean): 53.5 (no cataract), 61 (cataract) Sex: 100% Female | β-carotene, vitamin A, and vitamin E | Only 2031 people developed cataract. Most of them were smokers, had high BMI, and reported a history of hypertension, diabetes, and high cholesterol. Higher dietary intakes of L/Z (p = 0.04) and vitamin E (p = 0.03) significantly decreased the risk of cataract. |
| [102] | - | Prospective study | 5 years | Patients and healthy subjects N = 400 Age range: 50–86 years Sex: Both male and Female | No supplement was provided. Serum concentrations of individual carotenoids, α- and γ-tocopherol were determined | Serum carotenoids did not have significant association with nuclear cataract prevention (p = 0.13). |
| [103] | - | Cohort study | 10 years | Patients and healthy subjects N = 5925 Age range: 43–84 years Sex: Both male and Female | Intake of L and Z was assessed using a food frequency questionnaire | L (intake in the distant past) had protective influence on the development of nuclear cataracts (p = 0.002). |
| [104] | - | Prospective study | 8 years | Patients and healthy subjects N = 36,644 Age range: 45–75 years Sex: 0% Female | Dietary questionnaire was used to assess | A statistically significant lower risk of cataract in higher intakes of L and Z was observed (p = 0.03). No impact of other carotenoids (α-carotene, β-carotene, lycopene, and β-cryptoxanthin) are found in cataract prevention |
| [105] | - | Prospective cohort study | 12 years | Patients and healthy subjects N = 77,466 Age range: 45–71 years | Food frequency questionnaire was used to assess | Foods rich in carotenoids (L and Z) decreased the risk of cataracts (22%) (p = 0.04). |
| [106] | NCT00000145 | Clinic-based, baseline cross-sectional and prospective cohort study | 9.6 years | Patients N = 3115 Age range: 55–80 years Sex: Both male and Female | Food frequency questionnaires | No significant association of L plus Z intake with the development of nuclear or cortical lens opacity. |
| [107] | NCT00000145 | 11-center age-related eye disease study double-masked clinical trial | 6.3 years | Patients N = 4757 Age range: 55–80 years Sex: 56% Female | G1: Antioxidants (β-carotene 15 mg, vitamin C 500 mg; vitamin E, 400 IU) G2: Antioxidants (vitamin C, 500 mg; vitamin E, 400 IU; and β-carotene, 15 mg) + zinc 80 mg + copper 2 mg G3: Zinc 80 mg + copper 2 mg G4: Placebo | No statistically significant effect was observed on the progression or prevention of cataract, age-related macular degeneration, age-related lens opacities, or visual acuity loss. |
| [108] | NCT00345176 | Randomized, double-masked, controlled clinical trial, cohort study. | 5 years | Patients N = 4203 Age range: 50–80 years Sex: 56.8% Female | G1: Control group G2: L 10 mg and Z 2 mg G3: Docosahexaenoic acid (DHA 350 mg) and eicosapentaenoic acid (EPA 650 mg) G4: L 10 mg and Z 2 mg, docosahexaenoic acid (DHA 350 mg) and eicosapentaenoic acid (EPA 650 mg) | Carotenoid supplementation did not have any significant positive or negative impact on the high mortality rate observed in people having age-related macular disease. |
| [109] | - | Randomized, double-masked, placebo-controlled trial | 12 years | Patients and healthy subjects N = 22,071 Age range: 40–84 years Sex: 0% Female | β-carotene (50 mg) or placebo | β-carotene supplementation had no overall beneficial or harmful effect on cataract or cataract extraction. |
| [110] | NCT00000479 | Randomized, double masked, placebo-controlled trial | 2.1 years | Patients and healthy subjects N = 39,876 Age: ≥45 years Sex: 100% Female | G1: β-carotene G2: Placebo | No large beneficial or harmful effect was found on the development of cataract. |
| [111] | - | Multi-centered, prospective, double-masked, randomized, placebo-controlled trial | 3 years | Patients N = 445 Age (mean ± SD): 66.2 ± 8.9 Sex: 59.3% Female | G1: 6 mg β-carotene, 200 mg α-tocopherol acetate (Vitamin E) and 250 mg ascorbic acid G2: Placebo | A small, statistically significant reduction in the progression of age-related cataract was observed after three years of treatment (p = 0.048). |
| [112] | NCT00342992 | Randomized, double-blind, placebo-controlled clinical trial | 5 to 8 years (median 6.6 years) | Patients N = 1828 Age range: 50–69 years Sex: 0% Female | G1: α-tocopherol 50 mg/day, G2: β-carotene 20 mg/day, G2: A combination of the two G4: placebo | No influence of supplementation on cataract prevalence. |
| [113] | NCT0140845 | Prospective, randomized, double-masked multicenter study | 24 months | Patients N = 126 Age (mean ± SD): 75.3 ± 7.6 Sex: 58.7% Female | G1: Carotenoids (5 mg of L and 1 mg of Z) + (560 mg of DHA, 420 mg GLA, 80 mg of vitamin C, 10 mg of vitamin E, 2 mg of vitamin B6, 200 g of Vitamin B9, 1 g of vitamin B12, 10 mg of Zinc) G2: Placebo + (560 mg of DHA, 420 mg GLA, 80 mg of vitamin C, 10 mg of vitamin E, 2 mg of vitamin B6, 200 g of Vitamin B9, 1 g of vitamin B12, 10 mg of Zinc) | Carotenoid supplementation did not improve MPOD, which was exacerbated by cataract and age-related macular degeneration. |
| [114] | - | Randomized, double-blind, placebo-controlled supplementation study | 2 years | Patients N = 17 Age range: 55–73 years Sex: 86.7% Female | G1: 12 mg of all-trans-lutein, 3 mg of 13/15-cis-lutein, 3.3 mg of alpha-tocopherol G2: 100 mg of alpha-tocopherol G3: Placebo (0.06 mg of alpha-tocopherol, 0.23 mg of gamma-tocopherol, 500 mg of corn oil) | L has beneficial effect on age-related cataracts, whereas alpha-tocopherol was not beneficial. |
| [115] | NCT01269697 | Phase 3, double-blind, randomized clinical trial | 6 months | Healthy subjects N = 120 Age range: 40–70 years Age (mean ± SD): 56.7 ± 6.6 Sex: 71.7% Female | G1: L 5 mg, Z 1 mg, vitamin C (90 mg), vitamin E (15 mg), zinc (7.5 mg), copper (<0.5 mg), and resveratrol (0.5 mg), 33 mg of fish oil (50% ω-3) G2: Placebo | An increase in plasma L and Z concentrations was observed (p < 0.005) but no elevation of MPOD was found. |
| [116] | NCT00029289 | Double-masked randomized placebo-controlled clinical trial with a crossover design | 24 weeks | Patients N = 34 Age (mean ± SD): 49.2 ± 9 Sex: 61.8% Female | G1: L 10 mg/d for 12 weeks, 30 mg/d for next 12 weeks, followed by placebo for 24 weeks G2: Placebo 24 weeks, followed by L 10 mg/d for 12 weeks, followed by 30 mg/d for next 12 weeks | Significantly improved visual field (p = 0.038), slightly improved visual acuity. L 10–30 mg/day for up to 6 months was safe. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. https://doi.org/10.3390/antiox9111046
Johra FT, Bepari AK, Bristy AT, Reza HM. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants. 2020; 9(11):1046. https://doi.org/10.3390/antiox9111046
Chicago/Turabian StyleJohra, Fatima Tuj, Asim Kumar Bepari, Anika Tabassum Bristy, and Hasan Mahmud Reza. 2020. "A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease" Antioxidants 9, no. 11: 1046. https://doi.org/10.3390/antiox9111046
APA StyleJohra, F. T., Bepari, A. K., Bristy, A. T., & Reza, H. M. (2020). A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants, 9(11), 1046. https://doi.org/10.3390/antiox9111046







