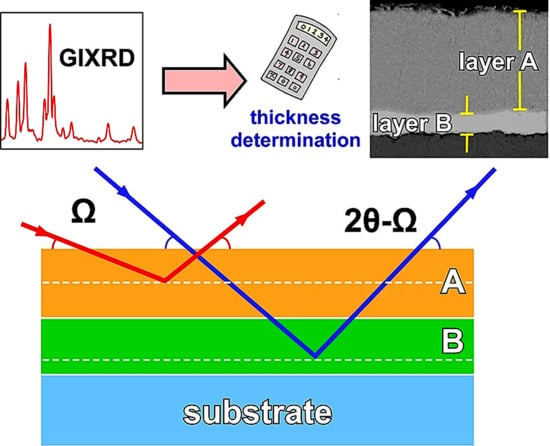
Application of X-rays Diffraction for Identifying Thin Oxide Surface Layers on Zinc Coatings
Abstract
:1. Introduction
2. Theoretical Calculations of the Penetration Depth of X-rays
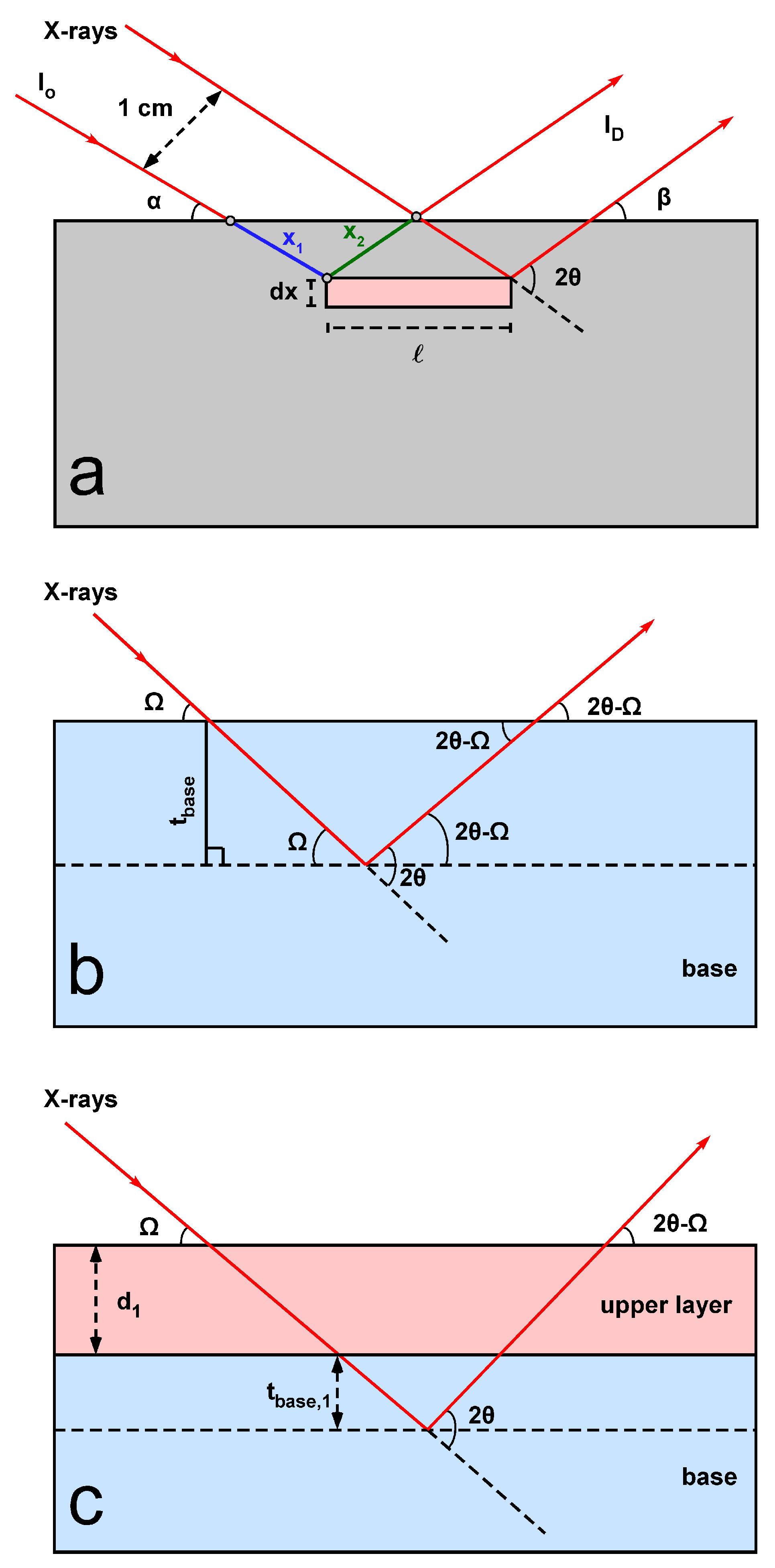
2.1. Calculation of the Penetration Depth of X-rays in BB-XRD
2.2. Penetration Depth of X-rays in GI-XRD
2.3. Penetration Depth of X-rays in a Multilayered Material
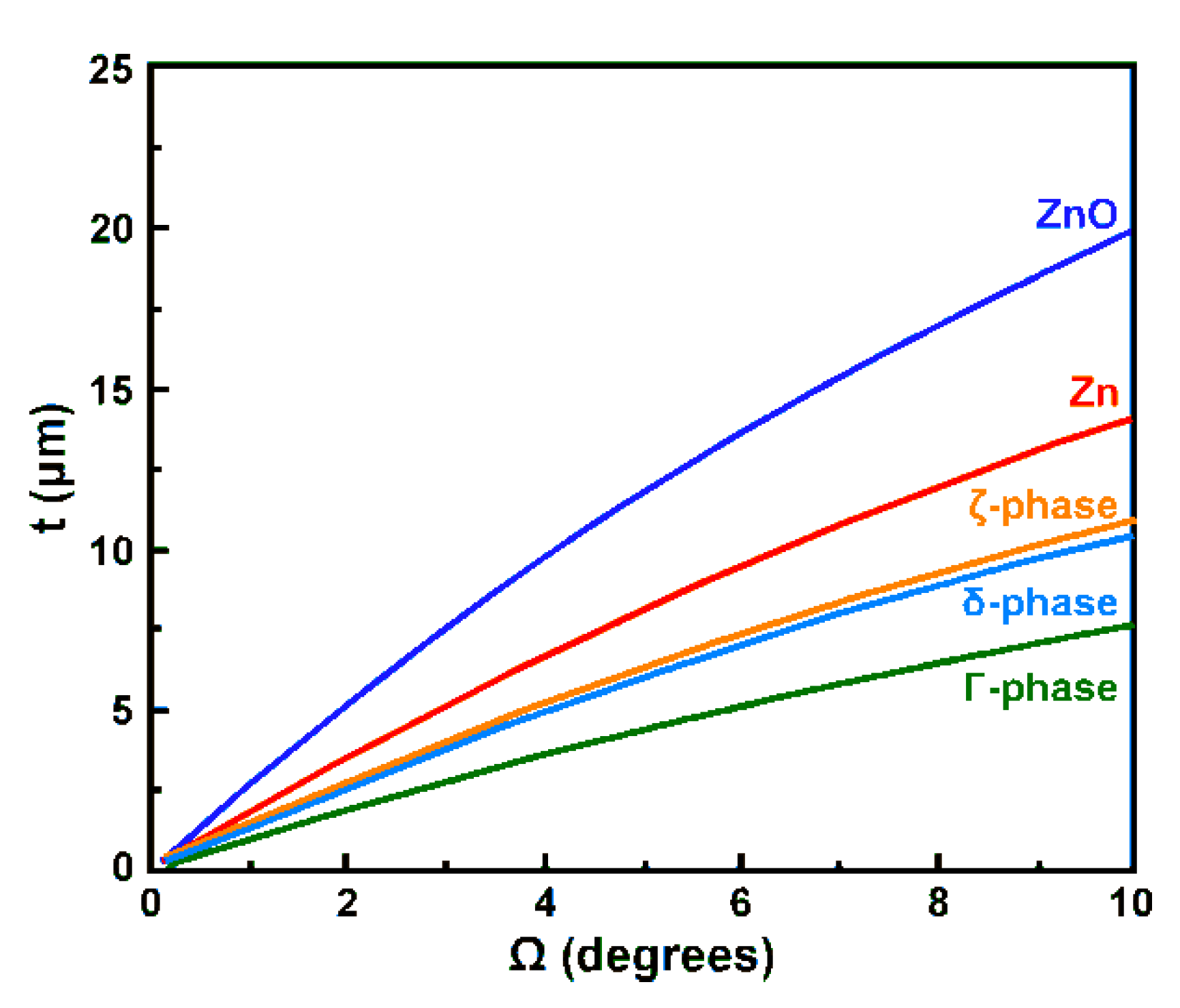
2.4. Evaluation of the Penetration Depth of X-rays in Particular Zn Compounds
3. Experimental Details
3.1. Growth Processes
3.2. Characterization of Coatings
4. Results and Discussion
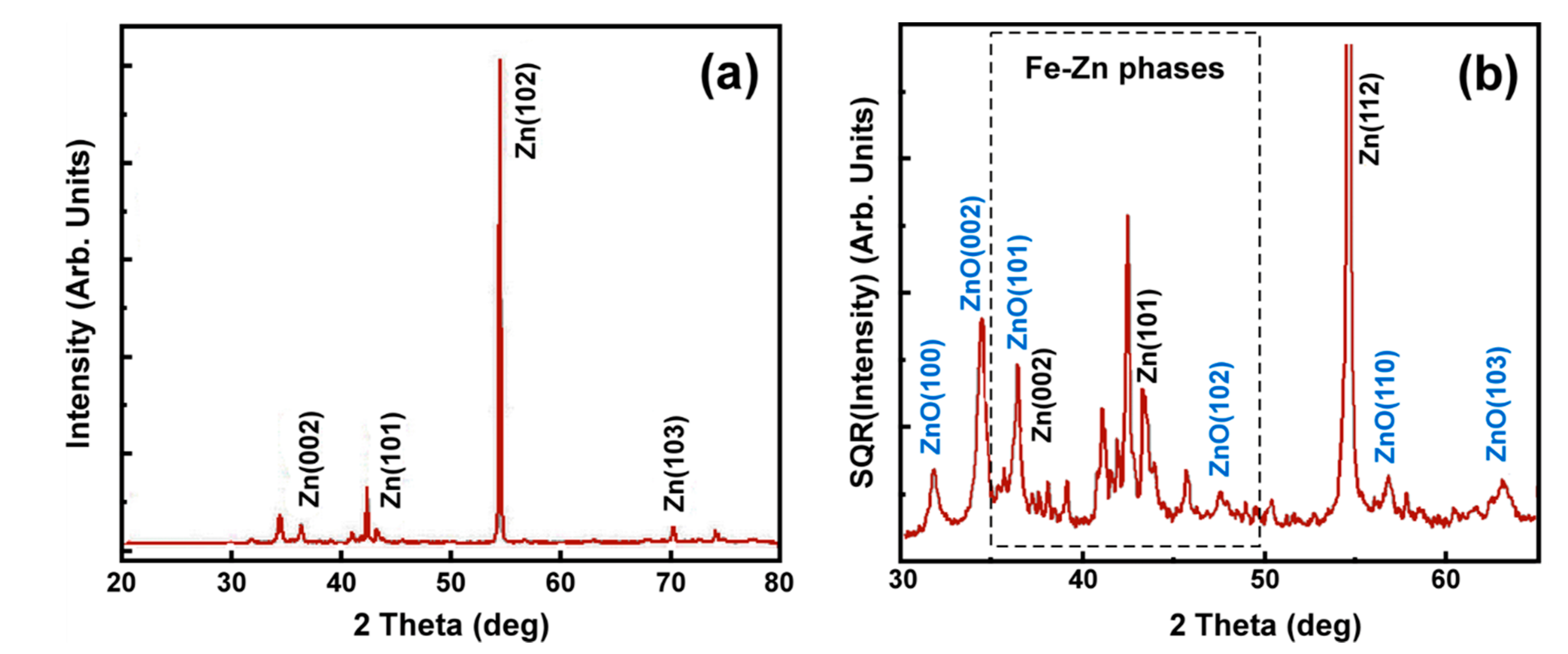
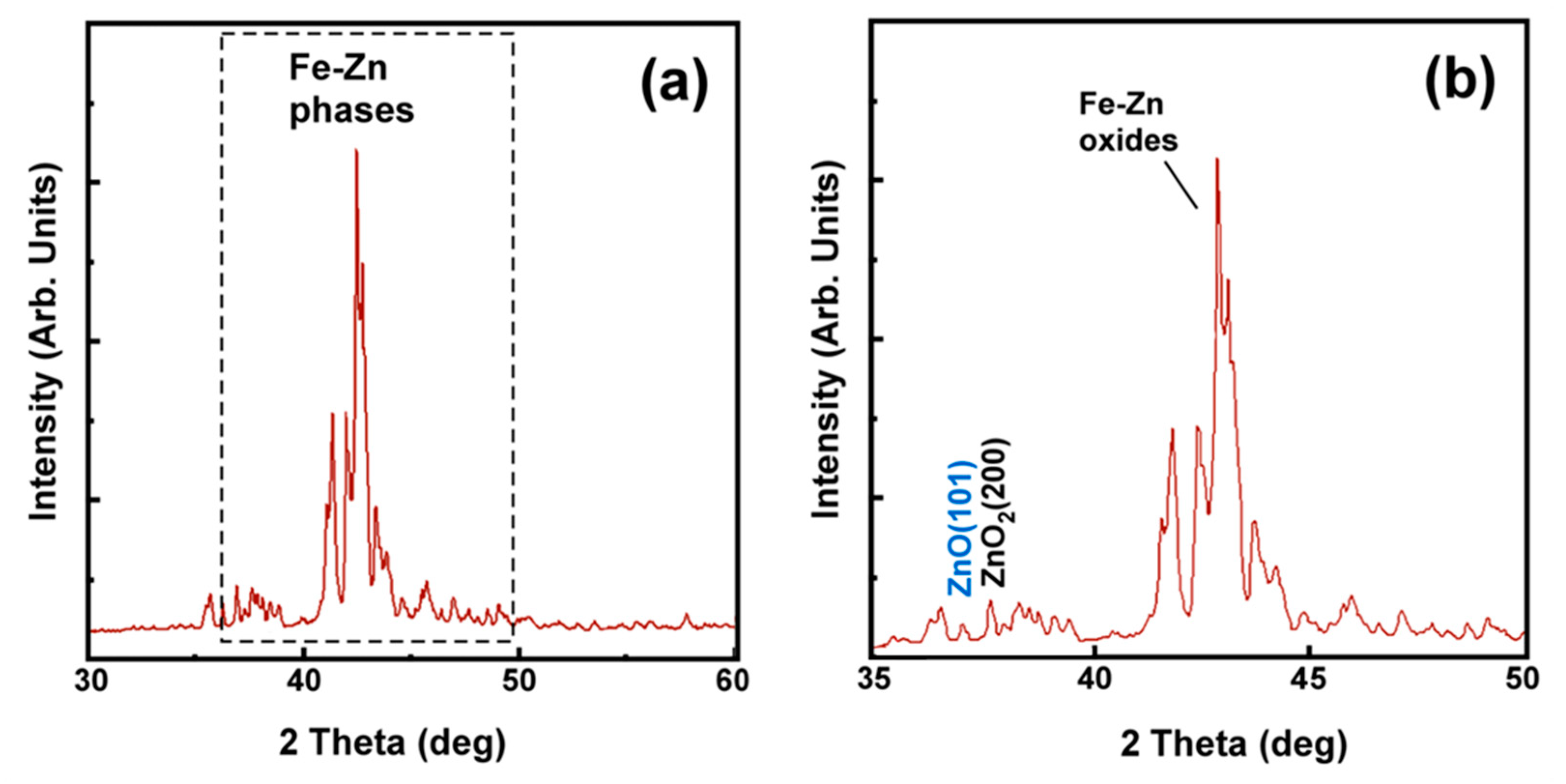
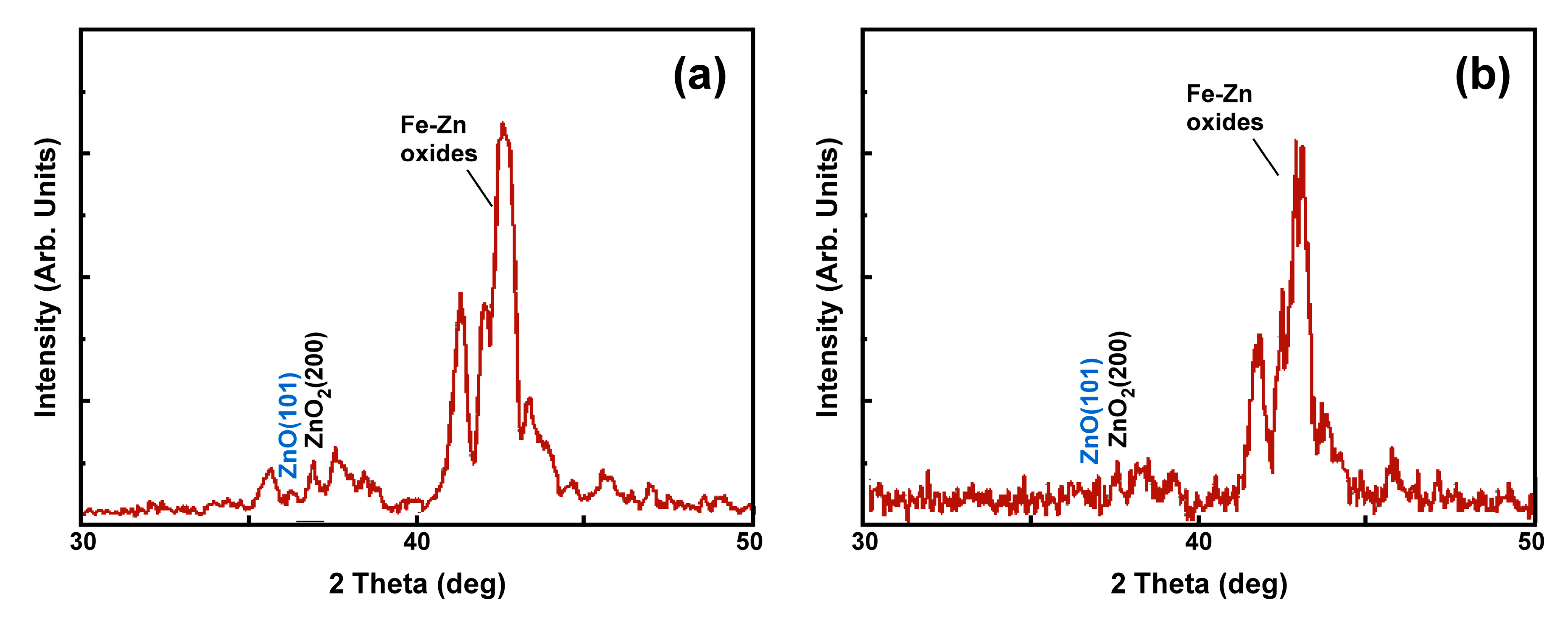
4.1. Hot-Dip Galvanized Coatings
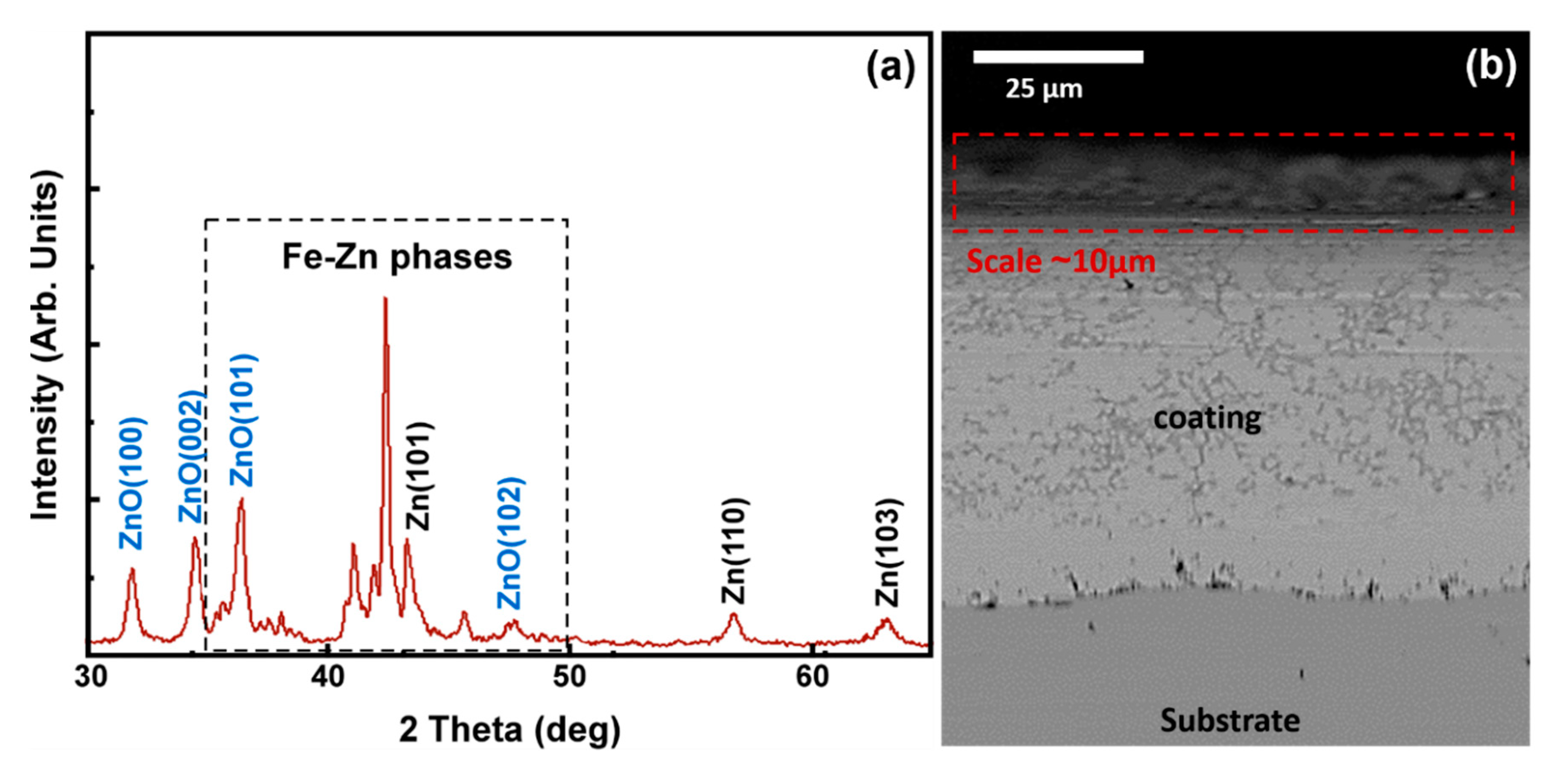
4.2. Characterization of Pack Cementation Coatings
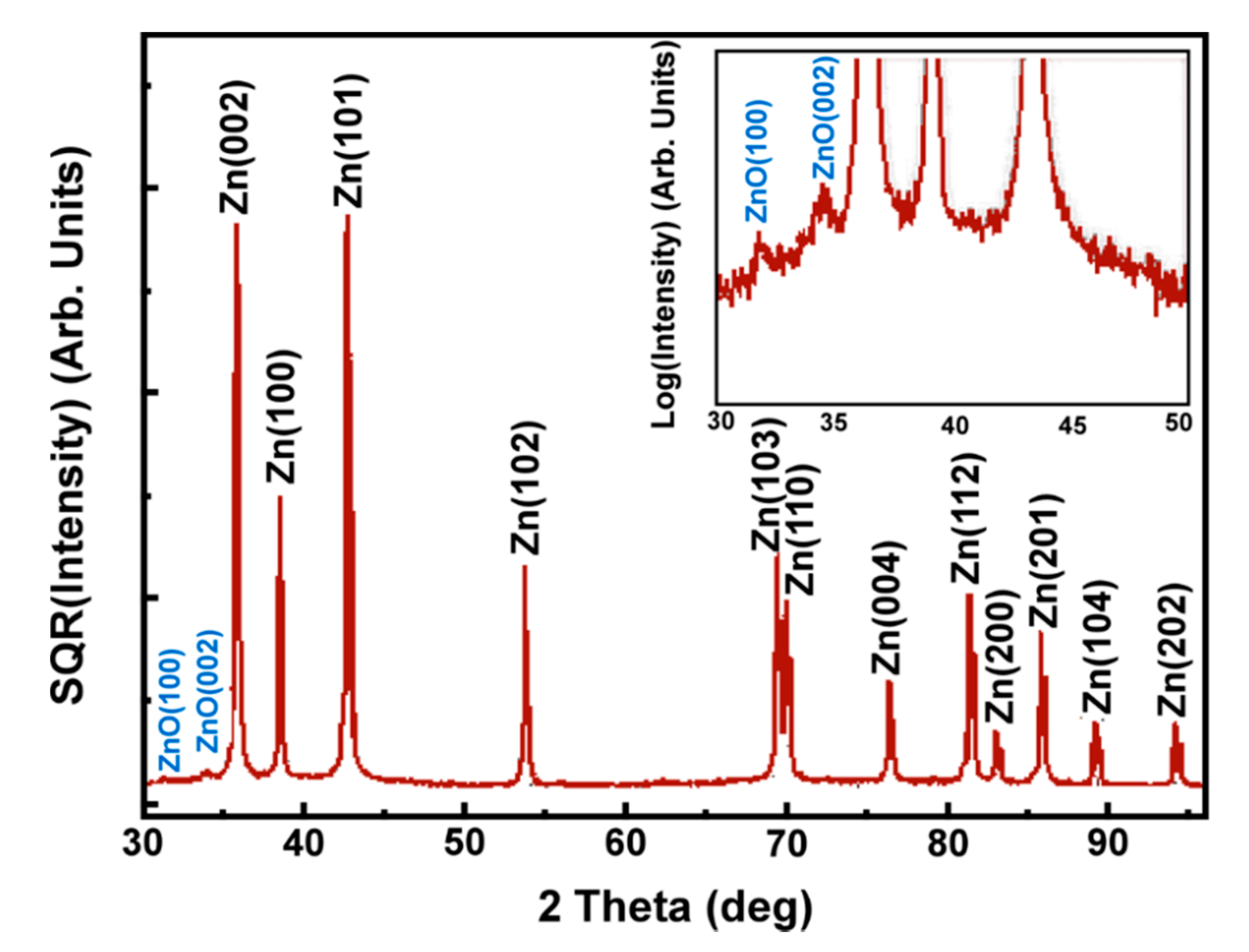
4.3. Characterization of Thermal Sprayed Zn Coatings
4.4. Experimental Verification of the Theoretical Calculations
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Vourlias, G.; Pistofidis, N.; Stergioudis, G.; Tsipas, D. The effect of alloying elements on the crystallization behavior and on the properties of galvanized coatings. Cryst. Res. Technol. 2004, 39, 23–29. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Bai, X.; Wang, H.; Xu, S.; Song, S. Microstructure, mechanical properties, abrasive wear, and corrosion behavior in molten zinc of boride-based coatings in situ synthesized by an HVOF spraying process. Coatings 2019, 9, 665. [Google Scholar] [CrossRef] [Green Version]
- Thierry, D.; LeBozec, N.; Le Gac, A.; Persson, D. Long-term atmospheric corrosion rates of hot dip galvanised steel and zinc-aluminium-magnesium coated steel. Mater. Corros. 2019, 70, 2220–2227. [Google Scholar] [CrossRef]
- Pistofidis, N.; Vourlias, G.; Chaliampalias, D.; Pavlidou, E.; Chrissafis, K.; Stergioudis, G.; Polychroniadis, E.K.; Tsipas, D. DSC study of the deposition reactions of zinc pack coatings up to 550 °C. J. Therm. Anal. Calorim. 2006, 54, 191–194. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Pei, L.; Sheng, H. Improved corrosion resistance of hydroxyapatite coating on carbon fiber by applying SiC interlayer. Appl. Surf. Sci. 2020, 512, 145692. [Google Scholar] [CrossRef]
- He, J.; Guo, X.; Qiao, Y. Oxidation behavior and adhesion performance of TiSi2–NbSi2 composite coating prepared via magnetron sputtering and then pack cementation. J. Alloys Compd. 2020, 820, 153425. [Google Scholar] [CrossRef]
- Vourlias, G.; Pistofidis, N.; Chaliampalias, D.; Patsalas, P.; Stergioudis, G.; Tsipas, D. A comparative study of the structure and the corrosion behavior of zinc coatings deposited with various methods. Surf. Coat. Technol. 2006, 200, 6594–6600. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Miyazaki, F.; Yamasaki, M.; Yamagata, Y.; Kobayashi, N.; Muraoka, K. Coating qualities deposited using three different thermal spray technologies in relation with temperatures and velocities of spray droplets. Coatings 2017, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Curry, N.; VanEvery, K.; Snyder, T.; Markocsan, N. Thermal conductivity analysis and lifetime testing of suspension plasma-sprayed thermal barrier. Coatings 2014, 4, 630–650. [Google Scholar] [CrossRef]
- Kahar, S.; Singh, A.; Vala, U.; Desai, A.; Smit Desai, S. Thermal sprayed coating using zinc: A review. Int. Res. J. Eng. Technol. 2020, 7, 6497–6503. [Google Scholar]
- Milanti, A.; Koivuluoto, H.; Vuoristo, P.; Bolelli, G.; Bozza, F.; Lusvarghi, L. Microstructural characteristics and tribological behavior of HVOF-sprayed novel fe-based alloy coatings. Coatings 2014, 4, 98–120. [Google Scholar] [CrossRef]
- Vourlias, G.; Pistofidis, N.; Chrissafis, K.; Stergioudis, G. Zinc coatings for oxidation protection of ferrous substrates: Part I. Macroscopic examination of the coating oxidation. J. Therm. Anal. Calorim. 2007, 90, 769–775. [Google Scholar] [CrossRef]
- Vourlias, G.; Pistofidis, N.; Chrissafis, K.; Stergioudis, G. Zinc coatings for oxidation protection of ferrous substrates: Part II. Microscopic and oxidation mechanism examination. J. Therm. Anal. Calorim. 2007, 90, 777–782. [Google Scholar] [CrossRef]
- Yamada, H.; Suryanarayanan, R. Calculation of the penetration depth of X-rays in intact pharmaceutical film-coated tablets by microdiffractometry. Pharm. Res. 2006, 23, 2149–2157. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Pistofidis, N.; Pavlidou, E.; Tsipas, D.; Stergioudis, G.; Vourlias, G. Resistance of different Zn coatings at elevated temperature air environments. Surf. Eng. 2016, 32, 53–60. [Google Scholar] [CrossRef]
- Kolaklieva, I.; Kakanakov, R.; Chaliampalias, D.; Vourlias, G.; Cholakova, T.; Pashinski, C.; Chitanov, V.; Bahchedjiev, C.; Petkov, N.; Polychroniadis, E. Mechanical, structural and thermal properties of multilayered gradient nanocomposite coatings. J. Nano Res. 2012, 17, 193–202. [Google Scholar] [CrossRef]
- Riyas, A.H.; Shibli, S.M.A. Assessment of the role of ζ- and δ-phases in ZrO2–Al2O3 composite based hot dip zinc galvanized coating by layer wise tuning. Appl. Surf. Sci. 2019, 481, 972–986. [Google Scholar] [CrossRef]
- Kania, H.; Saternus, M.; Kudláček, J.; Svoboda, J. Microstructure characterization and corrosion resistance of zinc coating obtained in a Zn–AlNiBi galvanizing bath. Coatings 2020, 10, 758. [Google Scholar] [CrossRef]
- Yang, T.; He, Y.; Chen, Z.; Zheng, W.; Wang, H.; Li, L. Effect of dew point and alloy composition on reactive wetting of hot dip galvanized medium manganese lightweight steel. Coatings 2020, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.; Yang, W.; Baik, K.-H.; Kim, Y.; Lee, S.; Park, J.S. Growth kinetics and isothermal oxidation behavior of aluminide pack coatings on a multiphase Mo–Si–B alloy. Oxid. Met. 2019, 92, 423–437. [Google Scholar] [CrossRef]
- Pistofidis, N.; Vourlias, G.; Chaliampalias, D.; Chrysafis, K.; Stergioudis, G.; Polychroniadis, E.K. On the mechanism of formation of zinc pack coatings. J. Alloys Compd. 2006, 407, 221–225. [Google Scholar] [CrossRef]
- Chaia, N.; Cossu, C.M.F.A.; Ferreira, L.M.; Parrisch, C.J.; Cotton, J.D.; Coelho, G.C.; Nunes, C.A. Protective aluminide coating by pack cementation for Beta 21-S titanium alloy. Corros. Sci. 2019, 160, 108165. [Google Scholar] [CrossRef]
- Chaia, N.; Cury, P.L.; Rodrigues, G.; Coelho, G.C.; Nunes, C.A. Aluminide and silicide diffusion coatings by pack cementation for Nb–Ti–Al alloy. Surf. Coat. Technol. 2020, 389, 125675. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Pistofidis, N.; Vourlias, G. Effect of temperature and zinc concentration on zinc coatings deposited with pack cementation. Surf. Eng. 2008, 24, 259–263. [Google Scholar] [CrossRef]
- Guo, X.; Planche, M.-P.; Chen, J.; Liao, H. Relationships between in-flight particle characteristics and properties of HVOF sprayed WC–CoCr coatings. J. Mater. Process. Technol. 2014, 214, 456–461. [Google Scholar] [CrossRef]
- Ce, N.; Paul, S. Thermally sprayed aluminum coatings for the protection of subsea risers and pipelines carrying hot fluids. Coatings 2016, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Lin, Y.; Ke, P.; Wang, S.; Zhang, S.; Zhen, Z.; Ge, L. Influence of process parameters on high velocity oxy-fuel sprayed Cr3C2–25%NiCr. Coatings 2017, 7, 98. [Google Scholar] [CrossRef]
- Papanastasiou, K. Study of Zinc Coatings in Correlation with the Penetration Depth of X-rays-Applications. Ref. No. GRI-2011-6023. Master’s Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 7 July 2010. [Google Scholar]
- Angerer, P.; Mann, R.; Gavrilovic, A.; Nauera, G.E. In situ X-ray diffraction study of the electrochemical reaction on lead electrodes in sulphate electrolytes. Mater. Chem. Phys. 2009, 114, 983–989. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Shah, S. Thickness measurement of thin films by X-ray absorption. J. Appl. Phys. 1991, 69, 499–501. [Google Scholar] [CrossRef]
- Huaqiang, W.; Bin, L.; Wei, M.; Xingtao, L.; Kun, T. Computed depth profile method of X-ray diffraction and its application to Ni/Pd films. Surf. Coat. Technol. 2002, 149, 198–205. [Google Scholar]
- Doerner, M.F.; Brennan, S. Strain distribution in thin aluminum films using X-ray depth profiling. J. Appl. Phys. 1988, 63, 126–131. [Google Scholar] [CrossRef]
- Eisenberger, P.; Marra, W.C. X-ray diffraction study of the Ge(001) reconstructed surface. Phys. Rev. Lett. 1981, 46, 1081–1084. [Google Scholar] [CrossRef]
- Skrzypek, S.J.; Baczmanski, A.; Ratuszek, W.; Kusior, E. New approach to stress analysis based on grazing-incidence X-ray diffraction. J. Appl. Crystallogr. 2001, 34, 427–435. [Google Scholar] [CrossRef]
- Hubbell, J.H.; McMaster, W.H.; Del Grande, N.K.; Mallett, J.H. X-ray crosssections and attenuation coefficients. In IUCr International Tables for X-ray Crystallography; Kynoch Press: Birmingham, UK, 1974; pp. 47–49. [Google Scholar]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall Inc.: New York, NY, USA, 2001; pp. 466–468. [Google Scholar]
- Korb, L.J.; Olson, D.L. Hot Dip Coatings, Corrosion. In ASM Handbook; ASM International: New York, NY, USA, 1992; Volume 13, pp. 975–1009. [Google Scholar]
- Korb, L.J.; Olson, D.L. CVD/PVD Coatings, Corrosion. In ASM Handbook; ASM International: New York, NY, USA, 1992; Volume 13, pp. 1035–1042. [Google Scholar]
- Tucker, R.C. Thermal Spray Coatings in Surface Engineering. In ASM Handbook; ASM International: New York, NY, USA, 2000; Volume 5, pp. 1446–1471. [Google Scholar]
- Pistofidis, N.; Vourlias, G.; Stergioudis, G.; Polychroniadis, E.K.; Tsipas, D. Corrosion Protection: Processes, Management and Technologies; Nova Publishing, Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009; Chapter 1; pp. 1–38. [Google Scholar]
- Vourlias, G.; Pistofidis, N.; Stergioudis, G.; Pavlidou, E.; Tsipas, D. Influence of alloying elements on the structure and on the corrosion resistance of the galvanized coatings. Phys. Status Solidi (A) Appl. Res. 2004, 201, 1518–1527. [Google Scholar] [CrossRef]
- Vourlias, G.; Pistofidis, N.; Chaliampalias, D.; Pavlidou, E.; Stergioudis, G.; Polychroniadis, E.K.; Tsipas, D. Zinc deposition with pack cementation on low carbon steel substrates. J. Alloys Compd. 2006, 416, 125–130. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Vourlias, G.; Pistofidis, N.; Stergioudis, G.; Polychroniadis, E.K. A morphological and microstructural study of flame-sprayed zinc coatings on low-alloyed steels as a contribution to explaining their corrosion resistance. Phys. Status Solidi (A) Appl. Res. 2008, 205, 1566–1571. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards (JCPDS). Powder Diffraction Files; International Centre for Diffraction Data (ICDD): Newton Square, PA, USA, 2003. [Google Scholar]



| Phase | μ (cm−1) | θ (ο) | t (μm) |
|---|---|---|---|
| η-phase (Zn) | 430.300 | 21.616 | 29.540 |
| 18.148 | 24.970 | ||
| 41.051 | 52.650 | ||
| ζ-phase FeZn13 | 549.890 | 20.749 | 22.227 |
| 21.294 | 22.784 | ||
| 44.394 | 43.918 | ||
| δ-phase FeZn10 | 576.350 | 21.138 | 21.586 |
| 20.544 | 21.006 | ||
| 20.892 | 21.346 | ||
| Γ-phase Fe3Zn10 | 749.701 | 21.338 | 15.796 |
| 21.800 | 16.122 | ||
| 20.709 | 15.351 | ||
| ZnO | 287.973 | 18.128 | 37.276 |
| 15.886 | 32.793 | ||
| 17.210 | 35.447 |
| Fe | C | Mn | S | P | Si | Al |
|---|---|---|---|---|---|---|
| Balance | 0.11 | 0.55 | 0.012 | 0.016 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vourlias, G. Application of X-rays Diffraction for Identifying Thin Oxide Surface Layers on Zinc Coatings. Coatings 2020, 10, 1005. https://doi.org/10.3390/coatings10101005
Vourlias G. Application of X-rays Diffraction for Identifying Thin Oxide Surface Layers on Zinc Coatings. Coatings. 2020; 10(10):1005. https://doi.org/10.3390/coatings10101005
Chicago/Turabian StyleVourlias, George. 2020. "Application of X-rays Diffraction for Identifying Thin Oxide Surface Layers on Zinc Coatings" Coatings 10, no. 10: 1005. https://doi.org/10.3390/coatings10101005
APA StyleVourlias, G. (2020). Application of X-rays Diffraction for Identifying Thin Oxide Surface Layers on Zinc Coatings. Coatings, 10(10), 1005. https://doi.org/10.3390/coatings10101005