Spirogyra Oil-Based Biodiesel: Response Surface Optimization of Chemical and Enzymatic Transesterification and Exhaust Emission Behavior
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Spirogyra Oil
2.2. Biodiesel Production and Optimization
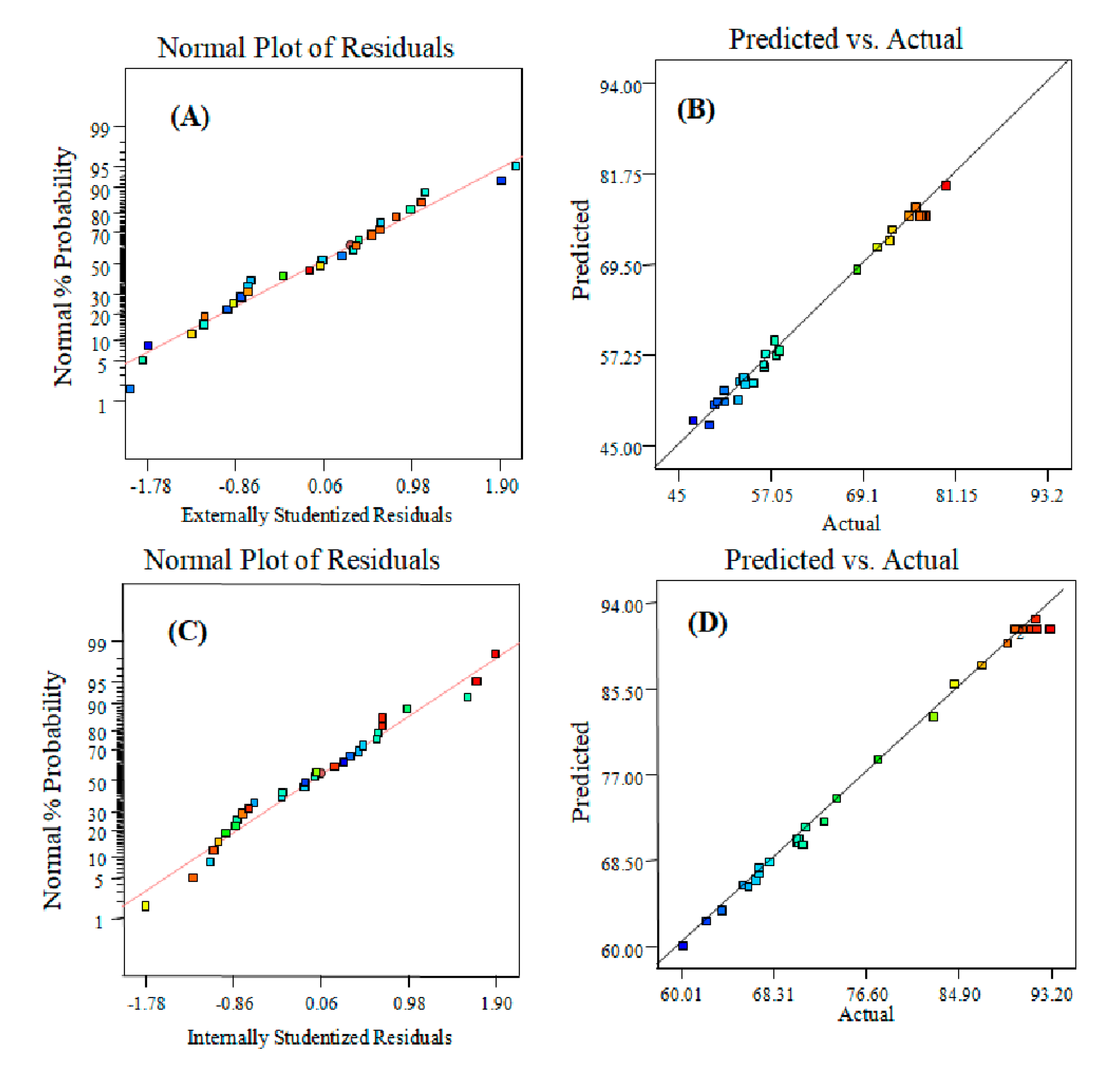
2.2.1. Summary Statistics of Selected Model
2.2.2. Optimized Reaction Parameters and Optimum Yield
2.2.3. Analysis of Variance (ANOVA)
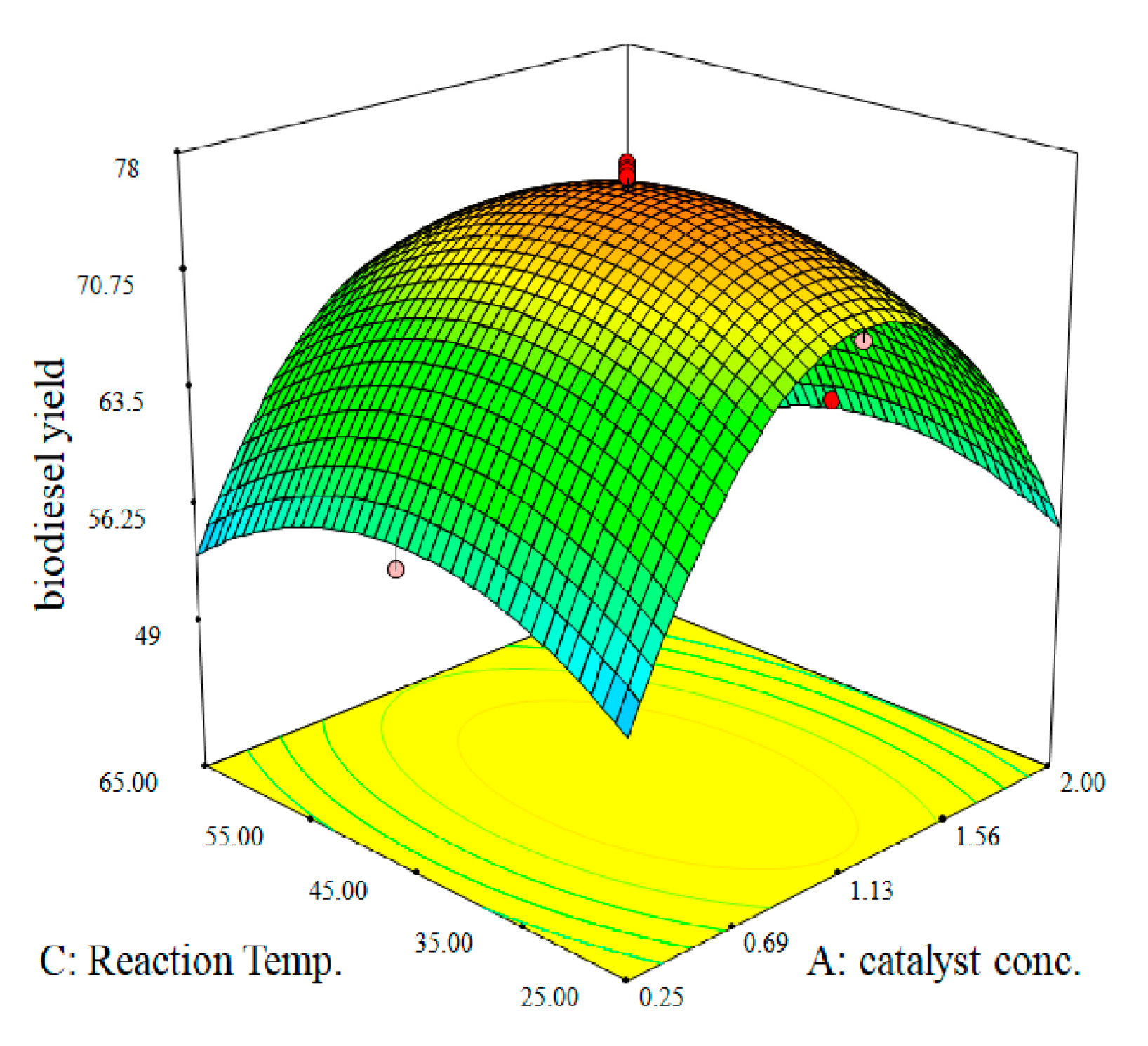
2.2.4. Response Surface Plots for Chemical Transesterification
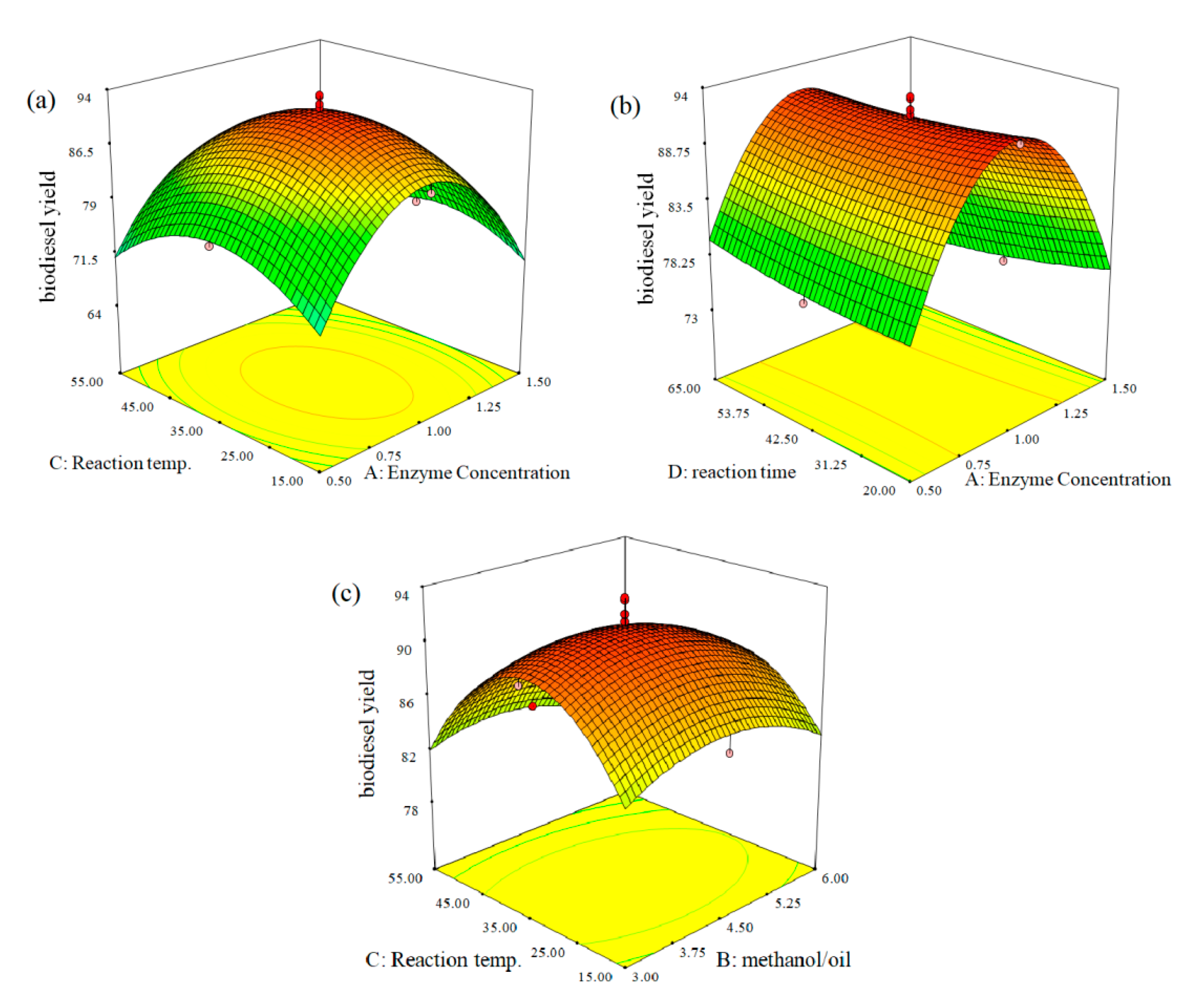
2.2.5. Response Surface Plots for Enzymatic Transesterification of Algal Oil
2.3. Fuel Properties of Resultant Biodiesel
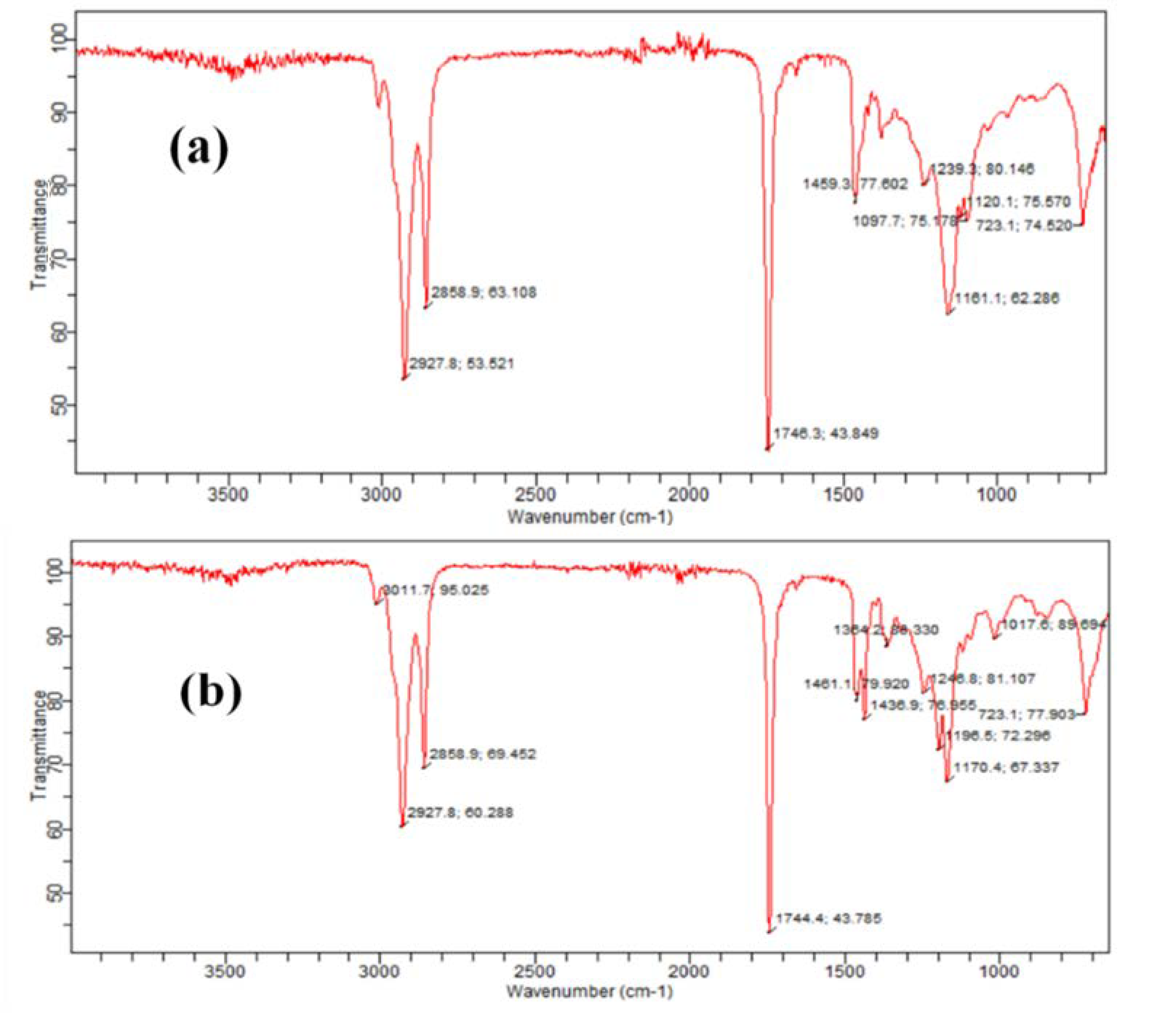
2.4. Characterization of Spirogyra Oil-based Biodiesel
2.5. Emission Profiling
3. Materials and Methods
3.1. Extraction of Spirogyra Oil
3.2. Physico-Chemical Characterization of Spirogyra crassa Oil
3.3. Experimental Design for Transesterification
3.4. Statistical Optimization
3.5. Biodiesel Characterization
3.6. Fuel Properties of Produced Biodiesel
3.7. Exhaust Emission Profiling
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hossain, S.Z.; Razzak, S.A.; Al Shater, A.F.; Moniruzzaman, M.; Hossain, M.M. Recent advances in enzymatic conversion of microalgal lipids into biodiesel. Energy Fuels 2020, 34, 6735–6750. [Google Scholar] [CrossRef]
- Meena, R.A.A.; Banu, J.R.; Kannah, R.Y.; Yogalakshmi, K.; Kumar, G. Biohythane production from food processing wastes–Challenges and perspectives. Bioresour. Technol. 2020, 298, 122449. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Takala, J.; Hiltunen, E.; Qin, L.; Xu, Z.; Qin, X.; Yuan, Z. Scale-up potential of cultivating Chlorella zofingiensis in piggery wastewater for biodiesel production. Bioresour. Technol. 2013, 137, 318–325. [Google Scholar] [CrossRef]
- Roy, T.; Sahani, S.; Sharma, Y.C. Study on kinetics-thermodynamics and environmental parameter of biodiesel production from waste cooking oil and castor oil using potassium modified ceria oxide catalyst. J. Clean. Prod. 2020, 247, 119166. [Google Scholar] [CrossRef]
- Gusniah, A.; Veny, H.; Hamzah, F. Activity and stability of immobilized lipase for utilization in transesterification of waste cooking oil. Bull. Chem. React. Eng. Catal. 2020, 15, 242–252. [Google Scholar] [CrossRef]
- Nagarajan, S.; Chou, S.K.; Cao, S.; Wu, C.; Zhou, Z. An updated comprehensive techno-economic analysis of algae biodiesel. Bioresour. Technol. 2013, 145, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Tran, K.-Q.; Giselrød, H.R. Towards sustainable production of biofuels from microalgae. Int. J. Mol. Sci. 2008, 9, 1188–1195. [Google Scholar] [CrossRef]
- Shafiq, F.; Mumtaz, M.W.; Mukhtar, H.; Touqeer, T.; Raza, S.A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Response surface methodology approach for optimized biodiesel production from waste chicken fat oil. Catalysts 2020, 10, 633. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, M.F. Importance of algae oil as a source of biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Deng, X.; Li, Y.; Fei, X. Microalgae: A promising feedstock for biodiesel. Afr. J. Microbiol. Res. 2009, 3, 1008–1014. [Google Scholar]
- Delrue, F.; Setier, P.-A.; Sahut, C.; Cournac, L.; Roubaud, A.; Peltier, G.; Froment, A.-K. An economic, sustainability, and energetic model of biodiesel production from microalgae. Bioresour. Technol. 2012, 111, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Umdu, E.S.; Tuncer, M.; Seker, E. Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour. Technol. 2009, 100, 2828–2831. [Google Scholar] [CrossRef] [PubMed]
- Rajakumari, K.; Abirla, M. Fuels from algae: A review. Res. J. Pharm. Technol. 2019, 12, 403–406. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Production of biodiesel by enzymatic transesterification of waste sardine oil and evaluation of its engine performance. Heliyon 2017, 3, e00486. [Google Scholar] [CrossRef]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-lipase as surface functionalized nano biocatalyst for the production of biodiesel using waste cooking oil as feedstock: Characterization and process optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Kumar, R.R.; PraveenKumar, R.; Chakravarthy, M.; Yogendran, D.; Jayamuthunagai, J. Biodiesel production from different algal oil using immobilized pure lipase and tailor made rPichia pastoris with Cal A and Cal B genes. Bioresour. Technol. 2016, 213, 69–78. [Google Scholar] [CrossRef]
- Saydut, A.; Kafadar, A.B.; Tonbul, Y.; Kaya, C.; Aydin, F.; Hamamci, C. Comparison of the biodiesel quality produced from refined sunflower (Helianthus annuus L) oil and waste cooking oil. Energy Explor. Exploit. 2010, 28, 499–512. [Google Scholar] [CrossRef]
- Stanley, S.A.; MR, A.P.; Anitha, A. Studies on the extraction and characterisation of microalgal oil. Natl. J. ChemBiosis 2010, 1, 1–3. [Google Scholar]
- Mat Yasin, M.H.; Mamat, R.; Yusop, A.F.; Rahim, R.; Aziz, A.; Shah, L.A. Fuel physical characteristics of biodiesel blend fuels with alcohol as additives. Procedia Eng. 2013, 53, 701–706. [Google Scholar] [CrossRef]
- Pathak, V. Algal oil potential as a biofuel and food supplement. Res. J. Chem. Sci. 2015, 6, 6–10. [Google Scholar]
- Ojo-Awo, A.P. Potentials of selected microalgae oiil as a possible replacement for fish oils and edible oils. J. Agric. Ecol. Res. Int. 2015, 2, 230–236. [Google Scholar]
- Renita, A.A.; Amarnath, D.J.; Sivasubramanian, S.A. Study on the optimization of algal biodiesel reaction parameters using response surface methodology. Int. J. Chem. Eng. Appl. 2012, 3, 311. [Google Scholar] [CrossRef][Green Version]
- Ashokkumar, V.; Agila, E.; Sivakumar, P.; Salam, Z.; Rengasamy, R.; Ani, F.N. Optimization and characterization of biodiesel production from microalgae Botryococcus grown at semi-continuous system. Energy Convers. Manag. 2014, 88, 936–946. [Google Scholar] [CrossRef]
- Barabas, I.; Todoruţ, I. Biodiesel Quality, Standards and Properties. In Biodiesel Quality, Emissions and By-Products; Gisela, M., Ed.; Intechopen: London, UK, 2011; ISBN 978-953-307-784-0. Available online: http://www.intechopen.com/books/biodiesel-quality-emissions-and-by-products/biodiesel-qualitystandards-and-properties (accessed on 15 July 2020).
- Ahmad, M.; Ahmed, S.; Ul-Hassan, F.; Arshad, M.; Khan, M.A.; Zafar, M.; Sultana, S. Base catalyzed transesterification of sunflower oil biodiesel. Afr. J. Biotechnol. 2010, 9, 8630–8635. [Google Scholar]
- Ahmad, F.; Khan, A.U.; Yasar, A. Transesterification of oil extracted from different species of algae for biodiesel production. Afr. J. Environ. Sci. Technol. 2013, 7, 358–364. [Google Scholar]
- Owolabi, R.; Osiyemi, N.; Amosa, M.; Ojewumi, M. Biodiesel from household/restaurant waste cooking oil (WCO). J. Chem. Eng. Process. Technol. 2011, 2, 1000112. [Google Scholar]
- PS, S.S. A method for production and characterization of biodiesel from green micro algae. Int. J. Bio-Sci. Bio-Technol. 2014, 6, 111–122. [Google Scholar]
- Peter, S.K.; Ganswindt, R.; Neuner, H.P.; Weidner, E. Alcoholysis of triacylglycerols by heterogeneous catalysis. Eur. J. Lipid Sci. Technol. 2002, 104, 324–330. [Google Scholar] [CrossRef]
- El-Shimi, H.; Attia, N.K.; El-Sheltawy, S.; El-Diwani, G. Biodiesel production from Spirulina-Platensis microalgae by in-situ transesterification process. J. Sustain. Bioenergy Syst. 2013, 3, 224–233. [Google Scholar] [CrossRef]
- Prakash, S.S.L. Evaluation of biodiesel production from microalgae collected from fresh water habitat. Int. J. Fundam. Appl. Sci. 2015, 4, 56–60. [Google Scholar]
- Gumus, S.; Ozcan, H.; Ozbey, M.; Topaloglu, B. Aluminum oxide and copper oxide nanodiesel fuel properties and usage in a compression ignition engine. Fuel 2016, 163, 80–87. [Google Scholar] [CrossRef]
| Sr. No. | Property | Value |
|---|---|---|
| 1 | Acid value before acid transesterification | 16.67 ± 3.53 mg KOH/g |
| 2 | Acid value after acid transesterification | 1.23 ± 0.53 mg KOH/g |
| 3 | Iodine value | 117.67 ± 13.01 g I2/100g |
| 4 | Saponification value | 165.33 ± 13.20 mg KOH/g |
| 5 | Density | 0.86 ± 0.05 g/cm3 |
| 6 | Peroxide value | 4.63 ± 0.25 meq/kg |
| Models | Catalyst | Selected Models | Model Significant (p-values) | R-Squared | Adjust R-Squared | Lack of Fit |
|---|---|---|---|---|---|---|
| a | NaOCH3 | Quadratic | (<0.0001) | 0.9933 | 0.9871 | 0.0843 |
| b | NOVOZYME-435 | Quadratic | (<0.0001) | 0.9954 | 0.99911 | 0.6693 |
| Model | Catalysts | Catalyst Conc.% | Methanol:Oil | Reaction Time (Min/Hrs) | Reaction Temp °C | Biodiesel Yield (%) |
|---|---|---|---|---|---|---|
| a | NaOCH3 | 1.13 | 6:1 | 95 min | 45 | 77.3 ± 1.27 |
| b | NOVOZYME-435 | 1 | 4.5:1 | 42.5 h | 35 | 93.2 ± 1.06 |
| Source | Df | SS(MS) a | SS(MS) b | f value (p value) a | f value (p value) b |
|---|---|---|---|---|---|
| Model | 14 | 3633.03(259.5023) | 3648.775(260.63) | 160.0411 (<0.0001) | 232.81(<0.0001) |
| A-Enzyme Concentration | 1 | 8.268(8.268) | 64.22(64.22) | 5.099(0.0393) | 57.37(<0.0001 |
| B-methanol/oil | 1 | 10.125(10.125) | 20.06(20.056) | 6.244(0.0246) | 17.91(0.0007) |
| C-Reaction temp. | 1 | 42.32(42.32) | 48.02(48.02) | 26.099(0.0001) | 42.89(<0.0001) |
| D-reaction time | 1 | 38.427(38.427) | 3.83(3.83) | 23.698(0.0002) | 3.42(0.0843) |
| AB | 1 | 3.0625(3.062) | 5.18(5.18) | 1.888(0.1895) | 4.62(0.0843) |
| AC | 1 | 28.09(28.09) | 22.33(22.33) | 17.323(0.0008) | 19.94(0.0005) |
| AD | 1 | 0.2025(0.202) | 28.36(28.36) | 0.124(0.7287) | 25.33(0.0001) |
| BC | 1 | 1.96(1.96) | 8.56(8.56) | 1.208(0.2889) | 7.64(0.0145) |
| BD | 1 | 0.9025(0.9025) | 0.031(0.031) | 0.556(0.4672) | 0.027(0.8708) |
| CD | 1 | 0.01(0.01) | 0.005(0.005) | 0.0061(0.9384) | 0.005(0.9444) |
| A2 | 1 | 795.216(795.216) | 565.09(565.09) | 490.428(<0.0001) | 504.78(<0.0001) |
| B2 | 1 | 17.103(17.103) | 15.79(15.79) | 10.548(0.0054) | 14.101(0.019) |
| C2 | 1 | 84.749(84.749) | 125.81(125.81) | 52.267(<0.0001) | 112.383(<0.0001) |
| D2 | 1 | 17.930(17.93) | 0.88(0.88) | 11.058(0.0046) | 0.782(0.3902) |
| Residual | 15 | 24.322(1.62) | 16.7923(1.119) | ||
| Lack of fit | 10 | 21.368(2.14) | 10.1173(1.011) | 3.62(0.0843) | 0.76(0.6693) |
| Pure error | 5 | 2.953(0.59) | 6.675(1.335) | ||
| core total | 29 | 3657.355 | 3665.567 |
| Sr. No. | Property | Value |
|---|---|---|
| 1 | Kinematic viscosity | 5.87 ± 2.20 mm2/mL |
| 2 | Density | 0.85 6 ± 0.03 g/cm3 |
| 3 | Flash Point | 125.67 ± 2.11 °C |
| 4 | Pour Point | −19.67 ± 0.8 °C |
| 5 | Fire Point | 138.66 ± 2.52 °C |
| 6 | Cloud Point | −13.00 ± 1 °C |
| Blends | NOx (ppm) | CO (ppm) | HC (ppm) |
|---|---|---|---|
| D100 | 146.53 ± 5.95 | 605.33 ± 6.92 | 68.36 ± 1.99 |
| S5 | 150.37 ± 3.63 | 580.11 ± 4.47 | 63.24 ± 1.72 |
| S20 | 165.58 ± 5.10 | 506.27 ± 8.95 | 52.73 ± 2.65 |
| S40 | 177.56 ± 2.69 | 445.26 ± 5.07 | 49.95 ± 1.50 |
| S100 | 192.50 ± 4.59 | 402.70 ± 5.55 | 42.25 ± 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, S.; Mumtaz, M.W.; Mukhtar, H.; Touqeer, T.; Anjum, M.K.; Rashid, U.; Wan Ab Karim Ghani, W.A.; Choong, T.S.Y. Spirogyra Oil-Based Biodiesel: Response Surface Optimization of Chemical and Enzymatic Transesterification and Exhaust Emission Behavior. Catalysts 2020, 10, 1214. https://doi.org/10.3390/catal10101214
Sohail S, Mumtaz MW, Mukhtar H, Touqeer T, Anjum MK, Rashid U, Wan Ab Karim Ghani WA, Choong TSY. Spirogyra Oil-Based Biodiesel: Response Surface Optimization of Chemical and Enzymatic Transesterification and Exhaust Emission Behavior. Catalysts. 2020; 10(10):1214. https://doi.org/10.3390/catal10101214
Chicago/Turabian StyleSohail, Saqib, Muhammad Waseem Mumtaz, Hamid Mukhtar, Tooba Touqeer, Muhammad Kafeel Anjum, Umer Rashid, Wan Azlina Wan Ab Karim Ghani, and Thomas Shean Yaw Choong. 2020. "Spirogyra Oil-Based Biodiesel: Response Surface Optimization of Chemical and Enzymatic Transesterification and Exhaust Emission Behavior" Catalysts 10, no. 10: 1214. https://doi.org/10.3390/catal10101214
APA StyleSohail, S., Mumtaz, M. W., Mukhtar, H., Touqeer, T., Anjum, M. K., Rashid, U., Wan Ab Karim Ghani, W. A., & Choong, T. S. Y. (2020). Spirogyra Oil-Based Biodiesel: Response Surface Optimization of Chemical and Enzymatic Transesterification and Exhaust Emission Behavior. Catalysts, 10(10), 1214. https://doi.org/10.3390/catal10101214








