Abstract
Enteral nutrition (EN) is considered the first feeding route for critically ill patients. However, adverse effects such as gastrointestinal complications limit its optimal provision, leading to inadequate energy and protein intake. We compared the clinical outcomes of supplemental parenteral nutrition added to EN (SPN + EN) and EN alone in critically ill adults. Electronic databases restricted to full-text randomized controlled trials available in the English language and published from January 1990 to January 2019 were searched. The risk of bias was evaluated using the Jadad scale, and the meta-analysis was conducted using the MedCalc software. A total of five studies were eligible for inclusion in the systematic review and meta-analysis. Compared to EN alone, SPN + EN decreased the risk of nosocomial infections (relative risk (RR) = 0.733, p = 0.032) and intensive care unit (ICU) mortality (RR = 0.569, p = 0.030). No significant differences were observed between SPN + EN and EN in the length of hospital stay, hospital mortality, length of ICU stay, and duration of mechanical ventilation. In conclusion, when enteral feeding fails to fulfill the energy requirements in critically ill adult patients, SPN may be beneficial as it helps in decreasing nosocomial infections and ICU mortality, in addition to increasing energy and protein intakes with no negative effects on other clinical outcomes.
1. Introduction
It is well established that enteral nutrition (EN) is considered the preferred feeding route for critically ill patients who cannot maintain a volitional intake of food [,,,]. Early EN (within 24–48 h of intensive care unit (ICU) admission) is recommended for patients with a functional gastrointestinal tract [,]. However, several complications limit the use of enteral feeding in critically ill patients, leading to suboptimal nutritional intake. These complications include diarrhea, vomiting, aspiration, and feeding interruptions [,,]. A prospective multicenter study in 201 units from 26 countries with 3390 critically ill patients revealed that, on an average, only 61.2% and 57.6% of the prescribed calories and protein, respectively, were delivered to the patients []. A systematic review and meta-analysis of randomized controlled trials (RCTs) comparing the effects of EN and parenteral nutrition (PN) in critically ill patients reported no difference in the mortality rate, whereas EN alone decreased the incidence of bloodstream infections and the length of hospital stay, however, it did increase gastrointestinal complications []. Since malnutrition is prevalent among these patients, providing insufficient energy and protein may further worsen their already poor nutritional status [,].
Nonetheless, current guidelines for critically ill adults recommend early EN and the initiation of PN when nutritional goals are not met [,]. Supplementing EN with a partial dose of PN, also known as supplemental parenteral nutrition (SPN), is a strategy that might improve the nutritional intake of patients in the ICU []. However, according to a few previous meta-analyses on the effects of SPN and EN included trials that compared early versus late PN, some patients were initially administered PN, and EN was added later [], and combining SPN and EN led to adverse outcomes and resulted in inconsistent conclusions. Previous systematic reviews and meta-analyses of RCTs comparing the effects of combined SPN and EN with EN alone in critically ill patients reported no difference in in-hospital mortality, length of ICU stay, and duration of ventilatory support. However, evidence of the effects of SPN on other outcomes, such as nutritional intake, is yet to be investigated []. Evidence on the use of SPN is limited, and further research is warranted [].
Thus, the objective of this review and meta-analysis was to evaluate and compare the clinical outcomes of using SPN as a supplement to EN (SPN + EN) versus EN alone on ICU mortality, length of ICU stay, length of hospital stay, duration of mechanical ventilation, nosocomial infections, and protein and energy intakes in adult patients in the ICU. For an accurate assessment of the effects of SPN, SPN + EN was defined as either EN with SPN provided to the patient on day one in the ICU or SPN provided to patients already receiving EN; therefore, trials that provided EN to patients who previously received significant calories from PN were excluded.
2. Materials and Methods
2.1. Protocol and Registration
We followed the Cochrane Handbook for Systematic Reviews of Intervention in addition to the PRISMA guidelines [,]. The protocol for this systematic review and meta-analysis was registered in PROSPERO (CRD42019121888) [].
2.2. Eligibility Criteria
Type of studies: Full-text RCT articles available in the English language published from January 1990 to January 2019 were considered.
Type of participants: Studies on adults (≥16 years old) who were critically ill or admitted to the ICU were included.
Types of interventions: Studies comparing the effects of SPN + EN (only when SPN is added with or after EN) versus EN alone were included.
Outcomes:
Primary Outcomes
ICU mortality, length of ICU stay, length of hospital stay, duration of mechanical ventilation, and nosocomial infections
Secondary Outcomes
The effects of SPN + EN on protein and energy intakes were assessed as secondary outcomes and were included when they were available in the same studies along with the clinical outcomes.
2.3. Search Strategy
The electronic databases PubMed, EMBASE, Google Scholar, Scopus, and Cochrane Central Register of Controlled Trials were searched systematically for eligible studies during the period between September 2019 and January 2020. Other sources of grey literature such as clinicaltrials.gov, ProQuest, and European Society for Clinical Nutrition and Metabolism (ESPEN) congress abstracts, were also searched for possibly related articles. The search was restricted to full-text articles available in the English language that were published from January 1990 to January 2019. The following search terms were used: “supplemental parenteral nutrition”, “combined enteral and parenteral nutrition,” “enteral nutrition”, “ICU”, “critical care”, “critically ill adult”, randomized controlled trial” and their derivatives. Each term was used separately and in combination with other terms (see Appendix A for the search strategy). Any possibly related citations were added to the citation manager (Mendeley) for further abstract screening. Filters were applied while searching to limit the date and language of publication. The process was performed separately by two independent researchers (D.J.A. and F.J.A.) and was repeated for all databases.
2.4. Study Selection
While screening abstracts, all studies involving SPN in adult patients were included for a full-text assessment. Full-text articles were evaluated by two researchers (M.M.A.A. and G.S.A.) for conformance with the eligibility criteria and were checked by a third researcher when necessary (M.M.A.A.).
2.5. Data Collection Process
Two researchers (D.J.A. and F.J.A.) were involved in extracting the data independently, which were then checked by a third researcher (M.M.A.A. or G.S.A.) for any missing information. Discrepancies were resolved based on maximum votes (three out of four votes). Investigators used two pre-set tables to extract data. While the first table was used for extracting the characteristics of studies, the second table was used for extracting the outcomes, including ICU mortality, length of ICU stay, length of hospital stay, duration of mechanical ventilation, hospital-acquired infections, and energy and protein intakes. The authors of the corresponding studies were contacted through email to confirm the data when needed.
2.6. Quality Assessment
The risk of bias was examined independently by two researchers (D.J.A. and F.J.A.) using the Jadad scale, a three-criteria appraisal form that focuses on randomization, blinding, and accounts for all patients []. The scale had a maximum score of five and was applied to all studies meeting the eligibility criteria. Studies were excluded if they scored less than three on the Jadad Scale.
2.7. Statistical Analysis
The systematic review analysis was carried out by applying meta-analysis for both continuous outcome variables (length of ICU stay, length of hospital stay, duration of mechanical ventilation, energy intake, and protein intake) and categorical outcome variables (ICU mortality and infection). Means, standard deviations, frequencies, and percentages were used to describe the outcome variables. Standardized mean difference (SMD) was calculated as a summary pooled statistic using the cutoffs as recommended by Cohen (pooled effect of 0.2, small; 0.5, medium; and, 0.8 and above, large). The statistical significance of SMD was assessed using Student’s t-test. For categorical outcome variables, pooled relative risk (RR) was used, where pooled RR < 1 shows a reduction in risk, and pooled RR > 1 shows an increase in risk. To identify heterogeneity in the pooled data, Cochran’s Q test (weighted sum of squares on a standardized scale) was used. Additionally, I2 was used to indicate the percentage of total variation across the studies included in the meta-analysis. A cutoff value of I2 > 50% was applied to rule out higher levels of unexplained variation in the effect sizes. Pooled estimates were obtained using both the fixed effect and random effect models. Statistical significance and precision of estimates were reported using p ≤ 0.05 and 95% confidence intervals. Forest plots were used to report the results (overall effect using both fixed and random effect models) of the studies included in the meta-analysis. All analyses were performed using MedCalc for Windows version 15.0 (MedCalc Software, Ostend, Belgium) [].
3. Results
3.1. Study Identification and Selection
The search strategy yielded a total of 163 articles (Figure 1), out of which 63 duplicate articles were removed. After screening the abstracts, 61 articles were omitted because they were either reviews or unrelated articles such as those dealing with pediatric patients or costs of SPN. After assessing the full-text articles, an additional 32 were excluded either because they were not RCTs available in full-text or they did not (a) include critically ill patients, (b) compare EN with SPN + EN, or (c) compare clinical outcomes. Details about the excluded RCTs are available in Table S1. Seven articles met the eligibility criteria and were assessed for the risk of bias using the Jadad Scale (Table S2). Of these, five articles scored three or more on the Jadad scale and were included in the systematic review and meta-analysis. The characteristics of the included studies are available in Table 1.
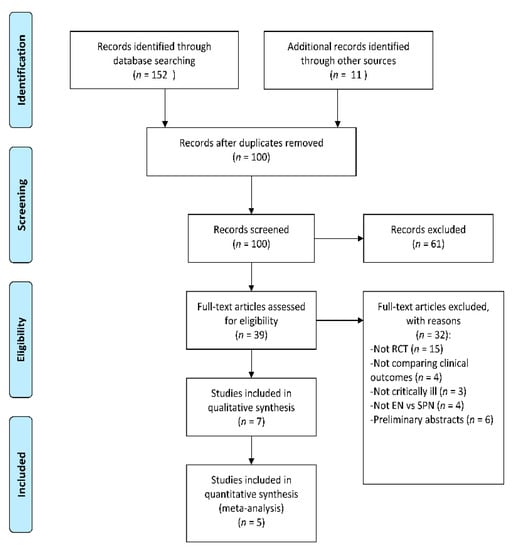
Figure 1.
Flow diagram of the search strategy. Randomized controlled trials (RCT), Supplemental parenteral nutrition (SPN), Enteral nutrition (EN).

Table 1.
Characteristics of the included studies.
3.2. Effect of SPN on Clinical Outcomes in Critically Ill Patients
3.2.1. ICU Mortality
The mortality events related to SPN + EN were lower than those related to EN (pooled RR = 0.569, z = −2.165, p = 0.030); that is, the risk of ICU mortality was reduced by 0.43 (43.1%) with SPN + EN as compared to EN alone (Table 2 and Figure 2a). Cochran’s Q value was not statistically significant (Q = 1.641, p = 0.650), and the I2 value (0.00%) revealed homogeneity across the four studies.

Table 2.
Meta-Analysis for the outcome variables: ICU mortality and presence of infection 1,2.
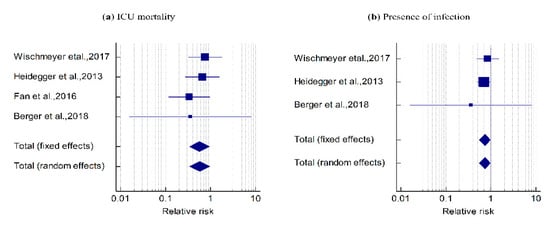
Figure 2.
Forest plot showing the effect of the SPN + EN on (a) ICU mortality: pooled RR = 0.569, z = −2.165, p = 0.030. The Cohran’s Q was not statistically significant (Q = 1.641, p = 0.650) and I2 = 0.00%. (b) The presence of infection events: pooled RR = 0.733, z = −2.145, p = 0.032. Q = 0.551, p = 0.759 and I2 value = 0.00%.
3.2.2. Presence of Infection
In three studies, the presence of infection events was lower with SPN + EN when compared to EN alone (pooled RR = 0.733, z = −2.145, p = 0.032), indicating that the risk of occurrence of infection was reduced by 0.267 (26.7%) with SPN + EN as compared to EN alone (Table 2 and Figure 2b). Cochran’s Q value was not statistically significant (Q = 0.551, p = 0.759) and the I2 value (0.00%) showed homogeneity across the three studies.
3.2.3. Length of Hospital Stay
In four studies, there was no statistical difference in hospital stay between SPN + EN and EN using fixed and random effects models (SMD = −0.083 t = −0.752, p = 0.452; Table 3 and Figure 3a). The overall effect was very small (−0.083 < 0.2). Cochran’s Q value was not statistically significant (0.5373, p = 0.911), and the I2 value (0.00%) indicated no heterogeneity among the four studies.

Table 3.
Meta-Analysis for the outcome variables: length of hospital stay, length of ICU stay, duration of mechanical ventilation, energy intake, and protein intake.
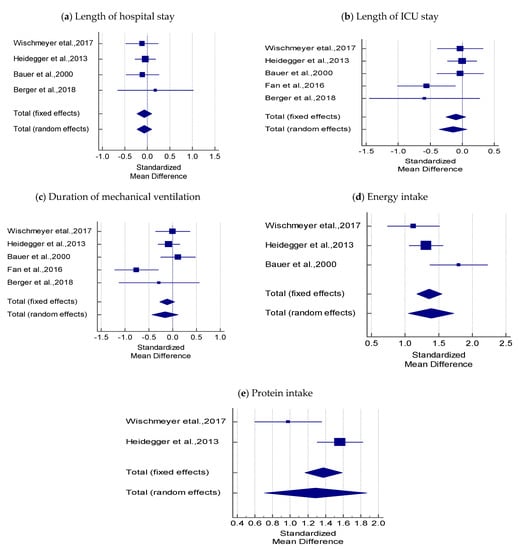
Figure 3.
Forest plots comparing the effect of SPN + EN on; (a) Hospital stay, (b) Length of ICU stay, (c) Duration of mechanical ventilation, (d) Energy intake, (e) Protein intake.
3.2.4. Length of ICU Stay
A fixed-effect criterion was considered because both Cohran’s Q (6.545) and I2 values (38.88%) were not significantly high. In five studies, the pooled estimate showed no significant difference in the mean length of ICU stay between the SPN + EN and EN groups (SMD = −0.100, t = −1.278; p = 0.202), and the overall effect was very small (−0.100 < 0.2; Table 3 and Figure 3b).
3.2.5. Duration of Mechanical Ventilation
The pooled SMD using the random-effects model showed no significant difference in the mean values of duration of mechanical ventilation between SPN + EN and EN (SMD = −0.159, t = −1.139, p = 0.255); the overall effect was very small (−0.159 < 0.2). Cochran’s Q value was statistically significant (Q = 10.195, p = 0.037), and the I2 value (60.77%) was high, indicating heterogeneity across the five studies (Table 3 and Figure 3c).
3.3. Effect of SPN on Energy and Protein Intake in Critically Ill Patients
3.3.1. Energy Intake
The pooled SMD by the random-effects model was used to infer that SPN + EN had higher mean values of energy intake when compared with EN (SMD = 1.391, t = 8.097, p < 0.001). The overall effect was 1.391 > 0.8. Cochran’ s Q value was statistically significant (Q = 5.719, p = 0.057) and the I2 value (65.03%) was high, indicating heterogeneity among the three studies (Table 3 and Figure 3d).
3.3.2. Protein Intake
Cochran’ s Q value (Q = 6.517, p = 0.011) was statistically significant and the I2 value (84.66%) was high, indicating heterogeneity across the two studies (Table 3 and Figure 3e). Hence, the pooled SMD using the random-effects model revealed that the mean protein intake was significantly higher in SPN + EN than in EN (SMD = 1.287, t = 4.371, p < 0.001). The overall effect was large (1.287 > 0.8).
4. Discussion
The current meta-analysis revealed that compared to EN alone, SPN + EN was not associated with an increase in the lengths of hospital stay, ICU stay, and mechanical ventilation (Figure 3a–c). However, SPN + EN was associated with a decrease in ICU mortality and hospital-acquired infections (Figure 2) without adversely affecting other clinical outcomes. Moreover, combining EN with SPN improved the protein and energy intakes in critically ill adult patients (Figure 3d,e). These findings demonstrate the benefits of SPN + EN in situations where enteral feeding alone fails to fulfill the energy requirements of critically ill patients.
We addressed the methodological limitations of previous meta-analyses of RCTs that compared the effects of SPN + EN with EN alone [,]. For example, we included only high-quality RCTs and excluded RCTs scoring less than three on the Jadad Scale. In addition, our meta-analysis only included studies comparing SPN + EN with EN alone, while previous meta-analyses included studies comparing early and late PN combined with EN [,]. For example, in one study that included in both the analyses, the Early versus Late Parenteral Nutrition in Critically ill adults (EPaNIC) study, early PN administration (within the first day of ICU admission) was associated with an increased risk of nosocomial infections as well as longer durations of mechanical ventilation and hospital stay []. However, the EPaNIC study differed from studies included in the current meta-analysis in that both the intervention and standard groups received PN to supplement insufficient energy intake from EN. The intervention group received PN early whereas in the standard group, PN was initiated after day eight. In the intervention group, the patients received 800 kcal from glucose infusion before starting EN on day three. In the intervention group, some patients did not receive SPN when EN was sufficient []. Our meta-analysis included studies where PN was initiated along with EN within 48–72 h of hospital admission [,,], while in two studies, PN was initiated on day 4 [,].
In contrast with the recommendations of the American Society of Parenteral and Enteral Nutrition, our meta-analysis revealed that SPN + EN could be beneficial in critically ill patients for increasing their protein and energy intakes as well as decreasing the risk of ICU mortality and nosocomial infections with no adverse effects on other clinical outcomes even when initiated before day eight []. This recommendation is different from the recent ESPEN guidelines [], where SPN is recommended for critically ill adults and its efficacy should be weighted depending on the case. Several studies have shown that the administration of adequate energy in critically ill patients improved clinical outcomes, which may explain the decrease in the rates of nosocomial infections associated with SPN + EN [,,]. The SPN Swiss study by Pradelli et al. revealed that every 1000 kcal reduction in the cumulative energy deficit was linked to a 10% decrease in the risk of nosocomial infections []. In addition, the medical savings per avoided infection were CHF 63,048 []. Thus, SPN + EN providing adequate levels of protein and energy is also a cost-saving strategy that reduces expenses associated with infections.
The small number of studies (n = 5) is a limitation of the current meta-analysis. This was because only a few RCTs have compared the effects of SPN + EN to EN alone [,,,,,,]. Therefore, more studies are needed to confirm the results of the current systematic review. Additionally, the included studies had different categories of ICU patients (burn, trauma, and others), and the responses to the interventions were different in each category. Moreover, several cofounding factors that interfere with the effects of SPN + EN and could have influenced the results of the analysis were not accounted for. These include the type of enteral formula used, the form of lipids used in the PN solution, and the equations used to estimate the energy requirement. Energy targets in the included studies ranged from to 20–30 kcal/kg using either the actual, ideal, or adjusted body weight [,,,]. The energy target was validated by indirect calorimetry in only one study []. Although the target energy intake seems similar between studies, it was not individualized based on each patient’s needs. Individualization of energy based on indirect calorimetry is recommended to avoid overfeeding [].
Despite these limitations, the current meta-analysis is of clinical importance because it highlights the potential benefits of SPN + EN, especially in cases where EN alone is insufficient. A few trials have reported that PN is not associated with increased mortality [,]. In the CALORIES randomized controlled multicenter trial, no significant differences in infectious complications and 30-day and 90-day mortality were reported between patients on EN or PN []. In addition,, in the NUTRIREA-2 study, there was no significant difference between the two groups with respect to mortality (measured by day 28) and ICU-acquired infections []. Furthermore, EN was associated with a higher risk of digestive complications []. Thus, the benefits of adding PN to EN might outweigh its risk when added at the right time and in the right amount. EN should be provided during the first 24–48 h of admission, but if it does not fulfill the nutritional energy requirement by day four, SPN should be considered.
5. Conclusions
When EN fails to fulfill the energy requirements in critically ill patients, SPN might be considered as it helps in (a) increasing the energy and protein intake and (b) decreasing nosocomial infections and ICU mortality without a significant increase in in-hospital mortality and the lengths of hospital stay, ICU stay, and mechanical ventilation. To obtain the maximum benefits from SPN, it should be delayed until at least day four after the initiation of EN to allow EN to progress sufficiently and decrease the amount of SPN needed.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/10/2968/s1, Table S1. Excluded Studies [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,], and Table S2. Quality assessment of the selected studies by the Jadad Scale.
Author Contributions
Conceptualization, M.M.A.A. and D.J.A.; methodology, F.J.A.; software, G.S.A.; validation, M.M.A.A., D.J.A., and F.J.A.; formal analysis, G.S.A.; investigation, D.J.A.; resources, F.J.A.; data curation, D.J.A.; writing—original draft preparation, D.J.A.; writing—review and editing, G.S.A.; visualization, F.J.A.; supervision, M.M.A.A.; project administration, D.J.A.; funding acquisition, M.M.A.A. Generally, D.J.A. and M.M.A.A. had equal contribution as first author, the first mention is for D.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia through the project number IFKSURP-1439-078.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-1439-078. Furthermore, the authors appreciate the free statistical and editing services of the investigator support unit in Prince Naif Bin Abdulaziz Health Research Center, King Saud University.
Conflicts of Interest
All authors declare that they have no conflicts of interest concerning the research, authorship, or publication of this article.
Appendix A. Pubmed and Embase Search Strategy
Pubmed search strategy
#1 intensive care units [MeSH Terms] OR critical care [Title/Abstract] OR critical illness [Title/Abstract] OR critically ill [Title/Abstract] OR intensive care [Title/Abstract] OR ICU [Title/Abstract]
#2 parenteral nutrition [MeSH Terms] OR parenteral nutrition [Title/Abstract] OR parenteral, nutrition [Title/Abstract] OR combined enteral and parenteral nutrition [Title/Abstract] OR supplemental parenteral nutrition [Title/Abstract] OR PN [Title/Abstract]
#3 enteral nutrition [MeSH Terms] OR enteral nutrition [Title/Abstract] OR enteral, nutrition [Title/Abstract] OR tube feeding [Title/Abstract] OR enteral feeding [Title/Abstract] OR EN [Title/Abstract]
#4 randomized controlled trial [MeSH Terms] OR randomized controlled trial [Title/Abstract] OR clinical trial [Title/Abstract] OR study [Title/Abstract]
#5 humans [MeSH Terms] AND adult [MeSH Terms] NOT animals [MeSH Terms]
#6 #1 AND #2 AND #3 AND #4 AND #5
For Embase, the search strategy was:
#1 ‘critically ill patient’/exp OR ‘intensive care unit’/exp OR ‘intensive care’/exp OR ‘critical illness’/exp
#2 ‘critically ill’:ti,ab,kw OR ‘intensive care unit’:ti,ab,kw OR ‘icu’:ti,ab,kw OR ‘critical illness’:ti,ab,kw
#3 #1 OR #2
#4 ‘enteric feeding’/exp OR ‘enteric feeding’:ti,ab,kw OR ‘enteral, nutrition’:ti,ab,kw
#5 ‘parenteral nutrition’/exp OR ‘parenteral nutrition’:ti,ab,kw OR ‘parenteral, nutrition’:ti,ab,kw OR ‘combined enteral and parenteral nutrition’:ti,ab,kw OR ‘supplemental parenteral nutrition’:ti,ab,kw
#6 #4 AND #5
#7 ‘crossover-procedure’/exp OR ‘double-blind procedure’/exp OR ‘randomized controlled trial’/exp OR ‘single-blind procedure’/exp
#8 ‘random*’:ti,ab,kw OR ‘blind*’:ti,ab,kw OR ‘placebo’:ti,ab,kw
#9 7 OR 8
#10 [adult]/lim OR [middle aged]/lim OR [aged]/lim OR [very elderly]/lim)
#11 [humans]/lim
#12 #11 AND #12
#13 #3 AND #6 AND #9 AND #12
References
- Casas, M.; Mora, J.; Fort, E.; Aracil, C.; Busquets, D.; Galter, S.; Jauregui, C.E.; Ayala, E.; Cardona, D.; Gich, I.; et al. Total enteral nutrition vs. total parenteral nutrition in patients with severe acute pancreatitis. Rev. Esp. Enferm. Dig. 2007, 99, 264–273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kudsk, K.A.; Croce, M.A.; Fabian, T.C.; Minard, G.; Tolley, E.A.; Poret, H.A.; Kuhl, M.R.; Brown, R.O. Enteral Versus Parenteral Feeding Effects on Septic Morbidity After Blunt and Penetrating Abdominal Trauma. Ann. Surg. 1992, 215, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kalfarentzos, F.; Kehagias, J.; Mead, N.; Kokkinis, K.; Gogos, C.A. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: Results of a randomized prospective trial. Br. J. Surg. 1997, 84, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Windsor, A.C.J.; Kanwar, S.; Li, A.G.K.; Barnes, E.; Guthrie, J.A.; Spark, J.I.; Welsh, F.; Guillou, P.J.; Reynolds, J.V. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut 1998, 42, 431–436. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter Enter Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Montejo, J.C. Enteral Nutrition-Related Gastrointestinal Complications in Critically Ill Patients: A Multicenter Study. The Nutritional and Metabolic Working Group of the Spanich Society of Intensive Care Medicine and Coronary Units. Crit. Care Med. 1999, 27, 1447–1453. [Google Scholar] [CrossRef]
- Luft, V.C.; Beghetto, M.G.; de Mello, E.D.; Polanczyk, C.A. Role of enteral nutrition in the incidence of diarrhea among hospitalized adult patients. Nutrition 2008, 24, 528–535. [Google Scholar] [CrossRef]
- Uozumi, M.; Sanui, M.; Komuro, T.; Iizuka, Y.; Kamio, T.; Koyama, H.; Mouri, H.; Masuyama, T.; Ono, K.; Lefor, A.K. Interruption of enteral nutrition in the intensive care unit: A single-center survey. J. Intensive Care 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Wang, M.; Day, A.G. The prevalence of iatrogenic underfeeding in the nutritionally “at-risk” critically ill patient: Results of an international, multicenter, prospective study. Clin. Nutr. 2015, 34, 659–666. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, K.; Cui, W.; Hong, Y.; Zhang, Z. The effect of enteral versus parenteral nutrition for critically ill patients: A systematic review and meta-analysis. J. Clin. Anesth. 2018, 51, 62–92. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, N.P.; Mazloom, Z.P.; Zand, F.M.; Rezaianzadeh, A.M.M.P.; Amini, A.M. Nutritional Assessment in Critically Ill Patients. Iran J. Med. Sci. 2016, 41, 171–179. [Google Scholar] [PubMed]
- Shpata, V.; Kreka, M.; Mjekaj, E.; Naço, M.; Gjyzari, A.; Soxhuku, A. Malnutrition affects negatively the outcome of intensive care unit ( ICU ) patients. Eur. J. Anaesthesiol. 2013, 30, 189. [Google Scholar] [CrossRef]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef] [PubMed]
- Ridley, E.J.; Davies, A.R.; Parke, R.; Bailey, M.; McArthur, C.; Gillanders, L.; Copper, J.; McGuinness, S. Supplemental parenteral nutrition versus usual care in critically ill adults: A pilot randomized controlled study. Crit. Care 2018, 22, 12. [Google Scholar] [CrossRef]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Cromphaut, S.V.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus Late Parenteral Nutrition in Critically Ill Adults. N. Engl. J. Med. 2011, 365, 506–517. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21714640 (accessed on 12 October 2019). [CrossRef]
- Jialinga, S.; Liyinga, W.; Rongzhia, H.; Liangb, L. Effect of combined parenteral and enteral nutrition versus enteral nutrition alone for critically ill patients. Medicine 2018, 97, e11874. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2011; Available online: http://handbook-5-1.cochrane.org/ (accessed on 10 September 2019).
- Al Sharif, D.; Abulmeaty, M.; Al Sharif, F. The Effect of Supplemental Parenteral Nutrition Versus Enteral Nutrition Alone on Clinical Outcomes for Adults Critically Ill Patients: Systematic Review of Randomized Controlled Trials. Report No.: CRD42019121888. 2019. Available online: https://www.crd.york.ac.uk/prospero/#myprospero (accessed on 27 February 2019).
- Jadad, A.R.; Moore, R.A.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- MedCalc Software bv. MedCalc Statistical Software Version 15.0; MedCalc Software bv: Ostend, Belgium, 2016; Available online: https://www.medcalc.org (accessed on 10 October 2019).
- Bauer, P.; Charpentier, C.; Bouchet, C.; Nace, L.; Raffy, F.; Gaconnet, N. Parenteral with enteral nutrition in the critically ill. Intensive Care Med. 2000, 26, 893–900. [Google Scholar] [CrossRef]
- Berger, M.M.; Pantet, O.; Jacquelin-Ravel, N.; Charrière, M.; Schmidt, S.; Becce, F.; Audran, R.; Spertini, F.; Tappy, L.; Picard, C. Supplemental parenteral nutrition improves immunity with unchanged carbohydrate and protein metabolism in critically ill patients: The SPN2 randomized tracer study. Clin. Nutr. 2018, 38, 2408–2416. [Google Scholar] [CrossRef]
- Fan, M.; Wang, Q.; Fang, W.; Jiang, Y.; Li, L.; Sun, P.; Wang, Z. Early Enteral Combined with Parenteral Nutrition Treatment for Severe Traumatic Brain Injury: Effects on Immune Function, Nutritional Status and Outcomes. Chin. Med. Sci. J. 2016, 31, 213–220. [Google Scholar] [CrossRef]
- Heidegger, C.P.; Berger, M.M.; Graf, S.; Zingg, W.; Darmon, P.; Costanza, M.C.; Thibault, R.; Pichard, C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet 2013, 381, 385–393. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Hasselmann, M.; Kummerlen, C.; Kozar, R.; Kutsogiannis, D.J.; Karvellas, C.J.; Besecker, B.; Evans, D.K.; Preiser, J.; Gramlich, L.; et al. A randomized trial of supplemental parenteral nutrition in underweight and overweight critically ill patients: The TOP-UP pilot trial. Crit. Care 2017, 21, 1–14. [Google Scholar] [CrossRef]
- Lewis, S.R.; Schofield-Robinson, O.J.; Alderson, P.; Smith, A.F. Enteral versus parenteral nutrition and enteral versus a combination of enteral and parenteral nutrition for adults in the intensive care unit. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Compher, C.; Chittams, J.; Sammarco, T.; Nicolo, M.; Heyland, D.K. Greater Protein and Energy Intake May Be Associated With Improved Mortality in Higher Risk Critically Ill Patients: A Multicenter, Multinational Observational Study. Crit. Care Med. 2017, 45, 156–163. [Google Scholar] [CrossRef]
- Alberda, C.; Gramlich, L.; Jones, N.; Jeejeebhoy, K.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med. 2009, 35, 1728–1737. [Google Scholar] [CrossRef]
- Singer, P.; Anbar, R.; Cohen, J.; Shapiro, H.; Shalita-Chesner, M.; Lev, S.; Grozovski, E.; Theilla, M.; Frishman, S.; Madar, Z. The tight calorie control study (TICACOS): A prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011, 37, 601–609. [Google Scholar] [CrossRef]
- Pradelli, L.; Graf, S.; Pichard, C.; Berger, M.M. Supplemental parenteral nutrition in intensive care patients: A cost saving strategy. Clin. Nutr. 2018, 37, 573–579. [Google Scholar] [CrossRef]
- Dunham, C.M.; Frankenfield, D.; Belzberg, H.; Wiles, C.; Cushing, B.; Grant, Z. Gut failure--predictor of or contributor to mortality in mechanically ventilated blunt trauma patients? J. Trauma 1994, 37, 30–34. [Google Scholar] [CrossRef]
- Abrishami, R.; Ahmadi, A.; Abdollahi, M.; Moosivand, A.; Khalili, H.; Najafi, A.; Gholami, K.; Hamishehkar, H.; Peivandi Yazdi, A.; Mojtahedzadeh, M. Comparison the inflammatory effects of early supplemental parenteral nutrition plus enteral nutrition versus enteral nutrition alone in critically ill patients. J. Fac. Pharm. 2010, 18, 103–106. [Google Scholar]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Bear, D.E.; Segaran, E.; Beale, R.; Bellingan, G.; Leonard, R.; Mythen, M.g.; Rowan, K.M. Trial of the Route of Early Nutritional Support in Critically Ill Adults. N. Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef]
- Reignier, J.; Boisramé-Helms, J.; Brisard, L.; Lascarrou, J.B.; Hssain, A.A.; Anguel, N.; Argaud, L.; Asehnoune, K.; Asfar, P.; Bellec, F.; et al. Enteral versus parenteral early nutrition in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Allingstrup, M.J.; Kondrup, J.; Wiis, J.; Claudius, C.; Pedersen, U.G.; Hein-Rasmussen, R.; Jensen, T.H.; Lange, T.; Anders, P. Early goal-directed nutrition versus standard of care in adult intensive care patients: The single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017, 43, 1637–1647. [Google Scholar] [CrossRef]
- Berger, M.; Brancato, V.; Graf, S.; Heidegger, C.; Darmon, P.; Pichard, C. OP027 SPN study. Supplemental parenteral nutrition (PN) to reach energy target does not compromise glucose control. Clin. Nutr. Suppl. 2011, 6, 11–12. [Google Scholar] [CrossRef]
- Berger, M.M.; Pantet, O.; Jacquelin-Ravel, N.; Charrière, M.; Schmidt, S.; Becce, F.; Audran, R.; Spertini, F.; Tappy, L.; Pichard, C. Supplemental parenteral nutrition does not alter carbohydrate and protein metabolism and improves immunity: The SPN2 randomized trial. Clin. Nutr. 2018, 37, S169. [Google Scholar] [CrossRef]
- Caccialanza, R.; Cereda, E.; Caraccia, M.; Klersy, C.; Nardi, M.; Cappello, S.; Borioli, V.; Turri, A.; Imarisio, I.; Lasagna, A.; et al. Early 7-day supplemental parenteral nutrition improves body composition and muscle strength in hypophagic cancer patients at nutritional risk. Support Care Cancer 2019, 27, 2497–2506. [Google Scholar] [CrossRef]
- Chao, P.C.C.; Lin, C.-F.F.; Chuang, H.-J.J. Parenteral nutrition combined with enteral feeding improves the outcome of cancer patients. Asia Pac. J. Clin. Nutr. 2017, 26, 1032–1038. [Google Scholar]
- Deegan, H.; Dent, S.; Keefe, L.; Drover, J.; Heyland, D. Supplemental parenteral nutrition in the critically ill patient: A retrospective study. Clin. Intensive Care 1999, 10, 131–136. [Google Scholar] [CrossRef]
- Elke, G.; Schädler, D.; Engel, C.; Bogatsch, H.; Frerichs, I.; Ragaller, M.; Scholz, J.; Brunkhorst, F.M.; Loffler, M.; Reinhart, K.; et al. Current practice in nutritional support and its association with mortality in septic patients-Results from a national, prospective, multicenter study. Crit Care Med. 2008, 36, 1762–1767. [Google Scholar] [CrossRef]
- El-Sayed, N.; El-Reweny, E.; Doha, M. Evaluation of the Effect of Enteral Versus Combined Entero-Parenteral Nutrition on Critically Ill Geriatric Patients. J. Am. Sci. 2015, 11, 1–12. [Google Scholar]
- Gavri, C.; Kokkoris, S.; Vasileiadis, I.; Oeconomopoulou, A.C.; Kotanidou, A.; Nanas, S.; Routsi, C. Route of nutrition and risk of blood stream infections in critically ill patients; a comparative study. Clin. Nutr. ESPEN 2016, 12, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Graf, S.; Berger, M.M.; Clerc, A.; Brancato, V.; Heidegger, C.P.; Pichard, C. PP001-MON SPN study. Supplemental Parenteral nutrition (SPN) to reach energy target does not compromise glucose control. Clin. Nutr. Suppl. 2012, 7, 138–139. [Google Scholar] [CrossRef]
- Heidegger, C.P.; Graf, S.; Thibault, R.; Darmon, P.; Berger, M.; Pichard, C. Supplemental Parenteral Nutrition (SPN) in intensive care unit (ICU) patients for optimal energy coverage improved clinical outcome. Clin. Nutr. Suppl. 2011, 6, 2–3. [Google Scholar] [CrossRef]
- Heyland, D.K.; Cahill, N.E.; Wang, M.; Kutsogiannis, J.; Alberda, C.; Gramlich, L. Benefit of supplemental parenteral nutrition in the critically ill patient? Results of a multicenter observational study. Crit. Care 2010, 14, P557. [Google Scholar] [CrossRef][Green Version]
- Hsu, M.-H.H.; Yu, Y.-E.E.; Tsai, Y.-M.M.; Lee, H.-C.C.; Huang, Y.-C.C.; Hsu, H.-S.S. Combined enteral feeding and total parenteral nutritional support improves outcome in surgical intensive care unit patients. J. Chin. Med. Assoc. 2012, 75, 459–463. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yen, C.E.; Cheng, C.H.; Jih, K.S.; Kan, M.N. Nutritional status of mechanically ventilated critically ill patients: Comparison of different types of nutritional support. Clin. Nutr. 2000, 19, 101–107. [Google Scholar] [CrossRef]
- Kutsogiannis, J.; Alberda, C.; Gramlich, L.; Cahill, N.E.; Wang, M.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. Early use of supplemental parenteral nutrition in critically ill patients: Results of an international multicenter observational study. Crit. Care Med. 2011, 39, 2691–2699. [Google Scholar] [CrossRef]
- Lidder, P.; Flanagan, D.; Fleming, S.; Russell, M.; Morgan, N.; Wheatley, T.; Rahamin, J.; Shaw, S.; Lewis, S. Combining enteral with parenteral nutrition to improve postoperative glucose control. Br. J. Nutr. 2010, 103, 1635–1641. [Google Scholar] [CrossRef]
- Mazaherpur, S.; Khatony, A.; Abdi, A.; Pasdar, Y.; Najafi, F. The effect of continuous enteral nutrition on nutrition indices, compared to the intermittent and combination enteral nutrition in traumatic brain injury patients. J. Clin. Diagn. Res. 2016, 10, JC01–JC05. [Google Scholar] [CrossRef]
- Nagata, S.; Fukuzawa, K.; Iwashita, Y.; Kabashima, A.; Kinoshita, T.; Wakasugi, K.; Maehara, Y. Comparison of enteral nutrition with combined enteral and parenteral nutrition in post-pancreaticoduodenectomy patients: A pilot study. Nutr. J. 2009, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nataloni, S.; Gentili, P.; Marini, B.; Guidi, A.; Marconi, P.; Busco, F.; Pelaia, P. Nutritional assessment in head injured patients through the study of rapid turnover visceral proteins. Clin. Nutr. 1999, 18, 247–251. [Google Scholar] [CrossRef]
- Oertel, M.F.; Hauenschild, A.; Gruenschlaeger, J.; Mueller, B.; Scharbrodt, W.; Boeker, D.-K.K. Parenteral and enteral nutrition in the management of neurosurgical patients in the intensive care unit. J. Clin. Neurosci. 2009, 16, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Probst, P.; Keller, D.; Steimer, J.; Gmür, E.; Haller, A.; Imoberdorf, R.; Ruhlin, M.; Gelpke, H.; Breitenstein, S. Early combined parenteral and enteral nutrition for pancreaticoduodenectomy—Retrospective cohort analysis. Ann. Med. Surg. 2016, 6, 68–73. [Google Scholar] [CrossRef]
- Sena, M.J.; Utter, G.H.; Cuschieri, J.; Maier, R.V.; Tompkins, R.G.; Harbrecht, B.G.; Moore, E.E.; O’Keefe, G.E. Early Supplemental Parenteral Nutrition Is Associated with Increased Infectious Complications in Critically Ill Trauma Patients. J. Am. Coll. Surg. 2008, 207, 459–467. [Google Scholar] [CrossRef]
- Singh, A.; Chen, M.; Li, T.; Yang, X.L.; Li, J.Z.; Gong, J.P. Parenteral nutrition combined with enteral nutrition for severe acute pancreatitis. ISRN Gastroenterol. 2012, 2012, 791383. [Google Scholar] [CrossRef]
- Theilla, M.; Kagan, I.; Makalde, M.; Rattanachaiwong, S.; Cohen, J.; Singer, P. The place of supplemental parenteral nutrition in critically ill transplanted patients: A One year retrospective study. Clin. Nutr. 2018, 37, S170. [Google Scholar] [CrossRef]
- Thibault, R.; Heidegger, C.P.; Methot, C.; Maisonneuve, N.; Jolliet, P.; Romand, J.; Darmon, P.; Pichard, C. P026 Supplemental parenteral nutrition (SPN) in ICU patients for early coverage of energy target: 100 first patients preliminary report. Clin. Nutr. Suppl. 2009, 4, 36. [Google Scholar] [CrossRef]
- Thibault, R.; Heidegger, C.P.; Graf, S.; Marin Caro, M.; Darmon, P.; Brancato, V.; Berger, M.M.; Picard, C. PP228 Supplemental parenteral nutrition (SPN) in ICU patients for early coverage of energy target:second preliminary report of a bi-centric, prospective, controlled, randomized study. Clin. Nutr. Suppl. 2010, 5, 112. [Google Scholar] [CrossRef]
- Titova, Y.; Petrikov, S.; Klychnikova, E.; Tazina, E.; Godkov, M.; Solodov, A.; Krylov, V.; Ryk, A. PP019-MON: Dynamics of Serum Triglycerides and Oxidative Stress Markers During Supplemental Parenteral Nutrition in Critically Ill Patients with Intracranial Hemorrhage. Clin. Nutr. 2014, 33, S136–S137. [Google Scholar] [CrossRef]
- Vallejo, K.P.; Martínez, C.M.; Matos-Adames, A.A.; Fuchs-Tarlovsky, V.; Nogales, G.C.C.; Paz, R.E.R.; Perman, M.I.; Toulson, M.i.; Correia, D. Current clinical nutrition practices in critically ill patients in Latin America: A multinational observational study. Crit. Care 2017, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.Y.; Wang, W.T.; Chen, C.Y.; Chu, Z.D.; Liu, X.J.; Liu, X.J. Early enteral and parenteral nutrition on immune functions of neurocritically ill patients. J. Biol. Regul. Homeost. Agents 2016, 30, 227–232. [Google Scholar] [PubMed]
- Zhu, X.H. Effect of early enteral combined with parenteral nutrition in patients undergoing pancreaticoduodenectomy. World J. Gastroenterol. 2013, 19, 5889. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).