Fucoidan from Ascophyllum nodosum Suppresses Postprandial Hyperglycemia by Inhibiting Na+/Glucose Cotransporter 1 Activity
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of Fucoidans from Various Sources
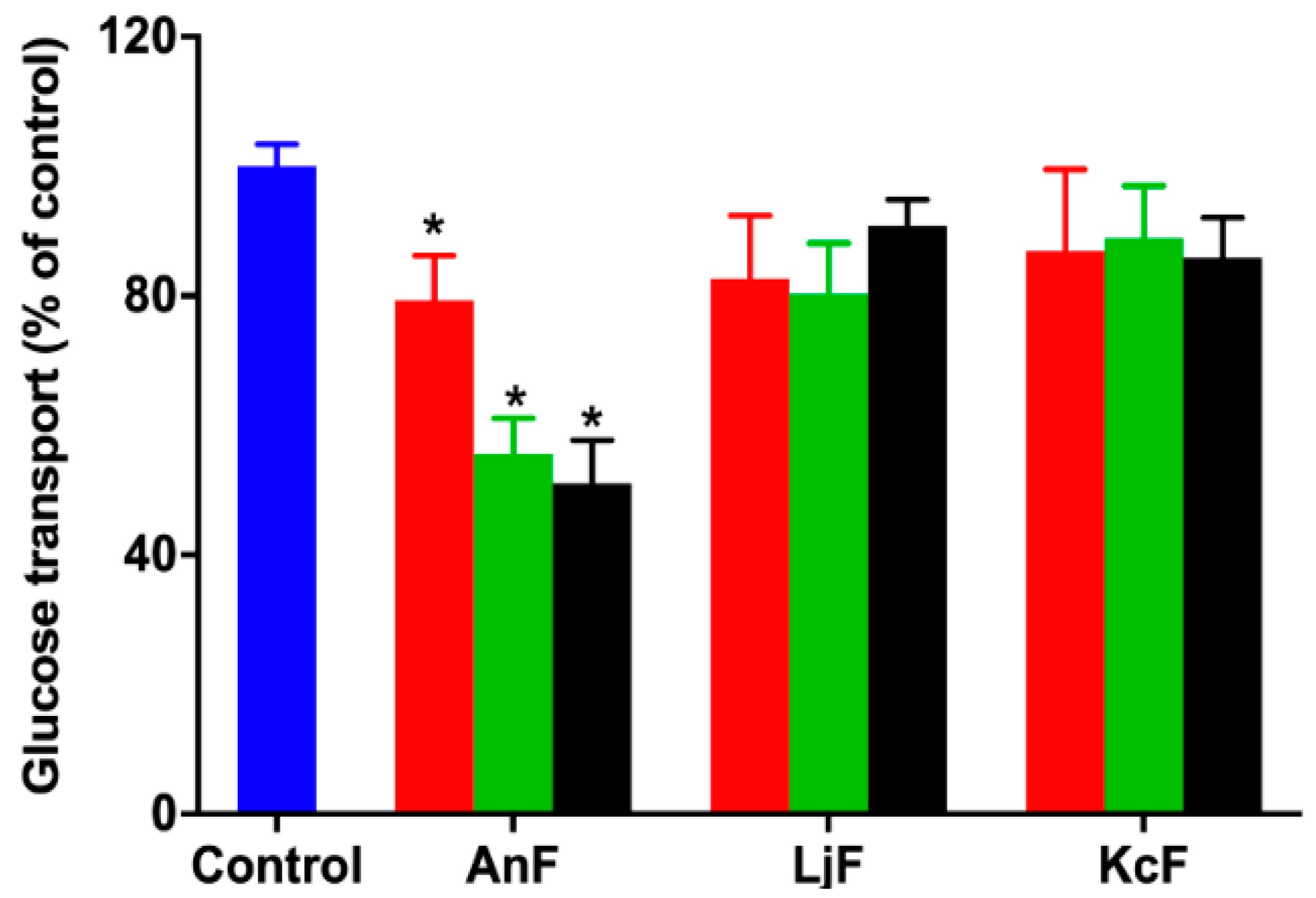
2.2. Effect of Fucoidans on Inhibiting Glucose Transport in a Caco-2 Monolayer Cell Model
2.3. Effect of Fucoidans on Intestinal Glucose Uptake Using an Everted Gut Sac Model
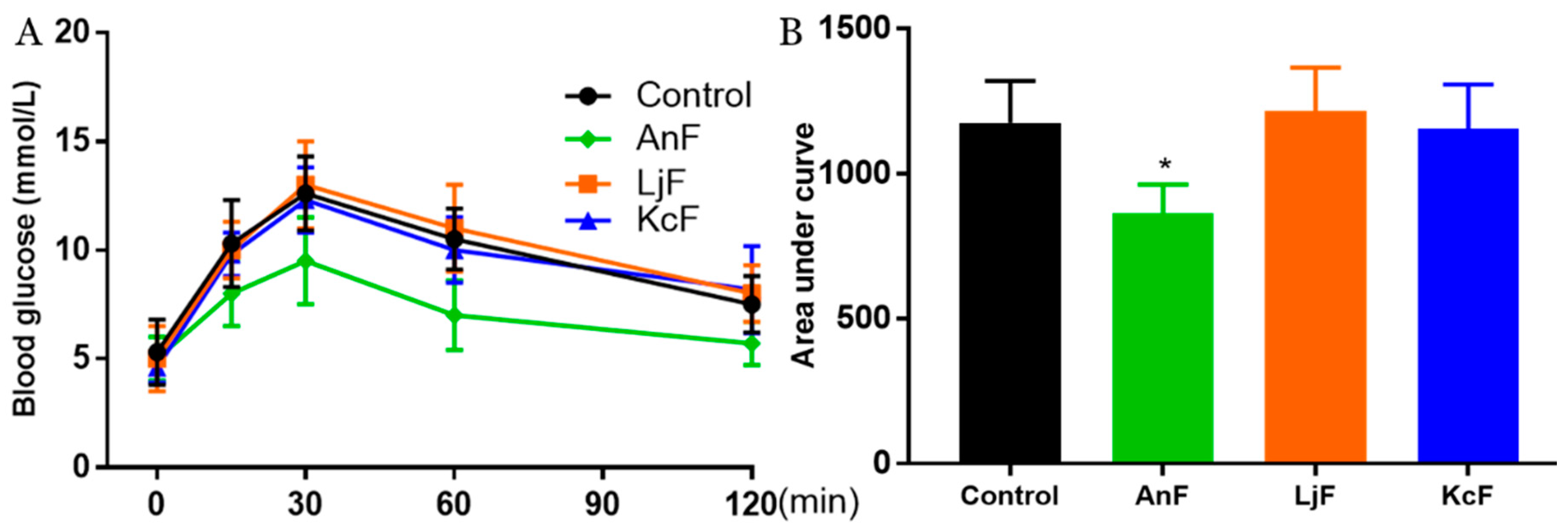
2.4. Effects of Fucoidans on OGTT in Kunming Mice
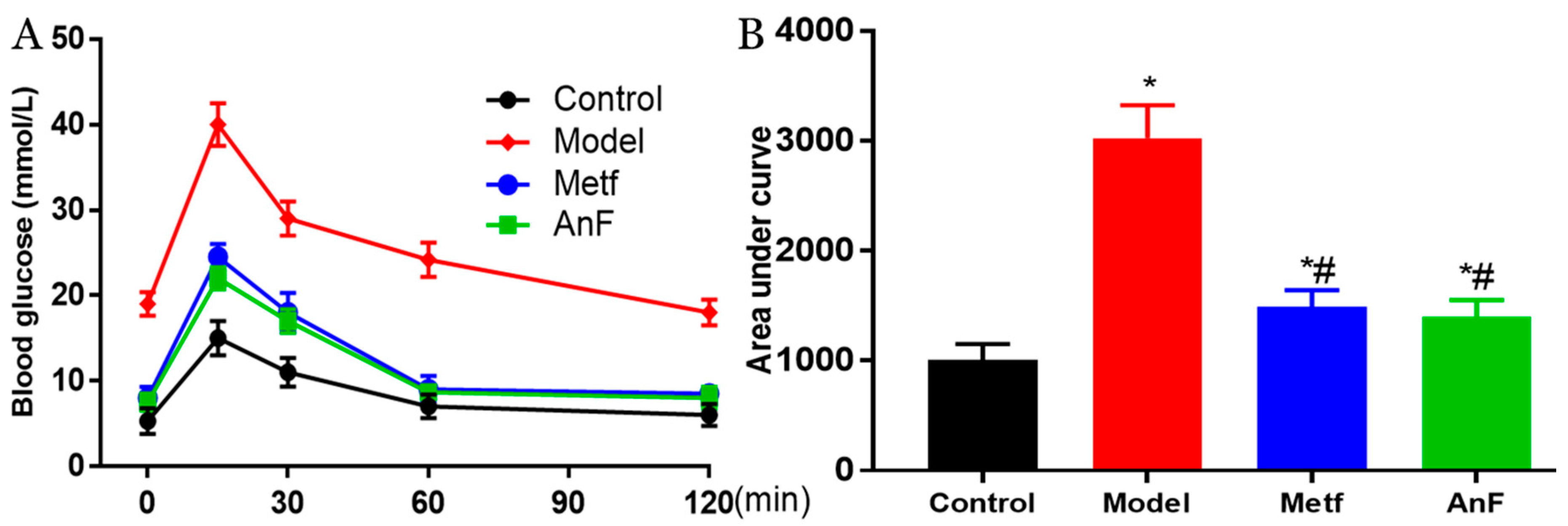
2.5. Effects of AnF on the OGTT in db/db Mice
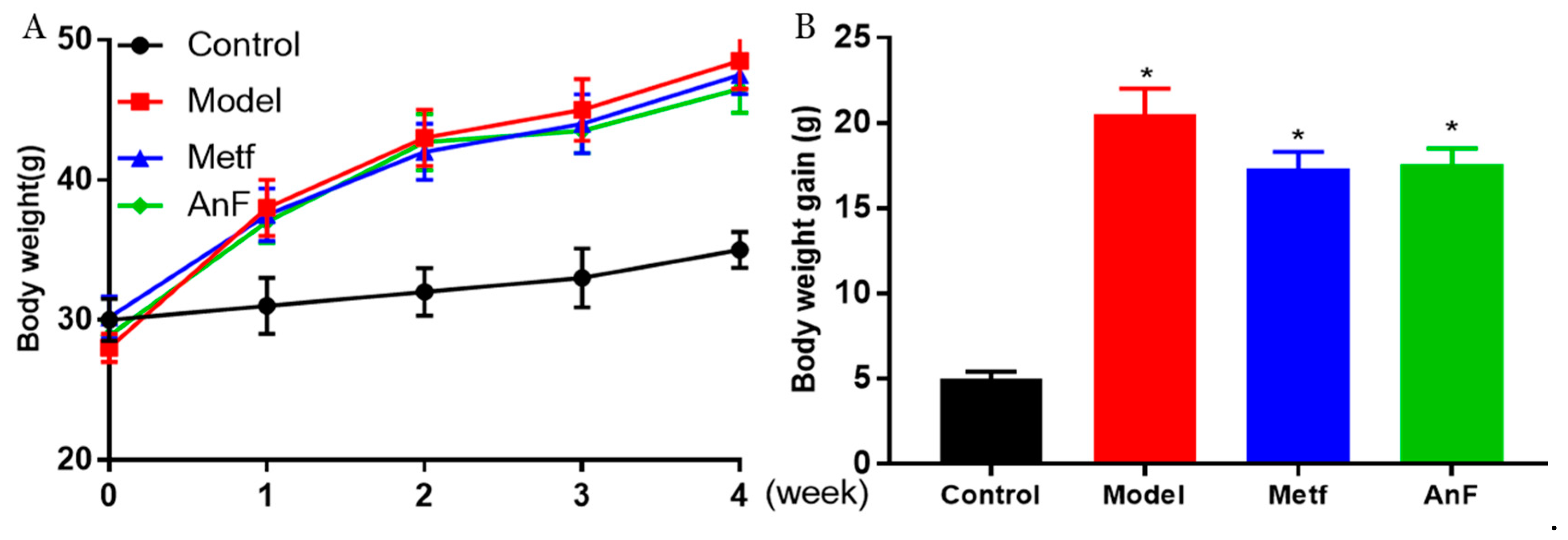
2.6. Effects of AnF on Body Weight in db/db Mice
2.7. Effects of AnF on Glucose-Insulin Homeostasis in db/db Mice
2.8. Effects of AnF on GLP-1 Level in db/db Mice
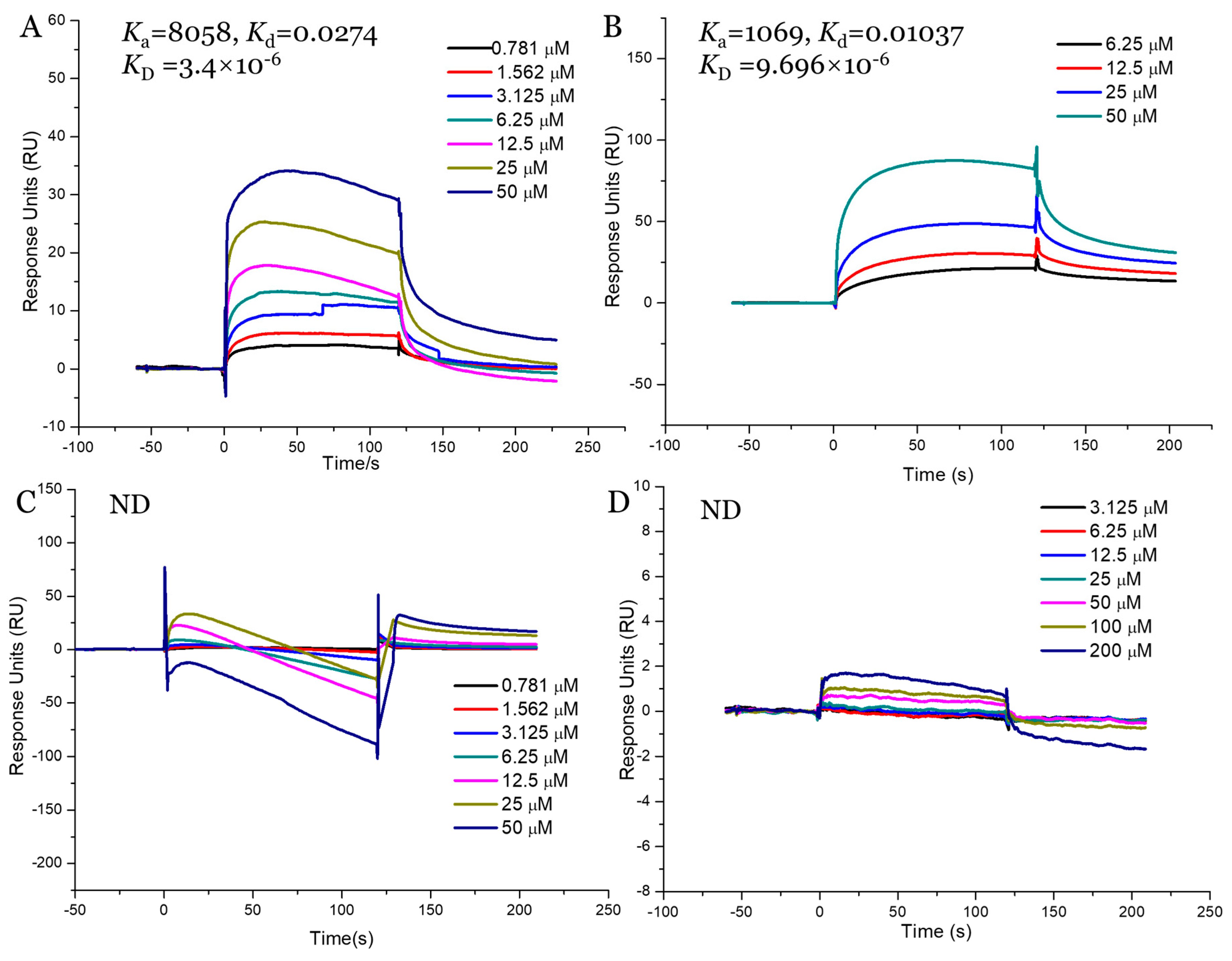
2.9. Interaction Study between Fucoidans and SGLT1
3. Discussion
4. Materials and Methods
4.1. Preparation of Fucoidans
4.2. Physicochemical Properties Analysis of Fucoidans
4.3. Effects of Fucoidans on Glucose Absorption Using Caco-2 Monolayer Model
4.4. Effects of Fucoidans on OGTT in Kunming Mice and Glucose Transport Using Everted Gut Sac Model
4.5. Effects of AnF on Alleviating Hyperglycemia in db/db Mice
4.6. Effects of AnF on Biochemical Indexes in db/db Mice
4.7. Binding Kinetics Analysis of Interaction between SGLT1 and Fucoidans
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- DeFronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [Green Version]
- Loghmani, E. Diabetes mellitus: Type 1 and type 2. In Guidelines for Adolescent Nutrition Services; Scientific Research Publishing: Irvine, CA, USA, 2005; pp. 167–182. [Google Scholar]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Han, J.S.; Kim, H.J.; Jeon, Y.J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Rizvi, A.A.; Spinas, G.A.; Rini, G.B.; Berneis, K. Glucose lowering and anti-atherogenic effects of incretin-based therapies: GLP-1 analogues and DPP-4-inhibitors. Expert Opin. Investig. Drugs 2009, 18, 1495–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, P.E.; El-kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002, 51, 434–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuno, K.; Fujimori, Y.; Takemura, Y.; Hiratochi, M.; Itoh, F.; Komatsu, Y.; Fujikura, H.; Isaji, M. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J. Pharmacol. Exp. Ther. 2007, 320, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Dobbins, R.L.; Greenway, F.L.; Chen, L.; Liu, Y.; Breed, S.L.; Andrews, S.M.; Wald, J.A.; Walker, A.; Smith, C.D. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am. J. Physiol.-Gastr. Liver Physiol. 2015, 308, 946–954. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Bailey, C.J.; Del Prato, S.; Barnett, A.H. Management of type 2 diabetes: New and future developments in treatment. Lancet 2011, 378, 182–197. [Google Scholar] [CrossRef]
- Guthrie, R.M. Evolving therapeutic options for type 2 diabetes mellitus: An overview. Postgrad. Med. 2012, 124, 82–89. [Google Scholar] [CrossRef]
- Deng, R. A review of the hypoglycemic effects of five commonly used herbal food supplements. Recent Pat. Food Nutr. Agric. 2012, 4, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Fawaz, A.; Hoi-Man, C.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932. [Google Scholar]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Bangert, A.; Kottra, G.; Geillinger, K.E.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol. Nutr. Food Res. 2014, 58, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Vrhovac, I.; Eror, D.B.; Klessen, D.; Burger, C.; Breljak, D.; Kraus, O.; Radović, N.; Jadrijević, S.; Aleksic, I.; Walles, T. Localizations of Na+-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflügers Arch.-Eur. J. Physiol. 2015, 467, 1881–1898. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.G.; Turk, E.; Lostao, M.P.; Kerner, C.; Wright, E.M. Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nat. Genet. 1996, 12, 216–220. [Google Scholar] [CrossRef]
- Turk, E.; Zabel, B.; Mundlos, S.; Dyer, J.; Wright, E. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 1991, 350, 354–356. [Google Scholar] [CrossRef]
- Ina, S.; Hamada, A.; Nakamura, H.; Yamaguchi, Y.; Kumagai, H.; Kumagai, H. Rice (Oryza sativa japonica) albumin hydrolysates suppress postprandial blood glucose elevation by adsorbing glucose and inhibiting Na+-d-glucose cotransporter SGLT1 expression. J. Funct. Foods 2020, 64, 103603. [Google Scholar] [CrossRef]
- Müller, U.; Stübl, F.; Schwarzinger, B.; Sandner, G.; Iken, M.; Himmelsbach, M.; Schwarzinger, C.; Ollinger, N.; Stadlbauer, V.; Höglinger, O. In vitro and in vivo inhibition of intestinal glucose transport by guava (Psidium guajava) extracts. Mol. Nutr. Food Res. 2018, 62, 1701012. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.C.; Zhao, S.; Yang, B.Y.; Wang, Q.H.; Kuanga, H.X. Anti-diabetic polysaccharides from natural sources: A review. Carbohydr. Polym. 2016, 148, 86–97. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, X.; Jiang, H.; Cai, C.; Li, G.Y.; Hao, J.J.; Yu, G.L. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, Z.M.; Xu, X.; Jiang, H.; Cai, C.; Yu, G.L. Odd-numbered agaro-oligosaccharides alleviate type 2 diabetes mellitus and related colonic microbiota dysbiosis in mice. Carbohydr. Polym. 2020, 240, 116261. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Jiang, H.; Zhang, N.; Cai, C.; Li, G.Y.; Hao, J.J.; Yu, G.L. Anti-diabetic activities of agaropectin-derived oligosaccharides from Gloiopeltis furcata via regulation of mitochondrial function. Carbohydr. Polym. 2020, 229, 115482. [Google Scholar] [CrossRef] [PubMed]
- Necyk, C.; Zubach-Cassano, L. Natural health products and diabetes: A practical review. Can. J. Diabetes 2017, 41, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Chen, C.; Wang, S.K.; Sun, G.J. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int. J. Biol. Macromol. 2015, 77, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.J.; Zhao, R.X. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albana, C.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Armida, D.I.; Antonio, P.; Licia, T.; Nicola, T.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar]
- Shang, Q.S.; Song, G.R.; Zhang, M.F.; Shi, J.J.; Xu, C.Y.; Hao, J.J.; Li, G.Y.; Yu, G.L. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Shang, Q.S.; Shan, X.D.; Cai, C.; Hao, J.J.; Li, G.Y.; Yu, G.L. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Wang, X.L.; Shan, X.D.; Dun, Y.L.; Cai, C.; Hao, J.J.; Li, G.Y.; Cui, K.Y.; Yu, G.L. Anti-metabolic syndrome effects of fucoidan from Fucus vesiculosus via reactive oxygen species-mediated regulation of JNK, Akt, and AMPK signaling. Molecules 2019, 24, 3319. [Google Scholar] [CrossRef] [Green Version]
- Shan, X.D.; Liu, X.; Hao, J.J.; Cai, C.; Fan, F.; Dun, Y.L.; Zhao, X.L.; Liu, X.X.; Li, C.X.; Yu, G.L. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef]
- Foley, S.A.; Szegezdi, E.; Mulloy, B.; Samali, A.; Tuohy, M.G. An unfractionated fucoidan from Ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J. Nat. Prod. 2011, 74, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.D.; Lv, Y.J.; Liu, X.X.; Zhao, X.L.; Jiao, G.L.; Tai, W.J.; Wang, P.P.; Zhao, X.; Cai, C.; Yu, G.L. Structural study of sulfated fuco-oligosaccharide branched glucuronomannan from Kjellmaniella crassifolia by ESI-CID-MS/MS. J. Carbohydr. Chem. 2015, 34, 303–317. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Song, H.F.; Li, P.C. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, A.R.; Conradi, R.A.; Burton, P.S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm. Res. 1990, 7, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Kipp, H.; Khoursandi, S.; Scharlau, D.; Kinne, R.K. More than apical: Distribution of SGLT1 in Caco-2 cells. Am. J. Physiol.-Cell Physiol. 2003, 285, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Erickson, R.H.; Gum, J.R.; Yoshioka, M.; Gum, E.; Kim, Y.S. Biosynthesis of alkaline phosphatase during differentiation of the human colon cancer cell line Caco-2. Gastroenterology 1990, 98, 1199–1207. [Google Scholar] [CrossRef]
- Wright, E.M. Intestinal sugar transport. In Physiology of the Gastrointestinal Tract; Academic Press: Manhattan, NY, USA, 1994; pp. 1751–1772. [Google Scholar]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-mohizea, A.M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Butt, A.G. Glucose transport into everted sacs of the small intestine of mice. Adv. Physiol. Educ. 2013, 37, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef]
- Kim, K.T.; Rioux, L.E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Mar. Drugs 2015, 13, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- McCulloch, D.K.; Robertson, R.P. Pathogenesis of Type 2 Diabetes Mellitus; UpTo-Date [consultado 12 Fev 2013]; Available online: http://www.uptodate.com/contents/pathogenesis-of-type-2-diabetes-mellitus (accessed on 10 October 2019).
- Gribble, F.M. The gut endocrine system as a coordinator of postprandial nutrient homoeostasis. Proc. Nutr. Soc. 2012, 71, 456–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriya, R.; Shirakura, T.; Ito, J.; Mashiko, S.; Seo, T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Hjøllund, K.R.; Deacon, C.F.; Holst, J.J. Dipeptidyl peptidase-4 inhibition increases portal concentrations of intact glucagon-like peptide-1 (GLP-1) to a greater extent than peripheral concentrations in anaesthetised pigs. Diabetologia 2011, 54, 2206–2208. [Google Scholar] [CrossRef]
- Drucker, D.J. Incretin action in the pancreas: Potential promise, possible perils, and pathological pitfalls. Diabetes 2013, 62, 3316–3323. [Google Scholar] [CrossRef] [Green Version]
- Powell, D.R.; Smith, M.; Greer, J.; Harris, A.; Zhao, S.; Dacosta, C.; Mseeh, F.; Shadoan, M.K.; Sands, A.; Zambrowicz, B. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J. Pharmacol. Exp. Ther. 2013, 345, 250–259. [Google Scholar] [CrossRef]
- Shibazaki, T.; Tomae, M.; Ishikawa-Takemura, Y.; Fushimi, N.; Itoh, F.; Yamada, M.; Isaji, M. KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J. Pharmacol. Exp. Ther. 2012, 342, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Timo, R.; Volker, V. Development of SGLT1 and SGLT2 inhibitors. Diabetes 2018, 61, 2079–2086. [Google Scholar]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul. Fibrinolysis 2009, 20, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Ponce, N.M.A.; Pujol, C.A.; Damonte, E.B.; Flores, M.A.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohyd. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Dodgson, K.; Price, R. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, T.T.; Furneaux, R.H. Chemical methods for the analysis of sulphated galactans from red algae. Carbohydr. Res. 1991, 210, 277–298. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Blodgett, A.B.; Kothinti, R.K.; Kamyshko, I.; Petering, D.H.; Kumar, S.; Tabatabai, N.M. A fluorescence method for measurement of glucose transport in kidney cells. Diabetes Technol. Ther. 2011, 13, 743–751. [Google Scholar] [CrossRef] [Green Version]
- He, Y.L.; Wang, Y.; Bullock, J.M.; Deacon, C.F.; Holst, J.J.; Dunning, B.E.; Ligueros-Saylan, M.; Foley, J.E. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J. Clin. Pharmacol. 2007, 47, 633–641. [Google Scholar] [CrossRef]
- Nagy, C.; Einwallner, E. Study of in vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). JoVE-J. Vis. Exp. 2018, 131, e56672. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Wang, L.H.; Yang, C.D.; Jiang, H.; Li, M.M.; Xu, D.; Li, G.Y.; Li, C.X.; Yu, G.L. Chemoenzymatic synthesis of heparan sulfate mimetic glycopolymers and their interactions with the receptor for advanced glycation end-product. ACS Macro Lett. 2019, 8, 1570–1574. [Google Scholar] [CrossRef] [Green Version]
- Terada, Y.; Seto, H.; Hoshino, Y.; Murakami, T.; Shinohara, S.; Tamada, K.; Miura, Y. SPR study for analysis of a water-soluble glycopolymer interface and molecular recognition properties. Polym. J. 2017, 49, 255–262. [Google Scholar] [CrossRef]



| Sample | Source | Linkage Mode | SO42− (%) | MW (kDa) | Monosaccharide Composition (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Man | GlcN | Rha | GlcA | Glc | Gal | Xyl | Fuc | |||||
| AnF | Ascophyllum nodosum | 1→3/1→4 | 22.7 | 210 | 7.5 | 0.6 | 0.3 | 3.3 | 8.0 | 3.8 | 17.9 | 58.5 |
| LjF | Laminaria japonica | 1→3 | 26.0 | 200 | 9.3 | 2.0 | 3.9 | 4.1 | 5.1 | 19.6 | 4.1 | 52.0 |
| KcF | Kjellmaniella crassifolia | 1→3 | 27.6 | 940 | 11.0 | 1.4 | 0.8 | 6.3 | 1.7 | 3.9 | 2.9 | 72.1 |
| FvF [31] | Fucus vesiculosus | 1→3/1→4 | 26.3 | 1039 | ND | ND | ND | ND | 1.0 | 1.9 | 2.3 | 94.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, X.; Wang, X.; Jiang, H.; Cai, C.; Hao, J.; Yu, G. Fucoidan from Ascophyllum nodosum Suppresses Postprandial Hyperglycemia by Inhibiting Na+/Glucose Cotransporter 1 Activity. Mar. Drugs 2020, 18, 485. https://doi.org/10.3390/md18090485
Shan X, Wang X, Jiang H, Cai C, Hao J, Yu G. Fucoidan from Ascophyllum nodosum Suppresses Postprandial Hyperglycemia by Inhibiting Na+/Glucose Cotransporter 1 Activity. Marine Drugs. 2020; 18(9):485. https://doi.org/10.3390/md18090485
Chicago/Turabian StyleShan, Xindi, Xueliang Wang, Hao Jiang, Chao Cai, Jiejie Hao, and Guangli Yu. 2020. "Fucoidan from Ascophyllum nodosum Suppresses Postprandial Hyperglycemia by Inhibiting Na+/Glucose Cotransporter 1 Activity" Marine Drugs 18, no. 9: 485. https://doi.org/10.3390/md18090485





