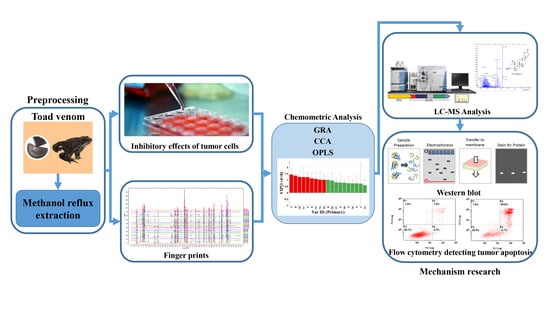
1. Introduction
Lung cancer is one of the most common cancers in humans with high incidence and mortality rate. Non-Small Cell Lung Carcinoma (NSCLC), the most common form of lung cancer, comprises of approximately 85% of all cases of lung cancer [
1,
2]. Natural medicines have shown attractive potential for preventing and treating diseases for centuries and have contributed to the development of modern medicine [
3]. Toad venom, the dried white secretion from the postauricular and skin glands of Bufo gargarizans cantor, is well known for treating many kinds of cancer [
4]. According to traditional records, the main efficacy of toad venom is in detoxification, as an analgesic, etc. [
5]. The medicine currently used in clinical practice in China are mainly complexes that have originated from toad venom [
6,
7,
8]. Modern medicines composed of toad venom include Huachansu injection, and Shexiang Baoxin Pills, among others. Huachansu injection, prepared from a water extract of dried toad venom, has long been used to treat various cancers of the digestive system [
9,
10]. It has also shown a reversal effect on multi-drug resistance (MDR) of acute myeloid leukemia cells [
11]. In combination with chemotherapy, Huachansu injection enhances curative effects and diminishes the side-effects of chemotherapy [
12]. Shexiang Baoxin Pills, which are composed of Moschus, Bufonis Venenum, etc., have been commonly used for cardiovascular diseases, like unstable angina pectoris [
13,
14]. Because of the complexity of toad venom, both of these medicines have shown drug-related adverse effects, including cardiac toxicity, hematologic toxicities, mucocutaneous toxicities, and gastrointestinal toxicities, thus limiting their use [
15]. There is, therefore, a great need to distinguish the active ingredients in toad venom and further study its underlying molecular mechanism.
Bufogenin and bufotoxin, as major components of toad venom, are considered to be the main bioactive constituents, which exert various pharmacological effects with different mechanisms. Ma [
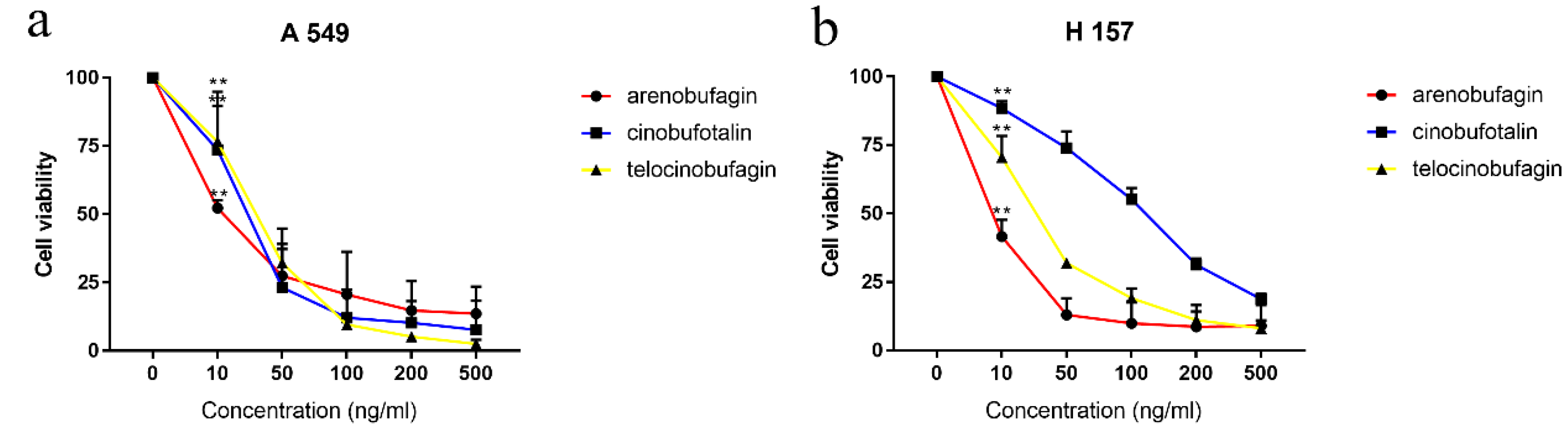
16] reported that arenobufagin has anticancer influences on several non-small-cell lung cancer (NSCLC) cells through activation of Noxa (the pro-apoptosis protein)-related signaling pathways and promotes apoptotic cell death in human NSCLC cells. Kai [
17] held that cinobufotalin showed obvious inhibitory effects against lung cancer cells without inducing significant cell apoptosis, and Zhang [
18] discovered that arenobufagin induced apoptosis and autophagy in human hepatoma carcinoma cells through PI3K/AKt/mTOR pathway inhibition. As shown by these studies, different bufadienolides conducted antitumor functions by regulating different cell signaling pathways. As a result, different bufadienolides have distinct inhibitory effects on diverse kinds of cancer, which awaits further exploration and utilization. It is thus urgent to identify the active ingredients in toad venom.
Spectrum-effect relationship analysis is an effective method to clarify active components in complex mixtures. By combining the characteristic fingerprint and pharmacodynamics information processed by chemometric methods, effective components can be screened.
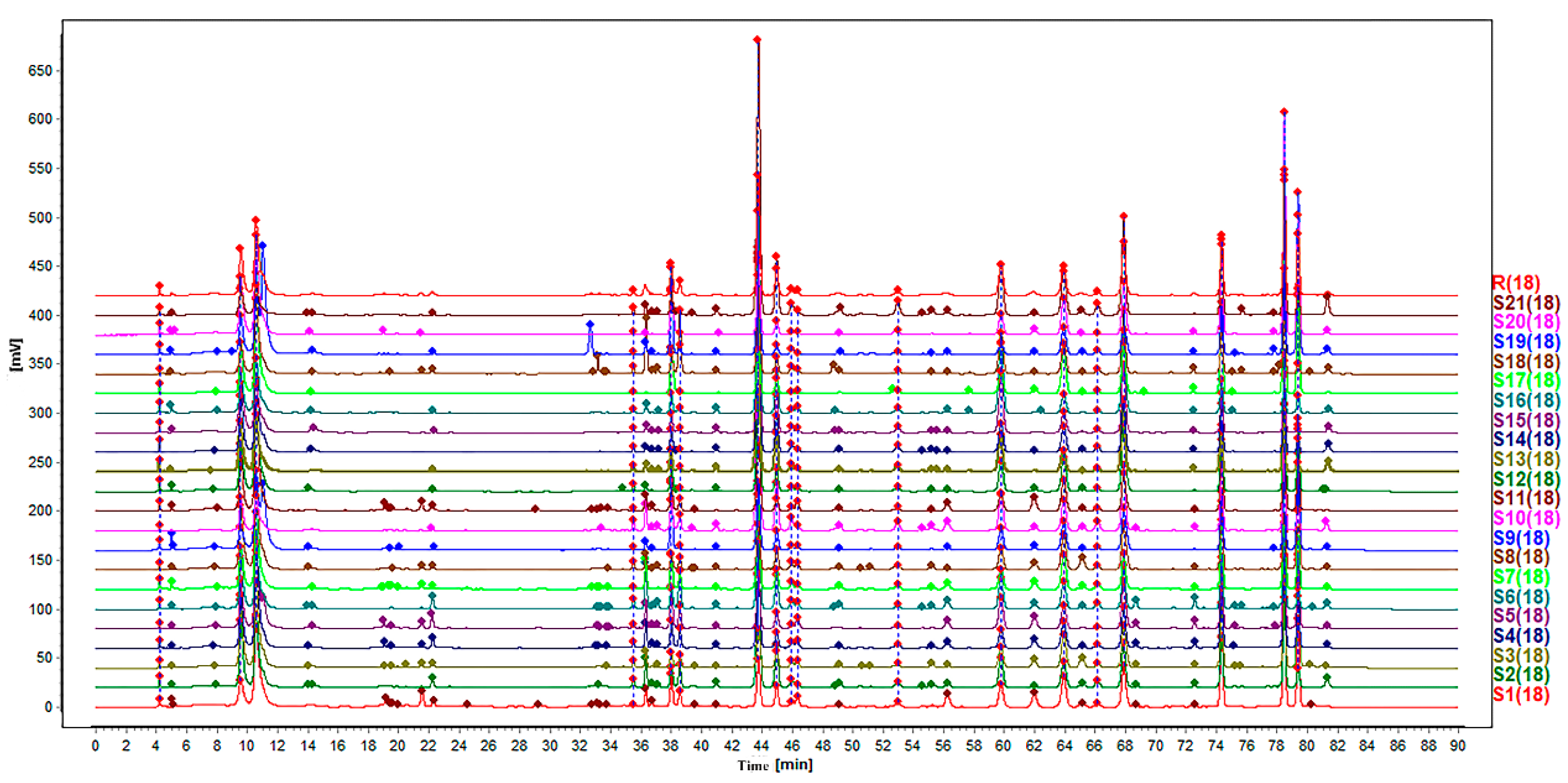
Chromatographic fingerprint is an effective method to evaluate the consistency and quality of traditional Chinese medicines (TCMs), which could reveal the chemical characteristics of samples to a certain extent [
19]. Multiple techniques including HPLC, gas chromatography (GC), etc. have been used to construct specific fingerprints for recognition of complex compounds of TCMs. Among them, HPLC is a broadly applied method owing to its high sensitivity and accessibility [
20]. HPLC-MS is an analytical technique mainly used for identification of chemical structures.
While fingerprint analysis is a useful method for chemical analysis of complex matrices [
21], it does not involve the identification of components that play leading roles in pharmacology activity. In this paper, with the aim to research the correlation between the biological activity of toad venom and the fingerprint, multivariate chemometrics techniques (including orthogonal partial least squares (OPLS), canonical correlation analysis (CCA), and gray relationship analysis (GRA)) were employed.
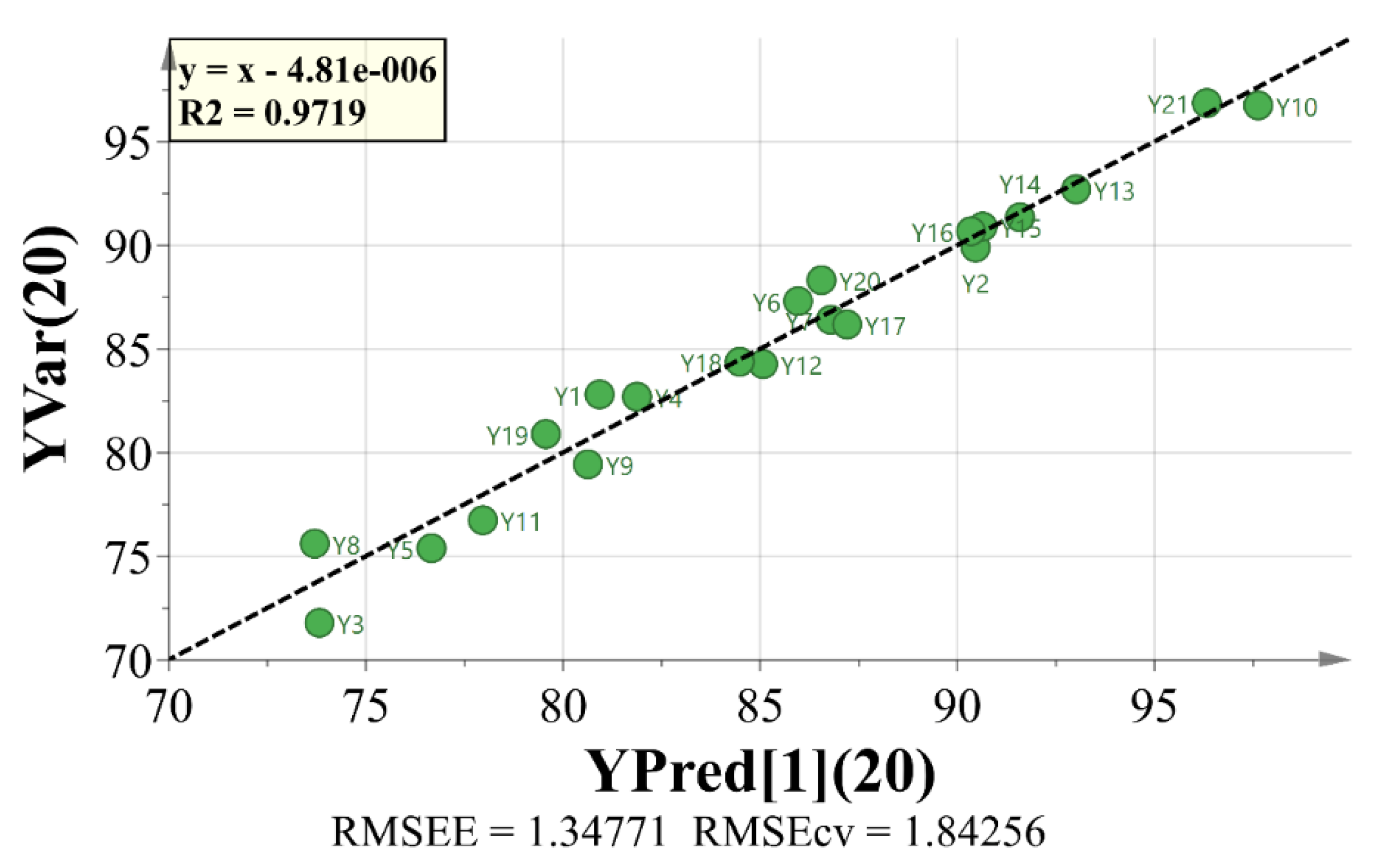
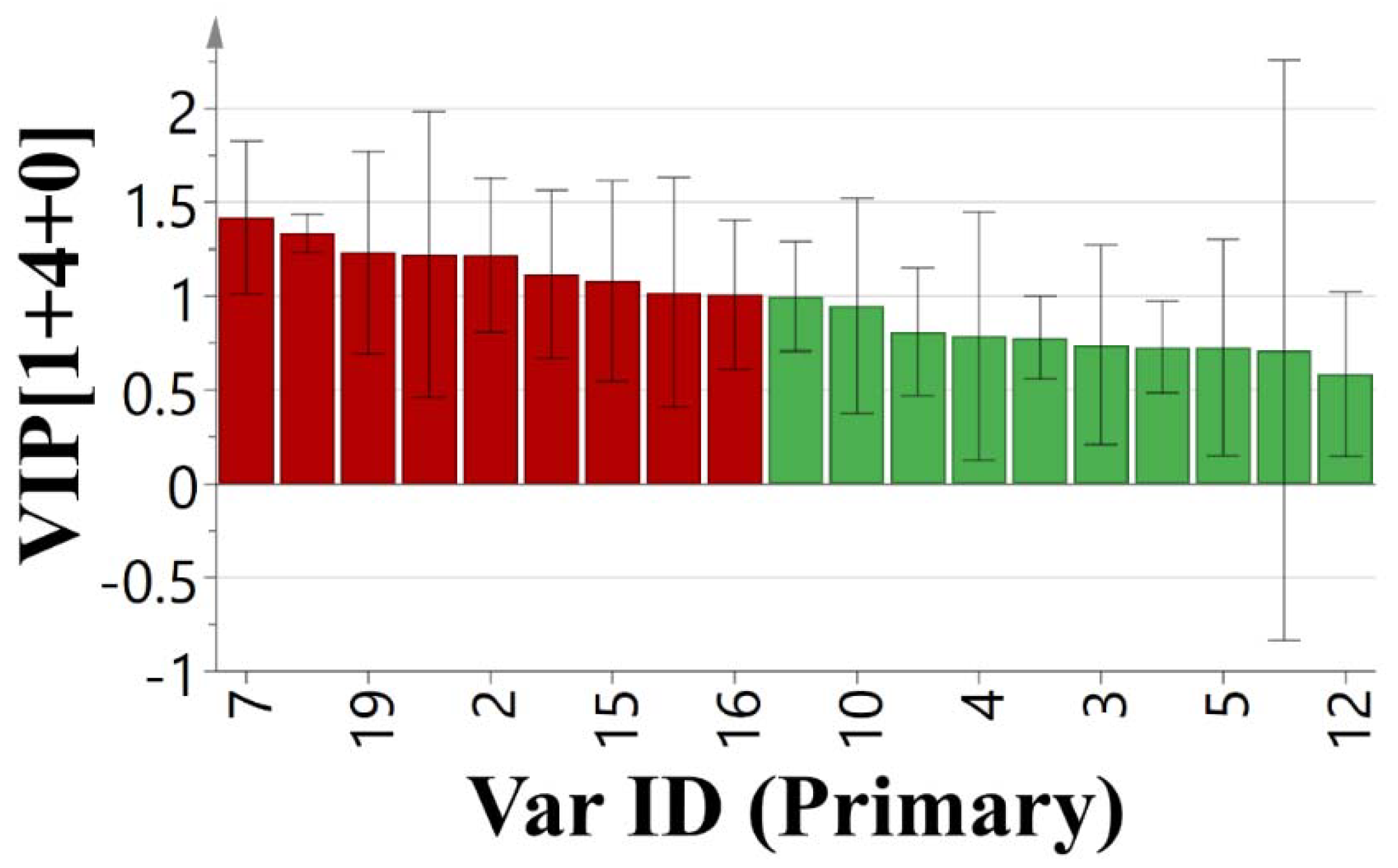
PCA is a method to analyze and simplify data set by reducing its dimensionality, and keeping the largest contribution to the variance of the data set [
22,
23]. OPLS is a generic method to build a model of the observed data in order to analyze the relationship between two groups of variables and selecting the key variables [
24]. Processed by OPLS, variable importance in projection (VIP) reflects the loading weights of each independent variable to the dependent variable. When VIP > 1, the independent variable is a significant factor in interpreting the dependent variable [
24]. GRA, which originated from the grey system theory proposed by Deng [
25] in the 1980s, is suitable for manipulating complex interrelationships between multiple factors and variables. GRA results can be provided as a ranking sequence that reflects the order of correlation among dependent and independent factors [
26]. Correlation analysis is a statistic analytical method to research the linear relation between variables; the correlation coefficients are used to measure the degree of correlation of the variables [
19].
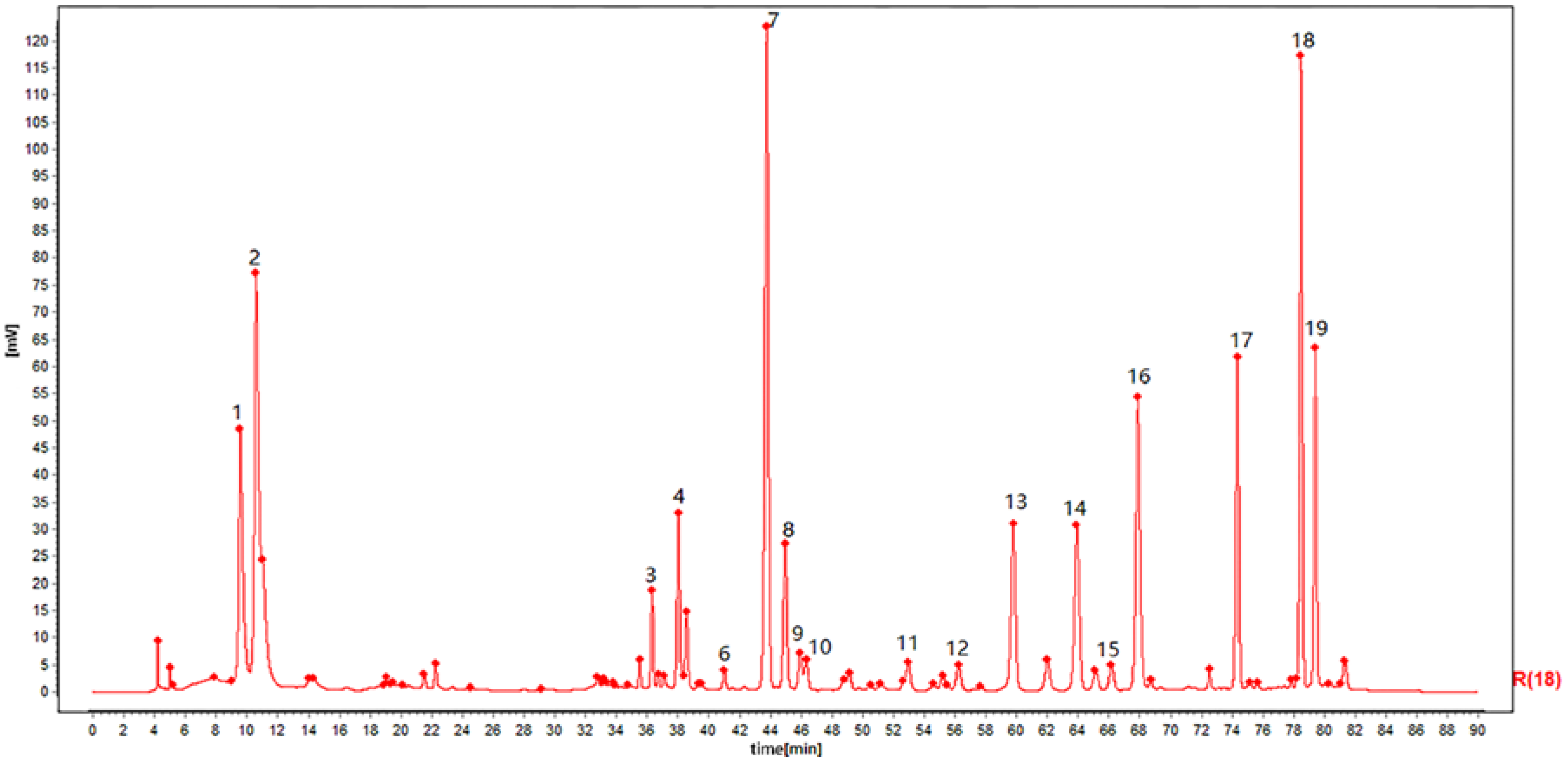
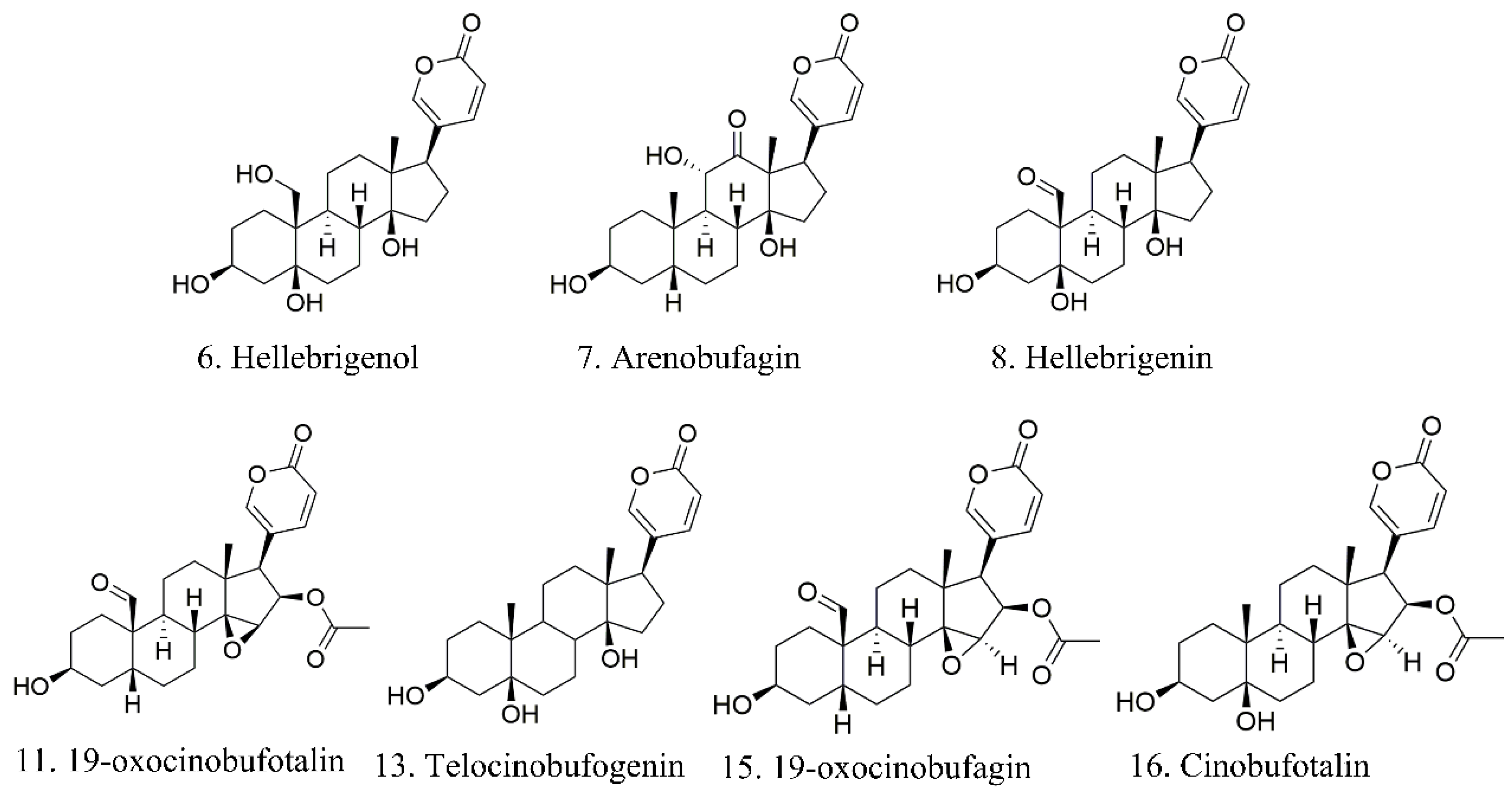
These analysis methods were used to establish the spectrum-effect relationship between the peak area of the fingerprint and the results of anti-cancer activity study, respectively. We isolated seven bufadienolides (hellebrigenol, arenobufagin, hellebrigenin, 19-oxo-cinobufotalin, telocinobufogenin, 19-oxo-cinobufagin, cinobufotalin) and evaluated the inhibitory effect of the compounds with relatively high content on two non-small cell lung cancer cells in vitro. Herein, we found out the key pharmacophores of bufadienolides and further investigated the possible mechanism of arenobufagin, which is most significant correlated with antitumor activity.
3. Materials and Methods
3.1. Materials and Reagents
Twenty-one batches of toad venom derived from Bufo gargarizans Cantor were collected from Jiangsu China. The reference substances, including arenobufagin, telocinobufogenin, and cinobufotalin were provided by Yuanye Bio-Technology Co, Ltd. (Shanghai, China). The purity of all the substances was detected to be higher than 98% by HPLC-DAD. HPLC grade acetonitrile, formid acid, and ammonium formate were obtained from Aladdin Bio-Chem Technology Co, Ltd. (Shanghai, China). Ultra water was prepared by using a Milli-Q plus system (Millipore, Billerica, MA, USA).
3.2. Cell Culture
Human No-Small Cell Lung Cancer cell lines were obtained from Cobioer Biosciences (Nanjing, China). Cell lines A549 and H157 was cultured in RPMI-1640 supplemented with 10% foetal bovine serum in an incubator with 5% CO2 at 37°C. Cell lines were authenticated by short-tandem repeat genotyping performed by the Shanghai Bio Wing Applied Biotechnology Company (Shanghai, China), displaying identical morphology as cells provided by ATCC.
3.3. Preparation of Extracts and Standard Solutions
Each batch of toad venom was ground to 40 mesh, and precisely measured powder (5 g) was immersed in methanol (50 mL) and then extracted thrice by reflux for 1 h. The extract was filtered, combined, and concentrated in a rotary evaporator by evaporation and vacuum (60 °C water bath) and then dried in a vacuum freeze-drying machine. For the HPLC analysis, the precisely measured final filtrate was diluted with methanol to 25 mL, and then the mixture was filtered through a 0.22 μm filter before HPLC analysis. The final concentration was 2 mg/mL.
3.4. Instrumentation and Analysis Conditions
3.4.1. HPLC Conditions
In our previous works, the HPLC analysis method with satisfactory selectivity and efficiency was established [
30]. The HPLC analysis was performed with the Agilent 1200 system (Agilent Technologies, Tokyo, Japan) with an X Bridge reverse phase C18 column (4.6 × 250 mm, 5μm). The mobile phase was composed of 0.3% Acetic Acid-10mmol ammonium acetate water (A) and acetonitrile (B). The gradient program was 97–95% A for 0-7 min, 95–95% A for 7–11 min, 95–85% A for 11–13, 85–85% A for 13–25 min, 85–76% A for 25–27 min, 76–72% A for 24–45 min, 72-68% A for 45–60 min, 68–50% A for 60–75 min, and 50–97% A for 75–90 min. The flow rate was 0.7 mL/min with a sample injection volume of 10 μL. Temperature was maintained at 30 °C and detection wavelength was set at 296 nm.
3.4.2. HPLC-MS Conditions
HPLC-MS analysis was conducted by AB Triple TOF 5600 plus Mass spectrometer (AB SCIEX, Framingham, USA) in positive electrospray ionization (ESI) mode with the liquid chromatography system (Waters Corp., Milford, MA, USA). Specific experimental methods and conditions were conducted as we reported previously [
30]. The accurate mass and compounds’ structure was calculated by Peak View Software (AB SCIEX, version 1.2.0.3).
3.4.3. Flow-Cytometric Analysis
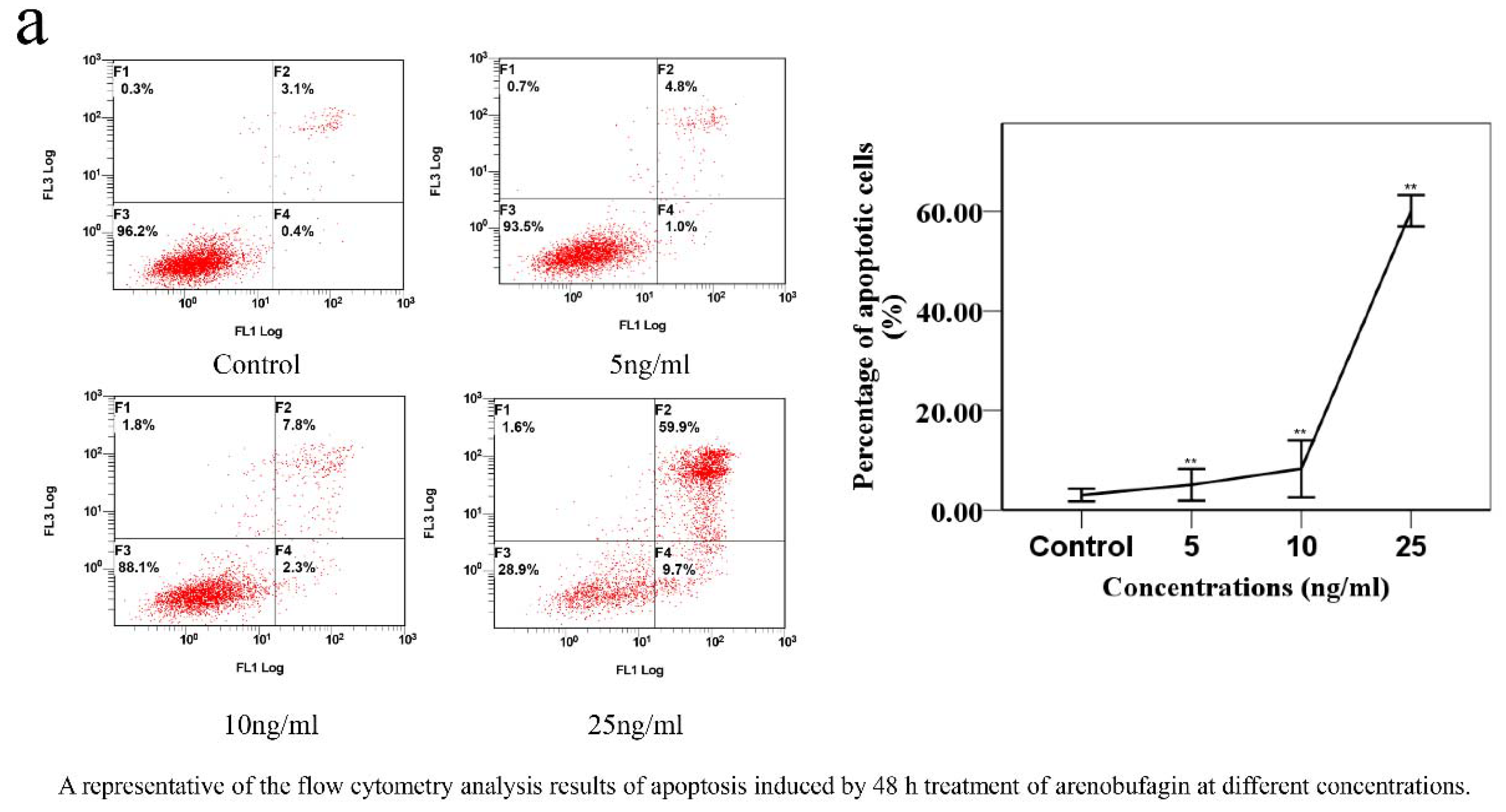
Apoptosis degree was measured by Annexin V staining (Biosharp Biotechnology (Shanghai, China)). Firstly, A549 cells were incubated with the tested compound (arenobufagin) for 48 h, then collected from six-well plates, washed once with cold PBS (4C), and centrifuged (2000 rpm for 5 min) before being suspended with a 300 μL binding buffer. Annexin V-FITC (6 μL) was then added and the cells were stained in the dark for 15 min before adding 6 μL propidium iodide and 300 μL binding buffer. Apoptosis quantification was counted by flow cytometry (FC 500MCL, Beckman Coulter, Indianapolis, IN, USA).
3.4.4. Western Blot Analysis
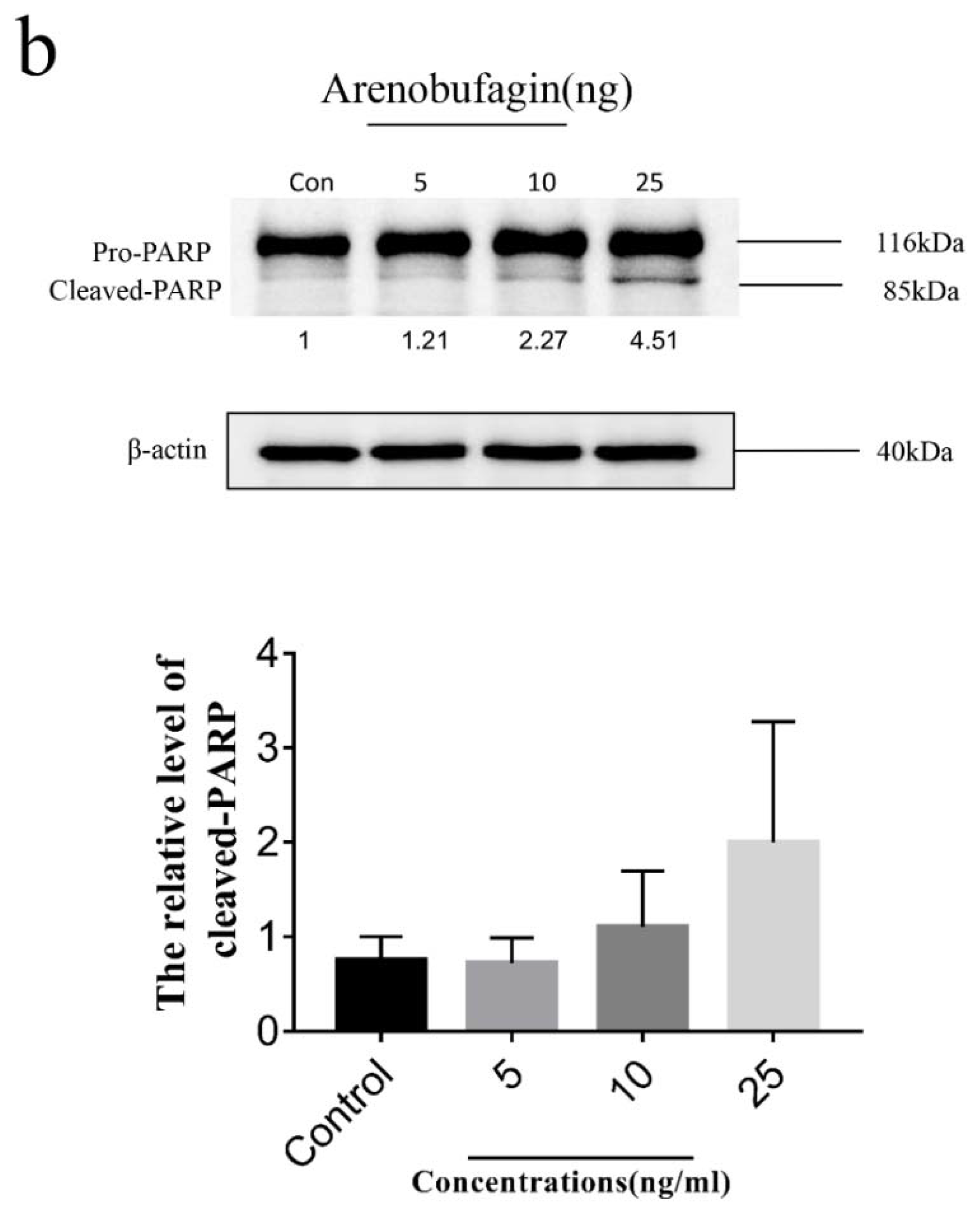
The cell samples were treated with arenobufagin (0, 5, 10, 25 ng) for 48 h. After incubation, the cells were suspended in lysis buffer (Tris-HCl, NaCl, EDTA, EGTA, NP-40 and PMSF) (Beyotime Institute of Biotechnology) on ice for 30 min and vortexed for 60 s, then centrifuged at 16,000 rpm at 4 °C for 20 min. Protein content was quantified by BCA assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Protein samples with appropriate concentration were loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel in a 2 h run under 100 V. Subsequently, the proteins were transferred to polyvinylidene fluoride (PVDF) membranes by wet transfer electrophoresis. The membranes were blocked with skimmed milk before being incubated with the primary detection antibody PARP (Cell Signaling Technology, 1:1000), after being washed by TBS-T for 30 min. The membranes were then probed with counterpart secondary antibodies (Cell Signaling, 1:5000), and visualized by chemiluminescence (Bio-Rad, Hercules, CA, USA).
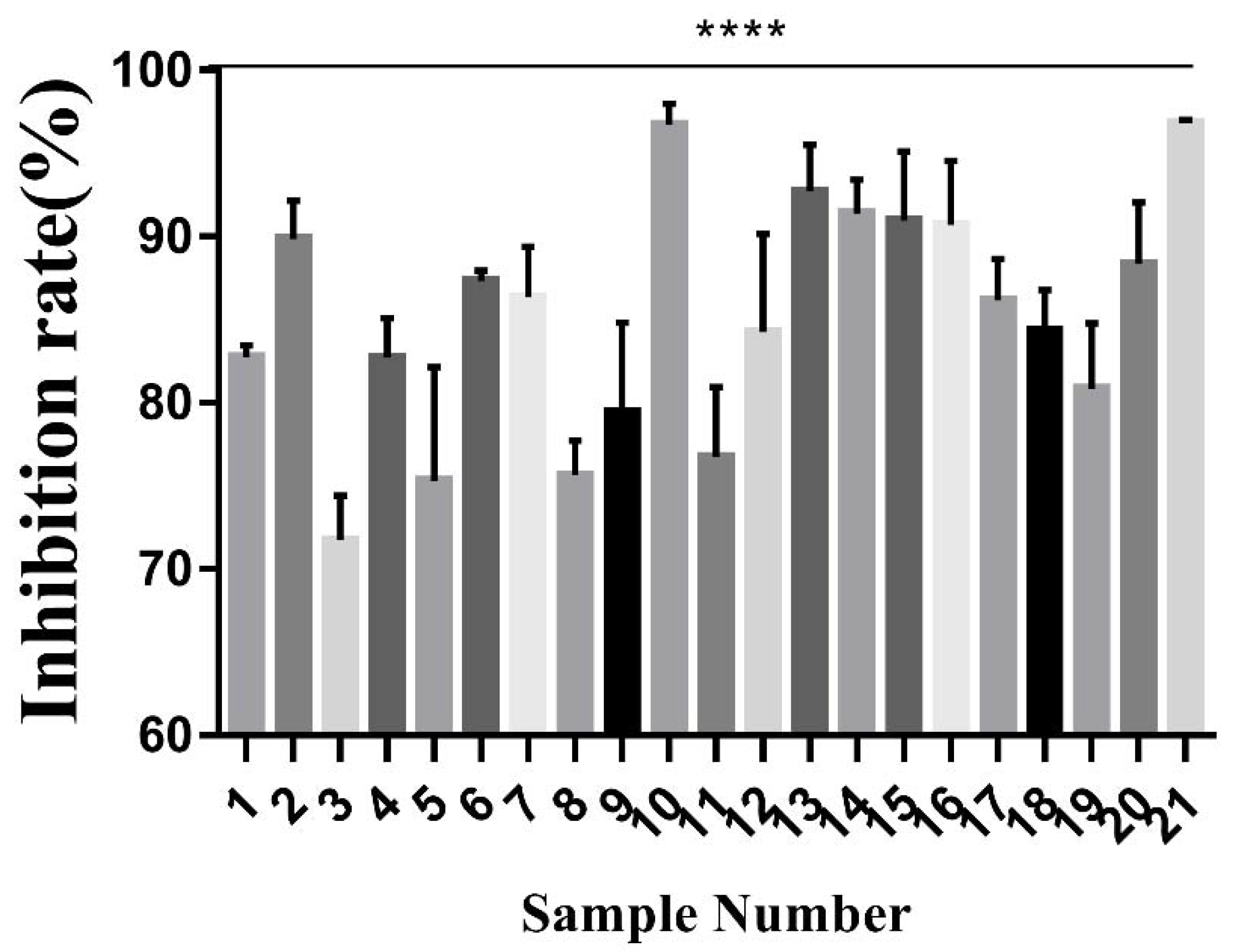
3.5. Cytotoxicity Assay
A-549 cell line were cultured in RPMI 1640 medium with 10% foetal bovine serum, 1% penicillin and streptomycin, and kept at 37 °C in an incubator with a 5% CO2 atmosphere. After being cultured for 24 h, the extracts were added and the cells were incubated for 72 h; 20 μL MTT (5 mg/mL) was then added and the cells were incubated for 4 h. 150 μL of DMSO was used to dissolve formazan crystals that were formed. Absorbance of the solution was measured with a spectrophotometer (Synergy 2, BioTek, Winooski, VT, USA) at 490 nm. The inhibition ratio was calculated by the following formula:
3.6. Spectrum-Effect Relationship Analysis
Based on the tested spectral and pharmacodynamic data, gray relational analysis, orthogonal partial least square (OPLS) regression, and pearson correlation analysis were applied to establish the spectrum-effect relationship and screen variables.
3.7. Statistical Analysis
Data were expressed as means ± standard error (SE). Statistical analysis was performed using Graph Pad computer software Version 7.00. The levels of significant difference were set at p < 0.05, p < 0.01.