A Coolant Circulation Cooling System Combining Aluminum Plates and Copper Rods for Li-Ion Battery Pack
Abstract
1. Introduction
2. Theoretical Analysis
3. Experiment and Result Analysis
3.1. Experimental Design
- (1)
- Preparation stage: all lithium-ion batteries were connected in series in groups, and the discharging experiments were carried out by the charging and discharging tester. Then, six lithium-ion batteries with stable performance and consistent power were chosen. In order to avoid excessive local temperature and reduce the average temperature time during charging and discharging, six lithium-ion batteries were connected in series with an interval of 10 mm.
- (2)
- Environmental simulation stage: after completing the assembly of the experimental model in the thermostat and installing thermocouples to a battery pack for measuring the temperature, the thermostat began to work and the ambient temperature required was set. We waited for another 8 h until the temperature in thermostat reached the set temperature, and then discharging experiments began to be conducted.
- (3)
- Charging and discharging test stage: in order to protect the power battery, all the charging experiments in this paper adopted the charging mode of constant current and constant voltage (CC-CV). The discharging experiments were carried out at the current of 144 A, the discharging capacity was 70 Ah, and the end-of-discharge voltage was 15 V.
- (4)
- Data processing stage: the value of temperature at the center of the single battery was selected to represent the temperature of the battery, and then the value of the temperature of each battery was added to calculate the average value, which represents the value of temperature of the whole battery pack.
3.2. Temperature Rise of Battery Pack in Natural State
3.3. Temperature Rise of Battery Pack in Coolant Circulation Cooling System (CCCS)
3.3.1. Design of CCCS
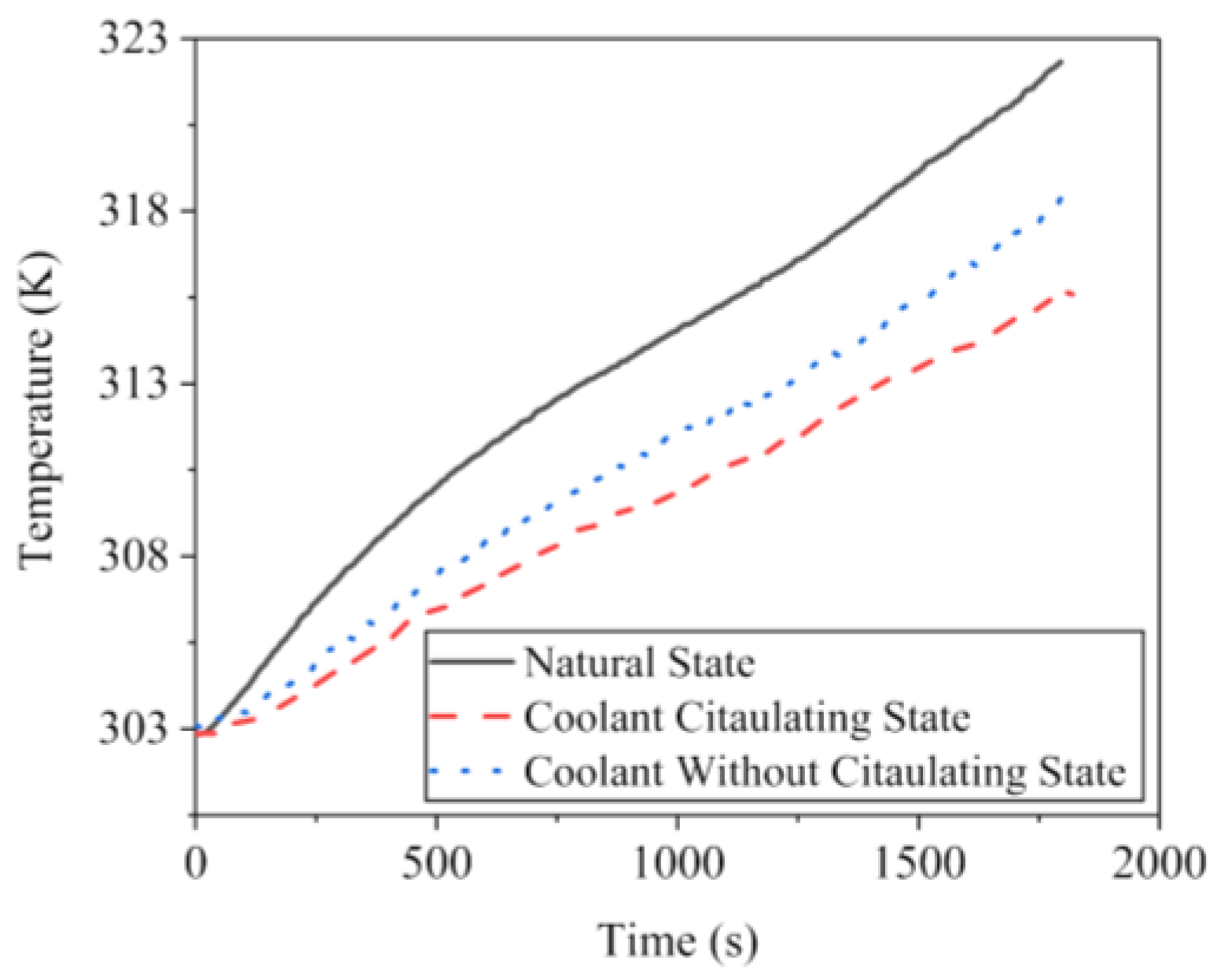
3.3.2. Temperature Rise of Battery Pack in Coolant Circulating State
3.3.3. Temperature Rise of Battery Pack in Coolant without Circulating State
4. Simulation and Result Analysis
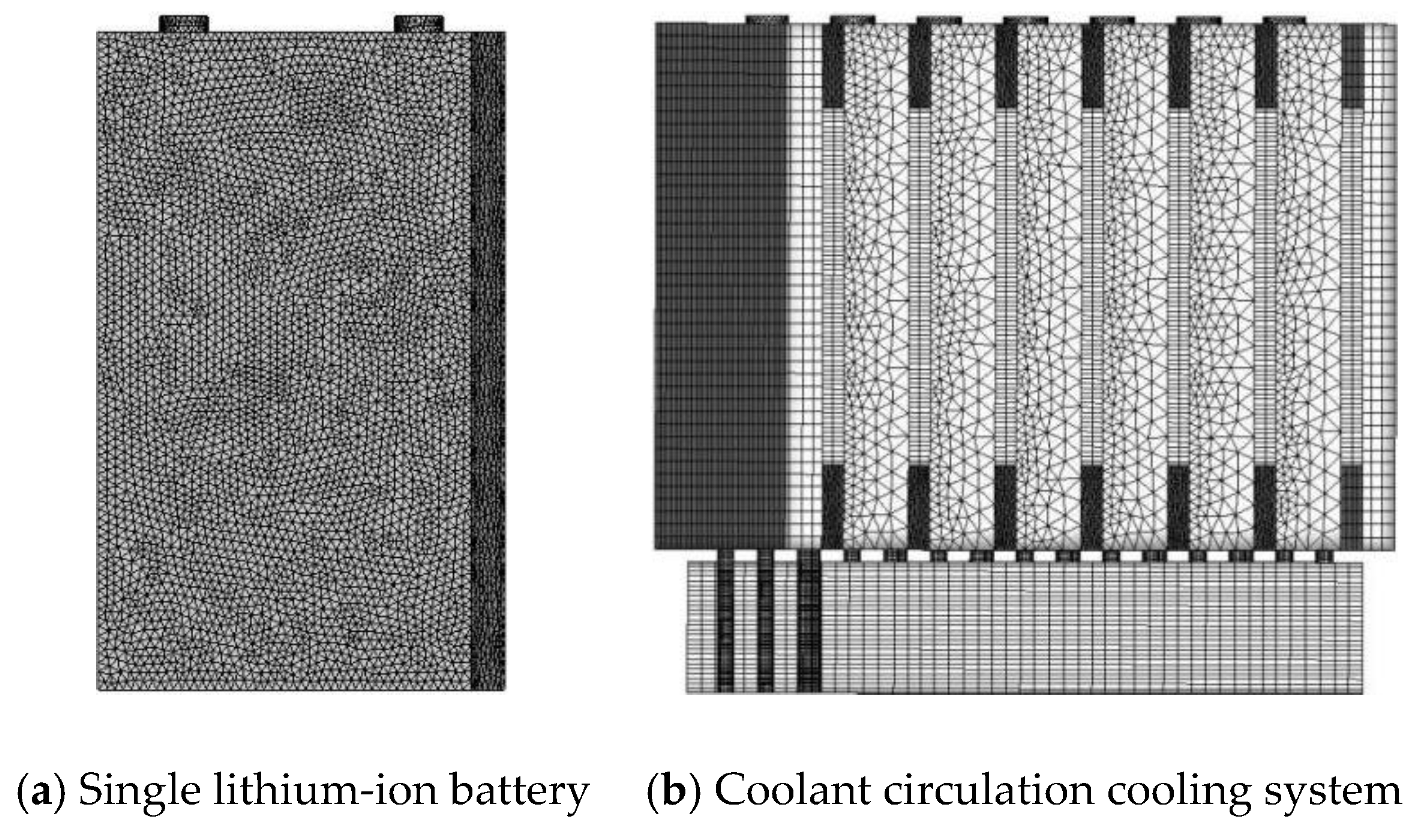
4.1. Simulation Model Design
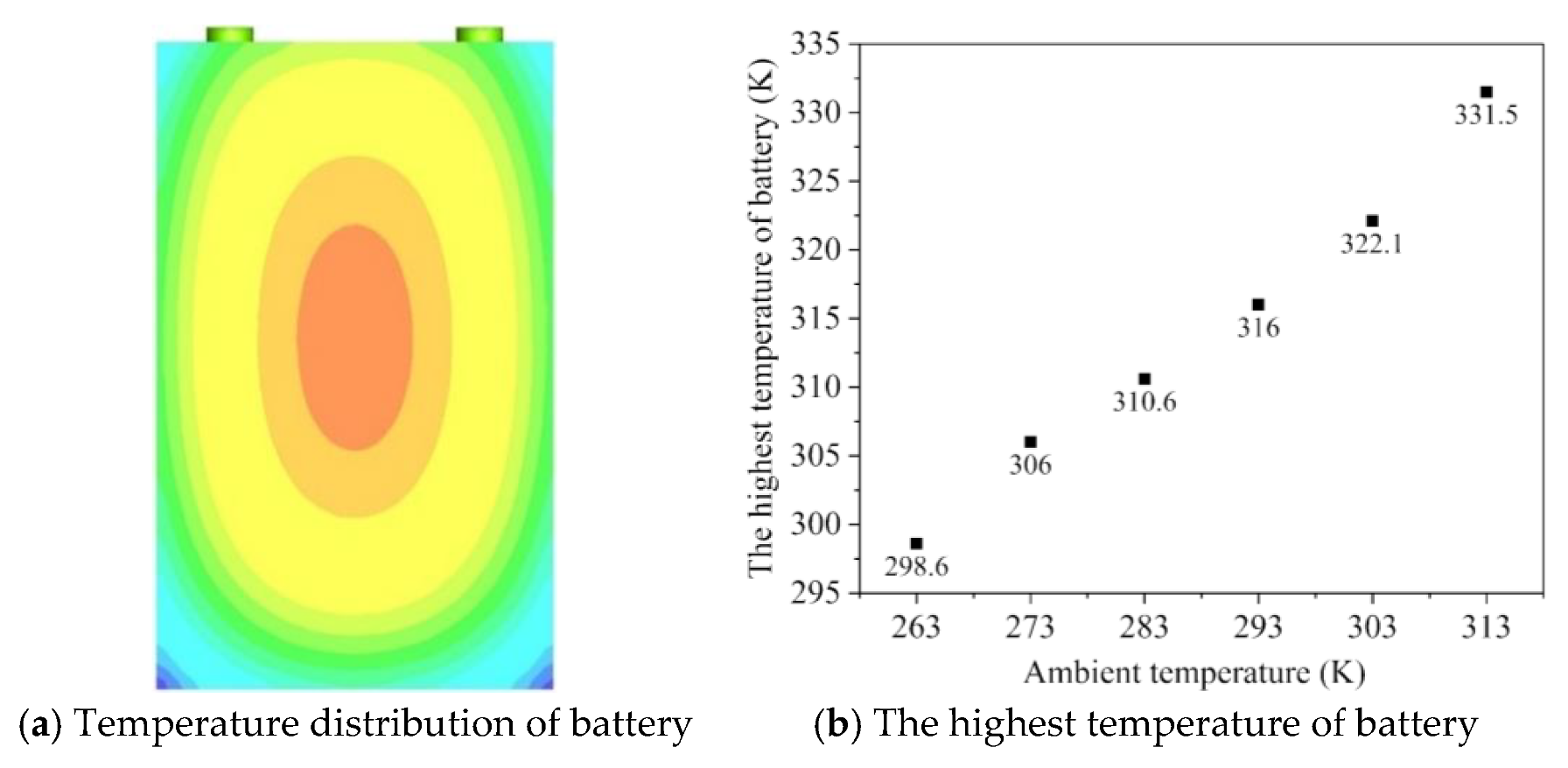
4.2. Simulation of Temperature Rise of Battery Pack in Natural State
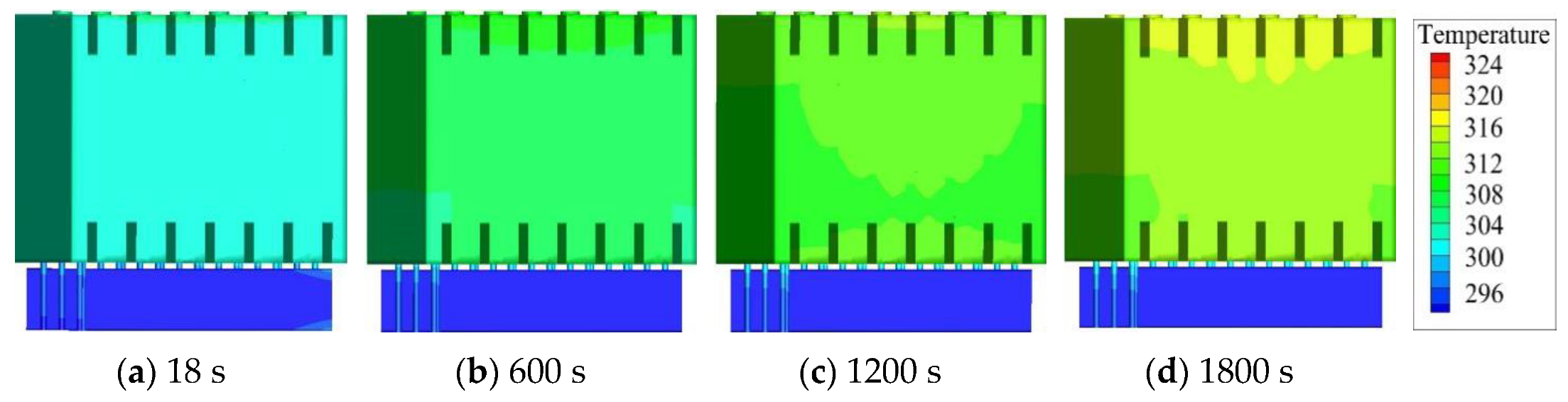
4.3. Simulation with CCCS
4.4. Simulation with Optimized CCCS
5. Discussion and Conclusions
5.1. Discussion
5.1.1. Comparison of Experiment and Simulation Results in the Natural State
5.1.2. Comparison of Experiment and Simulation Results Adding CCCS
5.2. Conclusions
- (1)
- Working in the natural state, a lithium-ion battery pack can discharge within its safe temperature range in the condition where ambient temperature is below 303 K.
- (2)
- Adding the CCCS with coolant not circulating, the temperature of lithium-ion battery pack can be controlled in a reasonable range at the ambient temperature of 303 K. When the CCCS with coolant circulating, the temperature has reduced further, and is still in a safe range until the ambient temperature goes over 313 K.
- (3)
- When the ambient temperature reaches 313 K, the temperature of the battery pack is close to the upper limit of the safe-use range. The CCCS can be optimized by increasing the diameter of the copper rod to reduce the temperature further.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, W.X.; Yang, X.Q.; Zhang, G.Q.; Ke, X.F.; Wang, Z.Y.; Situ, W.F.; Li, X.X.; Zhang, J.Y. An experiment study of thermal management system using copper mesh-enhanced composite phase change materials for power battery pack. Energy 2016, 113, 909–916. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, Z.; Wang, B.; Gao, H.B.; Chen, S.R.; Zong, S.C.; Luo, K.X. A Li-Ion Battery Management System Combining a Heat Pipe and Thermoelectric Cooler. Energies 2020, 13, 841. [Google Scholar] [CrossRef]
- Wang, X.M.; Xie, Y.Q.; Day, R.; Wu, H.W.; Hu, Z.L.; Zhu, J.Q.; Wen, D.S. Performance analysis of a novel thermal management system with composite phase material for a lithium-ion battery pack. Energy 2018, 156, 154–168. [Google Scholar] [CrossRef]
- Wang, Q.S.; Ping, P.; Zhao, X.J. Thermal runaway caused fire and explosion of lithium ion battery. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Feng, X.N.; Lu, L.G.; Ouyang, M.G.; Li, J.Q.; He, X.M. A 3D thermal runaway propagation model for a large format lithium ion battery module. Energy 2016, 115, 194–208. [Google Scholar] [CrossRef]
- Teng, H.; Ma, Y.; Yeow, K.; Thelliez, M. Thermal Characterization of a Li-ion Battery Module Cooled through Aluminum Heat-Sink Plates. Int. J. Passeng. Cars 2011, 4, 1331–1342. [Google Scholar] [CrossRef]
- Wang, T.; Tseng, K.J.; Zhao, J.Y.; Wei, Z.B. Thermal investigation of lithium-ion battery module with different cell arrangement structures and forced air-cooling strategies. Appl. Energy 2014, 134, 229–238. [Google Scholar] [CrossRef]
- Wang, T.; Tseng, K.J.; Zhao, J.Y. Development of efficient air-cooling strategies for lithium-ion battery module based on empirical heat source model. Appl. Therm. Eng. 2015, 90, 521–529. [Google Scholar] [CrossRef]
- Adams, D.T.; Berdichevsky, G.; Colson, T.; Hebert, A.; Kohn, S.; Lyons, D.; Mendez, N.J.; Straubel, J.B.; West, D.; Simpson, A. Battery Pack Thermal Management System. U.S. Patent 20090023056A1, 22 January 2009. [Google Scholar]
- Lan, C.J.; Xu, J.; Qiao, Y.; Ma, Y.B. Thermal management for high power lithium-ion battery by minichannel aluminum tubes. Appl. Therm. Eng. 2016, 101, 284–292. [Google Scholar] [CrossRef]
- Zhao, C.R.; Sousa, A.C.M.; Jiang, F.M. Minimization of thermal non-uniformity in lithium-ion battery pack cooled by channeled liquid flow. Int. J. Heat Mass Transf. 2019, 129, 660–670. [Google Scholar] [CrossRef]
- Jarrett, A.; Kim, I.Y. Design optimization of electric vehicle battery cooling plates for thermal performance. J. Power Sources 2011, 196, 10359–10368. [Google Scholar] [CrossRef]
- Wang, S.N.; Li, Y.H.; Li, Y.Z.; Mao, Y.F.; Zhang, Y.N.; Guo, W.; Zhong, M.L. A forced gas cooling circle packaging with liquid cooling plate for the thermal management of Li-ion batteries under space environment. Appl. Therm. Eng. 2017, 123, 929–939. [Google Scholar] [CrossRef]
- Deng, S.S.; Li, K.N.; Xie, Y.; Wu, C.X.; Wang, P.Z.; Yu, M.; Li, B.; Deng, J.T. Heat Pipe Thermal Management Based on High-Rate Discharge and Pulse Cycle Tests for Lithium-Ion Batteries. Energies 2019, 12, 3143. [Google Scholar] [CrossRef]
- Arora, S.S.; Kapoor, A.; Shen, W.X. A novel thermal management system for improving discharge/charge performance of Li-ion battery packs under abuse. J. Power Source 2018, 378, 759–775. [Google Scholar] [CrossRef]
- Qian, S.T. The Design of Heat Radiation Structure and Simulation of Cooling System for EV’S Li-ion Power Battery. Master’s Thesis, Zhejiang University of Technology, Hangzhou, China, 2013. [Google Scholar]
- Park, H. A design of air flow configuration for cooling lithium ion battery in hybrid electric vehicles. J. Power Sources 2013, 239, 30–36. [Google Scholar] [CrossRef]
- Arun, M.D.; Dean, H.; Krishna, N.M.; Manickam, M. Design, Development and Thermal Analysis of Reusable Li-Ion Battery Module for Future Mobile and Stationary Application. Energies 2020, 13, 1477. [Google Scholar]
- Noboru, S. Thermal behavior analysis of lithium-ion batteries for electric and hybrid vehicles. J. Power Sources 2001, 99, 70–77. [Google Scholar]
- Jiang, C. Research of Thermal Characteristics of Battery Pack in Pure Electric Vehicle. Master’s Thesis, Hefei University of Technology, Hefei, China, 2015. [Google Scholar]
- Bernardi, D.; Pawlikowski, E.; Newman, J. A general balance for battery systems. Electrochem. Soc. 1985, 132, 1. [Google Scholar] [CrossRef]
- Rodrigues, M.T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperature. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, Y.H.; Yang, Y.J.; Lv, W.; Lian, G.; Golberg, D.; Wang, X.; Zhao, X.; Ding, Y. A MoS2/Carbon hybrid anode for high-performance Li-ion batteries at low temperature. Nano Energy 2020, 70, 104550. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhang, N.; Zhang, M. Simulation Analysis of Lithium-ion Battery Thermal Management in EV. J. Therm. Sci. Technol. 2016, 15, 40–45. [Google Scholar]











| Number Order | Item | Specification |
|---|---|---|
| 1 | Size (L × W× H) | 135 × 30 × 220.8 mm |
| 2 | Rated capacity | 72 Ah |
| 3 | Rated voltage | 3.2 V |
| 4 | Normal working voltage | 2.5–3.65 V |
| 5 | Maximum discharging current | 144 A |
| 6 | Safe working temperature | 253–323 K |
| Component | Length (mm) | Thickness (mm) | Height (mm) |
|---|---|---|---|
| Aluminum plate | 135 | 10 | 150 |
| Copper rod | Diameter 6 mm | 245 | |
| Heat sink | 30 | 30 | 25 |
| Water tank | 256 | 135 | 60 |
| Material | Density (kg·m−3) | Specific Heat Capacity (J·kg−1·K−1) | Thermal Conductivity (W·m−1·K−1) |
|---|---|---|---|
| Li-ion Battery | 1990 | 990 | 10 |
| Aluminum Plate | 2719 | 871 | 202 |
| Copper Rod | 7900 | 390 | 401 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Xia, Z.; Gao, H.; Wen, J.; Chen, S.; Dang, M.; Gu, S.; Zhang, J. A Coolant Circulation Cooling System Combining Aluminum Plates and Copper Rods for Li-Ion Battery Pack. Energies 2020, 13, 4296. https://doi.org/10.3390/en13174296
Zhang C, Xia Z, Gao H, Wen J, Chen S, Dang M, Gu S, Zhang J. A Coolant Circulation Cooling System Combining Aluminum Plates and Copper Rods for Li-Ion Battery Pack. Energies. 2020; 13(17):4296. https://doi.org/10.3390/en13174296
Chicago/Turabian StyleZhang, Chuanwei, Zhan Xia, Huaibin Gao, Jianping Wen, Shangrui Chen, Meng Dang, Sujing Gu, and Jianing Zhang. 2020. "A Coolant Circulation Cooling System Combining Aluminum Plates and Copper Rods for Li-Ion Battery Pack" Energies 13, no. 17: 4296. https://doi.org/10.3390/en13174296
APA StyleZhang, C., Xia, Z., Gao, H., Wen, J., Chen, S., Dang, M., Gu, S., & Zhang, J. (2020). A Coolant Circulation Cooling System Combining Aluminum Plates and Copper Rods for Li-Ion Battery Pack. Energies, 13(17), 4296. https://doi.org/10.3390/en13174296




