Zinc Therapy in Early Alzheimer’s Disease: Safety and Potential Therapeutic Efficacy
Abstract
1. Introduction
1.1. Non-ceruloplasmin Copper Typifies a Subset of Alzheimer’s Disease Patients
1.2. Non-ceruloplasmin Copper, Astrocytes and Alzheimer’s Disease Axis
2. The Zinc-copper Connection in Microglial Cell Function and Alzheimer’s Disease
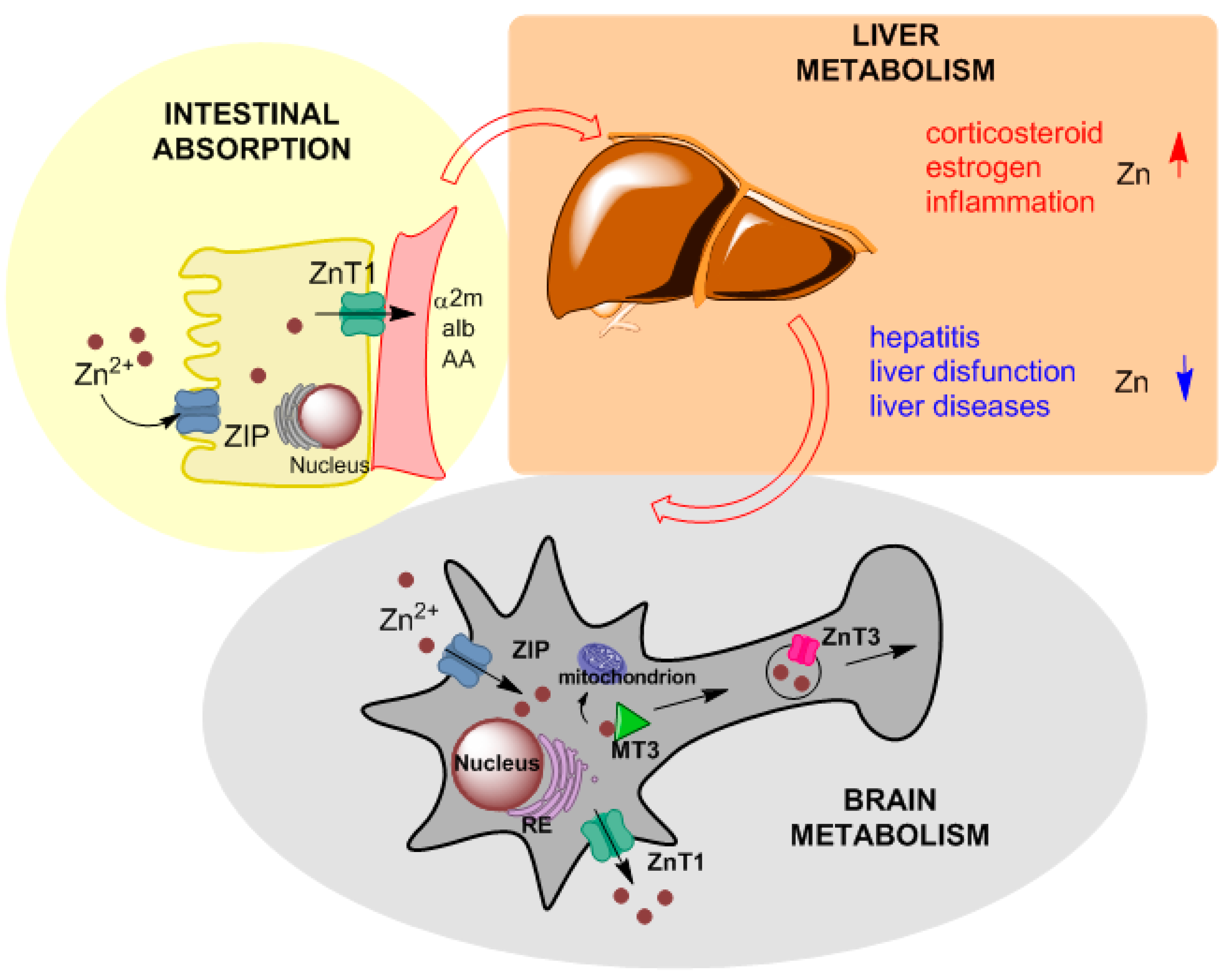
2.1. Zinc in Physiology
2.2. Zinc Clinical Information
3. Zinc Therapy Indications and Dosage
3.1. Rational of Zinc Therapy in Alzheimer’s Disease
3.2. Early Studies on Zinc Therapy in Alzheimer’s Disease
3.3. Zinc Therapy: Dosage, Adverse Drug Reactions and Preventive Measures
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- European Association for Study of The Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.A.; Schilsky, M.L.; American Association for Study of Liver, D. Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhou, R.; Yang, G.; Shi, Y. Analysis of 138 pathogenic mutations in presenilin-1 on the In Vitro production of Abeta42 and Abeta40 peptides by gamma-secretase. Proc. Natl. Acad. Sci. USA 2017, 114, E476–E485. [Google Scholar] [CrossRef]
- Walshe, J.M.; Clinical Investigations Standing Committee of the Association of Clinical Biochemists. Wilson’s disease: The importance of measuring serum caeruloplasmin non-immunologically. Ann. Clin. Biochem. 2003, 40, 115–121. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Masters, C.L.; Selkoe, D.J. Biochemistry of amyloid beta-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006262. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef]
- Kepp, K.P. Ten Challenges of the Amyloid Hypothesis of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 447–457. [Google Scholar] [CrossRef]
- Bush, A.I.; Tanzi, R.E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 2008, 5, 421–432. [Google Scholar] [CrossRef]
- Kepp, K.P. Alzheimer’s disease due to loss of function: A new synthesis of the available data. Prog. Neurobiol. 2016, 143, 36–60. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P.; Squitti, R. Copper imbalance in Alzheimer’s disease: Convergence of the chemistry and the clinic. Coord. Chem. Rev. 2019, 397, 168–187. [Google Scholar] [CrossRef]
- Sensi, S.L.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Volitakis, I.; Adlard, P.A.; Duce, J.A.; Masters, C.L.; Cherny, R.A.; Bush, A.I. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Barbati, G.; Rossi, L.; Ventriglia, M.; Dal Forno, G.; Cesaretti, S.; Moffa, F.; Caridi, I.; Cassetta, E.; Pasqualetti, P.; et al. Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF [beta]-amyloid, and h-tau. Neurology 2006, 67, 76–82. [Google Scholar] [CrossRef]
- Squitti, R.; Ghidoni, R.; Simonelli, I.; Ivanova, I.D.; Colabufo, N.A.; Zuin, M.; Benussi, L.; Binetti, G.; Cassetta, E.; Rongioletti, M.; et al. Copper dyshomeostasis in Wilson disease and Alzheimer’s disease as shown by serum and urine copper indicators. J. Trace Elem. Med. Biol. 2018, 45, 181–188. [Google Scholar] [CrossRef]
- Hoogenraad, T.U. Paradigm shift in treatment of Alzheimer’s disease: Zinc therapy now a conscientious choice for care of individual patients. Int. J. Alzheimers Dis. 2011, 2011, 492686. [Google Scholar] [CrossRef]
- Pal, A.; Prasad, R. Recent discoveries on the functions of astrocytes in the copper homeostasis of the brain: A brief update. Neurotox. Res. 2014, 26, 78–84. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Dewachter, I.; Walter, J.; Klockgether, T.; Van Leuven, F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflammation 2005, 2, 22. [Google Scholar] [CrossRef]
- Olabarria, M.; Noristani, H.N.; Verkhratsky, A.; Rodriguez, J.J. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia 2010, 58, 831–838. [Google Scholar] [CrossRef]
- Frost, G.R.; Li, Y.M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Michaelides, C.; Walker, T.A.; Ekonomou, A.; Suessmilch, M.; Sriskanthanathan, A.; Abraha, S.; Parkes, A.; Parkes, H.G.; Geraki, K.; et al. Regional Distributions of Iron, Copper and Zinc and Their Relationships With Glia in a Normal Aging Mouse Model. Front Aging Neurosci. 2019, 11, 351. [Google Scholar] [CrossRef]
- Bishop, G.M.; Dang, T.N.; Dringen, R.; Robinson, S.R. Accumulation of non-transferrin-bound iron by neurons, astrocytes, and microglia. Neurotox. Res. 2011, 19, 443–451. [Google Scholar] [CrossRef]
- Matyash, V.; Kettenmann, H. Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 2010, 63, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Karch, C.M.; Cruchaga, C.; Goate, A.M. Alzheimer’s disease genetics: From the bench to the clinic. Neuron 2014, 83, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Rebeck, G.W.; Reiter, J.S.; Strickland, D.K.; Hyman, B.T. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron 1993, 11, 575–580. [Google Scholar] [CrossRef]
- Cash, J.G.; Kuhel, D.G.; Basford, J.E.; Jaeschke, A.; Chatterjee, T.K.; Weintraub, N.L.; Hui, D.Y. Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J. Biol. Chem. 2012, 287, 27876–27884. [Google Scholar] [CrossRef]
- Hamon, R.; Homan, C.C.; Tran, H.B.; Mukaro, V.R.; Lester, S.E.; Roscioli, E.; Bosco, M.D.; Murgia, C.M.; Ackland, M.L.; Jersmann, H.P.; et al. Zinc and zinc transporters in macrophages and their roles in efferocytosis in COPD. PLoS ONE 2014, 9, e110056. [Google Scholar] [CrossRef]
- Fredenburgh, J.C.; Leslie, B.A.; Stafford, A.R.; Lim, T.; Chan, H.H.; Weitz, J.I. Zn2+ mediates high affinity binding of heparin to the alphaC domain of fibrinogen. J. Biol. Chem. 2013, 288, 29394–29402. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Fink, J.K.; Hedera, P. Diagnosis and treatment of Wilson’s disease. Semin. Neurol. 1999, 19, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Gower-Winter, S.D.; Levenson, C.W. Zinc in the central nervous system: From molecules to behavior. Biofactors 2012, 38, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Masters, B.A.; Quaife, C.J.; Erickson, J.C.; Kelly, E.J.; Froelick, G.J.; Zambrowicz, B.P.; Brinster, R.L.; Palmiter, R.D. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J. Neurosci. 1994, 14, 5844–5857. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.; Meloni, G.; Talmard, C.; Vasak, M.; Faller, P. Zinc release of Zn-metallothionein-3 induces fibrillar type amyloid-beta aggregates. Metallomics 2010, 2, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Sonois, V.; Delaine, T.; Guilloreau, L.; Gillet, A.; Teissie, J.; Faller, P.; Vasak, M. Metal swap between Zn7-metallothionein-3 and amyloid-beta-Cu protects against amyloid-beta toxicity. Nat. Chem. Biol. 2008, 4, 366–372. [Google Scholar] [CrossRef]
- Ventriglia, M.; Brewer, G.J.; Simonelli, I.; Mariani, S.; Siotto, M.; Bucossi, S.; Squitti, R. Zinc in Alzheimer’s Disease: A Meta-Analysis of Serum, Plasma, and Cerebrospinal Fluid Studies. J. Alzheimers Dis. 2015. [Google Scholar] [CrossRef]
- Corona, C.; Masciopinto, F.; Silvestri, E.; Viscovo, A.D.; Lattanzio, R.; Sorda, R.L.; Ciavardelli, D.; Goglia, F.; Piantelli, M.; Canzoniero, L.M.; et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. 2010, 1, e91. [Google Scholar] [CrossRef]
- Lyubartseva, G.; Parkin, S. Bis(tripyrazol-1-ylmethane)nickel(II) tetra-cyanidonickelate(II) dihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m1530. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, T.; Yu, D.; Feng, W.Y.; Nie, Y.X.; Stoltenberg, M.; Danscher, G.; Wang, Z.Y. Elevation of zinc transporter ZnT3 protein in the cerebellar cortex of the AbetaPP/PS1 transgenic mouse. J. Alzheimers Dis. 2010, 20, 323–331. [Google Scholar] [CrossRef]
- Choi, D.W.; Weiss, J.H.; Koh, J.Y.; Christine, C.W.; Kurth, M.C. Glutamate neurotoxicity, calcium, and zinc. Ann. N. Y. Acad. Sci. 1989, 568, 219–224. [Google Scholar] [CrossRef]
- Koh, J.Y.; Suh, S.W.; Gwag, B.J.; He, Y.Y.; Hsu, C.Y.; Choi, D.W. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 1996, 272, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A. A potential role for alterations of zinc and zinc transport proteins in the progression of Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors 2012, 38, 107–113. [Google Scholar] [CrossRef]
- Hoogenraad, T. T. Wilson’s Disease; Intermed Medical Publishers: Amsterdam/Rotterdam, The Netherlands, 2001. [Google Scholar]
- Avan, A.; de Bie, R.M.A.; Hoogenraad, T.U. Wilson’s Disease Should Be Treated with Zinc rather than Trientine or Penicillamine. Neuropediatrics 2017, 48, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Yuzbasiyan-Gurkan, V.; Grider, A.; Nostrant, T.; Cousins, R.J.; Brewer, G.J. Treatment of Wilson’s disease with zinc: X. Intestinal metallothionein induction. J. Lab. Clin. Med. 1992, 120, 380–386. [Google Scholar] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agron, E.; Launer, L.J.; Grodstein, F.; Bernstein, P.S.; Age-Related Eye Disease Study 2 Research Group. Effect of Omega-3 Fatty Acids, Lutein/Zeaxanthin, or Other Nutrient Supplementation on Cognitive Function: The AREDS2 Randomized Clinical Trial. JAMA 2015, 314, 791–801. [Google Scholar] [CrossRef]
- Hoogenraad, T.U.; van Hattum, J. Treatment of Wilson’s disease. Dtsch. Med. Wochenschr. 1987, 112, 942. [Google Scholar]
- Oelshlegel, F.J., Jr.; Brewer, G.J. Absorption of pharmacologic doses of zinc. Prog. Clin. Biol. Res. 1977, 14, 299–316. [Google Scholar]
- Brewer, G.J.; Dick, R.D.; Johnson, V.D.; Brunberg, J.A.; Kluin, K.J.; Fink, J.K. Treatment of Wilson’s disease with zinc: XV long-term follow-up studies. J. Lab. Clin. Med. 1998, 132, 264–278. [Google Scholar] [CrossRef]
- Alzheimer’s-Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Alzflorum, Network for a Cure. Exposure, Exposure, Exposure? At CTAD, Aducanumab Scientists Make a Case Go to Another Part. Series—Clinical Trials on Alzheimer’s Disease 2019. Available online: https://www.alzforum.org/news/conference-coverage/exposure-exposure-exposure-ctad-aducanumab-scientists-make-case (accessed on 4 May 2020).
- Klein, G.; Delmar, P.; Voyle, N.; Rehal, S.; Hofmann, C.; Abi-Saab, D.; Andjelkovic, M.; Ristic, S.; Wang, G.; Bateman, R.; et al. Gantenerumab reduces amyloid-beta plaques in patients with prodromal to moderate Alzheimer’s disease: A PET substudy interim analysis. Alzheimers Res. Ther. 2019, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Alzforum. Topline Result for First DIAN-TU Clinical Trial: Negative on Primary. Available online: https://www.alzforum.org/news/research-news/toplineresult-first-dian-tu-clinical-trial-negative-primary (accessed on 10 February 2020).
- Lahiri, D.K. There is no Failure, Only Discovery-the Year Ahead for CARving New Paths. Curr. Alzheimer Res. 2020, 17, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Aizenstein, H.J.; Nebes, R.D.; Saxton, J.A.; Price, J.C.; Mathis, C.A.; Tsopelas, N.D.; Ziolko, S.K.; James, J.A.; Snitz, B.E.; Houck, P.R.; et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008, 65, 1509–1517. [Google Scholar] [CrossRef]
- Sperling, R.; Mormino, E.; Johnson, K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron 2014, 84, 608–622. [Google Scholar] [CrossRef]
- Barnard, N.D.; Bush, A.I.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35 (Suppl. S2), S74–S78. [Google Scholar] [CrossRef]
- Drew, S.C.; Barnham, K.J. The heterogeneous nature of Cu2+ interactions with Alzheimer’s amyloid-beta peptide. Acc. Chem. Res. 2011, 44, 1146–1155. [Google Scholar] [CrossRef]
- Drew, S.C.; Noble, C.J.; Masters, C.L.; Hanson, G.R.; Barnham, K.J. Pleomorphic copper coordination by Alzheimer’s disease amyloid-beta peptide. J. Am. Chem. Soc. 2009, 131, 1195–1207. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; La Penna, G. Metal ions and intrinsically disordered proteins and peptides: From Cu/Zn amyloid-beta to general principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef]
- Hureau, C.; Dorlet, P. Coordination of redox active metal ions to the APP protein and to the amyloid-beta peptides involved in alzheimer’s disease. Part2:how cu(II) binding sites depend on changes in abeta sequences. Coord. Chem. Rev. 2012, 256, 2175–2187. [Google Scholar] [CrossRef]
- Kepp, K.P. Alzheimer’s disease: How metal ions define β-amyloid function. Coord. Chem. Rev. 2017, 351, 127–157. [Google Scholar] [CrossRef]
- Squitti, R.; Malosio, M.L.; Rongioletti, M.C.A.; Tecchio, F. Copper involvement in glutamatergic transmission in physiology and disease as revealed by magnetoencephalography/electroencephalography (MEG/EEG) studies. Aging Clin. Exp. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.; Harris, C.; Ralle, M.; Duffy, M.; Murchison, C.; Quinn, J.F. Modulation of tau phosphorylation by environmental copper. Transl. Neurodegener. 2014, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Tan, L.; Wang, H.F.; Ma, J.; Liu, J.; Tan, M.S.; Sun, J.H.; Zhu, X.C.; Jiang, T.; Yu, J.T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimers Dis. 2015, 47, 565–581. [Google Scholar] [CrossRef]
- Constantinidis, J. The hypothesis of zinc deficiency in the pathogenesis of neurofibrillary tangles. Med. Hypotheses 1991, 35, 319–323. [Google Scholar] [CrossRef]
- Constantinidis, J. Hypothesis regarding amyloid and zinc in the pathogenesis of Alzheimer disease: Potential for preventive intervention. Alzheimer Dis. Assoc. Disord. 1991, 5, 31–35. [Google Scholar] [CrossRef]
- Constantinidis, J. Treatment of Alzheimer’s disease by zinc compounds. Drug Dev Res 1992, 27, 1–14. [Google Scholar] [CrossRef]
- Burnet, F.M. A possible role of zinc in the pathology of dementia. Lancet 1981, 1, 186–188. [Google Scholar] [CrossRef]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s Disease: How Far Have We Come in the Clinic? J. Alzheimers Dis. 2018, 62, 1369–1379. [Google Scholar] [CrossRef]
- Potocnik, F.C.; van Rensburg, S.J.; Park, C.; Taljaard, J.J.; Emsley, R.A. Zinc and platelet membrane microviscosity in Alzheimer’s disease. The In Vivo effect of zinc on platelet membranes and cognition. S. Afr. Med. J. 1997, 87, 1116–1119. [Google Scholar] [PubMed]
- Van Rhijn, A.; Macintyre, F.; Corrigan, F.M.; Watt, C.; Ijomah, G.; Skinner, E.R. Plasma lipoprotein profiles and the distribution of high-density lipoprotein subfractions in the elderly: The effect of Alzheimer’s disease and multi-infarct dementia. Biochem. Soc. Trans. 1990, 18, 324. [Google Scholar] [CrossRef] [PubMed]
- Crapper McLachlan, D.R.; Dalton, A.J.; Kruck, T.P.; Bell, M.Y.; Smith, W.L.; Kalow, W.; Andrews, D.F. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337, 1304–1308. [Google Scholar] [CrossRef]
- Squitti, R.; Rossini, P.M.; Cassetta, E.; Moffa, F.; Pasqualetti, P.; Cortesi, M.; Colloca, A.; Rossi, L.; Finazzi-Agro, A. D-penicillamine reduces serum oxidative stress in Alzheimer’s disease patients. Eur. J. Clin. Investig. 2002, 32, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.W.; Bush, A.I.; Mackinnon, A.; Macfarlane, S.; Mastwyk, M.; MacGregor, L.; Kiers, L.; Cherny, R.; Li, Q.X.; Tammer, A.; et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch. Neurol. 2003, 60, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Maylor, E.A.; Simpson, E.E.; Secker, D.L.; Meunier, N.; Andriollo-Sanchez, M.; Polito, A.; Stewart-Knox, B.; McConville, C.; O’Connor, J.M.; Coudray, C. Effects of zinc supplementation on cognitive function in healthy middle-aged and older adults: The ZENITH study. Br. J. Nutr. 2006, 96, 752–760. [Google Scholar]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef]
- Faux, N.G.; Ritchie, C.W.; Gunn, A.; Rembach, A.; Tsatsanis, A.; Bedo, J.; Harrison, J.; Lannfelt, L.; Blennow, K.; Zetterberg, H.; et al. PBT2 rapidly improves cognition in Alzheimer’s Disease: Additional phase II analyses. J. Alzheimers Dis. 2010, 20, 509–516. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Rowe, C.C.; Barnham, K.J.; Cherny, R.; Woodward, M.; Bozinosvski, S.; Salvado, O.; Bourgeat, P.; Perez, K.; Fowler, C.; et al. A randomized, exploratory molecular imaging study targeting amyloid beta with a novel 8-OH quinoline in Alzheimer’s disease: The PBT2-204 IMAGINE study. Alzheimers Dement. 2017, 3, 622–635. [Google Scholar] [CrossRef]
- Albert, M.; Smith, L.A.; Scherr, P.A.; Taylor, J.O.; Evans, D.A.; Funkenstein, H.H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int. J. Neurosci. 1991, 57, 167–178. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Portet, F.; Ousset, P.J.; Visser, P.J.; Frisoni, G.B.; Nobili, F.; Scheltens, P.; Vellas, B.; Touchon, J.; MCI Working Group of the European Consortium on Alzheimer’s Disease. Mild cognitive impairment (MCI) in medical practice: A critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 714–718. [Google Scholar] [CrossRef]
- Delli Pizzi, S.; Punzi, M.; Sensi, S.L.; Alzheimer’s Disease Neuroimaging, I. Functional signature of conversion of patients with mild cognitive impairment. Neurobiol. Aging 2019, 74, 21–37. [Google Scholar] [CrossRef]
- Squitti, R.; Bressi, F.; Pasqualetti, P.; Bonomini, C.; Ghidoni, R.; Binetti, G.; Cassetta, E.; Moffa, F.; Ventriglia, M.; Vernieri, F.; et al. Longitudinal prognostic value of serum "free" copper in patients with Alzheimer disease. Neurology 2009, 72, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Ghidoni, R.; Siotto, M.; Ventriglia, M.; Benussi, L.; Paterlini, A.; Magri, M.; Binetti, G.; Cassetta, E.; Caprara, D.; et al. Value of serum nonceruloplasmin copper for prediction of mild cognitive impairment conversion to Alzheimer disease. Ann. Neurol. 2014, 75, 574–580. [Google Scholar] [CrossRef]
- Czlonkowska, A.; Litwin, T.; Karlinski, M.; Dziezyc, K.; Chabik, G.; Czerska, M. D-penicillamine versus zinc sulfate as first-line therapy for Wilson’s disease. Eur. J. Neurol. 2014, 21, 599–606. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Grover, D.K.; LeClaire, V.; Tseng, M.; Wicha, M.; Pienta, K.; Redman, B.G.; Jahan, T.; Sondak, V.K.; et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000, 6, 1–10. [Google Scholar] [PubMed]
- Linder, M.C.; Houle, P.A.; Isaacs, E.; Moor, J.R.; Scott, L.E. Copper regulation of ceruloplasmin in copper-deficient rats. Enzyme 1979, 24, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Pettingell, W.H.; Multhaup, G.; d Paradis, M.; Vonsattel, J.P.; Gusella, J.F.; Beyreuther, K.; Masters, C.L.; Tanzi, R.E. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 1994, 265, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Cuajungco, M.P.; Lees, G.J. Zinc and Alzheimer’s disease: Is there a direct link? Brain Res. Rev. 1997, 23, 219–236. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Lees, G.J. Zinc metabolism in the brain: Relevance to human neurodegenerative disorders. Neurobiol. Dis. 1997, 4, 137–169. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Cuajungco, M.P. Is zinc the link between compromises of brain perfusion (excitotoxicity) and Alzheimer’s disease? J. Alzheimers Dis. 2005, 8, 155–160. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
| Manifestations | Movement disorder, liver disease, low ceruloplasmin (<200 mg/L), high serum non-ceruloplasmin copper (>100 μg/L),* increased urinary copper (>100 μg/L), Kayser-Fleischer ring |
| Cause of symptoms | Increased non-ceruloplasmin bound (free) copper * (>100 μg/L) |
| Aim of medication | Normalization of serum and urine free copper levels * |
| Choice of medication | Zinc (100–200 mg daily of elemental zinc) |
| Monitoring treatment | Normalization of serum non-ceruloplasmin copper levels * (<100 μg/L), normalization of urinary copper (<100 μg/L) |
| Authors, year | Treatment | Sample Size | Design | Clinical Outcomes |
|---|---|---|---|---|
| Van Rhijn et al. 1990 [75] | Zinc-sulphate and sodium selenite | A total of 15 AD | Dietary supplementation study | Improved performance on the anomalous sentences repetition test, colored progressive matrices, graded naming test and digit copying test |
| Crapper McLachlan et al. 1991 [76] | Desferrioxamine mesylate (metal chelator) | A total of 48 AD | Single-blind trial | Slowing of clinical deterioration, increased activities of daily living in the treatment group |
| Constantinidis 1992 [71] | Zinc-hydrogenaspartate | 5 presenile AD 5 senile AD | Follow-on study | Improved memory, understanding, communication and social interaction in 8 patients |
| Potocnik et al. 1997 [74] | Zinc-methionine | 4 AD | Open-labelled pilot study (12 months) | Improved performance on the cognitive tests |
| Squitti et al. 2002 [77] | D-penicillamine (Copper chelator) | A total of 18 AD (treated: placebo 1:1) | Double-blind, placebo-controlled 6 months trial | No widespread benefit on cognitive outcomes; placebo patients did not worsen in the 24 weeks follow-up; peroxides in serum from patients taking D-penicillamine decreased by 29% with respect to their t0 evaluation found (F1,16 = 4·52; P = 0·049). |
| Ritchie et al. 2003 [78] | Clioquinol | A total of 36 probable AD (treated: placebo 1:1) | Double-blind, placebo-controlled, parallel group randomized 36 weeks study | No effect on cognitive outcomes at any of the time points assessed. Significant improvements in MMSE when the groups were stratified by their level of impairment at baseline; plasma Aβ declined in the treated group and increased in the placebo group |
| Maylor et al. 2006 [79] | Zinc-gluconate (Zenith) | 387 healthy older adults | Randomized double-blind placebo-controlled 6 months study | Few significant benefits in visual memory, working memory, attention and reaction time were obtained using the Cambridge Automated Neuropsychological |
| Lannfelt et al. 2008 [80] | PBT2 (copper/zinc ionophore) | A total of 78 Early AD (treated: placebo 1:1 | Phase II, double-blind, randomized, placebo-controlled 12 months trial | No significant difference in the Neuropsychiatric Test Battery (NTB); dose-dependent reduction of Aβ concentrations in the CSF and positively impacted on two executive function component tests showing significant improvement over placebo in the PBT2 250 mg group: category fluency test (2.8 words, 0.1 to 5.4; p = 0.041) and trail making part B (–48.0 s, –83.0 to –13.0; p = 0.009). |
| Faux et al. 2010 [81] | PBT2 (copper/zinc ionophore) | 40 AD | Phase II double-blind, randomized, placebo-controlled 12 weeks trial | Improvement on NTB Composite or Executive Factor z-scores |
| Brewer 2012 [44] | Zinc (Adeona) 150 mg/day | A total of 42 AD mild-moderate AD patients (treated: placebo 1:1) | Phase II double-blind, randomized, placebo-controlled 12 weeks trial. Primary outcome: ADAS-Cog | No significant effect on primary clinical outcome; post hoc analyses limiting the analysis to those patients aged 70 years and older (14 zinc treated patients vs. 15 placebo patients) revealed statistically significant better cognition scores in the zinc-treated patients vs. controls in ADAS-Cog (p = 0.037) and CDR SOB (P ¼ 0.032), with near significant results in MMSE (p = 0.07) |
| Villemagne et al 2017 [82] | PBT2 (copper/zinc ionophore) | 40 AD (12-month double-blind phase) (placebo = 15, PBT2 = 25), and 27 subjects12-month (placebo = 11, PBT2 = 16) | A randomized, exploratory molecular imaging study targeting amyloid beta with PBT2 in AD, 12-month phase in a double-blind and a 12-month open label extension phase trial design | There was no significant difference between PBT2 and controls at 12 months, likely due to the large individual variances over a relatively small number of subjects |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squitti, R.; Pal, A.; Picozza, M.; Avan, A.; Ventriglia, M.; Rongioletti, M.C.; Hoogenraad, T. Zinc Therapy in Early Alzheimer’s Disease: Safety and Potential Therapeutic Efficacy. Biomolecules 2020, 10, 1164. https://doi.org/10.3390/biom10081164
Squitti R, Pal A, Picozza M, Avan A, Ventriglia M, Rongioletti MC, Hoogenraad T. Zinc Therapy in Early Alzheimer’s Disease: Safety and Potential Therapeutic Efficacy. Biomolecules. 2020; 10(8):1164. https://doi.org/10.3390/biom10081164
Chicago/Turabian StyleSquitti, Rosanna, Amit Pal, Mario Picozza, Abofazl Avan, Mariacarla Ventriglia, Mauro C. Rongioletti, and Tjaard Hoogenraad. 2020. "Zinc Therapy in Early Alzheimer’s Disease: Safety and Potential Therapeutic Efficacy" Biomolecules 10, no. 8: 1164. https://doi.org/10.3390/biom10081164
APA StyleSquitti, R., Pal, A., Picozza, M., Avan, A., Ventriglia, M., Rongioletti, M. C., & Hoogenraad, T. (2020). Zinc Therapy in Early Alzheimer’s Disease: Safety and Potential Therapeutic Efficacy. Biomolecules, 10(8), 1164. https://doi.org/10.3390/biom10081164







