Exercise- and Cold-Induced Human PGC-1α mRNA Isoform Specific Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Testing
2.2. Experimental Trials
2.3. Muscle Biopsies
2.4. mRNA Extraction and cDNA Synthesis
2.5. RT-qPCR
2.6. Statistical Analyses
3. Results
3.1. Experimental Trials
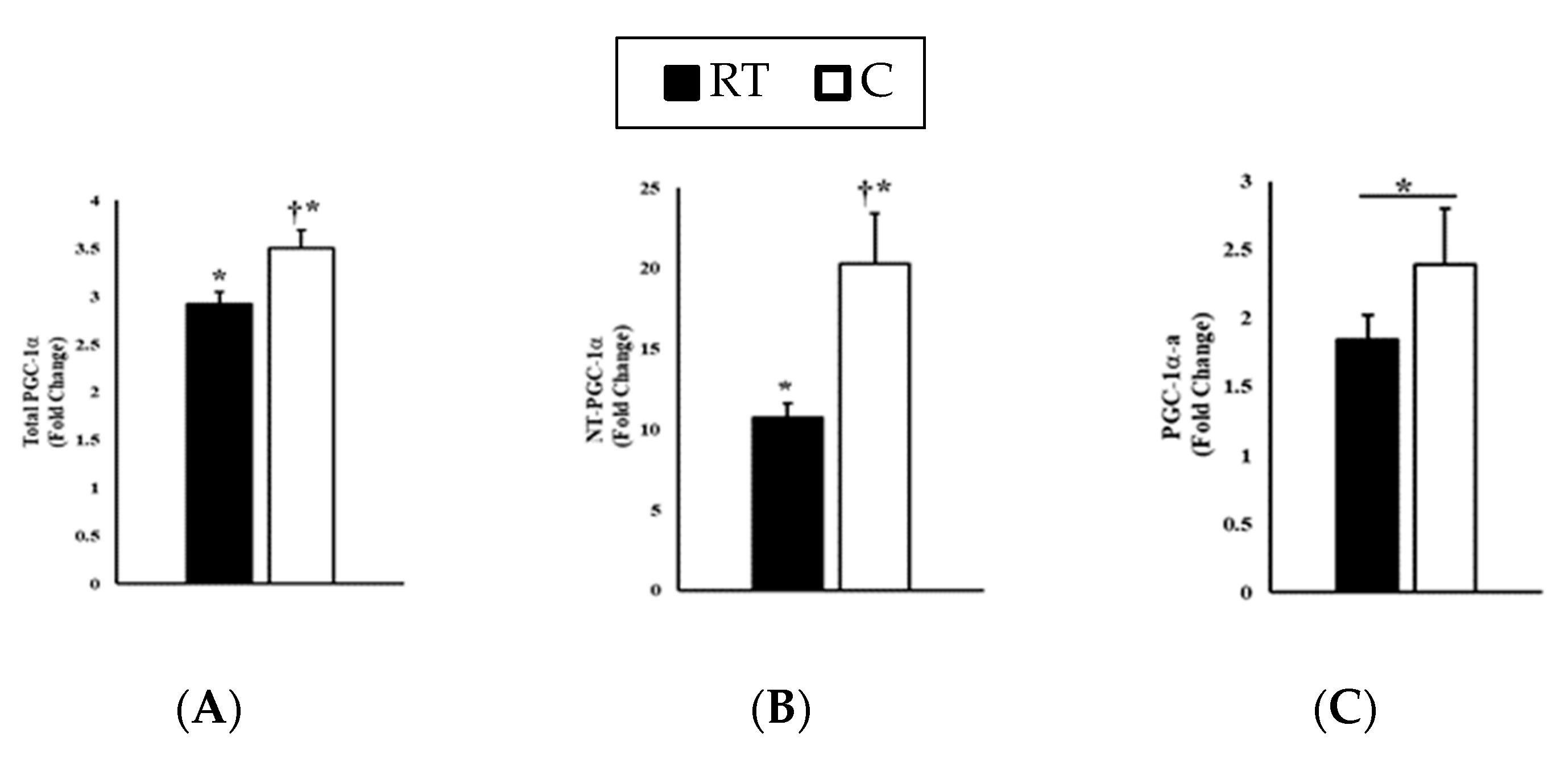
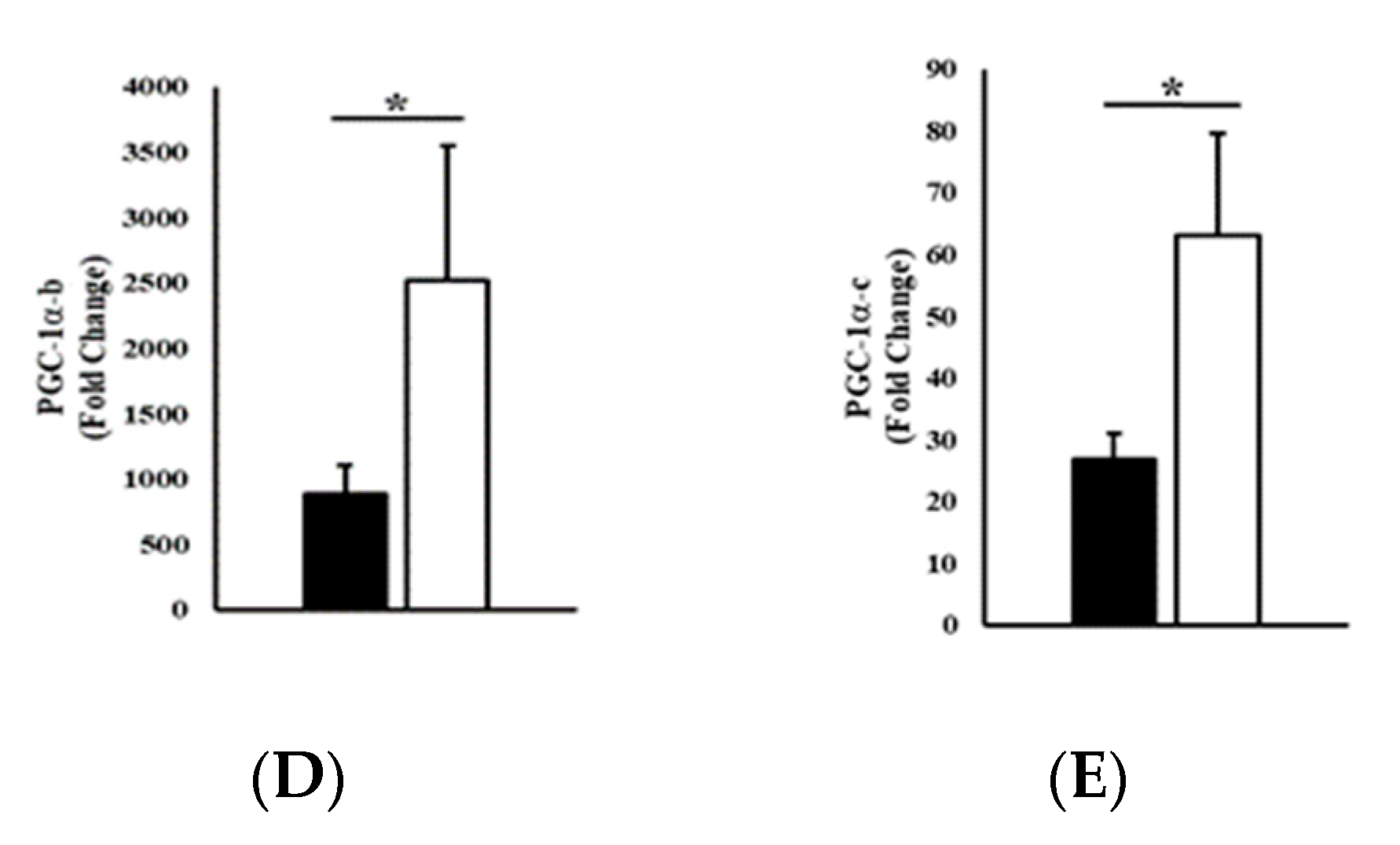
3.2. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Witte, M.; de Vries, H.; van der Valk, P.; Witte, M.; van Horssen, J.; Geurts, J. Mitochondrial dysfunction: A potential link between neuroinflammation and neurodegeneration? Mitochondrion 2010, 10, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Derbré, F.; Tresguerres, J.A.; Gomez-Cabrera, M.C.; Nascimento, A.L.; Gratas-Delamarche, A.; Martinez-Bello, V.E.; Fuentes, T.; Viña, J.; Sanchis-Gomar, F.; Monsalve, M. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1a to exercise training. Age 2012, 34, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Newman, H.; Battino, M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology viaoxidative stress and mitochondrial dysfunction? Periodontology 2000 2014, 64, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Irrcher, I.; Ljubicic, V.; Joseph, A.M. Coordination of metabolic plasticity in skeletal muscle. J. Exp. Biol. 2006, 209, 2265–2275. [Google Scholar] [CrossRef]
- Slivka, D.; Tucker, T.J.; Dumke, C.; Cuddy, J.; Ruby, B. Human mRNA Response to Exercise and Temperature. Int. J. Sports Med. 2012, 33, 94–100. [Google Scholar] [CrossRef]
- Slivka, D.; Dumke, C.; Hailes, W.; Cuddy, J.; Heesch, M.; Ruby, B. Effects of post-exercise recovery in a cold environment on muscle glycogen, PGC-1a, and downstream transcription factors. Cryobiology 2013, 66, 250–255. [Google Scholar] [CrossRef]
- Shute, R.; Heesch, M.; Laursen, T.; Slivka, D. Local Muscle Cooling Does Not Impact Expression of Mitochondrial-Related Genes. J. Therm. Biol. 2017, 67, 35–39. [Google Scholar] [CrossRef]
- Shute, R.; Heesch, M.; Zak, R.; Kreiling, J.; Slivka, D. Effect of Exercise in a Cold Environment on Transcriptional Control of PGC-1a. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R850–R857. [Google Scholar] [CrossRef]
- Opichka, M.; Shute, R.; Marshall, K.; Slivka, D. Effects of Exercise in a cold environment on gene expression for mitochondrial biogenesis and mitophagy. Cryobiology 2019, 90, 47–53. [Google Scholar] [CrossRef]
- Shute, R.; Marshall, K.; Opichka, M.; Schnitzler, H.; Ruby, B.; Slivka, D. Effects of 7 °C environmental temperature acclimation during a 3-week training period. J. Appl. Physiol. 2020, 128, 768–777. [Google Scholar] [CrossRef]
- Martinez-Redondo, V.; Jannig, P.R.; Correia, J.C.; Ferreira, D.M.; Cervenka, I.; Lindvall, J.M.; Sinha, I.; Izadi, M.; Pettersson-Klein, A.T.; Agudelo, L.Z.; et al. Peroxisome Proliferator-activated Receptor gamma Coactivator-1 alpha Isoforms Selectively Regulate Multiple Splicing Events on Target Genes. J. Biol. Chem. 2016, 291, 15169–15184. [Google Scholar] [CrossRef]
- Zhang, Y.; Perwitz, N.; Burk, D.; Henagan, T.; Boudreau, A.; Klein, J.; Gettys, T.; Kralli, A.; Shin, J.; Adamson, A.; et al. Alternative mRNA Splicing Produces a Novel Biologically Active Short Isoform of PGC-1a. J. Biol. Chem. 2009, 284, 32813–32826. [Google Scholar] [CrossRef] [PubMed]
- Thom, R.; Arany, Z.; Thom, R.; Safdar, A.; Jang, C.; Rowe, G. Hypoxic Induction of Vascular Endothelial Growth Factor (VEGF) and Angiogenesis in Muscle by Truncated Peroxisome Proliferator-activated Receptor g Coactivator (PGC)-1a. J. Biol. Chem. 2014, 289, 8810–8817. [Google Scholar] [CrossRef] [PubMed]
- Tadaishi, M.; Miura, S.; Kai, Y.; Kano, Y.; Oishi, Y.; Ezaki, O. Skeletal Muscle-Specific Expression of PGC-1α-b, an Exercise-Responsive Isoform, Increases Exercise Capacity and Peak Oxygen Uptake. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Chang, J.; Wen, X.; Zhang, Y.; Zhang, P.; Zhang, Y.; Wu, J.; Wang, J.; Gettys, T. Effect of exercise intensity on isoform-specific expressions of NT-PGC-1 a mRNA in mouse skeletal muscle. BioMed Res. Int. 2014. [Google Scholar] [CrossRef]
- Miura, S.; Kawanaka, K.; Kai, Y.; Tamura, M.; Goto, M.; Shiuchi, T.; Minokoshi, Y.; Ezaki, O. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology 2007, 148, 3441–3448. [Google Scholar] [CrossRef]
- Miura, S.; Kai, Y.; Kamei, Y.; Ezaki, O. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to beta2-adrenergic receptor activation and exercise. Endocrinology 2008, 149, 4527–4533. [Google Scholar] [CrossRef]
- Tadaishi, M.; Miura, S.; Kawanaka, K.; Koshinaka, K.; Ezaki, O.; Oishi, Y.; Nagata, J.; Kai, Y. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1a mRNA: A role of b2-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E341–E349. [Google Scholar] [CrossRef]
- Thomas, T.R.; Etheridge, G.L. Hydrostatic weighing at residual volume and functional residual capacity. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 49, 157–159. [Google Scholar] [CrossRef]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1980, 9, 480–491. [Google Scholar]
- Leick, L.; Plomgaard, P.; Grønløkke, L.; AlAbaiji, F.; Wojtaszewski, J.; Pilegaard, H. Endurance exercise induces mRNA expression of oxidative enzymes in human skeletal muscle late in recovery. Scand. J. Med. Sci. Sports 2010, 20, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen, M.; Kyröläinen, H.; Ahtiainen, J.; Hulmi, J.; Laine, T.; Häkkinen, K.; Nindl, B.; Selänne, H.; Kainulainen, H.; Taipale, R.; et al. PGC-1 isoforms and their target genes are expressed differently in human skeletal muscle following resistance and endurance exercise. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real Time Quantitative PCR and the 2-DCT. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.; Jensen, J.; Ørntoft, T. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.M.; Sessler, D.I.; Birnbauer, F.; Illievich, U.M.; Spiss, C.K. Thermoregulatory Vasoconstriction Impairs Active Core Cooling. Anesthesiology 1995, 82, 870–876. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef]
- Olesen, J.; Kiilerich, K.; Pilegaard, H. PGC-1α-mediated adaptations in skeletal muscle. Pflüg. Arch. Eur. J. Physiol. 2010, 460, 153–162. [Google Scholar] [CrossRef]
- Chang, J.S.; Fernand, V.; Zhang, Y.; Shin, J.; Jun, H.J.; Joshi, Y.; Gettys, T.W. NT-PGC-1alpha protein is sufficient to link beta3-adrenergic receptor activation to transcriptional and physiological components of adaptive thermogenesis. J. Biol. Chem. 2012, 287, 9100–9111. [Google Scholar] [CrossRef]
- Popov, D.V.; Bachinin, A.V.; Lysenko, E.A.; Miller, T.F.; Vinogradova, O.L. Exercise-induced expression of peroxisome proliferator-activated receptor γ coactivator-1α isoforms in skeletal muscle of endurance-trained males. J. Physiol. Sci. 2014, 5, 317–323. [Google Scholar] [CrossRef]
- Ydfors, M.; Fischer, H.; Mascher, H.; Blomstrand, E.; Norrbom, J.; Gustafsson, T. The truncated splice variants, NT-PGC-1alpha and PGC-1alpha4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol. Rep. 2013, 1. [Google Scholar] [CrossRef]
- Nader, G.A.; von Walden, F.; Liu, C.; Lindvall, J.; Gutmann, L.; Pistilli, E.E.; Gordon, P.M. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J. Appl. Physiol. (1985) 2014, 116, 693–702. [Google Scholar] [CrossRef] [PubMed]
| Variable | Participants |
|---|---|
| Age (y) | 24.3 ± 5.7 |
| Height (m) | 179.4 ± 5.4 |
| Weight (kg) | 82.5 ± 13.9 |
| Body Composition (%) | 18.5 ± 6.2 |
| VO2 peak (mL/kg/min) | 45.6 ± 7.2 |
| Transcript | Strand | Sequence |
|---|---|---|
| Total PGC-1α | FWD | AGCCTCTTTGCCCAGATCTT |
| RVS | GGCAATCCGTCTTCATCCAC | |
| Total NT-PGC-1α | FWD | TCACACCAAACCCACAGAGA |
| RVS | CTGGAAGATATGGCACAT | |
| PGC-1α-a | FWD | ATGGAGTGACATCGAGTGTGCT |
| RVS | GAGTCCACCCAGAAAGCTGT | |
| PGC-1α-b | FWD | CTATGGATTCAATTTTGAAATGTGC |
| RVS | CTGATTGGTCACTGCACCAC | |
| PGC-1α-c | FWD | TGAAAGTGAGTATCAGGAGGCA |
| RVS | CTGATTGGTCACTGCACCAC | |
| GAPDH | FWD | ACATCGCTCAGACACCATG |
| RVS | TGTAGTTGAGGTCAATGAAGGG | |
| B2M | FWD | GGACTGGTCTTTCTATCTCTTGT |
| RVS | ACCTCCATGATGCTGCTTAC | |
| RPS18 | FWD | GTTCCAGCATATTTTGCGAGT |
| RVS | GTCAATGTCTGCTTTCCTCAAC |
| Variable | RT | C |
|---|---|---|
| Chamber Temperature (°C) | 20.1 ± 0.2 | −1.7 ± 1.5 * |
| Chamber Humidity (%) | 66.8 ± 4.2 | 71.7 ± 6.6 |
| Core Temperature (°C) | 38.41 ± 0.48 | 39.04 ± 0.90 * |
| Skin Temperature (°C) | 33.2 ± 1.4 | 28.1 ± 1.4 * |
| Sweat Rate (L/min) | 0.86 ± 0.21 | 0.47 ± 0.38 * |
| HR (bpm) | 160 ± 16 | 155 ± 13 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larson, C.; Opichka, M.; McGlynn, M.L.; Collins, C.W.; Slivka, D. Exercise- and Cold-Induced Human PGC-1α mRNA Isoform Specific Responses. Int. J. Environ. Res. Public Health 2020, 17, 5740. https://doi.org/10.3390/ijerph17165740
Larson C, Opichka M, McGlynn ML, Collins CW, Slivka D. Exercise- and Cold-Induced Human PGC-1α mRNA Isoform Specific Responses. International Journal of Environmental Research and Public Health. 2020; 17(16):5740. https://doi.org/10.3390/ijerph17165740
Chicago/Turabian StyleLarson, Camille, Megan Opichka, Mark L. McGlynn, Christopher W. Collins, and Dustin Slivka. 2020. "Exercise- and Cold-Induced Human PGC-1α mRNA Isoform Specific Responses" International Journal of Environmental Research and Public Health 17, no. 16: 5740. https://doi.org/10.3390/ijerph17165740
APA StyleLarson, C., Opichka, M., McGlynn, M. L., Collins, C. W., & Slivka, D. (2020). Exercise- and Cold-Induced Human PGC-1α mRNA Isoform Specific Responses. International Journal of Environmental Research and Public Health, 17(16), 5740. https://doi.org/10.3390/ijerph17165740






